#oncogenic pathways
Explore tagged Tumblr posts
Text
youtube
#Carcinogenesis#tumor initiation#cancer progression#oncogenesis#genetic mutations#epigenetic alterations#oncogenic pathways#tumor suppressor genes#DNA damage repair#cell proliferation#metastatic cascade#angiogenesis#inflammation and cancer#tumor microenvironment#oxidative stress#apoptosis resistance#cellular transformation#mutagenesis#carcinogenic agents#chemoprevention.#Youtube
0 notes
Text
Though "poker faces", psoriasis and skin diseases show their last CARD: 1 4 Myc should be enough
Our skin, the body’s largest organ–provides the first line of defense against infections and many other threats to our health. Decades of research has shown that a wide range of diseases can occur, or become worse, when the skin cannot form an effective barrier. Millions of people are affected by eczema, psoriasis and other inflammatory skin disorders. Now, experts in human genetics and asthma…
#c-Myc#CARD14#cellular signaling#chronic eczema#proto-oncogene#psoriasis#signaling pathway#skin barrier
0 notes
Text
Drug resistance in multiple myeloma: When cancer cells say "NO" to treatment
Drug resistance is like a game of cat and mouse. Cancer cells are the cat, and researchers are the mouse. The cat is always trying to find new ways to catch the mouse, but the mouse is always trying to find new ways to avoid getting caught.
Read More
#multiple myeloma#molecular mechanisms#signaling pathways#Health#stem cell transplantation#oncogenes#Lifestyle#cancer cells#gene mutations#drug resistance#cellular environment#gene expression
0 notes
Text
Reference saved in our archive
Covid's making it difficult for people's immune systems to suppress cancer. There was a recent small-scale mouse study showing "tumor shrinkage" in a small percentage of those transgenic mice. This is a much larger analysis of the oncogenic potential of covid in humans. Guess which story the news has been reporting.
Abstract The 2019 outbreak of SARS-CoV-2 has caused a major worldwide health crisis with high rates of morbidity and death. Interestingly, it has also been linked to cancer, which begs the issue of whether it plays a role in carcinogenesis. Recent studies have revealed various mechanisms by which SARS-CoV-2 can influence oncogenic pathways, potentially promoting cancer development. The virus encodes several proteins that alter key signaling pathways associated with cancer hallmarks. Unlike classical oncogenic viruses, which transform cells through viral oncogenes or by activating host oncogenes, SARS-CoV-2 appears to promote tumorigenesis by inhibiting tumor suppressor genes and pathways while activating survival, proliferation, and inflammation-associated signaling cascades. Bioinformatic analyses and experimental studies have identified numerous interactions between SARS-CoV-2 proteins and cellular components involved in cancer-related processes. This review explores the intricate relationship between SARS-CoV-2 infection and cancer, focusing on the regulation of key hallmarks driving initiation, promotion and progression of cancer by viral proteins. By elucidating the underlying mechanisms driving cellular transformation, the potential of SARS-CoV-2 as an oncovirus is highlighted. Comprehending these interplays is essential to enhance our understanding of COVID-19 and cancer biology and further formulating strategies to alleviate SARS-CoV-2 influence on cancer consequences.
#mask up#wear a mask#public health#pandemic#wear a respirator#covid 19#covid#still coviding#coronavirus#sars cov 2#cancer
51 notes
·
View notes
Text
Anti-Lung Cancer Effect and Mechanism of Ginsenosides
Anti-Lung Cancer Effect and Mechanism of Ginsenosides in Biomedical Journal of Scientific & Technical Research
ung cancer is the leading cause of cancer-related mortality around the world, with an estimated 1.8 million death [1], posing a serious threat to human health. In recent years, plant-derived natural active ingredients become a research hotspot, and show the advantages of rich variety and low toxicity. Ginsenosides, derived from the plants of Araliaceous family, including ginseng (Panax ginseng C. A. Meyer) notoginseng (Panax notoginseng (Burkill) F. H. Chen ex C. H.) and American ginseng (Panax quiquefolium L.), are responsible for most of the pharmacological activities of ginseng. Moreover, some ginsenosides have shown great anti-cancer activity [2]. In recent years, some studies have reported that ginsenosides performed their anti-lung cancer effects by affecting some signaling pathway and down-regulating various oncogenic proteins, however no specific reviews conduct by now. So the purpose of this review is to provide a summary of the anti-cancer effects and the potential mechanisms about ginsenosides of some most recent findings. We will hope that this review may provide valuable insights into application of ginsenosides for treatment of lung cancer.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01/ Follow on Blogger :https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#Journal on medical science#Journal on Medical Genetics#American medical journal#Biomedical Journal Impact Factor#open access journals of biomedical science
0 notes
Text

YH STUDENT ID: Nakajima Shouma
AKA The Lab Rat — Lives in research buildings. Always present, never acknowledged.
Birthdate & age: August 27, 1994 (30)
School year: 1st year PhD student
Program: Biomedical Science (PhD)
Please refer to our student handbook for any questions!
Introduction
Hasekura Foundation researcher Nakajima Shoma (MS) doesn’t have a “prototypical” approach of a cancer researcher. He has taken a unique perspective on researching the disease, as fit for a virologist, a scientist who specializes in the study of viruses. “I’ve spent my entire academic career closely examining viruses. The pathways that cells utilize to protect themselves against viral infection, including responses to, are necessary to defend themselves from cancer,��� he says. The cell’s defense mechanism has furthered his perspective on cancer, and is connected to understanding the development of cancer.
Tumor virology is closely related to Nakajiima’s research, a field that studies viruses that cause cancer. How they interact with each other is dependent on the host cells. From studying oncogenic viruses in cancer, Nakajima has found fundamental importance in looking into cancer biology. “I was able to assess oncogenes and tumor-suppressing genes,” informs the thirty-year-old. “Some of these viruses I began engineering for bio-virology therapy, by using, of course, oncolytic viruses that can restrictively infect and kill cancerous cells.”
At the 2024 Hasekura-Kinjo Assembly, Nakajima Shoma presented an update regarding his work at a lecture. The Hasekura-Kinjo Assembly is a conference funded by researchers to network with peers in cancer research, to share prospects, and engage with established researchers. Co-funded by the Uchida Sakiko Memorial Cancer Research Fund, Nakajima leads a small team of researchers at the Yamase Cancer Center in Kyoto, Japan. His research focuses on DNA sequences as aftermaths from viral infections. “I’d like to thank the foundation for supporting my findings,” Nakajima says. “It’s been nothing but transformative, and it’s the first grant that funded our laboratory, so I’d like to thank everyone involved for making it happen.”
Nakajima Shoma’s king's pin on cell defense mechanisms against viral infections and their embryonic relevance to cancer is suggestive of his knowledge and exploration of how disordering said pathways may contribute to tumor development. His studies are indicative of his ability to harness therapeutic practices. Thus, Nakajima’s work evaluates viral pathogens interplaying with host cellular mechanisms in the wake of oncogenesis.
0 notes
Text
Entrectinib: Uses, Interactions, Mechanism of Action
Understanding Entrectinib: Uses, Mechanism of Action, and Drug Interactions
The development of targeted cancer therapies has revolutionized the approach to oncology. Among these innovative agents, entrectinib stands out as a selective inhibitor designed to target specific genetic alterations that drive cancer progression. With its ability to address multiple tumor types driven by particular gene fusions, entrectinib represents a shift toward personalized medicine in oncology.
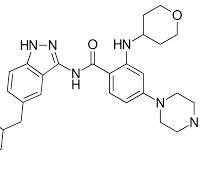
In this blog, we will explore the key clinical uses of entrectinib, its mechanism of action, and potential drug interactions clinicians and patients should be aware of.
What Is Entrectinib?
Entrectinib is an oral small-molecule tyrosine kinase inhibitor (TKI) developed to target specific gene rearrangements that are known to promote cancer growth. It belongs to a class of medications that selectively inhibit the activity of certain proteins involved in cell signaling pathways that regulate cell proliferation and survival.
Entrectinib is notable for its ability to cross the blood-brain barrier, making it effective for cancers that have metastasized to the central nervous system (CNS), a common and difficult-to-treat site of disease in many malignancies.
Clinical Uses of Entrectinib
Entrectinib is primarily used to treat patients whose tumors harbor specific genetic alterations, namely:
NTRK Gene Fusion-Positive Solid Tumors Entrectinib is indicated for the treatment of solid tumors with a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, regardless of tumor type. These fusions lead to the production of abnormal TRK fusion proteins, which drive uncontrolled cellular growth. Entrectinib is used in cases where:
There are no satisfactory alternative treatments available.
The cancer has metastasized or is inoperable.
ROS1-Positive Non-Small Cell Lung Cancer (NSCLC) In NSCLC, gene rearrangements involving ROS1 are found in a small subset of patients. These gene fusions activate oncogenic signaling pathways, making entrectinib a rational choice as a ROS1 inhibitor.
The use of entrectinib in these contexts exemplifies the growing trend of tissue-agnostic cancer therapies, which are guided more by genetic markers than by the cancer’s site of origin.
Mechanism of Action
Entrectinib acts by selectively inhibiting the tyrosine kinases encoded by the following genes:
NTRK1, NTRK2, and NTRK3: These genes encode for TRKA, TRKB, and TRKC proteins, respectively. When abnormally fused with other genes, these proteins can trigger constitutive activation of downstream signaling pathways like MAPK, PI3K-AKT, and PLCγ, leading to unchecked cell division.
ROS1: A receptor tyrosine kinase implicated in oncogenic fusion events in lung cancer.
ALK (Anaplastic Lymphoma Kinase): Entrectinib also has activity against ALK, though this is not its primary approved indication.
By blocking the ATP-binding site of these kinases, entrectinib halts downstream signaling that drives proliferation and survival of tumor cells. The compound's ability to penetrate the CNS further allows it to combat brain metastases, a significant advantage over some earlier TKIs.
Pharmacokinetics and Administration
Entrectinib is administered orally and is generally taken once daily. It undergoes metabolism primarily in the liver, predominantly via the CYP3A4 enzyme, a feature that has important implications for potential drug interactions.
The half-life of entrectinib is approximately 20 hours, supporting its once-daily dosing. Peak plasma concentrations are typically reached within 4 to 6 hours after administration.
Potential Drug Interactions
Given its metabolism via CYP3A4, entrectinib is susceptible to several significant drug interactions. These include:
1. CYP3A4 Inhibitors
Drugs that inhibit CYP3A4 can increase plasma levels of entrectinib, potentially raising the risk of toxicity. Examples include:
Ketoconazole
Itraconazole
Clarithromycin
Grapefruit juice (a dietary source)
2. CYP3A4 Inducers
Conversely, drugs that induce CYP3A4 can decrease entrectinib levels, reducing its efficacy. Examples include:
Rifampin
Carbamazepine
Phenytoin
St. John’s Wort (a common herbal supplement)
Co-administration with strong inducers is generally discouraged.
3. QT Interval-Prolonging Drugs
Entrectinib may modestly prolong the QT interval, a measurement of cardiac electrical activity. Concomitant use with other QT-prolonging drugs (e.g., certain antiarrhythmics, antipsychotics, or antibiotics) may increase the risk of arrhythmias.
4. P-glycoprotein (P-gp) Substrates
Entrectinib may inhibit P-glycoprotein, potentially increasing exposure to drugs that are P-gp substrates, such as digoxin.
Side Effects and Monitoring
Common side effects of entrectinib include:
Fatigue
Dizziness
Nausea and vomiting
Constipation
Weight gain
Anemia
More serious adverse effects can include cardiomyopathy, QT prolongation, central nervous system effects (e.g., cognitive disturbances), and skeletal fractures, particularly in pediatric populations. Regular monitoring of cardiac function, liver enzymes, and neurologic status is advised during therapy.
Conclusion
Entrectinib is a potent, targeted therapy designed for cancers driven by specific gene fusions, notably involving NTRK and ROS1. Its effectiveness across tumor types, ability to cross the blood-brain barrier, and relatively well-tolerated safety profile make it a valuable tool in modern oncology.
However, like all targeted therapies, its use must be guided by appropriate molecular diagnostics, and potential drug interactions must be carefully managed.
URL: For more information, visit Vasista Pharma: Entrectinib: Uses, Interactions, Mechanism of Action
0 notes
Text
Exploring BRAF and FLT3 Mutations: Advanced Detection with PCR Kits for Precision Oncology

Genetic mutation detection plays a crucial role in providing targeted treatments and personalized patient care. Among the many genetic alterations studied, BRAF and FLT3 mutations are particularly significant in various cancers, including melanoma, colorectal cancer, and acute myeloid leukemia (AML). The availability of highly sensitive and specific molecular diagnostic tools, like the BRAF Mutations Kit, BRAF PCR Kit, FLT3-ITD PCR Kit, and FLT3-TKD PCR Kit, has revolutionized how clinicians approach diagnosis and treatment planning.
The BRAF gene encodes a protein that is part of the MAPK/ERK signaling pathway, which regulates cell division and differentiation. Mutations in the BRAF gene, especially the V600E mutation, are known to drive oncogenic processes in several malignancies. Detecting these mutations early is crucial, as patients with BRAF mutations often benefit from targeted therapies that inhibit the aberrant BRAF protein function. The BRAF Mutations Kit by 3B BlackBio Biotech is designed to detect common mutations in the BRAF gene with exceptional accuracy. Based on real-time PCR technology, this kit provides a quick and reliable method to identify mutations like V600E, V600K, and V600D, which are clinically relevant for treatment decisions.
The BRAF PCR Kit offers several advantages, including high sensitivity, specificity, and a simple workflow that can be seamlessly integrated into diagnostic laboratories. It allows oncologists to determine mutation status efficiently, helping to tailor treatment regimens that can improve patient outcomes significantly. As targeted therapies become a cornerstone of cancer treatment, the ability to accurately identify BRAF mutations with the help of advanced PCR kits is more critical than ever.
While BRAF mutations are closely associated with solid tumors, mutations in the FLT3 gene are primarily linked with hematological malignancies, notably AML. FLT3 encodes a receptor tyrosine kinase that is crucial for the proliferation and differentiation of hematopoietic stem cells. Mutations like internal tandem duplications (ITD) and tyrosine kinase domain (TKD) point mutations result in constitutive activation of the FLT3 receptor, leading to uncontrolled cell proliferation and poor prognosis in AML patients. Therefore, precise detection of these mutations is vital for prognosis, risk stratification, and treatment planning.
The FLT3-ITD PCR Kit by 3B BlackBio Biotech is a specialized tool designed to detect FLT3-ITD mutations effectively. Using a real-time PCR approach, the kit enables rapid and highly sensitive detection of internal tandem duplications within the FLT3 gene. The presence of FLT3-ITD mutations often indicates an aggressive disease course and is associated with an increased risk of relapse, thus making it essential to incorporate FLT3 mutation testing into the diagnostic workflow for AML patients. Early detection with the FLT3-ITD PCR Kit can help in identifying high-risk patients who may benefit from more aggressive or alternative therapeutic strategies.
Additionally, another significant alteration in the FLT3 gene is the point mutation in the tyrosine kinase domain, known as FLT3-TKD mutation. This mutation involves a change at codon D835 and is also associated with adverse outcomes in AML. The FLT3-TKD PCR Kit provides an efficient method to detect this specific mutation with high sensitivity. Both the FLT3-ITD and FLT3-TKD PCR Kits enable comprehensive FLT3 mutation analysis, offering clinicians a complete picture of a patient's genetic profile and guiding therapy choices such as the use of FLT3 inhibitors.
One of the standout features of the FLT3-ITD and FLT3-TKD PCR kits from 3B BlackBio Biotech is their compatibility with standard real-time PCR instruments, making them highly accessible for most clinical laboratories. These kits are optimized for rapid turnaround times, allowing clinicians to make timely decisions that could impact the patient’s prognosis significantly. Moreover, they come with ready-to-use reagents, standardized protocols, and internal controls, ensuring reliability and reproducibility across different laboratories.
The importance of mutation detection in oncology cannot be overstated. As precision medicine advances, identifying actionable genetic alterations becomes the first and most critical step toward offering targeted therapies that can improve survival rates and quality of life. Kits like the BRAF Mutations Kit, BRAF PCR Kit, FLT3-ITD PCR Kit, and FLT3-TKD PCR Kit provide healthcare professionals with the tools needed to perform high-quality molecular testing and deliver personalized patient care.
When evaluating patients with melanoma, colorectal cancer, or AML, incorporating robust mutation detection strategies with trusted tools ensures better clinical management. The development of highly specialized diagnostic kits by companies like 3B BlackBio Biotech continues to empower oncologists and pathologists, facilitating precision medicine and bringing hope to patients battling complex malignancies.
The BRAF PCR Kit enables the rapid identification of critical BRAF mutations, while the FLT3-ITD and FLT3-TKD PCR Kits offer comprehensive FLT3 mutation analysis essential for AML management. As the field of oncology moves towards more personalized treatment approaches, leveraging these advanced diagnostic tools becomes indispensable for delivering better patient outcomes and shaping the future of cancer therapy.
0 notes
Text

Is Liver Cancer Hereditary? Exploring Genetic Risk Factors
Liver cancer is a serious condition affecting thousands of people worldwide. While environmental factors such as hepatitis infection and alcohol use are well-known contributors, there's growing interest in understanding whether liver cancer can be inherited. So, is liver cancer hereditary? Let’s explore the genetic risk factors and the role they play in liver cancer development.
Understanding Liver Cancer
Liver cancer begins in the cells of the liver. The most common type is hepatocellular carcinoma (HCC), which originates in the main liver cells (hepatocytes). Other types include intrahepatic cholangiocarcinoma and hepatoblastoma (more common in children).
Genetic vs. Environmental Causes
Cancer can be caused by genetic mutations (changes in your DNA) or external risk factors such as:
Chronic hepatitis B or C infections
Excessive alcohol use
Fatty liver disease (especially linked to obesity and diabetes)
Aflatoxin exposure (a toxin from mold-contaminated food)
But genetics can also increase your susceptibility to these environmental triggers or even cause mutations on their own.
Can Liver Cancer Be Inherited?
In most cases, liver cancer is not directly inherited, but certain genetic conditions and inherited syndromes can increase your risk. Here are a few:
1. Hemochromatosis
A genetic disorder that causes the body to absorb too much iron. Excess iron gets stored in organs including the liver, increasing the risk of cirrhosis and liver cancer.
2. Alpha-1 Antitrypsin Deficiency
This inherited condition affects the liver's ability to process certain proteins, leading to liver damage and possibly cancer.
3. Glycogen Storage Diseases
A group of inherited disorders that affect the way the body processes glycogen and can increase the risk of liver tumors.
4. Family History
Having a first-degree relative (parent, sibling, child) with liver cancer may slightly raise your risk. While it’s unclear whether this is due to shared genes, shared environments, or both, studies show a measurable increase in risk.
Genetic Mutations in Liver Cancer
In some people, mutations in tumor suppressor genes like TP53 or oncogenes such as CTNNB1 are involved in the development of liver cancer. These mutations can sometimes be inherited or can occur spontaneously due to other factors like viral infections.
Common Genetic Changes in Liver Cancer:
TP53 Mutation – Often associated with exposure to aflatoxins
CTNNB1 Mutation – Involved in WNT signaling pathway
TERT Promoter Mutations – Common in hepatocellular carcinoma
Should You Get Genetic Testing?
Genetic testing is not routinely recommended for everyone with liver cancer. However, if you have a strong family history of liver disease, cancer at a young age, or known inherited syndromes, your doctor might suggest testing.
Testing can:
Help identify your risk
Guide screening and prevention strategies
Inform family members about their risk
What Can You Do If You Have a Genetic Risk?
If you have a family history of liver cancer or a known genetic condition:
Get regular liver check-ups (ultrasounds, blood tests for AFP)
Avoid alcohol and tobacco
Get vaccinated against hepatitis B and treated for hepatitis C
Maintain a healthy weight and diet
Limit exposure to toxins and contaminated foods
Conclusion
While liver cancer is mostly linked to environmental factors, certain inherited conditions and genetic mutations can increase your risk. Knowing your family history and understanding these risks can empower you to take proactive steps toward prevention and early detection.
If you're concerned about your risk, talk to a healthcare provider or genetic counselor.
📌 Key Takeaways
Liver cancer is not typically inherited, but genetic conditions like hemochromatosis and alpha-1 antitrypsin deficiency can increase risk.
Mutations in genes like TP53 and CTNNB1 are linked to liver cancer.
Family history slightly increases your risk.
Early screening and lifestyle choices can help mitigate risk.
0 notes
Text
Molecular Oncology: The Interplay Between Viruses and Cancer
Cancer remains one of the most challenging diseases in modern medicine, affecting millions of people worldwide. While traditional treatments like chemotherapy, radiation, and surgery have been the cornerstone of cancer management, they often come with significant side effects and are not always effective. In recent years, the field of molecular oncology has emerged as a game-changer in understanding and treating cancer at the genetic and cellular level. Molecular oncology focuses on identifying the specific genetic mutations and molecular pathways that drive cancer growth, allowing researchers and clinicians to develop highly targeted therapies. These advancements have paved the way for precision medicine, where treatments are tailored to the unique genetic profile of a patient's tumor. With the advent of next-generation sequencing, targeted therapies, immunotherapy, and liquid biopsies, molecular oncology is fundamentally transforming how we diagnose, treat, and manage cancer.
The molecular oncology market is poised for significant growth over the next decade, driven by advancements in genomic research, precision medicine, and targeted cancer therapies. Valued at USD 2.3 billion in 2023, the industry is projected to expand at a CAGR of 11.0% from 2024 to 2034, reaching over USD 7.4 billion by 2034.
Key factors fueling this growth include the rising prevalence of cancer, increasing adoption of next-generation sequencing (NGS), and the growing demand for personalized treatment approaches. Additionally, innovations in liquid biopsy, biomarker discovery, and AI-driven oncology research are expected to further accelerate market expansion, making molecular oncology a cornerstone of future cancer care.
The Molecular Basis of Cancer
Cancer is fundamentally a disease of the genome, arising from genetic mutations and epigenetic changes that disrupt normal cellular functions. These changes can be inherited or acquired due to environmental factors such as smoking, UV radiation, chemical exposure, and viral infections. At the heart of cancer development are three key molecular components: oncogenes, tumor suppressor genes, and DNA repair mechanisms.
Oncogenes are genes that normally play a role in cell growth and division. When mutated or overexpressed, they become hyperactive, leading to uncontrolled cell proliferation. Examples include the KRAS gene, which is frequently mutated in lung, colorectal, and pancreatic cancers, and the MYC gene, which is implicated in aggressive cancers like Burkitt lymphoma. In contrast, tumor suppressor genes act as the body’s defense mechanisms, preventing uncontrolled cell growth. When these genes are mutated or deleted, they lose their ability to regulate cell division and apoptosis, leading to cancer progression. The TP53 gene, often referred to as the "guardian of the genome," is the most commonly mutated tumor suppressor in human cancers. It plays a critical role in DNA repair, cell cycle regulation, and apoptosis. When TP53 is inactivated, damaged cells survive and proliferate, increasing the risk of tumor development.
Another crucial aspect of cancer biology is DNA repair mechanisms. The human body constantly repairs DNA damage through various pathways, such as mismatch repair (MMR) and homologous recombination repair (HRR). Defects in these repair systems lead to genomic instability and increase cancer susceptibility. For instance, mutations in BRCA1 and BRCA2 impair the DNA repair process, significantly raising the risk of breast and ovarian cancers. In addition to genetic mutations, epigenetic modifications—such as changes in DNA methylation and histone modifications—can also contribute to cancer progression. These changes can silence tumor suppressor genes or activate oncogenes, further driving malignancy.
Advancements in Molecular Oncology
Next-Generation Sequencing (NGS) and Genomic Profiling
The development of next-generation sequencing (NGS) has revolutionized the field of molecular oncology. This technology allows scientists to analyze entire genomes, transcriptomes, and epigenomes in a high-throughput manner, identifying key mutations and molecular alterations in individual tumors. NGS has led to the discovery of actionable mutations in various cancers, enabling oncologists to select the most effective targeted therapies. For example, in lung cancer, NGS can detect mutations in EGFR, ALK, and ROS1, guiding the use of specific inhibitors like erlotinib, crizotinib, and lorlatinib. Genomic profiling has also been instrumental in classifying tumors into molecular subtypes, leading to more precise treatment decisions and improved patient outcomes.
Targeted Therapies: Precision Medicine in Action
One of the biggest breakthroughs in molecular oncology is the development of targeted therapies, which attack cancer cells based on their specific genetic alterations while sparing healthy cells. Unlike chemotherapy, which indiscriminately kills rapidly dividing cells and causes significant side effects, targeted therapies are designed to interfere with specific molecular pathways that drive cancer growth.
One of the earliest success stories in targeted therapy was imatinib (Gleevec), which transformed the treatment of chronic myeloid leukemia (CML) by inhibiting the BCR-ABL fusion protein. Since then, numerous targeted therapies have been developed. Tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, are highly effective in treating EGFR-mutant lung cancer. HER2-targeted monoclonal antibodies, like trastuzumab (Herceptin), have dramatically improved survival rates for HER2-positive breast cancer patients. PARP inhibitors, such as olaparib and rucaparib, are now widely used for BRCA-mutated ovarian and breast cancers by exploiting their defective DNA repair mechanisms.
Immunotherapy and Its Role in Molecular Oncology
While targeted therapies have significantly improved treatment outcomes, immunotherapy has emerged as another revolutionary approach in molecular oncology. Unlike traditional treatments that directly attack cancer cells, immunotherapy enhances the body’s immune system to recognize and destroy cancer cells. One of the most important breakthroughs in this field is the development of immune checkpoint inhibitors, which block proteins like PD-1, PD-L1, and CTLA-4, preventing cancer cells from evading immune detection. Drugs like pembrolizumab (Keytruda) and nivolumab (Opdivo) have been life-changing for patients with melanoma, lung cancer, and other malignancies.
Another promising innovation is CAR-T cell therapy, which involves genetically modifying a patient’s T cells to recognize and attack cancer cells. CAR-T therapy has shown remarkable success in treating blood cancers like leukemia and lymphoma, with long-term remissions in some patients. As research continues, scientists are exploring ways to extend the benefits of immunotherapy to solid tumors and minimize immune-related side effects.
Liquid Biopsies: A Non-Invasive Approach to Cancer Detection
A major challenge in oncology has been the difficulty of obtaining tumor tissue for genetic analysis. Traditional biopsies are invasive and sometimes not feasible, especially for deep-seated tumors. Liquid biopsies offer a revolutionary solution by detecting circulating tumor DNA (ctDNA), tumor-derived exosomes, and other biomarkers in the blood. This technology allows for early cancer detection, treatment monitoring, and identification of drug resistance without requiring invasive procedures. Liquid biopsies are particularly useful for tracking minimal residual disease (MRD) and detecting relapse earlier than imaging techniques. Companies like Guardant Health and Foundation Medicine have developed commercial liquid biopsy tests that are transforming cancer care.
Challenges and Future Directions
Despite the remarkable progress in molecular oncology, several challenges remain. One major issue is tumor heterogeneity, where different parts of the same tumor may have distinct genetic profiles, making treatment more complicated. Drug resistance is another obstacle, as cancer cells can evolve and develop mechanisms to evade targeted therapies. Additionally, while molecular testing and precision medicine have improved outcomes, not all patients have access to these cutting-edge treatments due to cost and availability issues.
Looking ahead, researchers are working to identify new biomarkers for early cancer detection, develop combination therapies to prevent resistance, and integrate artificial intelligence (AI) and machine learning to analyze complex genomic data for better treatment decisions. The future of molecular oncology lies in personalized, data-driven cancer treatment, bringing us closer to more effective and less toxic cancer therapies.
0 notes
Text
youtube
#piR-31115#PIWIL4#HSP90AA1#triple-negative breast cancer#MDA-MB-231#cancer cell migration#TNBC#metastasis#cancer research#oncogenic pathways#molecular oncology#heat shock proteins#cancer biomarkers#tumor progression#cancer cell biology#therapeutic targets#cancer therapeutics#tumor microenvironment#breast cancer mechanisms#oncology innovation.#Youtube
0 notes
Text
Understanding c-MET Non-Small Cell Lung Cancer Market: Causes, Symptoms, Diagnosis, and Treatment Options
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, comprising approximately 85% of all lung cancer cases. Within the realm of NSCLC, c-MET-positive tumors represent a significant subset associated with specific molecular and clinical characteristics. This article provides a detailed exploration of c-MET-positive NSCLC, including its causes, signs and symptoms, diagnostic approaches, and treatment options, drawing insights from the latest market research and developments.
c-MET, also known as hepatocyte growth factor receptor (HGFR), is a proto-oncogene that plays a crucial role in cell growth, migration, and invasion. In NSCLC, the c-MET gene can become dysregulated, leading to aberrant signaling pathways that promote tumor growth and metastasis. Overexpression or mutation of the c-MET gene has been implicated in aggressive tumor behavior and resistance to standard therapies.
c-MET-positive NSCLC often presents with distinct clinical features and may require specialized treatment approaches to effectively manage the disease. Identifying and targeting c-MET alterations is essential for optimizing therapeutic strategies and improving patient outcomes.
Causes of c-MET Non-Small Cell Lung Cancer
The exact causes of c-MET-positive NSCLC are multifaceted and involve a combination of genetic and environmental factors:
1. Genetic Mutations: Mutations or amplifications in the c-MET gene can lead to the overexpression of the c-MET protein, driving tumor growth and progression. These genetic alterations can result from inherited genetic predispositions or acquired mutations.
2. Environmental Factors: Exposure to carcinogens, such as tobacco smoke, asbestos, and environmental pollutants, is a well-established risk factor for lung cancer. These environmental factors can contribute to the development of genetic mutations and aberrant signaling pathways, including those involving c-MET.
3. Preexisting Lung Conditions: Chronic lung conditions, such as chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis, can increase the risk of developing lung cancer. These conditions may contribute to the dysregulation of c-MET signaling.
4. Family History: A family history of lung cancer or other cancers can increase the risk of developing NSCLC, including c-MET-positive tumors. Genetic predisposition and familial cancer syndromes may play a role in c-MET dysregulation.
Signs and Symptoms
The symptoms of c-MET-positive NSCLC are similar to those of other NSCLC subtypes, though the presence of c-MET alterations may influence the disease's clinical behavior. Common signs and symptoms include:
1. Persistent Cough: A chronic cough that does not improve with treatment may be an early sign of lung cancer. In c-MET-positive NSCLC, the cough may become more severe and persistent.
2. Shortness of Breath: Difficulty breathing or shortness of breath can occur as the tumor grows and obstructs the airways or lung tissue.
3. Chest Pain: Patients may experience localized chest pain or discomfort, which can be caused by the tumor's growth or invasion into surrounding tissues.
4. Hemoptysis: Coughing up blood or blood-streaked sputum may indicate advanced disease or tumor invasion.
5. Weight Loss and Fatigue: Unexplained weight loss and persistent fatigue are common systemic symptoms of cancer, including c-MET-positive NSCLC.
6. Recurrent Infections: Frequent respiratory infections or pneumonia may occur as the tumor obstructs or damages the lung tissue.
To know more about c-MET NSCLC Treatment market, visit: https://www.delveinsight.com/report-store/cmet-mnsclc-market
Diagnosis of c-MET Non-Small Cell Lung Cancer
The diagnosis of c-MET-positive NSCLC involves a combination of imaging studies, biopsy, and molecular testing:
1. Imaging Studies:
- Chest X-Ray: A preliminary imaging test that can reveal abnormalities in the lungs, such as masses or nodules.
- Computed Tomography (CT) Scan: Provides detailed cross-sectional images of the chest and can help identify the size, location, and extent of the tumor. CT scans are often used for staging and assessing tumor spread.
- Positron Emission Tomography (PET) Scan: PET scans can detect areas of increased metabolic activity associated with cancer, aiding in the detection of metastases.
2. Biopsy:
- Bronchoscopy: A procedure in which a flexible tube with a camera is inserted through the airways to obtain tissue samples from the tumor.
- Needle Biopsy: A needle is used to extract tissue samples from the tumor, often guided by imaging techniques.
3. Molecular Testing:
- c-MET Gene Testing: Identifies mutations or amplifications in the c-MET gene that are associated with tumor growth and progression. This testing is essential for determining the appropriate targeted therapy.
- Next-Generation Sequencing (NGS): Provides comprehensive genetic analysis to identify various mutations and alterations in the tumor, including c-MET.
Treatment Options for c-MET Non-Small Cell Lung Cancer
Treatment for c-MET-positive NSCLC often involves a combination of standard therapies and targeted approaches to address the specific molecular characteristics of the tumor:
1. Surgery: Surgical resection is often the first-line treatment for localized NSCLC. The goal is to remove the tumor and affected lung tissue. However, surgery may not be suitable for all patients, particularly those with advanced or metastatic disease.
2. Radiation Therapy: Radiation therapy uses high-energy rays to target and destroy cancer cells. It may be used as an adjuvant treatment following surgery or as a primary treatment for inoperable tumors.
3. Chemotherapy: Systemic chemotherapy involves the use of drugs to kill cancer cells throughout the body. It is often used for advanced or metastatic NSCLC and may be combined with other treatments.
4. Targeted Therapy:
- c-MET Inhibitors: Specific drugs targeting the c-MET pathway, such as crizotinib, cabozantinib, and tepotinib, are used to inhibit abnormal c-MET signaling and reduce tumor growth. These therapies are tailored to patients with c-MET gene mutations or amplifications.
- Combination Therapies: Combining c-MET inhibitors with other targeted agents or immunotherapies may enhance treatment efficacy and overcome resistance.
5. Immunotherapy: Immune checkpoint inhibitors, such as pembrolizumab and nivolumab, can help stimulate the immune system to recognize and attack cancer cells. While not specific to c-MET, these therapies may be used in conjunction with targeted treatments.
6. Clinical Trials: Participation in clinical trials may provide access to new and experimental treatments that are not yet widely available. Clinical trials are essential for advancing treatment options and improving outcomes for patients with c-MET-positive NSCLC.
Market Insights
The market for c-MET-positive NSCLC treatments reflects the growing demand for targeted therapies and advancements in personalized medicine:
- Market Size and Growth: The global market for c-MET-positive NSCLC treatments is expanding, driven by increasing prevalence, advances in molecular diagnostics, and the development of targeted therapies. Key market segments include pharmaceuticals, diagnostic tools, and personalized treatment solutions.
- Key Players: Leading companies involved in the c-MET NSCLC market include:
- Pfizer Inc.: Known for its development of targeted therapies and involvement in the treatment of lung cancer.
- Roche Holdings: Engaged in the development of targeted therapies and molecular diagnostics for NSCLC.
- Novartis Pharmaceuticals: Focuses on innovative treatments and research in oncology, including c-MET-targeted therapies.
- AstraZeneca: Contributes to research and development of targeted and combination therapies for lung cancer.
- Research and Development: Ongoing research aims to improve understanding of c-MET signaling, develop new targeted therapies, and enhance diagnostic capabilities. Innovations in drug development and molecular diagnostics are contributing to better management and treatment outcomes for patients with c-MET-positive NSCLC.
c-MET-positive NSCLC represents a significant subset of non-small cell lung cancer with distinct molecular and clinical characteristics. Understanding the causes, symptoms, and treatment options is crucial for effective management of this challenging condition. Advances in targeted therapies and molecular diagnostics offer hope for improved outcomes and personalized treatment strategies. The growing market for c-MET NSCLC treatments underscores the importance of continued research and innovation in addressing this complex and impactful form of lung cancer.
Download report @ https://www.delveinsight.com/sample-request/cmet-mnsclc-market
0 notes
Text
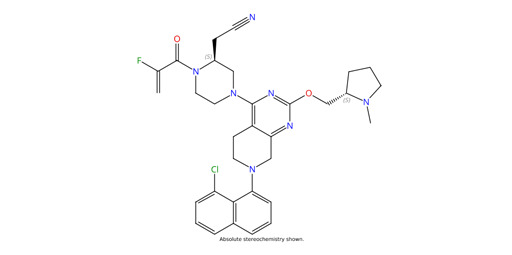
Adagrasib is an advanced investigational anti-cancer agent, specifically designed as a potent and selective inhibitor of the KRAS G12C mutation, which is prevalent in various cancers such as non-small cell lung cancer (NSCLC). This API (Active Pharmaceutical Ingredient) works by irreversibly binding to the mutant KRAS protein, thereby preventing its activation and subsequent oncogenic signaling pathways. This document delves into the pharmacodynamics, detailed mechanism of action, and emerging clinical applications of Adagrasib, offering insights into its potential role in targeted cancer therapies.
0 notes
Text
Deciphering the Ras/MAPK Signaling Pathway in the Progression and Treatment of Hepatocellular Carcinoma_Crimson Publishers

Abstract
Hepatocellular Carcinoma (HCC) is a serious health issue and its frequency is rapidly escalating throughout the world therefore researchers have focused more attention to the Ras/MAPK signaling pathway. The signaling pathways are linked to develop tumors and the Ras/MAPK pathway is one of these pathways, activated in 60% of HCCs with poor prognosis. A number of different proteins causes the abnormal regulation of the MAPK pathway in HCC. Ras, a small GTPase and Raf are the most commonly mutated oncogene supports the critical function of this pathway in oncogenesis. The genetic mutations leading to effector molecule to permanently activated in the Ras/MAPK signaling cascades. The inappropriate activation of this pathway is primarily due to the downregulation of various Ras/MAPK pathway inhibitors including RASSF proteins, GAPs, DUSP1, Spred and Sprouty proteins. The post-transcriptional or epigenetic processes downregulate these cancer suppressor genes. The aim of current study on the primary mutations resulting in aberrant activation of Ras/MAPK pathway and their role on the initiation and progression of HCC. It also offers an update on the various inhibitors to target this central signaling pathway including various Ras, Raf, MEK inhibitors in the context of HCC. Finally, we evaluate the available options for treatment in this context.
Read more about this article:
For more articles in Novel Approaches in Cancer Study
#cancer#breast cancer#crimson cancer#open access journal#cancer open access journal#crimsonpublishers#novel approaches in cancer study
0 notes