#Oxidative stress
Explore tagged Tumblr posts
Text
[oxygen atom voice] I'm essential to survival! I keep your cells healthy! But if you're not careful, I will also rip those cells apart looking for stealable electrons. Tee Hee! Best of luck, bitch.
#shitpost#shitposting#reactive oxygen species#oxidative stress#bad science#not really though#this is extremely sound science
8 notes
·
View notes
Text
youtube
#Spinal cord injury#neuroregeneration#nanomedicine#mitochondrial therapy#silica nanoparticles#dual-responsive nanoparticles#targeted drug delivery#neuroprotection#regenerative medicine#oxidative stress#inflammation control#apoptosis inhibition#cellular repair#tissue regeneration#advanced therapeutics#biomaterials#neuroscience research#precision medicine#theranostics#medical breakthrough.#Youtube
2 notes
·
View notes
Text
Will Humans Soon Live to 200? A Glimpse into the Future of Longevity. Humanity has always harbored a fascination with longevity, dreaming of a future where the bounds of life extend far beyond our current expectations. Recent scientific advancements suggest that this dream may soon transform into reality.
#Rapamycin#future#Metformindiet#Telomere#Oxidative Stress#Scienceexciseaging#DNA#InflammationAnti-#aging
2 notes
·
View notes
Text
Reclaim Vitality: The Science Behind Mitochondrial Biogenesis
Mitochondrial biogenesis is the cellular process of increasing the number of mitochondria, the organelles responsible for generating energy. This process is essential for maintaining cellular health and vitality, particularly in tissues with high energy demands, such as muscles. Mitochondrial biogenesis is often triggered by increased energy demand, usually resulting from exercise, caloric restriction, or the intake of specific nutrients.
Mitochondria are the energy producers of the cell, generating ATP, the energy currency of the cell, through oxidative phosphorylation. As cells face greater energy demands, they need more mitochondria to meet these requirements efficiently. The increase in mitochondrial numbers allows cells to produce more energy and better adapt to stress, thus enhancing overall health, recovery, and performance.
Key Factors Involved in Mitochondrial Biogenesis
Several molecular regulators drive mitochondrial biogenesis, with the most important being:
PGC-1α ActivationPGC-1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha) is recognized as the master regulator of mitochondrial biogenesis. This protein plays a pivotal role in controlling the transcription of nuclear genes that encode mitochondrial proteins. When activated by external stimuli like exercise, PGC-1α interacts with transcription factors like NRF-1 and NRF-2 to drive the production of new mitochondria. This results in increased mitochondrial DNA (mtDNA) replication and the synthesis of mitochondrial proteins necessary for energy production and cellular respiration.
AMPK & SirtuinsAMPK (AMP-activated protein kinase) is another critical regulator that responds to low energy levels within the cell (a high AMP ratio). It activates PGC-1α, which, in turn, increases the number of mitochondria. AMPK is activated during energy-demanding activities such as endurance exercise and fasting. Sirtuins (SIRT1) are a class of NAD+-dependent enzymes that also regulate mitochondrial biogenesis. Sirtuins, especially SIRT1, deacetylate PGC-1α, further activating it to promote the transcription of mitochondrial genes. Both AMPK and sirtuins respond to energy deprivation, whether through physical exertion or caloric restriction, helping cells increase energy efficiency and prolong cellular longevity.
Antioxidant Defense and Cellular ResilienceOne of the benefits of mitochondrial biogenesis is the enhancement of cellular resilience through improved antioxidant defences. Mitochondria are not only energy producers but also sources of reactive oxygen species (ROS), which can damage cells if not adequately managed. By increasing the number of healthy mitochondria, cells improve their ability to manage oxidative stress. New mitochondria are typically more efficient at energy production and less likely to produce excess ROS, reducing overall cellular damage. This process helps to protect cells from age-related decline and stress-induced damage.
How Mitochondrial Biogenesis Impacts Health and Performance
Mitochondrial biogenesis is essential for maintaining optimal energy production, particularly during periods of increased physical activity or stress. In muscle cells, the increased number of mitochondria leads to improved ATP generation, enhancing endurance and reducing fatigue during prolonged exercise. This is particularly important for athletes or individuals who engage in regular physical activity, as their muscles require a constant supply of energy for performance and recovery.
For general health, mitochondrial biogenesis supports metabolic efficiency and longevity. In metabolic disorders like type 2 diabetes and obesity, mitochondrial dysfunction often results in impaired energy metabolism and increased oxidative stress. By promoting mitochondrial biogenesis, cells can restore normal mitochondrial function, improving insulin sensitivity and energy balance. Furthermore, mitochondrial biogenesis may help reduce the risk of chronic diseases related to ageing by maintaining cellular energy production and reducing oxidative stress.
Beyond exercise and metabolic health, mitochondrial biogenesis is also a key factor in the body’s ability to adapt to various stressors, whether environmental or nutritional. The increase in mitochondrial capacity allows cells to better handle changes in energy demand, supporting recovery and cellular adaptation. For instance, during periods of caloric restriction, mitochondrial biogenesis helps the body use energy more efficiently, contributing to longer-term health benefits, including improved longevity and resistance to age-related diseases.
Supporting Mitochondrial Biogenesis with Nutraceuticals
In addition to lifestyle factors like exercise and caloric restriction, certain nutraceuticals can support mitochondrial biogenesis. Mitokatlyst™-E is one such product that targets mitochondrial function, optimising energy production, and promoting muscle recovery. By stimulating the molecular pathways involved in mitochondrial biogenesis, such products can enhance the body’s ability to adapt to stress, recover more efficiently, and improve overall cellular function.
Conclusion
Mitochondrial biogenesis is a vital process that supports energy production, cellular health, and adaptability to environmental and physical stressors. By regulating pathways such as PGC-1α, AMPK, and sirtuins, cells can increase mitochondrial content to meet higher energy demands, promote muscle recovery, and improve overall vitality. Products like Mitokatlyst™-E are designed to optimise mitochondrial function, helping the body adapt to stress and maintain optimal cellular health. By supporting mitochondrial biogenesis, we can improve energy efficiency, enhance physical performance, and promote long-term health and resilience.
#Mitochondrial biogenesis#Energy production#Cellular health#ATP generation#PGC-1α activation#AMPK activation#Sirtuins (SIRT1)#Antioxidant defense#Oxidative stress#Mitochondrial function#Muscle recovery#Physical performance#Metabolic efficiency#Insulin sensitivity#Nutraceuticals#Mitokatlyst™-E#Cellular resilience#Longevity#Endurance#Stress adaptation
1 note
·
View note
Text
Mammals are protected from oxidative stress through a series of elaborate prevention, interception and repair defence mechanisms (figure 12.20).
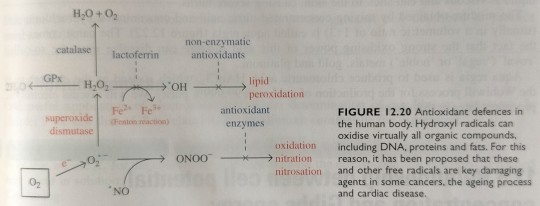
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#mammals#protection#oxidation#oxidative stress#prevention#interception#repair#defence mechanism#catalase#lactoferrin#antioxidant#lipid#peroxidation#enzymes#fenton reaction#superoxide dismutase#nitration#nitrosation#radicals
3 notes
·
View notes
Text
https://www.h-h-c.com/sleep-deprivation-and-oxidative-stress-hidden-health-risks/
0 notes
Text
The Role of Antioxidants in Skincare

Skincare is more than just moisturizing and cleansing—it’s about protecting your skin from the inside out. Every day, your skin is exposed to pollution, UV rays, and environmental toxins, all of which generate free radicals—unstable molecules that cause oxidative stress, collagen breakdown, and premature aging.
Antioxidants are powerful compounds that neutralize free radicals, protecting skin from damage and helping maintain a youthful, radiant appearance. Found in natural bar soaps, methylene blue skincare, face creams, and organic lip balms, antioxidants provide essential anti-aging and skin-repairing benefits.
Methylene blue is emerging as one of the most potent antioxidants, with studies showing its ability to enhance mitochondrial function, boost collagen production, and slow down visible signs of aging. Integrating antioxidant-rich skincare into a clean beauty routine ensures that skin stays protected, hydrated, and resilient.
This guide will explore how antioxidants work, why they’re essential for healthy skin, and how to incorporate them into your daily routine. From skincare gift sets to eco-friendly cleaning products, Nobiesse offers luxury, science-backed formulations that deliver effective antioxidant protection while maintaining a commitment to clean beauty and sustainability.
What Are Antioxidants and How Do They Work?
Antioxidants are natural molecules that prevent oxidative stress by neutralizing free radicals—unstable compounds produced by sun exposure, pollution, and environmental toxins. When free radicals build up, they damage skin cells, break down collagen, and accelerate the aging process.
Incorporating antioxidant-rich skincare products into your routine helps protect against these effects while promoting long-term skin health. Some of the most effective antioxidants include:
Methylene blue – A powerful, stable antioxidant that enhances cellular energy and supports collagen synthesis.
Vitamin C – Brightens skin and reduces hyperpigmentation caused by oxidative stress.
Vitamin E – Strengthens the skin barrier, locking in moisture and protecting against UV damage.
Coenzyme Q10 – Reduces fine lines and wrinkles while enhancing cell regeneration.
By pairing methylene blue skincare, organic lip balms, and antioxidant-rich face creams, you create a strong defense against premature aging. Additionally, reducing exposure to toxins found in household products—such as eco-friendly cleaning products, non-toxic laundry detergents, and fluoride-free toothpaste—minimizes oxidative damage, further preserving skin health and overall well-being.
The Link Between Antioxidants and Skin Aging
Aging is a natural process, but external factors like pollution, sun exposure, and stress can accelerate it. Free radicals weaken the skin’s structural proteins, leading to loss of elasticity, fine lines, and hyperpigmentation.
Antioxidants help counteract these effects, preventing oxidative damage and maintaining skin resilience. Key benefits include:
Reducing wrinkles and fine lines – By neutralizing free radicals, antioxidants slow down the breakdown of collagen and elastin.
Improving hydration and skin texture – A strong antioxidant barrier helps retain moisture, keeping skin smooth and plump.
Protecting against UV-induced damage – Some antioxidants, like methylene blue and vitamin C, provide natural photoprotection, minimizing sun-related aging.
Incorporating methylene blue face creams, fragrance-free deodorants, and antioxidant skincare gift sets into your routine enhances long-term skin health. Additionally, using eco-friendly cleaning products and non-toxic laundry detergents helps reduce overall environmental stressors, further slowing the aging process.
Why Methylene Blue is a Revolutionary Antioxidant
Among all antioxidants, methylene blue stands out for its ability to enhance skin longevity, repair cellular damage, and boost energy production at a molecular level. Unlike traditional antioxidants, methylene blue remains stable, providing long-lasting protection against oxidative stress.
Key Benefits of Methylene Blue in Skincare:
Stimulates collagen synthesis, reducing fine lines and sagging skin.
Enhances mitochondrial function, improving cellular repair and hydration.
Protects against environmental damage, neutralizing pollutants and toxins before they impact skin health.
When used in methylene blue cream, natural bar soaps, and organic lip balms, this ingredient delivers potent anti-aging and antioxidant benefits. Nobiesse’s methylene blue skincare formulations combine luxury with science, offering a clean, effective approach to skin protection and repair.
How to Build an Antioxidant-Rich Skincare Routine
To maximize antioxidant benefits, your skincare routine should focus on hydration, protection, and renewal.
Step-by-Step Antioxidant Routine:
Cleanse with a natural bar soap – Removes dirt while preserving the skin’s natural moisture barrier.
Apply a methylene blue face cream – Provides hydration and antioxidant protection against free radicals and pollution.
Use an organic lip balm – Shields lips from oxidative stress, dryness, and sun damage.
Switch to fragrance-free deodorants – Prevents irritation and eliminates exposure to synthetic chemicals.
Choose fluoride-free toothpaste – Reduces chemical intake and supports oral health.
By incorporating methylene blue skincare, non-toxic personal care products, and clean beauty essentials, you protect your skin from everyday stressors while enhancing long-term radiance and health.
Antioxidants and Men’s Skincare
While skincare is often marketed toward women, men's skincare is equally important. The skin is constantly exposed to pollution, sweat, and shaving-related irritation, all of which contribute to oxidative stress and premature aging. Antioxidants are essential for neutralizing these stressors and maintaining healthy, resilient skin.
Men’s skin tends to be thicker and oilier than women’s, which means it benefits from antioxidants that balance oil production while protecting against free radicals. A simple, antioxidant-rich routine includes:
Cleansing with a natural bar soap – Removes dirt and excess oil without stripping moisture.
Applying a methylene blue face cream – Provides deep hydration and anti-aging protection.
Using a fragrance-free deodorant – Eliminates odor without clogging pores with synthetic chemicals.
Incorporating organic lip balm – Keeps lips soft and protected from environmental stressors.
Men who shave regularly can also benefit from antioxidant skincare, as free radicals can worsen razor burn, inflammation, and dryness. Using eco-friendly cleaning products at home further reduces exposure to skin-irritating toxins, ensuring a holistic approach to skincare and wellness.
By integrating antioxidants into a clean beauty routine, men can experience clearer, healthier, and more youthful-looking skin for years to come.
Antioxidants for Hair and Scalp Health
Antioxidants aren’t just for skin health—they also play a crucial role in scalp and hair care. The scalp is an extension of the skin, meaning it is equally vulnerable to oxidative stress, pollution, and product buildup. Over time, free radical damage can lead to weakened hair follicles, dryness, and premature graying.
Using an antioxidant-rich haircare routine helps:
Protect against environmental pollutants, reducing scalp inflammation.
Strengthen hair follicles, promoting healthier, fuller hair growth.
Prevent oxidative stress, slowing hair thinning and early graying.
A natural shampoo bar infused with botanical antioxidants is ideal for cleansing the scalp without the harsh sulfates found in many commercial shampoos. Pairing this with a non-toxic laundry detergent helps prevent chemical residues from lingering on pillowcases, which can irritate the scalp and skin.
Methylene blue is especially beneficial for scalp health, as it improves circulation, hydration, and follicle strength. When combined with other antioxidants found in face creams, organic lip balms, and Skincare bundle sets, it creates a holistic, antioxidant-rich approach to hair and skin longevity.
The Role of Antioxidants in Lip Care
Lips are one of the most exposed and delicate areas of the skin, yet they are often overlooked in skincare routines. Constant exposure to UV rays, wind, and pollution leads to oxidative damage, dryness, and premature aging. Antioxidants are essential for protecting, hydrating, and repairing lip tissue.
How Antioxidants Benefit Lip Health:
Neutralize free radicals, preventing chapping, fine lines, and discoloration.
Boost moisture retention, keeping lips plump and hydrated.
Repair damaged skin, restoring smoothness and elasticity.
Using an organic lip balm infused with antioxidants—such as methylene blue, vitamin E, and shea butter—provides long-lasting hydration and environmental protection. Many commercial lip balms contain petroleum-based ingredients, which only provide temporary relief without true nourishment.
Pairing antioxidant lip care with a toxin-free lifestyle—including eco-friendly cleaning products, non-toxic laundry detergents, and fragrance-free deodorants—ensures that your entire routine supports overall skin health.
By integrating methylene blue skincare and antioxidant-rich organic lip balms, you create a complete defense against premature aging and environmental stressors.
Why Eco-Friendly Cleaning Products Matter for Skincare
Your skincare routine doesn’t stop at face creams and serums—the products you use in your home also affect your skin’s health. Many household cleaners contain harsh chemicals, synthetic fragrances, and toxic preservatives, which can irritate the skin, trigger allergies, and increase oxidative stress.
Switching to eco-friendly cleaning products minimizes chemical exposure, allowing antioxidants to work more effectively in repairing and protecting the skin. Common offenders in traditional cleaning products include:
Artificial fragrances, which can trigger skin irritation and respiratory issues.
Sulfates and harsh detergents, found in non-natural dish soaps and laundry detergents.
Parabens and preservatives, which can disrupt the skin’s natural barrier.
A holistic clean beauty lifestyle includes:
Using an eco-friendly dishwasher detergent, ensuring no chemical residues remain on your dishes.
Switching to non-toxic laundry detergent, preventing harsh additives from irritating skin through clothing.
Using a natural bar soap and fragrance-free deodorant, reducing exposure to synthetic skin irritants.
By reducing environmental toxins, you enhance the effectiveness of antioxidant skincare, keeping your skin healthy, balanced, and protected.
Skincare Gift Sets: The Best Way to Introduce Antioxidants
For those new to clean beauty and antioxidants, skincare gift sets offer a curated selection of high-performance, toxin-free essentials designed to nourish and protect the skin. These sets take the guesswork out of choosing products, making them perfect for:
Gifting loved ones who want to switch to clean beauty.
Building a complete antioxidant-rich skincare routine.
Exploring methylene blue skincare and its benefits.
A high-quality skincare gift set should include:
A methylene blue face cream, delivering antioxidant hydration and protection.
A natural bar soap, gently cleansing without disrupting the skin’s moisture barrier.
An organic lip balm, ensuring lips remain hydrated and shielded from oxidative stress.
Nobiesse’s luxury skincare gift sets provide an effortless way to transition into clean, antioxidant-rich beauty, ensuring glowing, healthy skin without exposure to harmful chemicals.
The Future of Antioxidants in Skincare
The skincare industry is continuously evolving, and antioxidants are leading the future of anti-aging science. As more research emerges, we are seeing advancements in antioxidant technology, sustainability, and clean beauty formulations.
What’s Next for Antioxidants in Skincare?
More stable and potent formulations – Ingredients like methylene blue are revolutionizing skincare by offering long-lasting, science-backed protection.
Integration of antioxidants into home care – More brands are incorporating antioxidant protection into non-toxic laundry detergents, kitchen hand soaps, and eco-friendly cleaning products.
Sustainability and biodegradable packaging – Clean beauty is moving toward more environmentally friendly formulations, reducing waste while ensuring optimal skin benefits.
Nobiesse is at the forefront of this antioxidant revolution, offering luxury, non-toxic skincare solutions that prioritize both performance and sustainability.
How to Maximize the Benefits of Antioxidants in Your Routine
Using antioxidant-rich skincare is a great step toward healthier, younger-looking skin, but to get the most out of these powerful ingredients, it’s essential to follow key best practices.
Tips for Maximizing Antioxidant Benefits:
Layer Your Antioxidants Correctly – Apply antioxidant serums or methylene blue face cream before sunscreen to enhance UV protection.
Use a Gentle Cleanser – Harsh cleansers strip the skin’s natural oils, making antioxidants less effective. A natural bar soap cleanses without disrupting the moisture barrier.
Stay Consistent – Antioxidants work best when used daily. Incorporate an organic lip balm, fragrance-free deodorant, and face cream into your routine for all-day protection.
Avoid Oxidative Stress – Reduce exposure to pollutants and synthetic chemicals by switching to eco-friendly cleaning products, non-toxic laundry detergent, and fluoride-free toothpaste.
Eat Antioxidant-Rich Foods – Pairing topical antioxidants with a nutrient-dense diet enhances skin repair and hydration from within.
By combining high-performance skincare with a clean lifestyle, you amplify the benefits of antioxidants, ensuring your skin remains radiant, resilient, and well-protected against environmental stressors.
Common Myths About Antioxidants in Skincare
Despite their growing popularity, there are many misconceptions about antioxidants in skincare. Let’s debunk some of the most common myths so you can make informed choices about your routine.
Myth #1: Antioxidants Are Only for Anti-Aging
While antioxidants are excellent for reducing wrinkles and fine lines, they benefit all skin types and ages by repairing skin, reducing inflammation, and preventing damage before signs of aging appear.
Myth #2: All Antioxidants Work the Same Way
Different antioxidants have unique benefits. Methylene blue is a cellular energy booster, while vitamin C brightens and coenzyme Q10 reduces fine lines. A combination of antioxidants offers the best protection.
Myth #3: You Only Need Antioxidants in Skincare
While topical antioxidants are crucial, they work best when paired with non-toxic habits. Using eco-friendly dishwasher detergent, non-toxic laundry detergent, and kitchen hand soap helps reduce skin exposure to oxidative stressors.
Myth #4: Antioxidants Replace Sunscreen
Although antioxidants protect against free radicals, they don’t block UV rays. Always use a broad-spectrum sunscreen in addition to methylene blue skincare and face cream for optimal protection.
By understanding the truth about antioxidants, you can create a skincare routine that truly enhances skin longevity and wellness.
Conclusion
Antioxidants are essential for maintaining youthful, healthy skin, as they combat oxidative stress, prevent collagen breakdown, and protect against environmental damage. Incorporating methylene blue skincare, face creams, and antioxidant-rich body care products into your routine helps shield the skin from premature aging while promoting long-term resilience.
To maximize antioxidant benefits, switch to clean, non-toxic alternatives, including natural bar soaps, fragrance-free deodorants, fluoride-free toothpaste, and eco-friendly cleaning products. Nobiesse’s luxury, antioxidant-infused skincare solutions offer a science-backed, toxin-free approach to beauty and wellness, ensuring radiance and longevity at every step.
Ready to transform your skincare routine? Explore Nobiesse’s antioxidant-rich skincare collection today.
#antioxidants in skincare#anti-aging#skin protection#free radicals#vitamin C#methylene blue skincare#skin barrier#oxidative stress#healthy skin
0 notes
Text
How Nutrition Impacts Your Skin: What to Eat for a Glow
Great skin starts from within. While skincare products play a crucial role in maintaining a healthy complexion, the food you eat has an equally, if not more significant, impact on your skin’s health and radiance. By nourishing your body with the right nutrients, you can tackle common skin concerns like dullness, dryness, and acne while promoting a natural glow. This blog will explore the…
#antioxidant packed foods#building blocks for skin repair#collagen boosting nutrients#healthy fats#hydrating foods#inflamamtion#nutrition impacts your skin#oxidative stress#probiotics for gut skin health#zinc rich foods
0 notes
Text

#health & fitness#joint stiffness#daily activities#mobility#Curcumitol-Q#dietary supplement#joint health#curcumin#turmeric#anti-inflammatory properties#Curcumin I#Curcumin II#Curcumin III#antioxidant properties#oxidative stress#inflammation#Quercetin#bioavailability#cartilage health#flexibility#immune function#cognitive health#digestive health#mood enhancement#cardiovascular health#health benefits.
0 notes
Text
#Molecular Hydrogen#Hydrogen Water#Antioxidant#Oxidative Stress#Hydration#Health Benefits#Dietary Supplements#Magnesium#Hydrogen-rich Water#Detoxification
0 notes
Text
youtube
#Carcinogenesis#tumor initiation#cancer progression#oncogenesis#genetic mutations#epigenetic alterations#oncogenic pathways#tumor suppressor genes#DNA damage repair#cell proliferation#metastatic cascade#angiogenesis#inflammation and cancer#tumor microenvironment#oxidative stress#apoptosis resistance#cellular transformation#mutagenesis#carcinogenic agents#chemoprevention.#Youtube
0 notes
Text
Uric Acid: Effects & Management

Uric acid plays a central role in metabolic health and oxidative stress regulation.
Elevated uric acid levels are linked to gout, metabolic syndrome, and cardiovascular diseases.
High fructose consumption is a major factor in uric acid overproduction and fat accumulation.
Copper deficiency and iron dysregulation contribute to oxidative stress, impacting uric acid metabolism.
Natural animal-based diets, including red meat, provide essential nutrients that regulate uric acid.
Introduction

Uric acid is a compound produced during the breakdown of purines, which are found in many foods and naturally occurring in the body.
While uric acid serves important antioxidant functions, excess levels can lead to health conditions such as gout and kidney stones.
Uric Acid Metabolism
Purine Breakdown and Uric Acid Production
Purines are substances found in both food and body tissues. When purines break down, uric acid is produced.
Most uric acid dissolves in the blood and is excreted by the kidneys. Problems arise when the body either produces too much uric acid or fails to excrete enough, leading to elevated serum uric acid levels.
Factors Influencing Uric Acid Levels
Several factors can influence uric acid levels in the body, including diet, kidney function, and metabolic processes.
High consumption of fructose is a key contributor to increased uric acid production. This occurs because fructose metabolism generates a large amount of uric acid, particularly in the liver.
Uric Acid and Fructose
Fructose, found in sugary beverages and high-fructose corn syrup, is metabolized differently than other sugars.
Unlike glucose, fructose undergoes rapid metabolism in the liver, leading to the depletion of ATP (the body’s energy currency) and the production of uric acid.
This process contributes to metabolic syndrome, fatty liver, and other health conditions. Reducing fructose intake is essential for lowering uric acid levels and improving metabolic health.
Iron Dysregulation and Oxidative Stress
The Role of Iron and Copper
Iron dysregulation, often exacerbated by copper deficiency, can lead to oxidative stress and metabolic disturbances.
Copper is critical in regulating iron and preventing its accumulation in tissues. When copper is deficient, iron builds up, leading to free radical damage and increased oxidative stress.
This oxidative stress further influences uric acid production and contributes to various health problems, including gout and cardiovascular disease.
Oxidative Stress and Uric Acid
Uric acid serves as an antioxidant in the bloodstream, but its overproduction, often triggered by factors like fructose consumption and iron dysregulation, can lead to harmful effects inside cells.
Intracellular uric acid promotes oxidative stress, inflammation, and fat accumulation, particularly in the liver.
This is a significant concern in metabolic disorders like non-alcoholic fatty liver disease (NAFLD).
Health Conditions Linked to Uric Acid

Gout
Gout is a painful condition caused by the accumulation of uric acid crystals in joints, leading to inflammation and discomfort.
While purine-rich foods are often blamed, the true drivers of elevated uric acid in gout are metabolic factors like fructose consumption, oxidative stress, and kidney function.
Addressing these underlying causes is key to managing gout effectively.
Metabolic Syndrome and NAFLD
Elevated uric acid levels are commonly seen in individuals with metabolic syndrome and non-alcoholic fatty liver disease (NAFLD).
These conditions are driven by insulin resistance, high carbohydrate intake, and fructose metabolism.
Lowering uric acid through dietary changes that reduce fructose and improve copper status can help mitigate these diseases.
Treatment and Management

Dietary Adjustments
Managing uric acid levels involves dietary changes focused on reducing fructose intake and optimizing nutrient balance.
Fructose, found in sugary drinks and processed foods, significantly contributes to uric acid overproduction.
Animal-based diets, particularly those rich in red meat, provide essential nutrients like copper and support metabolic health without contributing to uric acid-related problems.
Role of Medications
In some cases, medications like allopurinol are used to lower uric acid levels. These medications inhibit xanthine oxidase, an enzyme involved in uric acid production.
While effective, addressing the root causes through dietary and lifestyle changes is often the most sustainable approach.
Conclusion
Uric acid is a critical component of metabolic health, serving antioxidant functions in the body. However, when its levels become elevated due to factors like high fructose consumption, iron dysregulation, and oxidative stress, it can lead to conditions such as gout and metabolic syndrome. Prioritizing a nutrient-dense, animal-based diet and reducing fructose intake are essential strategies for managing uric acid levels and supporting overall health.
FAQs
What is the main cause of high uric acid levels?
Fructose consumption, not purine-rich foods, is a primary driver of high uric acid levels. It accelerates uric acid production during metabolism.
How does uric acid relate to gout?
Excess uric acid can form crystals in joints, leading to inflammation and gout. Managing fructose intake is key to reducing uric acid.
Does red meat cause high uric acid?
No. Red meat provides essential nutrients and does not significantly contribute to uric acid elevation. Carbohydrates and fructose are more likely culprits.
How can I lower my uric acid naturally?
Reduce fructose intake, optimize copper levels, and prioritize nutrient-dense foods like red meat to naturally lower uric acid levels.
What role does oxidative stress play in uric acid production?
Oxidative stress, often caused by iron dysregulation and fructose metabolism, increases uric acid production and contributes to metabolic diseases
.Research
Ayoub-Charette S, Liu Q, Khan TA, Au-Yeung F, Blanco Mejia S, de Souza RJ, Wolever TM, Leiter LA, Kendall C, Sievenpiper JL. Important food sources of fructose-containing sugars and incident gout: a systematic review and meta-analysis of prospective cohort studies. BMJ Open. 2019 May 5;9(5):e024171. doi: 10.1136/bmjopen-2018-024171. PMID: 31061018; PMCID: PMC6502023.
Bai, L., Zhou, J.-B., Zhou, T., Newson, R.B. and Cardoso, M.A., 2021. Incident gout and weight change patterns: a retrospective cohort study of US adults. Arthritis Research & Therapy, [online] 23(1). https://doi.org/10.1186/s13075-021-02461-7.
Basaranoglu, M., Basaranoglu, G., & Bugianesi, E. (2015). Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surgery and Nutrition, 4(2), 109-116. https://doi.org/10.3978/j.issn.2304-3881.2014.11.05
Cristina, M. (2023). Insulin and the kidneys: A contemporary view on the molecular basis. Frontiers in Nephrology, 3, 1133352. https://doi.org/10.3389/fneph.2023.1133352
El Ridi, R., & Tallima, H. (2017). Physiological functions and pathogenic potential of uric acid: A review. Journal of Advanced Research, 8(5), 487-493. https://doi.org/10.1016/j.jare.2017.03.003
Ghio, A.J., Ford, E.S., Kennedy, T.P. and Hoidal, J.R., 2005. The association between serum ferritin and uric acid in humans. Free Radical Research, [online] 39(3), pp.337–342. https://doi.org/10.1080/10715760400026088.
Goldberg, E. L., Asher, J. L., Molony, R. D., Shaw, A. C., Zeiss, C. J., Wang, C., Morozova-Roche, L. A., Herzog, R. I., Iwasaki, A., & Dixit, V. D. (2017). β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Reports, 18(9), 2077. https://doi.org/10.1016/j.celrep.2017.02.004
Jamnik, J., Rehman, S., Blanco Mejia, S., de Souza, R.J., Khan, T.A., Leiter, L.A., Wolever, T.M.S., Kendall, C.W.C., Jenkins, D.J.A. and Sievenpiper, J.L., 2016. Fructose intake and risk of gout and hyperuricemia: a systematic review and meta-analysis of prospective cohort studies. BMJ Open, [online] 6(10), p.e013191. https://doi.org/10.1136/bmjopen-2016-013191.
Kanbay, M., Segal, M., Afsar, B., Kang, D.-H., Rodriguez-Iturbe, B. and Johnson, R.J., 2013. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart, [online] 99(11), pp.759–766. https://doi.org/10.1136/heartjnl-2012-302535.
Lanaspa, M.A., Sanchez-Lozada, L.G., Cicerchi, C., Li, N., Roncal-Jimenez, C.A., Ishimoto, T., Le, M., Garcia, G.E., Thomas, J.B., Rivard, C.J., Andres-Hernando, A., Hunter, B., Schreiner, G., Rodriguez-Iturbe, B., Sautin, Y.Y. and Johnson, R.J., 2012. Uric Acid Stimulates Fructokinase and Accelerates Fructose Metabolism in the Development of Fatty Liver. PLoS ONE, [online] 7(10), p.e47948. https://doi.org/10.1371/journal.pone.0047948.
Lanaspa, M.A., Tapia, E., Soto, V., Sautin, Y. and Sánchez-Lozada, L.G., 2011. Uric Acid and Fructose: Potential Biological Mechanisms. Seminars in Nephrology, [online] 31(5), pp.426–432. https://doi.org/10.1016/j.semnephrol.2011.08.006.
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C. and Mollace, V., 2016. Regulation of uric acid metabolism and excretion. International Journal of Cardiology, [online] 213, pp.8–14. https://doi.org/10.1016/j.ijcard.2015.08.109.
Mainous, A.G., Knoll, M.E., Everett, C.J., Matheson, E.M., Hulihan, M.M. and Grant, A.M., 2011. Uric Acid as a Potential Cue to Screen for Iron Overload. The Journal of the American Board of Family Medicine, [online] 24(4), pp.415–421. https://doi.org/10.3122/jabfm.2011.04.110015.
Muscelli, E., 1996. Effect of insulin on renal sodium and uric acid handling in essential hypertension. American Journal of Hypertension, [online] 9(8), pp.746–752. https://doi.org/10.1016/0895-7061(96)00098-2.
Nakagawa, T., Lanaspa, M. A., & Johnson, R. J. (2019). The effects of fruit consumption in patients with hyperuricaemia or gout. Rheumatology, 58(7), 1133-1141. https://doi.org/10.1093/rheumatology/kez128
Pina, A.F., Borges, D.O., Meneses, M.J., Branco, P., Birne, R., Vilasi, A. and Macedo, M.P., 2020. Insulin: Trigger and Target of Renal Functions. Frontiers in Cell and Developmental Biology, [online] 8. https://doi.org/10.3389/fcell.2020.00519.
Rasool, M., Malik, A., Jabbar, U., Begum, I., Qazi, M.H., Asif, M., Naseer, M.I., Ansari, S.A., Jarullah, J., Haque, A. and Jamal, M.S., 2016. Effect of iron overload on renal functions and oxidative stress in beta thalassemia patients. Saudi Medical Journal, [online] 37(11), pp.1239–1242. https://doi.org/10.15537/smj.2016.11.16242.
Rho, Y.H., Zhu, Y. and Choi, H.K., 2011. The Epidemiology of Uric Acid and Fructose. Seminars in Nephrology, [online] 31(5), pp.410–419. https://doi.org/10.1016/j.semnephrol.2011.08.004.
Roman, Y. M. The Role of Uric Acid in Human Health: Insights from the Uricase Gene. Journal of Personalized Medicine, 13(9), 1409. https://doi.org/10.3390/jpm13091409
Singh, J.A., Reddy, S.G. and Kundukulam, J., 2011. Risk factors for gout and prevention: a systematic review of the literature. Current Opinion in Rheumatology, [online] 23(2), pp.192–202. https://doi.org/10.1097/bor.0b013e3283438e13.
Skøtt, P., Hother-Nielsen, O., Bruun, N.E., Giese, J., Nielsen, M.D., Beck-Nielsen, H. and Parving, H.-H., 1989. Effects of insulin on kidney function and sodium excretion in healthy subjects. Diabetologia, [online] 32(9). https://doi.org/10.1007/bf00274259.
So, A. and Thorens, B., 2010. Uric acid transport and disease. Journal of Clinical Investigation, [online] 120(6), pp.1791–1799. https://doi.org/10.1172/jci42344.
Wang, Y., Yang, Z., Wu, J., Xie, D., Yang, T., Li, H. and Xiong, Y., 2020. Associations of serum iron and ferritin with hyperuricemia and serum uric acid. Clinical Rheumatology, [online] 39(12), pp.3777–3785. https://doi.org/10.1007/s10067-020-05164-7.
Yamanaka H. [Alcohol ingestion and hyperuricemia]. Nihon Rinsho. 1996 Dec;54(12):3369-73. Japanese. PMID: 8976122.
0 notes
Text
Mitochondrial Dysfunction in Spinal Muscular Atrophy (SMA)
Introduction
Spinal Muscular Atrophy (SMA) is a severe neurodegenerative disorder that predominantly affects motor neurons, resulting in progressive muscle weakness and atrophy. The condition is caused by mutations in the survival motor neuron 1 (SMN1) gene, which leads to the loss of SMN protein, a critical factor for motor neuron survival. Although the primary defect lies in the motor neurons, increasing evidence suggests that mitochondrial dysfunction plays a pivotal role in the pathophysiology of SMA. Mitochondria, the powerhouse of the cell, are crucial for cellular energy production and regulation of various metabolic pathways. In the context of SMA, mitochondrial dysfunction has been linked to impaired cellular energy metabolism, oxidative stress, and neuronal death.
This article reviews the emerging role of mitochondrial dysfunction in SMA, examining its impact on motor neurons, the cellular processes involved, and the potential for mitochondrial-targeted therapies.
Mitochondrial Dysfunction in SMA: A Pathophysiological Overview
Mitochondria are essential organelles responsible for generating ATP through oxidative phosphorylation, controlling cellular metabolism, and mediating cell death mechanisms. In SMA, deficits in SMN protein affect multiple cellular pathways, including mitochondrial function. SMN is known to be involved in the biogenesis and maintenance of mitochondria. When its expression is reduced, mitochondrial dysfunction occurs in several ways, contributing to the progressive nature of SMA.
Impaired Mitochondrial Biogenesis
Mitochondrial biogenesis refers to the process by which new mitochondria are formed within cells. This process is tightly regulated by nuclear and mitochondrial signals, with the peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) being a key regulator of mitochondrial biogenesis. Studies in SMA models have shown that a reduction in SMN protein leads to downregulation of PGC-1α, resulting in decreased mitochondrial biogenesis. This reduced mitochondrial mass is particularly detrimental to motor neurons, which have high energy demands due to their long axonal projections and rapid neurotransmitter signaling.
Mitochondrial Dysfunction and ATP Production
Mitochondrial dysfunction in SMA results in decreased ATP production. ATP is required for essential cellular functions such as protein synthesis, ion transport, and maintaining membrane potential. In motor neurons, impaired ATP generation leads to cellular energy deficits that exacerbate neurodegeneration. Mitochondrial dysfunction also disrupts calcium homeostasis, as mitochondria play a central role in buffering intracellular calcium levels. Elevated intracellular calcium levels can activate enzymes that degrade cellular components, further contributing to cell death in motor neurons.
Oxidative Stress
One of the most significant consequences of mitochondrial dysfunction is the increased production of reactive oxygen species (ROS). Mitochondria are the main source of ROS in cells, and under normal conditions, the antioxidant defense systems neutralize these reactive molecules. However, in SMA, defective mitochondrial function leads to excessive ROS production, which overwhelms the cell’s ability to detoxify them. ROS are highly reactive and can damage cellular structures such as proteins, lipids, and DNA, ultimately contributing to oxidative stress and neuronal injury.
Mitochondrial Dynamics and Morphology
Mitochondrial morphology is highly dynamic, with the organelles undergoing fusion and fission events in response to cellular needs. In SMA, the balance between these processes is disrupted. Studies have shown that reduced SMN levels lead to an increase in mitochondrial fragmentation, a characteristic of mitochondrial dysfunction. Fragmented mitochondria are less efficient in energy production and more prone to damage. Additionally, the fragmented mitochondria in SMA models exhibit impaired mitochondrial transport along axons, further hindering motor neuron function.
Mitochondrial Quality Control
Mitochondrial quality control mechanisms, such as mitophagy, are critical for maintaining mitochondrial health. Mitophagy is the process by which damaged mitochondria are selectively degraded by autophagosomes. In SMA, defects in SMN protein affect the cellular machinery responsible for mitophagy, leading to the accumulation of dysfunctional mitochondria. This impairment in mitochondrial turnover accelerates neurodegeneration by allowing damaged mitochondria to persist, increasing oxidative stress, and triggering cellular apoptosis.
Mitochondrial Dysfunction in Different Types of SMA
SMA is classified into several types based on age of onset and severity, including Type I (Werdnig-Hoffmann disease), Type II, Type III, and Type IV. Mitochondrial dysfunction is observed in all types, but its extent varies depending on the severity of the disease.
SMA Type I
This is the most severe form of SMA, typically presenting in infants before six months of age. These children experience profound muscle weakness and may not survive beyond the first two years of life without intervention. In Type I, mitochondrial dysfunction is particularly pronounced, with severe mitochondrial fragmentation, impaired ATP production, and significant oxidative damage observed in motor neurons. The severity of mitochondrial dysfunction correlates with the extent of neurodegeneration in the spinal cord.
SMA Type II
Type II SMA presents later in infancy or early childhood, with affected individuals showing progressive muscle weakness but with a longer life expectancy compared to Type I. Mitochondrial dysfunction in Type II is still significant but less severe than in Type I. There is evidence of mitochondrial fragmentation and altered mitochondrial dynamics, but motor neurons in Type II patients may still retain some capacity for mitochondrial biogenesis and ATP production, contributing to the slower progression of the disease.
SMA Type III and IV
SMA Type III and IV are milder forms of the disease, with onset typically in childhood or adulthood. While mitochondrial dysfunction is present, it is less pronounced than in Type I and II. In these types, mitochondrial dynamics, ATP production, and oxidative stress are affected, but the clinical presentation is less severe, and individuals often experience a normal or near-normal life expectancy.
Conclusion
Mitochondrial dysfunction is a central feature of the pathophysiology of Spinal Muscular Atrophy (SMA). Reduced SMN protein leads to impaired mitochondrial biogenesis, altered mitochondrial dynamics, increased oxidative stress, and mitochondrial dysfunction. These defects contribute to the progressive degeneration of motor neurons and muscle weakness seen in SMA. Understanding the complex interplay between SMN deficiency and mitochondrial dysfunction provides valuable insights into the disease mechanisms and offers new avenues for therapeutic intervention. Mitochondrial-targeted approaches, including enhancing mitochondrial biogenesis, antioxidant therapy, and modulation of mitochondrial dynamics, hold promise for improving the quality of life and outcomes for SMA patients.
Ongoing research into mitochondrial dysfunction in SMA is crucial for identifying novel treatment strategies that can complement existing therapies and slow disease progression. As therapeutic options evolve, mitochondrial health will likely become an important consideration in the management of SMA, offering hope for more effective treatments in the future.

#Spinal Muscular Atrophy (SMA)#Mitochondrial Dysfunction#Motor Neurons#SMN1 Gene#SMN Protein#Neurodegeneration#Mitochondrial Biogenesis#Oxidative Stress#ATP Production#Mitochondrial Fragmentation#Reactive Oxygen Species (ROS)#Calcium Homeostasis#Mitochondrial Dynamics#Mitochondrial Transport#Mitophagy#Mitochondrial Quality Control#PGC-1α (Peroxisome Proliferator-Activated Receptor-Gamma Coactivator 1-Alpha)#Cellular Energy Metabolism#Mitochondrial-Targeted Therapies#Apoptosis.
0 notes
Text
Cordyceps – the most healing parasite in the world
by Dr.Harald Wiesendanger– Klartext What the mainstream media is hiding Traditional Chinese medicine knows it as one of the most powerful medicinal mushrooms of all: Cordyceps. What naturopaths have been saying about it for centuries has now been confirmed by numerous studies: it improves physical and mental performance, regulates the immune system, relieves pain, lowers high blood pressure –…
#cancer#Cordycepin#Cordyceps#Covid-19#desire#Harald Wiesendanger#impotence#joint pain#libido#medicinal mushroom CS-4#osteoarthritis#oxidative stress#radical scavenger#TCM#traditional Chinese medicine
0 notes
Text
Methylene Blue for Longevity: Benefits & How It Supports Aging

Longevity is not just about adding years to life—it’s about improving quality of life by maintaining youthful energy, cognitive sharpness, and skin vitality. As research into longevity expands, methylene blue has gained recognition for its anti-aging, mitochondrial-boosting, and antioxidant properties. Once used primarily in medicine and scientific research, this powerful compound is now making its way into skincare, wellness, and holistic health regimens.
Methylene blue has been studied for its ability to reduce oxidative stress, improve mitochondrial function, and enhance cognitive performance. These benefits make it a compelling option for those looking to optimize their health, skin, and energy levels. As part of a non-toxic skincare and wellness routine, methylene blue can help combat premature aging, promote skin rejuvenation, and support long-term well-being.
This article explores the science behind methylene blue for longevity, its impact on skin and overall health, and how integrating it into a clean beauty lifestyle with products like methylene blue cream, natural bar soap, and non-toxic home essentials can help you achieve radiant skin and lasting vitality. Nobiesse’s luxury skincare and eco-friendly products make this transition seamless, ensuring that longevity is supported inside and out.
What is Methylene Blue and How Does It Work?
Methylene blue is a synthetic compound with powerful antioxidant properties that has been widely used in medicine, biology, and chemistry for over a century. Recently, research has shown that it supports cellular energy production, reduces oxidative stress, and protects mitochondria, making it an exciting compound for longevity and skincare.
Mitochondria are the powerhouses of our cells, responsible for generating ATP (energy). As we age, mitochondrial efficiency declines, leading to fatigue, cognitive decline, and visible signs of aging. Methylene blue has been shown to:
Reduce oxidative stress by neutralizing free radicals that cause cellular damage and premature aging.
Enhance mitochondrial function, leading to better energy levels, improved cognitive performance, and enhanced skin repair.
Improve circulation and oxygen delivery, which may promote skin elasticity, hydration, and resilience.
Because of its unique ability to support cellular repair, methylene blue is now being incorporated into high-performance skincare products like face creams and serums, helping to combat fine lines, dullness, and environmental damage.
By integrating methylene blue skincare into a clean beauty regimen, alongside natural bar soap, fragrance-free deodorant, and eco-friendly cleaning products, individuals can protect their skin and body from premature aging while supporting overall longevity.
The Connection Between Methylene Blue and Longevity
Aging is a result of cumulative cellular damage, influenced by oxidative stress, mitochondrial dysfunction, and environmental toxins. Over time, these factors contribute to wrinkles, fatigue, cognitive decline, and reduced resilience in both the skin and body.
Methylene blue has been studied for its ability to combat the biological processes of aging by:
Protecting mitochondria from oxidative damage, ensuring higher energy production and better cellular repair mechanisms.
Reducing inflammation, which plays a key role in age-related diseases and skin damage.
Enhancing collagen production, helping skin remain firm, hydrated, and youthful-looking.
When used consistently, methylene blue skincare—such as face creams, serums, and anti-aging formulations—helps slow down visible signs of aging while protecting the skin from external aggressors like pollution and UV damage.
Beyond skincare, supporting longevity requires a holistic approach, including clean eating, proper hydration, regular exercise, and toxin-free living. Using eco-friendly cleaning products, natural shampoo bars, and fluoride-free toothpaste ensures that the body is not overburdened with toxins, promoting long-term wellness and vitality.
Methylene Blue in Skincare: Benefits for Youthful Skin
The skin is the body’s largest organ, acting as a barrier against pollution, UV rays, and environmental toxins. Over time, exposure to these elements leads to oxidative stress, collagen breakdown, and premature aging, resulting in fine lines, wrinkles, dullness, and loss of elasticity. Incorporating methylene blue into skincare offers a powerful anti-aging and skin-rejuvenating solution, helping to maintain a youthful, radiant complexion.
Methylene blue is a potent antioxidant that works by neutralizing free radicals, which cause cellular damage and accelerate the aging process. Studies have shown that methylene blue boosts collagen and elastin production, essential proteins that keep skin firm, smooth, and hydrated. Unlike synthetic anti-aging ingredients that may cause irritation, methylene blue is gentle yet effective, making it suitable for all skin types, including sensitive skin.
Key Benefits of Methylene Blue in Skincare:
Stimulates collagen production, reducing the appearance of fine lines and wrinkles while improving skin elasticity.
Enhances cellular energy (ATP production), speeding up skin repair and regeneration.
Strengthens the skin barrier, improving moisture retention and hydration levels.
Protects against environmental stressors, preventing sun damage, pollution-related aging, and oxidative damage.
Nobiesse’s methylene blue cream is expertly formulated to deliver these longevity-enhancing benefits. Designed to hydrate, repair, and protect the skin, this non-toxic, high-performance cream helps improve skin texture, resilience, and overall health.
For optimal results, pair methylene blue skincare with clean beauty essentials to create a comprehensive, non-toxic skincare routine:
Wash with a natural bar soap to cleanse gently without stripping the skin’s natural oils.
Apply fragrance-free deodorant, avoiding synthetic chemicals that clog pores or cause irritation.
Use an organic lip balm to keep lips hydrated and protected from environmental aggressors.
Incorporate eco-friendly cleaning products to create a toxin-free living environment, supporting long-term skin and overall health.
By integrating methylene blue skincare and non-toxic beauty products, individuals can protect their skin from premature aging, maintain hydration, and promote long-term resilience, achieving a healthier, more youthful glow naturally.
How to Incorporate Methylene Blue into Your Routine
For those looking to experience the full benefits of methylene blue, integrating it into a longevity-focused skincare and wellness routine is key. Methylene blue works best when paired with a holistic, non-toxic approach that supports skin renewal, hydration, and antioxidant protection from multiple angles.
The best way to incorporate methylene blue into your routine is through daily application of a high-quality methylene blue face cream. This ensures consistent antioxidant protection, hydration, and collagen support, leading to smoother, firmer skin over time. When used consistently, methylene blue enhances the skin’s natural repair processes, making it more resistant to wrinkles, sagging, and environmental stress.
Steps to Build a Methylene Blue Skincare Routine:
Cleanse with a natural bar soap – Start your routine with a gentle, toxin-free cleanser that effectively removes dirt and impurities without stripping essential moisture. A plant-based, non-toxic bar soap ensures skin stays balanced and hydrated.
Apply methylene blue cream – After cleansing, massage a small amount of methylene blue face cream onto clean skin. This step helps lock in moisture, strengthen the skin’s protective barrier, and provide antioxidant defense against aging.
Use organic lip balm – Keep lips soft and hydrated by avoiding petroleum-based formulas and opting for botanical-rich lip balms that nourish without harmful additives.
Switch to fluoride-free toothpaste – Oral health is a critical part of a non-toxic routine. A fluoride-free toothpaste free from harsh chemicals and synthetic additives helps maintain a healthier oral microbiome and overall wellness.
Opt for eco-friendly cleaning products – Household products can expose the skin to toxins. Using non-toxic laundry detergents, eco-friendly dishwasher detergents, and kitchen hand soaps reduces chemical exposure while promoting cleaner, healthier living spaces.
Hydrate and nourish from within – Drinking plenty of water, eating antioxidant-rich foods, and maintaining a balanced diet enhances the effects of methylene blue skincare, supporting glowing, healthy skin from the inside out.
By making these changes, individuals minimize their exposure to harmful chemicals, allowing their skin and body to function optimally. Non-toxic skincare and home essentials work together to enhance overall wellness, reduce inflammation, and promote youthful skin longevity.
When combined with a healthy lifestyle, methylene blue skincare offers a science-backed approach to anti-aging, ensuring radiance, resilience, and long-term skin health.
The Importance of Clean Beauty for Longevity
Skincare and longevity go hand in hand, but what you put on your skin matters just as much as what you put in your body. Many conventional beauty products contain parabens, sulfates, synthetic fragrances, and other toxic chemicals that contribute to inflammation, oxidative stress, and premature aging. Over time, these toxins accumulate in the body, increasing the burden on the skin and internal organs.
Clean beauty focuses on using high-performance, non-toxic ingredients that enhance skin health without harmful side effects. The goal is to reduce toxic exposure while delivering visible, long-lasting results.
A longevity-focused beauty routine includes:
Methylene blue skincare helps protect the skin from environmental damage and oxidative stress.
Natural bar soaps, free from synthetic detergents, to cleanse gently while preserving the skin’s microbiome.
Fragrance-free deodorant which eliminates aluminum and artificial scents that may cause irritation.
Eco-friendly cleaning products, ensuring that your home environment remains free from harmful residues that can be absorbed through the skin.
By making these changes, you support long-term skin health, reduce toxic buildup, and enhance overall well-being, contributing to a more youthful, radiant appearance at any age.
Methylene Blue and Men’s Skincare
Men's skincare is often overlooked in discussions about longevity, yet healthy, well-maintained skin plays a crucial role in overall well-being and confidence. The reality is that men’s skin ages differently than women’s, with increased oil production, larger pores, and thicker collagen layers. However, as men age, collagen production slows down, leading to wrinkles, dehydration, and irritation—especially from daily shaving.
Methylene blue skincare is an excellent addition to men’s skincare routines, offering:
Enhanced skin hydration and protection, keeping the skin smooth and resilient.
Anti-inflammatory properties, which help reduce irritation, redness, and razor burn.
Antioxidant benefits, preventing fine lines and environmental damage from pollution and UV rays.
A simple longevity-focused men’s skincare routine includes:
Cleansing with a natural bar soap to remove dirt and excess oil.
Applying a methylene blue cream to hydrate and protect the skin.
Using fragrance-free deodorant to avoid irritation and synthetic chemicals.
Incorporating organic lip balm to prevent dryness and chapping.
Nobiesse’s men’s skincare solutions provide a science-backed, non-toxic approach to maintaining youthful, healthy skin while embracing clean beauty principles.
Methylene Blue and Hair Care
Just as methylene blue supports skin longevity, it also plays a role in hair health and scalp vitality. Many hair care products contain sulfates, silicones, and artificial fragrances that strip the scalp of its natural oils, leading to dryness, irritation, and long-term damage.
Switching to a non-toxic haircare routine can improve hair quality, prevent premature graying, and support scalp health. Benefits of methylene blue in hair care include:
Improved scalp circulation, helping to promote healthier, fuller-looking hair.
Antioxidant protection, reducing the effects of UV damage and environmental stressors.
Reduced inflammation, making it ideal for those with sensitive scalps or dandruff concerns.
A clean haircare routine for longevity should include:
A natural shampoo bar, that cleanses without stripping the scalp of moisture.
Avoid harsh sulfates and parabens, which can weaken hair over time.
Use clean, nutrient-rich styling products that are free from synthetic chemicals.
By incorporating methylene blue and natural haircare solutions, individuals can promote stronger, healthier hair while maintaining a toxin-free beauty regimen.
Longevity-Focused Skincare Gift Sets
For those interested in transitioning to clean beauty and longevity-focused skincare, Skincare bundle sets provide a simple and effective way to explore high-performance, non-toxic products. These sets take the guesswork out of choosing clean beauty essentials, offering a curated selection of luxury skincare items that promote skin health, hydration, and rejuvenation.
A high-quality longevity skincare gift set typically includes:
Methylene blue face cream, providing deep hydration and antioxidant protection.
Natural bar soap, for gentle cleansing without synthetic chemicals.
Organic lip balm, to nourish and protect lips from dryness and environmental damage.
Eco-friendly skincare essentials, free from parabens, sulfates, and artificial additives.
Nobiesse’s luxury skincare gift sets make detoxing your beauty routine effortless, whether for personal use or as a thoughtful gift for someone looking to embrace non-toxic, high-performance beauty solutions.
Supporting Longevity Beyond Skincare: A Holistic Approach
While clean beauty and methylene blue skincare play an essential role in anti-aging and cellular protection, true longevity requires a holistic approach that supports the body inside and out.
To optimize health and longevity, consider these key lifestyle habits:
Maintain a clean environment by using eco-friendly cleaning products like non-toxic laundry detergent and kitchen hand soap to reduce exposure to synthetic chemicals and airborne toxins.
Choose fluoride-free toothpaste to avoid potential endocrine-disrupting compounds found in mainstream oral care products.
Prioritize hydration and nutrient-dense foods, including antioxidant-rich fruits and vegetables, which work synergistically with topical skincare for better results.
Incorporate stress-reducing activities like meditation, yoga, and deep breathing, which help lower inflammation and oxidative stress levels.
By making conscious choices in skincare, home products, and lifestyle habits, individuals can support long-term health, energy, and radiance, all of which contribute to a longer, more vibrant life.
How Methylene Blue Complements Non-Toxic Living
Choosing methylene blue skincare is just one part of adopting a clean, longevity-focused lifestyle. Many daily-use products contain hidden toxins, and exposure over time can accelerate aging and inflammation.
To minimize toxin exposure, consider making these swaps:
Switch to natural bar soap instead of synthetic liquid soaps that contain sulfates and artificial fragrances.
Use eco-friendly dishwasher detergent to avoid residues that can leave chemical traces on your dishes.
Opt for fragrance-free deodorant, eliminating aluminum and synthetic perfumes that disrupt the skin’s microbiome.
Incorporate methylene blue skincare into your routine for enhanced skin repair and protection.
By replacing chemical-laden products with non-toxic, high-performance alternatives, individuals can create a clean beauty and wellness regimen that supports cellular health, skin vitality, and overall longevity.
Conclusion
Methylene blue is emerging as a revolutionary ingredient in skincare and longevity science, offering antioxidant, anti-aging, and mitochondrial-boosting benefits. Its ability to enhance collagen production, protect against environmental stressors, and support cellular energy metabolism makes it an invaluable addition to high-performance beauty and wellness routines.
By combining methylene blue skincare with nontoxic beauty and home essentials, individuals can enhance their skin, energy levels, and overall well-being. A longevity-focused routine includes natural bar soaps, fragrance-free deodorants, eco-friendly cleaning products, and organic lip balms, ensuring that daily self-care remains clean, effective, and sustainable. Nobiesse is dedicated to delivering science-backed, non-toxic skincare solutions that promote long-term skin health and vitality. Ready to embrace clean beauty and longevity-enhancing skincare? Explore Nobiesse’s premium methylene blue skincare collection today.
#methylene blue#longevity#anti-aging#cellular health#mitochondrial support#cognitive function#oxidative stress#biohacking supplement
0 notes
Text
The Liposomal Advantage: Maximizing Vitamin C Benefits for Optimal Health
In the quest for optimal health, Vitamin C is often hailed as a superstar nutrient. Known for its powerful antioxidant properties and immune-boosting benefits, Vitamin C is essential for overall well-being. However, not all forms of Vitamin C are created equal. Enter liposomal Vitamin C, a revolutionary delivery system that maximizes the benefits of this vital nutrient. At NaturalFactors, we’re excited to share how this innovative approach can transform your health.
Understanding Liposomal Technology
So, what exactly is liposomal technology? Liposomes are tiny spherical structures made of phospholipids, the same components that make up our cell membranes. By encapsulating Vitamin C in liposomes, this technology protects the nutrient from degradation and enhances its absorption in the body. This means that more Vitamin C reaches your cells, providing you with greater benefits than traditional forms of supplementation.
Enhanced Absorption for Maximum Benefits
One of the most significant advantages of liposomal Vitamin C is its superior absorption rate. Traditional Vitamin C supplements, such as ascorbic acid, can sometimes cause gastrointestinal discomfort and have limited bioavailability. In contrast, liposomal Vitamin C bypasses these issues, allowing for a smoother and more effective absorption process.
Research has shown that liposomal formulations can increase the amount of Vitamin C that enters the bloodstream, meaning you can achieve optimal levels with a smaller dose. This makes liposomal Vitamin C not only more effective but also gentler on your stomach.
Immune Support Like Never Before
With the rise of seasonal colds and flu, supporting your immune system is more important than ever. Vitamin C plays a crucial role in various immune functions, including the production of white blood cells that combat infections. By choosing liposomal Vitamin C from NaturalFactors, you’re ensuring that your body receives the support it needs to fend off illnesses effectively.
The enhanced absorption of liposomal Vitamin C means that you’ll be providing your immune system with a steady supply of this essential nutrient. This can lead to quicker recovery times and improved overall health, especially during the colder months.
Antioxidant Powerhouse
Vitamin C is renowned for its antioxidant properties, which help neutralize free radicals in the body. These harmful compounds can lead to oxidative stress, contributing to chronic diseases and aging. By incorporating liposomal Vitamin C into your daily routine, you’re empowering your body to fight back against oxidative damage.
The liposomal delivery system ensures that a higher concentration of Vitamin C reaches your cells, maximizing its protective effects. This makes it a fantastic ally in your skincare regimen as well, promoting healthier, more radiant skin by combating environmental stressors.
Convenient and Versatile
Liposomal Vitamin C offers convenience and versatility. Available in liquid form, it can be easily added to smoothies, juices, or simply taken on its own. This flexibility makes it an ideal choice for those with busy lifestyles who still want to prioritize their health.
At NaturalFactors, we understand the importance of quality and convenience. Our liposomal Vitamin C is crafted with the highest standards, ensuring that you receive a premium product that supports your health goals.
Who Should Consider Liposomal Vitamin C?
Liposomal Vitamin C is suitable for a wide range of individuals. Whether you’re an athlete looking to enhance recovery, a busy professional wanting to boost immunity, or anyone interested in maintaining overall health, this form of Vitamin C can be a valuable addition to your daily routine.
Additionally, those who experience digestive discomfort with traditional Vitamin C supplements will find liposomal formulations to be a more pleasant alternative.
Conclusion
As we navigate the complexities of modern health, liposomal Vitamin C emerges as a powerful ally in achieving optimal wellness. With its enhanced absorption, immune support, and antioxidant properties, it’s a game-changer for anyone looking to elevate their health regimen. At NaturalFactors, we are committed to providing you with the highest quality liposomal Vitamin C, ensuring you get the maximum benefits from this essential nutrient. Embrace the liposomal advantage and take a significant step towards better health today!
#Vitamin C#liposomal Vitamin C#NaturalFactors#liposomal technology#enhanced absorption#immune support#antioxidant properties#oxidative stress#health benefits#digestive discomfort#quality supplements#wellness#superior absorption#health regimen#convenience#versatile#recovery#chronic diseases#radiant skin#premium product.
0 notes