#process validation
Text



we'll do fine.
#fionna and cake spoilers#what hits me a little is how similar fionna and simon's stories are in the case of finding nuance in their lives#when both have gone through their separate but still valid pain no matter the extent it had been#and its that they met each other they get to see how it compares and they're no less worth of the peace and fun they dreamed of#even in the form of simplicity and just being normal#“i wouldn't have met THE fionna and cake” “we wouldn't have met THE simon petrikov”#it hits me harder that after the dandelion scene would've been their last time seeing each other physically#and how assuring simon sounded when fionna didn't know what to do with the literal world in her hands#tho im sure prismo isnt that much of a rule jerk lol i still drew out the revelation anyway with this tiniest addition#also the fact fionna's world is influenced by simon's thought processes and conditions so now things are a little better for both of them#fionna the human#fionna campbell#simon petrikov#qiiarts#the lil flashback of#betty grof#fionna and cake#adventure time
35K notes
·
View notes
Text
Chef WK, lead charcuterie specialist in Alberta Canada
Table of contents
1. Control Program Requirements for Fermented Meat Products
2. Facility and Equipment Requirements
3. Starter Culture
4. Chemical Acidification
5. Water Activity Critical Limits
6. Time and Temperature for Fermented Products
7. Fermentation Done at a Constant Temperature
8. Examples of Degree-hours at constant room temperatures
9. Fermentation Done at Different Temperatures
10. Fermentation done at Different temperatures
11. What happens if fermentation fails to hit critical limit?
12. E. coli and Salmonella Control in Fermented Sausages
13. Options for E. coli validation
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
Control Program Requirements for Fermented Meat Products
The producer must have a program in place to assess the incoming product. This program should outline specifications for the incoming ingredients. This may include criteria including receiving temperature, farm/ supplier, lot code or packed on date, species/cut etc.
2. Facility and Equipment Requirements
Equipment used in the fermentation process must be included in the operator's prerequisite control programs. These must include the following elements:
Temperature in the fermentation, drying and smoking chambers must be uniform and controlled to prevent any fluctuation that could impact on the safety of the final product.
Fermentation, drying and smoking chambers must be equipped with a shatter resistant indicating thermometer, (or equivalent), with graduations of 1°C or less. If mercury thermometers are used, their mercury columns must be free from separations. All thermometers must be located such that they can be easily read.
Fermentation and smoking chambers must be equipped with a recording thermometer for determining degree-hours calculations in a reliable manner. Recording thermometers are also preferable in drying and aging rooms but, in these rooms, it may be sufficient to read and record the temperatures 2 times a day.
Drying and aging rooms must be equipped with humidity recorders in order to prevent uncontrolled fluctuations of the relative humidity. The only alternative to an automatic humidity recorder in these rooms would be for the company to manually monitor and record ambient humidity twice a day (morning and afternoon) every day with a properly calibrated portable humidity recorder.
For routine monitoring, accurate measurement electronic pH meters (± 0.05 units) should be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument as well as recommended sample preparation and testing be followed.
When the aw of a product is a critical limit set out in the HACCP plan for a meat product, accurate measurement devices must be employed. It is important that the manufacturer's instructions for use, maintenance and calibration of the instrument be followed.
3. Starter Culture
The operator must use a CFIA approved starter culture. This includes Freeze-dried commercially available culture as well as back-slopping (use of previously successful fermented meat used to inoculate a new batch). When performing back-slopping, the operator must have a control program in place to prevent the transmission of pathogens from when using the inoculum from a previous batch to initiate the fermentation process of a new batch. These must include:
The storage temperature must be maintained at 4°C or less and a pH of 5.3 or less.
Samples for microbiological analysis must be taken to ensure that the process is in line with the specifications.
The frequency of sampling is to be adjusted according to compliance to specifications.
Any batch of inoculum which has a pH greater than 5.3 must be analysed to detect at least Staphylococcus aureus. Only upon satisfactory results will this inoculum be permitted for use in back slopping.
This can be an expensive and a time exhaustive process and is generally avoided due to food safety concerns. AHS does not allow back-slopping.
[Chef WK was in communication with the U of A to get his method, a starter mix, studied.]
4. Chemical Acidification
If product is chemically acidified by addition of citric acid, glucono-delta-lactone or another chemical agent approved for this purpose, controls must be in place and records kept to ensure that a pH of 5.3 or lower is achieved by the end of the fermentation process. These acids are encapsulated in different coatings that melt at specific temperatures, which then release the powdered acids into the meat batter and directly chemically acidulate the protein.
Summer sausage is a very common chemically acidified product. The flavor profile tends to be monotone and lacking depth.
5. Water Activity Critical Limits
The aw may be reduced by adding solutes (salt, sugar) or removing moisture.
Approximate minimum levels of aw (if considered alone) for the growth of:
molds: 0.61 to 0.96
yeasts: 0.62 to 0.90
bacteria: 0.86 to 0.97
Clostridium botulinum: 0.95 to 0.97
Clostridium perfringens: 0.95
Enterobacteriaceae: 0.94 to 0.97
Pseudomonas fluorescens: 0.97
Salmonella: 0.92 - 0.95
Staphylococcus aureus: 0.86
parasites: Trichinella spiralis will survive at an aw of 0.93 but is destroyed at an aw of 0.85 or less.
The above levels are based on the absence of other inhibitory effects such as nitrite, competitive growth, sub-optimum temperatures, etc., which may be present in meat products. In normal conditions, Staphylococcus aureus enterotoxins are not produced below aw 0.86, although in vacuum packed products this is unlikely below aw 0.89.
6. Time and Temperature for Fermented Products
Certain strains of the bacteria Staphylococcus aureus are capable of producing a highly heat stable toxin that causes illness in humans. Above a critical temperature of 15.6°C, Staphylococcus aureus multiplication and toxin production can take place. Once a pH of 5.3 is reached, Staphylococcus aureus multiplication and toxin production are stopped.
Degree-hours are the product of time as measured in hours at a particular temperature multiplied by the "degrees" measured in excess of 15.6°C (the critical temperature for growth of Staphylococcus aureus). Degree-hours are calculated for each temperature used in the process. The limitation of the number of degree-hours depends upon the highest temperature in the fermentation process prior to the time that a pH of 5.3 or less is attained.
The operator is encouraged to measure temperatures at the surface of the product. Where this is not possible, the operator should utilize fermentation room temperatures. The degree hour calculations are based on fermentation room temperatures. Temperature and humidity should be uniform throughout the fermentation room.
A process can be judged as acceptable provided the product consistently reaches a pH of 5.3 using:
fewer than 665 degree-hours when the highest fermentation temperature is less than 33°C;
fewer than 555 degree-hours when the highest fermentation temperature is between 33° and 37°C; and
fewer than 500 degree-hours when the highest fermentation temperature is greater than 37°C.
This means that as the temperature increases, the amount of time that you have available to reach 5.3 or under is shorter. The warmer the temperature, the sharper the log growth phase of bacteria, which equates to more overshoot in lactic acid production, faster.
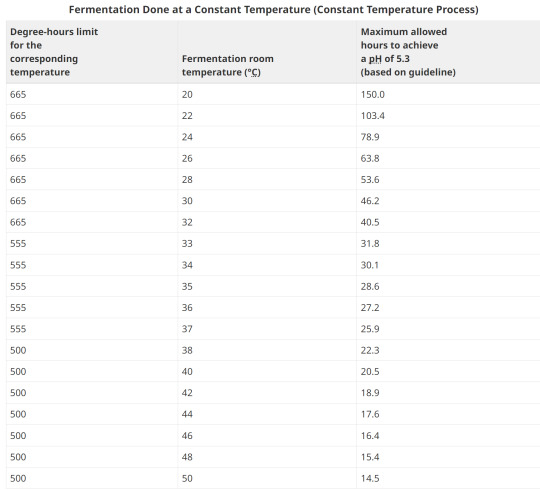
8. Examples of Degree-hours at constant room temperatures
Example 1:
Fermentation room temperature is a constant 26°C. It takes 55 hours for the pH to reach 5.3.
Degrees above 15.6°C: 26°C - 15.6°C = 10.4°C
Hours to reach pH of 5.3: 55
Degree-hours calculation: (10.4°C) x (55) = 572 degree-hours
The corresponding degree-hours limit (less than 33°C) is 665 degree-hours.
Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
Example 2:
Fermentation room temperature is a constant 35°C. It takes 40 hours for the pH to reach 5.3.
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C
Hours to reach pH of 5.3: 40
Degree-hours calculation: (19.4°C) x (40) = 776 degree-hours
The corresponding degree-hours limit (between 33 and 37°C) is 555 degree-hours.
Conclusion: Example 2 does not meet the guideline because its degree-hours exceed the limit
9. Fermentation Done at Different Temperatures
When the fermentation takes place at various temperatures, each temperature step in the process is analyzed for the number of degree-hours it contributes. The degree-hours limit for the entire fermentation process is based on the highest temperature reached during fermentation.
Example 1:
It takes 35 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for second 10 hours and 35°C for the final 15 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 35°C - 15.6°C = 19.4°C
Hours to reach pH of 5.3: 15
Degree-hours calculation: (19.4°C) x (15) = 291 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 291 = 519
The highest temperature reached = 35°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)Conclusion: Example 1 meets the guideline because its degree-hours are less than the limit.
10. Fermentation done at Different temperatures
Example 2:
It takes 38 hours for product to reach a pH of 5.3 or less. Fermentation room temperature is 24°C for the first 10 hours, 30°C for the second 10 hours and 37°C for the final 18 hours.
Step 1
Degrees above 15.6°C: 24°C - 15.6°C = 8.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (8.4°C) x (10) = 84 degree-hours
Step 2
Degrees above 15.6°C: 30°C - 15.6°C = 14.4°C
Hours to reach pH of 5.3: 10
Degree-hours calculation: (14.4°C) x (10) = 144 degree-hours
Step 3
Degrees above 15.6°C: 37°C - 15.6°C = 21.4°C
Hours to reach pH of 5.3: 18
Degree-hours calculation: (21.4°C) x (18) = 385.2 degree-hours
Degree-hours calculation for the entire fermentation process = 84 + 144 + 385.2 = 613.2
The highest temperature reached = 37°C
The corresponding degree-hour limit = 555 (between 33°C and 37°C)
Conclusion: Example 2 does not meet the guidelines because its degree-hours exceed the limit.
11. What happens if fermentation fails to hit critical limit?
What happens if the batch takes longer than degree-hours allows? For restaurant level production, it's always safer to discard the product. The toxin that Staph. Aureus produces is heat stable and cannot be cooked to deactivate. In large facilities that produce substantial batches, the operator must notify the CFIA of each case where degree-hours limits have been exceeded. Such lots must be held and samples of product submitted for microbiological laboratory examination after the drying period has been completed. Analyses should be done for Staphylococcus aureus and its enterotoxin, and for principal pathogens, such as E. coli O157:H7, Salmonella, and Clostridium botulinum and Listeria monocytogenes.
If the bacteriological evaluation proves that there are fewer than 104 Staphylococcus aureus per gram and that no enterotoxin or other pathogens are detected, then the product may be sold provided that it is labelled as requiring refrigeration.
In the case of a Staphylococcus aureus level higher than 104 per gram with no enterotoxin present the product may be used in the production of a cooked product but only if the heating process achieves full lethality applicable to the meat product.
In the case where Staphylococcus aureus enterotoxin is detected in the product the product must be destroyed.
12. E. coli and Salmonella Control in Fermented Sausages
Business' that manufacture fermented sausages are required to control for verotoxinogenic E. coli including E. coli O157:H7 and Salmonella when they make this type of product. This includes:
establishments which use beef as an ingredient in a dry or semi-dry fermented meat sausage;
establishments which store or handle uncooked beef on site;
Establishments which do not use beef and do not obtain meat ingredients from establishments which handle beef are not currently required to use one of the five options for the control of E. coli O157:H7 in dry/semi-dry fermented sausages.
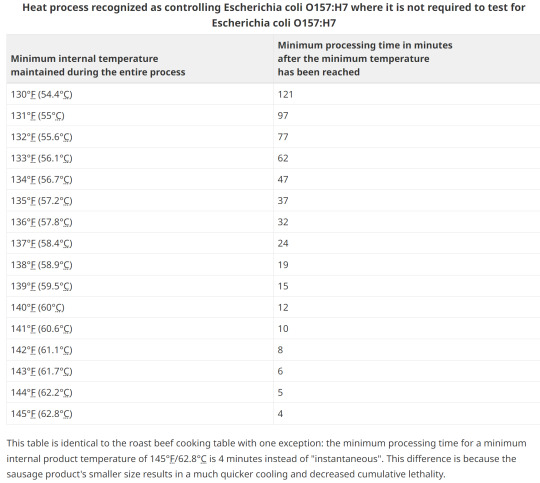
Any processed RTE product containing beef or processed in a facility that also processed beef, must be subjected to a heat treatment step to control E. coli O157:H7. Heating to an internal temperature of 71°C for 15 seconds or other treatment to achieve a 5D reduction is necessary. This is a CFIA requirement and is not negotiable.
Uncooked air dried products produced as RTE, must meet shelf stable requirements as detailed for Fermented-Dry products.
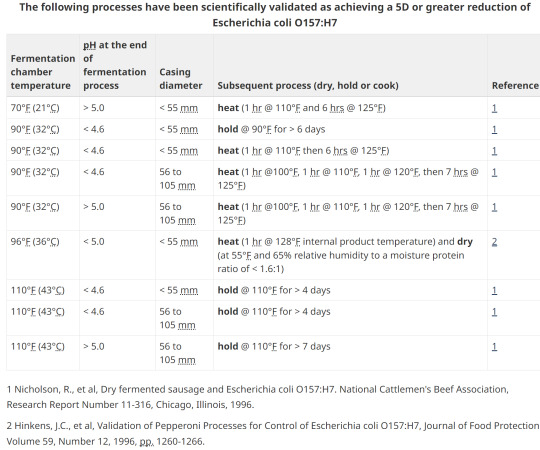
13. Options for E. coli validation
Without lab testing, the two main methods of validation are with heat treating by either low temp and a long duration, or various hotter processing temperatures for a shorter timeframe.
A challenge study to validate a process can take 1 year and over $100,000!
14. Option1; Heating
15. Option 2; pH, heating, holding, diameter
16. Safety and consistency
The aw and pH values are critical in the control of pathogens as well as to ensure shelf-stability in all semi-dry and dry fermented meat products. Each batch must be tested for aw and/or pH in order to verify that the critical limits are met.
Although aw measurement is mandatory only for shelf stable products, it is strongly recommended that the producer determine the aw values achieved for each product type they manufacture and for each product. Once this has been established, frequent regular checks should be made to ensure consistency. In the U.S., they rely on moisture to protein ratio and have set targets. This lab-tested value is a direct correlation of the % water to % meat protein and not aw. This gives more consistency to common names. For example, to legally call a product "jerky" it must have a MPR of 0.75:1 or lower.
Remember your ABCs:
Always be compliant.
-AND-
Documentation or it didn't happen.
(tags)
Charcuterie,Fermented Meat,Food Safety,Starter Culture,Chemical Acidification,Water Activity,Fermentation Process,Degree-Hours Method,Foodborne Pathogens,Meat Processing Guidelines,Chef WK Alberta Canada,Food Industry Standards,pH Critical Limits,Thermal Processing,Food Preservation,Food Microbiology,Sausage Fermentation,Charcuterie Expertise,Fermented Meats ,Food Safety Standards,Food Processing Guidelines,Starter Cultures,Chemical Acidification,Water Activity (a_w),Critical Limits,Degree-Hours Method,Foodborne Pathogens,Meat Processing Equipment,Processing Facility Requirements,Hazard Analysis and Critical Control Points (HACCP),Food Preservation Techniques,Temperature Control,Pathogen Reduction,Food Industry Compliance,Documentation Practices,Heat Treatment,pH Control,Food Stability,Consistency in Production,Microbial Testing,Real-time Monitoring,Process Validation,Regulatory Requirements,Verotoxigenic E. coli,Lethality Standards,Product Labelling,Spoilage Prevention,Enterotoxin Detection,Shelf-Stable Products,Moisture to Protein Ratio (MPR)
#Charcuterie#Fermented Meat#Food Safety#Starter Culture#Chemical Acidification#Water Activity#Fermentation Process#Degree-Hours#Meat Processing Guidelines#Thermal Processing#Food Preservation#Food Microbiology#Sausage Fermentation#Starter Cultures#Critical Limits#Meat Processing#Food Preservation Techniques#Temperature Control#Pathogen Reduction#Food Industry#Heat Treatment#pH Control#Food Stability#Microbial Testing#Real-time Monitoring#Process Validation#Spoilage Prevention#Enterotoxin Detection#Shelf-Stable Products#Moisture to Protein Ratio (MPR)
1 note
·
View note
Text
Why self-diagnosed autistics are valid

medical diagnosis can be expensive
humans are the experts on their own minds
family may prevent assessment
diagnosis criteria is a poor checklist of stereotypes
diagnosis criteria ignores gender, race, sexuality, culture & more
medical diagnosis confirms autism, but doesn't create it
discrimination within the medical profession may prevent diagnosis.
Assessment waiting lists often long
medical trauma may make assessment unfeasible
Neurodiverse Journeys
#self diagnosis#why it’s valid#I find it perfectly acceptable with doing the correct research#the diagnosis process can be complicated and sometimes not a very pleasant experience#i honestly think I had ADHD too but I have no idea if I am#actually autistic#self diagnosed adhd#neurodivergence#neurodiversity#actually neurodivergent#feel free to share/reblog#Neurodiverse Journeys (Facebook)#autism awareness month#autism acceptence month
6K notes
·
View notes
Text
Decoding Process Validation for FDA Compliance: Grab Our White Paper Now!
Unveil the significance of process validation through the eyes of FDA. 🌐🔒
📌 In the realm of pharmaceuticals and medical devices, meeting FDA standards is non-negotiable. Our illuminating white paper delves deep into the FDA's perspective on process validation, shedding light on the critical steps to ensure product quality, safety, and regulatory adherence.
🔬 Key Highlights:
- Explore the FDA's expectations for robust process validation protocols.
- Understand the pivotal role of data integrity and documentation.
- Gain insights into how Process Validation safeguards patient well-being.
- Learn from real-world case studies of successful FDA-compliant validations.
🚀 Elevate your compliance strategy and product excellence. Download the white paper from Compliance Group Inc today to gain a comprehensive understanding of how process validation aligns with FDA's vision for impeccable standards.
📥 Download Now - What Does Process validation Mean to the FDA? - Compliance Group Inc
#ProcessValidation #FDACompliance #QualityAssurance #ProductExcellence #DownloadToday
0 notes
Text

#this just appeared in my head and I had to do it#honestly very valid to go insane and do something unhinged after your partner of 20 years is still hung up over his ex and calls you boring#though daniel doesn't deserve any of this#IWTV is a process of continuously going “Oooft get out of this situation- you deserve better” about different people.#Armand#IWTV#interview with the vampire#amc iwtv#iwtv spoilers#funny#Daniel Molloy#Devil's minion#meme#armand iwtv
1K notes
·
View notes
Text
Shen Yuan getting transported into pidw isn't "the system punishing him for being a lazy internet hater," but instead representative of "step 1 of the creative process: getting so mad at something you decide to go write your own fucking book" in this essay I will
#svsss#scum villian self saving system#shen qingqiu#shen yuan#the fact that people think scum villain#-a series that examines and criticizes common tropes in fiction-#is somehow against criticism or being a little hater is wild to me#especially since shen qingqiu never gets punished for being a hater#heck- he's still a little hater by the end of the series#he mostly gets punished for treating life like a play and like he and the people around him are characters#(or in other words- he suffers for denying his own wants and emotions and his own sense of empathy)#I think some of y'all underestimate how much writing/art is inspired by creaters being little haters#like example off the top of my head-#the author of Iron Widow has been pretty vocal about the book being inspired by their hatred of Darling in the Franxx#I think my interpretation of Shen Yuan's transmigration is also supported by the fact that this series is an examines writing processes#side note- though i understand why people say Shen Yuan is lazy and think its a valid take it still doesnt sit right with me#i am probably biased because my own experiences with chronic pain and depression and isolation#but ya- i dont think Shen Yuan is lazy so much as he is deeply lonely and feels purposeless after denying parts of himself for 20ish years#like yall remember the online fandom boom from covid right?#being stuck completely alone in bed while feeling like shit for 20 days straight does shit to your brain#the fact that no one came to check on him + he wasn't exactly upset about leaving anyone behind supports the isolation interpretation too#+in the skinner demon arc he describes his life of being a faker/inability to stop being a faker now that he's Shen Qingqiu#as “so bland he's tempted to throw salt on himself” and “all he could do is lay around and wait for death” (<-paraphrasing)#bro wants to be doing stuff but is stuck in paralysis from repeatedly following scrips made by other people#another point on “Shen Yuan isn’t lazy” is just the sheer amount of studying that man does#also he did graduate college- how lazy can he really be#he doesnt know what hes doing but he at least tries to actively train his students#and he actually works on improving his own cultivation + spends quite a bit of time preping the mushroom body thing#+he's experiencing bouts of debilitating chronic pain throughout all this#but ya tldr: Shen Yuan's transmigration is an encouragement to write and not a punishment and also i dont think its fair to call him lazy
929 notes
·
View notes
Text

girl experiences gender euphoria and is immediately slammed by grief
for @litttlittt <3. this was supposed to be a portrait of caroline hill, but litta mentioned tim looking like janet when dressed as caroline and identity issues and angst and things spiraled
something about tim not knowing if he's his mother's child or bruce's or neither's.
figuring out the looks:

i wanted janet to have that poofy 70s hair
#tim drake#dc#bruce wayne#janet drake#sart#i'm picturing this as transfemme tim hence “he” and also compounding issues about bruce treating him as a daughter#which is exactly the gender validation tim wants and needs but isnt sure he deserves#but this also definitely works for trans tim#she gets to process that she looks like her mom!! and her mom will never get to know her daughter#would she have wanted to know her daughter? even though she showered tim with love when she was around#she barely knew her son#gender idk he's a girl 👍 hope that helps#i went down a rabbit hole looking up vintage dior necklaces -- hopefully something martha wayne wouldve worn#-- but dior necklaces are COMPLICATED#i almost drew pearls but i think that wouldve been too cruel to bruce lol#(a decent amount of my art--even when it's not femme tim--gets tagged 'gender' and i dont know what im going but im glad 👍)
3K notes
·
View notes
Text
Are you making these mistakes with FDA 21 CFR PART 820 made for medical devices
Manufacturers are expected to follow the quality system requirements described in FDA 21 CFR part 820 This document guides to govern the design, manufacture, packaging, labelling, storage, installation, and servicing of medical devices intended for human use. The requirements in 21 CFR Part 820 are meant to ensure the safety and efficacy of medical devices sold in the US marketplace.
FDA conducts regular inspections of medical device manufacturers to ensure compliance with these regulations. The inspection process, known as the Quality System Inspection Technique (QSIT), evaluates a company’s internal quality processes to determine whether they are in alignment with or in violation of these regulatory requirements. If any violations are discovered, the inspecting agent from FDA will issue in the form of 483 Inspectional Observations, Warning Letters what is applicable
Here are the most common mistakes companies run into with FDA 21 CFR Part 820:
1. CAPA Procedures and 21 CFR Part 820.100(a) CAPA not followed adequately and repeat failure is detected
2. Complaint Handling and CFR Part 820.198(a) Parameters described in procedure are not fulfilled and closure is not timely and adequate.
3. Nonconforming Product and CFR Part 820.90(a) Investigation is not proper, CAPA is not applied conclusion is not enough, preventive action is not proper, repeat failure is detected.
4. Purchasing Controls and CFR Part 820.50 control for specification and artwork s not controlled purchase found with old label artwork and specification. PO does not mention specification or art- work reference
5. Process Validation and CFR Part 820.75 System is not adequate, not followed, re-validation where defined is not done or done late, changes are not captured in validation. Protocols are not properly and timely approved before starting activities.
6. Quality Audit and CFR Part 820.22 Audit done randomly, annual plan is not available, Audit closer is not proper, audit compliance are not reviewed in Management review meeting.
7. Device History Records and CFR Part 820.184 Incomplete, completed in hurry just before audit, quality and regulatory are not in full control of DHR.
8. Design Validation and CFR Part 820.30(g) Protocol is not adequate; all aspects of validation are not covered. Documentation is not proper.
9 PMS (post marketing surveillance) System is not followed. Some reportable incidents are not informed to FDA. Some critical or major complaints related to product not covered in PMS
10 UDI (Unique Device Identification) system documentation and records are poorly maintained.
11. Production, process control and quality control and assurance: System inadequate, documentation is not timely, and records are filled late or improper. Critical values are not verified by another independent person.
IZiel adopts an analytical mindset thus enabling us to root out all possible non-conformances in a regulatory submission. IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) for USFDA Approvals.
0 notes
Photo
were you his boy? were you his number one boy?
#succession#kendall roy#logan roy#fanart#losing my kenstew privileges from#drawing logan roy after a kenstew episode#but i have feelings about this that needed processing#when you love an absence so sanctified and gigantified#you hold a sheet of paper like a relic#and study the angle of an ink line#was it real#was any of it real#do you feel validated by a ghost ken#i'm sure you do since you've been trying to do that your whole life#my#and now i can finally go watch barry
2K notes
·
View notes
Text
we need to see Rex's outright refusal to forgive Anakin for the carnage and trauma he caused the galaxy and the clones juxtaposed to Ahsoka's acceptance of the good in him living on in her
because while Ahsoka needed to make peace with Anakin and what he became to continue on in her journey...
Rex does not. Rex deserves to know the truth so he can realize that HE was the hero in his own story. HE was the one trying to save the galaxy from evil. and that Anakin is no longer deserving of the reverence and loyalty that Rex always gives him to this day. Rex and his brothers gave everything, including their lives, for the good of the galaxy and they do not owe Anakin any ounce of gratitude anymore.
I just want to see Rex turn his back on him because he knows loyalty is EARNED not given and that you can/should revoke your support of someone when they no longer stand for what you believe in.
#would just be nice to show that everyones journey to processing trauma and betrayal like this will look different#and everyone's reaction is equally as valid#ahsoka series#ahsoka spoilers#captain rex
622 notes
·
View notes
Text
ASOIAF discourse would be a lot more fun if we all realized that every single person who has been put in a position of leadership/rulership fails in one way or another. Jon and Dany failing is not an indictment on their abilities to lead or rule. They’re kids, they still have shit to figure out. Given “what was Aragorn’s tax policy”, I doubt GRRM will write a story that will feature the appearance of a most perfect ruler ever who will be a total success instead Jon and Dany who were tOtAl FlOpS. Especially if this person has no previous experience that has been detailed within the text itself. That’s not only antithetical to the series, but also not how you write a narrative.
#I don’t mean to be condescending but so many people missed the point of yg#he’s not here to be the perfect solution over jon and dany#he’s here to validate their arcs as heroes and leaders - sorry to say#they’re incredibly flawed individuals and they intimately failed in adwd but like literally so did everyone else#FAILURE IS A PROCESS OF LEARNING!!!#their stories aren’t over yet#grrm isn’t going to write yg being the most perfect king ever so we can be like ew jon and dany suck glad they died or whatever#he’s also not likely to be the third head of the dragon but I don’t want to get into that today#asoiaf#jon snow#daenerys targaryen#valyrianscrolls#anti reddit rant lmao#god I should stop visiting that subreddit it’s the worst snajbabsjan
292 notes
·
View notes
Text
Look at my favorite bone in the body, the C5 vertebra. Look how happy he is to support you. What a cute and friendly little fella :D

His siblings, C3-C7, are equally as cute (with varying levels of dopey looking “faces”). They want to support you all day so you can hold your head up high!

So remember, no need to feel down; you have a team of four typical C vertebrae (and three atypical) to support you!
#yes your professor did make this meme. Go study#Also you can detect C3-7 vertebrae from T by their bifurcated spinous process (present on C3-6 but not on C7 as an atypical vertebra)#their transverse foramen (not on C7 either)#and/or their small size… also no ribs attached! just a fun fact for you (now go study my sillies!)#C1 and C2 just look like sid the sloth and a turtle; go look them up. They’re C7’s fellow weirdos#academic validation#premed#med school#med studyblr#anatomy#stemblr#stem academia#Anatomy memes#Silly academia#studyblr#Anatomy meme
97 notes
·
View notes
Note
So I l’ve been getting into your ko crisis story lately (it all sounds rlly awesome btw) and i was curious - you’ve said in the tags in a previous ask that ash is comphet, so is that gonna play a major role in her character arc/journey in any way?
Yeah, the story basically covers her journey of redefining her identity as a queer Asian-American disabled woman in a sports-entertainment industry, which is a media landscape that specifically targets, exploits, and fetishizes people like her.
#ask me#itsthequeercryptid#i don't have much more to say on this cuz as of right now it's in like. early early development process and i'm not a queer woman so#i don't have much authority to say how i want this part of the story to go. even if it's my story i don't wanna set anything in stone yknow#but like. ashley's arc is realizing that she's been performing to appease people both in and out of her life. as both a daughter and a#pro boxer. and that she shouldn't have to force herself to be someone she's not. which in the story continuously hurts her#she doesn't need to validate her existence and find worthiness as a disabled and queer woman#especially to other people#one story beat i drafted is that ashley nearly gets s/a'd by a potential agent (keep in mind the project's inspired by psychological dramas#thrillers like utena and perfect blue) and it's one of the first big moments that causes her to have a crisis of identity#growing less and less comfortable in her skin - both organic and nonorganic - as she realizes how her body is perceived/ exploited/#/objectified as a vehicle for inflicting and absorbing violence. both physical and sexual. especially by men#and near the end of the story the beat is reflected/flipped when she comes together with noora and it's gentle and intimate#but again this is all very iffy. i'm not sure how appropriate this would be as a male writer.#i'm waiting to work with other writers who are more knowledgable on this before moving forward with anything
108 notes
·
View notes
Text
as a certified Diagnosed Autist(TM) i cannot stress enough that i am not only pro- self-diagnosis, but also pretty anti- legal medical diagnosis. it is, at best, a cruel hoop we have to jump through so privileged people will deign to give us what we need. don't fucking do that shit unless you have to, it was disgustingly expensive, fucking humiliating, infantilizing, and dehumanizing, and would probably actively cause problems in my life if i didn't have some really good allistic (-passing) people in my corner and also wasn't so fucking disabled that it mostly doesn't matter.
literally get that diagnosis if you need it for job/school accessibility shit or SSI or whatever, and otherwise dont tell the government SHIT about yourself. there is zero good reason for them to want that information. that's between you and the people you want in your life.
#as a side note: this goes for gender too#dont fucking get a special marker on your passport or whatever#trying to get ssi has made me realize how deeply cruel the system is#never reveal any vulnerability you have unless it's absolutely necessary#do not do this stuff for validation the government is not your friend and you should seek emotional fulfillment elsewhere#hm this post turned out a lot angrier than i meant#guess i'm still mad about how awful the process was#it wasnt even long it was just. so *impersonal.*#this woman talked to me for two hours. went down a fairly bigoted checklist.#didnt ask me my own opinion on much of anything. and then declared a bunch of her impressions as if they hold weight just bc shes allistic#like how i have 'identity issues' (am trans and dont want a romantic partner)#and thats just. my permanent record of diagnosis! this two hour conversation with a stranger! she doesnt fucking know me#we paid like $500 for that
118 notes
·
View notes
Text

#grief#trauma#coping#your feelings are valid#your pain is valid#self compassion#it's all part of the process#the only way out is through#glimmers#gratitude#healing#recovery
81 notes
·
View notes
Photo

what do you do when you meet a neutron star and it wants to watch television with you
#my art#ive been meaninf to sit down and paint this. for weeks#the murderbot diaries#murderbot#murderbot diaries#this is named start (star art) in my files lol#anyways. been thinking about feed presence and#the intimacy of knowing someone could kill you with no effort and still finding them comforting to have looming over your shoulder#and also i wrote my final term paper on the r-process and had to look at pictures of neutron stars for ages. and also that one screenshot#of the star and the cat and the cat says yeah im okay i just need some burgers to validate my gender identity#u know what im talking about#anyways
514 notes
·
View notes