#organic chemistry ii
Explore tagged Tumblr posts
Text

don’t care didn’t ask, attacking the electrophilic pussy carbon of a ketone or aldehyde
(I’ve reached my limit with studying mechanisms for orgo II)
#shitpost#organic chemistry#orgo#chemistry#organic chemistry ii#most of them are literally just attacking the electrophilic pussy of a ketone
24 notes
·
View notes
Text
After chemists first made gold-xenon bonds and realized they could make practically anything bond to anything by abusing the atoms enough.
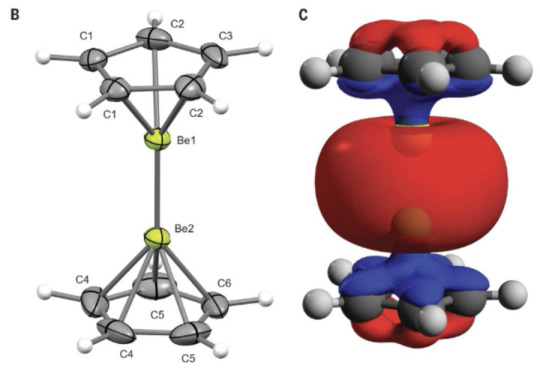
Poor berylliums getting all their electrons stolen
#cursed chemistry#mad science#science#chemistry#organic chemistry#Diberyllocene#tetraxenonogold (II)
70 notes
·
View notes
Text
I should be reading my book on organic chemistry because I have the exam in like a month and I haven't learned shit yet bc my professor fucking sucks. But why does the book have to be so... idk, it's not even really boring cause I do find it kinda interesting how different groups in molecules affect the reaction but like... the reality and act of having to actively try and learn all this, just SUCKS. I don't want to memorize all these stupid mechanisms and then when you adjust the temperature something totally different happens and I need to know that. Not even that, I need to be able to tell what reaction happens based on the reactants and like... I wouldn't mind knowing that, but I also wouldn't mind if I didn't but I'll fail my exam if I don't. I just really don't want to put in the work because I don't like it enough because it's fucking organic chemistry and of course I have to do FOUR FUCKING SEMESTERS of this shit and I'm already hating the first WHYYYYYY 😭😭
#and then biochemistry is thrown into the mix next semester which will probably also suck in addition to having organic chemistry II which is#supposedly worse than OC I which is what OC master student said!!! they like that field enough to get their masters degree in it! what the#fuck do you expect me to do then??#god i hate organic chemistry and its stupid fucking hexagons#anyways nerd rant over and I feel a teeny bit better now. so I guess I'm off to reading about the substitution of hydrogen on 1-alkines#or whatever#yey 😒
2 notes
·
View notes
Photo

#studyblr#notes#chemistry#chemistry notes#chem#chem notes#chemistry ex#chemistry example#chem ex#chem example#mcat#mcat studyblr#mcat chemistry#mcat chem#chemistry 1#chemistry 2#chemistry I#chemistry II#mcat chemistry example#organic chemistry#ochem#o-chem#o chem#ochem 1#ochem 2#organic chemistry 1#organic chemistry 2#mcat organic chemistry#orgo#reactions
6 notes
·
View notes
Text

In 1942, a team led by chemist Louis Fieser originally developed napalm for the US Chemical Warfare Service in a secret laboratory at Harvard University. The name “Napalm” is a portmanteau of two of the constituents of the original thickening and gelling agents: naphthenic acid and palmitic acid. The addition of these two helps gelatinize combustible materials like gasoline, which then sticks to surfaces and burns, causing more damage. Napalm was widely applied by the US during the Korean War for close air support. It also became an intrinsic element of US military action during the Vietnam War. Reportedly about 352,000 tonnes of napalm bombs were dropped in the region within 10 years. The image shows a girl named Phan Thị Kim Phúc at nine years of age running naked on a road after being severely burned on her back by a South Vietnamese napalm attack.

naphthenic acid
Naphthenic acids (NAs) are a mixture of several cyclopentyl and cyclohexyl carboxylic acids. NAs is mainly used in the soap, textile and leather industries. It can also be used in the manufacture of disinfectants and insecticides, or in the manufacture of dry agents and brighteners for the paint and coating industry. NAs soap is also a good emulsifier and it is widely used to prepare lubricating coolant for metal working.
palmitic acid
Palmitic acid, or hexadecanoic acid in IUPAC nomenclature, is the most common saturated fatty acid found in animals, plants, and microorganisms. As its name indicates, it is a major component of the oil from the fruit of oil palms.
#war history#war#korean war#vietnam war#world war ii#chemistry#organic chemistry#chemistry student#chemical weapons#information#factsmatter
2 notes
·
View notes
Text
oh wow hey it looks like i need to average a 92% on every quiz + the final for the rest of this physics class if i want to end with an A. that’s. wonderful really. just superb
#especially considering how i got a 50% on the last quiz 😍#fuck i Cannot let my first B in a class be for fucking gen physics I#i got As in organic chemistry I and II. how the fuck am i supposed to cope if i get a B in a level 111 physics class
1 note
·
View note
Text

only about a month away from moving back into my dorm, so here's a little behind-the-scenes to celebrate. behold two bio majors who are entrusted with handling scalpels

roommate took a stupid selfie and i photobombed it holding up my elvis themed cup. then i redrew it. and then i showed this redraw to my roommate and she said we should name each other Trapper and Hawkeye in our phones. but bc we sleep in the same room and spend all day at the same campus we really only text each other "are you out of class yet" "do you want to get lunch" "are you still in lab bc i want to get sandwiches" "i'm in the cafeteria where are you" "i'm omw to the caf is the food good today?" "no"
#this week was so hellish i'm like actually looking forward to the grueling class grind just to have something else to think about#and somewhere else to BE!!! bring on the Organic Chemistry II i'm ready i can handle it#shebbz irl#shebbz shoutz#mash
306 notes
·
View notes
Text
Velvet Chains (Part I)

Plot Overview:
Y/N Y/L/N is the heir to a powerful mafia empire, but she’s always preferred playing by her own rules. When tensions between her father’s Y/L/N family and the Stray Kids mafia escalate, she finds herself kidnapped by Bang Chan, the unpredictable leader of the rival gang. What starts as a strategic move to shake things up quickly turns into a high-stakes game of power, wit, and dangerous chemistry. Will Y/N outsmart Chan and reclaim control, or will she get swept up in his chaotic world?
Warnings: Mafia!BangChan, Mafia!AU, Violence, Kidnapping, Strong Language, Power Dynamics, Dark Themes, Flirting, Banter, High Tension, Smut(eventually)
PART II, PART III, PART IV, PART V, PART VI, FINAL PART
Author Note:
Hey everyone! So, after posting a poll on Tumblr, the results are in and… Chan won! 🎉 I guess y’all are as intrigued by his unpredictable charm as I am! 😏 So here we are, diving into the world of mafia intrigue with none other than Bang Chan. This story is going to be a wild ride, and I just couldn’t stop writing once I started (you know how it goes, right?). So, get ready for a few parts—yep, this one is going to be a series! 🤩
I hope you enjoy this story as much as I’m enjoying writing it. Expect plenty of tension, power plays, and some spicy moments to come. 😉
As always, please read the tags carefully and make sure this is your cup of tea before continuing!
Hope you all enjoy this as much as I loved writing it. Please feel free to leave your thoughts, comments, and feedback—I’d love to hear from you! 💖
⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⭒⋆⋆⭒⋆⭒⋆
Part I
The jazz bar wasn’t exactly your style, but you appreciated the quiet. As the only daughter of Victor Y/L/N, the man who controlled the northern sector with an iron fist, finding moments of peace was a rare commodity. Your father had built the Y/L/N empire on a foundation of precision, discipline, and cold, calculated power. For three generations, the Y/L/N mafia had ruled this part of the city, their influence expanding through smuggling, money laundering, and intricate political ties. Everything had been meticulously planned. Every move, every person, every resource—it was all part of the machine.
Victor Y/L/N wasn’t just feared—he was respected. A master strategist who never played by anyone else’s rules. His empire was a fortress, and you’d been raised to understand that you were part of it. You knew the stakes of the game, the cost of failure. You had a front-row seat to everything that happened in the world of organized crime, but instead of becoming the dutiful heir your father expected, you’d learned how to operate outside of his rigid control. You weren’t just another piece in his game of chess—you were the queen, always calculating your next move, never just following orders.
You were his greatest asset—and his greatest frustration.
Victor had raised you to understand power, to see the world in black and white. He taught you how to read people, how to dismantle an opponent without ever lifting a weapon. From the time you could walk, you’d been groomed for leadership. But you weren’t like him.
Victor saw the world as a chessboard, and every person was a piece to be moved or sacrificed. You, however, refused to stay on the board. You wanted freedom, independence. You wanted to be more than a pawn in his endless games of control.
“Emotion is a weakness,” he’d told you countless times. “Empathy will get you killed.”
But you didn’t believe him. You knew that in the right hands, emotion could be a weapon. And while Victor wanted you to be cold and calculating, you had something he didn’t: charisma. People followed your father out of fear. They followed you because they wanted to.
This difference had always been a point of contention between you.
Victor expected blind loyalty and obedience, but you questioned everything. When he ordered you to marry the son of an allied family to strengthen his position, you refused. When he tried to involve you in his dealings with corrupt politicians, you went behind his back to broker your own alliances.
You weren’t defiant for the sake of it—you were strategic. You understood the rules of the game, but you played by your own.
The silence of the bar was unsettling, though, as it contrasted with the world you’d known your entire life. The thrum of power, the constant buzz of danger—it had always been there, but tonight something felt different. The shadows seemed deeper than usual, and even the bartender’s hands shook as he poured your wine.
You glanced at the open notebook on the table in front of you, filled with coded notes about your father’s rivals, including one name that had come up more than any other recently—Bang Chan. You knew the Stray Kids mafia had been a thorn in your father’s side for years, but the tension had reached a boiling point lately. The southern sector had grown too powerful, too unpredictable. And now, it seemed they were coming for you.
The Y/L/N and Stray Kids mafias had been in conflict for years. At first, it was subtle: small skirmishes, intercepted shipments, whispers of betrayal. But as Bang Chan rose to power, the tension escalated into an all-out turf war.
Chan’s rise was meteoric. Where your father relied on tradition and loyalty, Chan built his empire with innovation and ambition. He recruited the best hackers, the most skilled fighters, and the most loyal men, creating a network that outpaced even the most established families. His crew—Stray Kids—was infamous for their unpredictability and efficiency.
Your father hated him, not just because of the territory disputes, but because Chan represented everything Victor despised: a new, disruptive power that didn’t play by the old rules.
You’d never met Bang Chan before, but you’d heard plenty about him. He was ruthless, charismatic, and maddeningly clever. If your father was a chess master, Chan was a wild card, someone who could flip the table and still win.
While the Y/L/N family’s strength lay in its calculated, methodical approach, the Stray Kids mafia relied on innovation and unpredictability.
Your notebook sat open on the table. You didn’t need to be here, but the idea of slipping away from under your father’s watchful eye always gave you a thrill. You lived for moments like this.
Until tonight.
The first thing you noticed was the bartender’s shaky hands as he poured your second glass of wine. Then came the eerie silence—the background chatter fading as patrons disappeared one by one. You leaned back, crossing your legs under the table, and glanced toward the shadowed corners of the room.
“Alright,” you murmured under your breath, reaching for the knife strapped to your thigh. “Let’s play.”
Two figures stepped into the dim light. Han Jisung and Lee Know. You recognized them immediately—not just from reputation, but from the detailed dossiers your father kept on the Stray Kids mafia.
The Stray Kids were brutal, unpredictable, and far more cunning than anyone gave them credit for.
Where your father’s mafia was cold and calculated, theirs was wild and ambitious. It was no wonder your father hated them.
Han and Lee Know approached with an air of casual confidence, but you could tell they weren’t taking any chances. You smiled, a sharp, mocking twist of your lips.
“Well, well. If it isn’t Chan’s errand boys. Did you get lost on the way to the kiddie pool?”
Han snorted, clearly amused. “She’s got jokes. I like her already.”
Lee Know’s eyes narrowed, his voice low and measured. “We can do this the easy way or the hard way, Y/N.”
You leaned forward, resting your chin on your hand. “Oh, honey. You’re adorable if you think either of those options work for me.”
Without warning, you lunged. The knife was in your hand in an instant, its blade glinting in the dim light. Lee Know blocked your strike, his movements quick and calculated, while Han stepped in to restrain your other arm.
“Cute,” Lee Know said, his grip like steel around your wrist. “But not smart.”
You twisted in his grasp, your knee coming up to narrowly miss Han’s side. “Don’t flatter yourself. I’m just getting started.”
Han laughed, despite himself. “She’s got fire. No wonder Chan’s so interested.”
That gave you pause. “Interested? Let me guess—he couldn’t find anyone else to stroke his ego, so he sent you two?”
Lee Know’s lips twitched, the ghost of a smirk. “You’ll find out soon enough.”
You laughed, though the sound was more to cover your growing irritation than anything else. “How cute. You think this is going to be easy?”
The two men didn’t answer. They moved quickly, forcefully, but you fought back with every ounce of your strength. You managed to strike one of them in the ribs before they overpowered you and pulled your hands behind your back. It was the usual dance—the struggle, the resistance. But you knew this wasn’t just about you. This was about your father’s empire, and if they were here for you, then it was time to face the consequences of your father’s years of making enemies.
As Lee Know tightened his grip on your wrist, you resisted the urge to lash out. This wasn’t about you—it was about your father. Victor Y/L/N had a way of making enemies, and it seemed Bang Chan had finally grown tired of playing nice. Not that you cared. You’d spent years trying to step out of Victor’s shadow, but his decisions had a way of dragging you back in.
"You do realize this is going to piss off my father,” you said, looking at Han. “Is that the plan, or is Chan just bored?”
Han didn’t seem fazed. “Bored? Nah. This is business, Y/N. Chan’s got a point to prove.”
You scoffed. “And you think kidnapping me will prove it?”
“Maybe. Maybe not,” Han said, his grin widening. “But it’ll get his message across.”
You couldn’t help but smile. “Well, don’t take it personally, boys. I’m not the one you should be worried about.”
Lee Know’s grip on your wrist tightened, but you barely noticed. It was the truth, after all. The moment your father found out, all hell would break loose.
The ride to the Stray Kids estate felt like hours, but you knew it was only a matter of time before you’d face Bang Chan. The southern sector and the northern sector had been in a delicate balance for years. Your father kept his enemies close, but Chan had always been an anomaly. He didn’t play by the same rules, and that made him dangerous.
You sat between Han and Lee Know, your hands loosely bound—just tight enough to make a statement but loose enough to mock.
“You know,” you said after a few minutes, breaking the silence, “this is a sloppy move for Chan. Kidnapping me? What’s the play? Ransom? Leverage? Or is he just looking for a date?”
Han snickered. “She’s quick.”
Lee Know didn’t look at you, his gaze fixed on the road ahead. “We’re not here to answer your questions, Y/N.”
“Of course not,” you replied smoothly. “That would require actual intelligence.”
Han turned to you, grinning. “You’re awfully bold for someone in your position.”
“Bold is just another word for better,” you said, tilting your head toward him. “Speaking of bold, is Chan still pretending he’s running the southern sector with brains, or has he admitted it’s all brawn and luck?”
Lee Know’s hand tightened on his knee, but Han seemed genuinely entertained. “I can’t wait for him to meet you.”
When you arrived at the mansion, Chan was waiting.
The estate was grand, modern, and cold—a stark contrast to the warmth of your father’s domain. The walls seemed to pulse with the quiet hum of power, and you could feel it as you were led inside. Chan was the type of man who demanded respect without saying a word. It was a quiet confidence that bordered on arrogance.
When he turned to face you, you couldn’t help but appreciate the way he commanded the room without a single movement. His gaze locked onto yours, and you stood your ground.
“Well, well,” you said, crossing your arms. “Let me guess. This is about my father. What, did he steal one of your shipments? Break one of your toys? Seems like a petty reason to kidnap me.”
Chan smirked, his hands sliding into his pockets. “Petty? No. Let’s call it… strategic. Your father’s been playing the same tired game for years. He doesn’t realize the board has changed.”
“And you think you’re the one changing it?” you shot back.
“I know I am,” he replied, his tone casual but sharp. “And you, Y/N, are far too smart to pretend otherwise.”
He smiled—a dangerous, predatory curve of his lips—as he walked toward you. “You’ve built quite the reputation for yourself. Smart, strategic, ruthless when you need to be. You’re not your father, though, are you?”
You bristled, stepping forward to meet his gaze head-on. “No, I’m not. I’m better.”
The room seemed to hold its breath.
Chan tilted his head, his smirk widening. “I see.” He gestured for Lee Know and Han to leave, his eyes never leaving yours. “You can drop the act, Y/N. I didn’t bring you here for ransom.”
“Then what?” you shot back. “You looking for a chess partner? Because I don’t play games I can’t win.”
Chan chuckled, low and dangerous. “Oh, I think you’ll find this game… worth playing.”
You crossed your arms, leaning closer to him. “And what makes you think I won’t burn your whole empire to the ground?”
He leaned in, his voice a soft whisper. “Because you’re too smart to destroy something you’ll want to rule.”
The tension crackled like electricity, but you didn’t flinch. This was a battle of wills, and you weren’t about to lose.
#bang chan#stray kids#skz smut#kpop smut#skz#lee know#han jisung#stray kids mafia#bang chan fanfic#bang chan smut#skz mafia
172 notes
·
View notes
Text



First Impressions 🎀
I've had 4 of my 5 classes so far (one doesn't start til october) and I definitely have some thoughts on them, so I thought I'd share my first impressions!
Principals of Accounting II:
the professor seems a bit disorganized, nervous, and semi chaotic but I like his energy
so many people in southern attire, I was a bit surprised
might take a decent amount of study time and energy to keep my grade up in the class, but I'm always up for a challenge and I definitely know I'm capable
gotta figure out a time efficient study routine for this class
Learning (Psychology)
professor has an accent, no idea where he's from, but it's a cool and thankfully understandable accent
feels very content heavy. I definitely want to buy the textbook for this class
need to find a way to stay awake, that's one of the classrooms that make me sleepy
that professor likes to talk for sure (1 hour and 15 min of just the syllabus? man's can talk)
Integrated Survey of Organic and Bio Chemistry
the professor is definitely a science guy, him being the professor makes perfect sense
i really like how he explains and teaches
appreciate that all the content needed is already posted to our class online and all the slides are available already
I love science based classes because I know how to succeed in them and this one seems no different
I have a friend in the class so that's nice!
Intro To Business (Online)
lots of content
lots to do
I gotta make sure I time manage for this class cause otherwise, I'm gonna fall behind
an introduction video?? why not just a discussion post?? ahhhhh
Overall, with working full time, this is going to be an incredibly stressful, incredibly busy semester, but I'm so up for the challenge, and I know I can do it. I just gotta study efficiently, time manage properly, and keep disciplined. I WILL be successful.
til next time lovelies 🩷
#pink pilates girl#pink pilates princess#self development#it girl#wonyoungism#mental health#self care#that girl#physical health#self love#study tips#college student#student life#studyblr#studyblr community#college studyblr#langblr#study community#langblr community#college life#university life#university student#studying#productivity#pink aesthetic#clean girl aesthetic#clean girl#pink academia#academic goals#academic validation
179 notes
·
View notes
Text
saturday, 4th of january, 2025. .𖥔 ݁ 🪐˖.𖥔 ݁ ˖


★ finished 'frog' from animal morphology.
★ finished revising:
(i) general organic chemistry.
(ii) hydrocarbons.
(iii) alcohol, phenol, ethers. (briefly)
(iv) phylum: chordata from animal kingdom + did examples from phylum: annelida → hemichordata.
★ studied 'respiration in plants' from plant physiology.
hi y'all! good day, got a lot done in biology! did a satisfactory amt. of chemistry too, but kind of neglected physics unfortunately :( i'll try to wake up early tmrw and do numericals since that's a weak point that i need to get done with. mentally felt okay, also worked out so there's that :) i think it's slowly dawning on me that NEET is exactly 4 months away, so i need to get my shit together and buckle up lol. i'll be having exams till the 19th, so after that i'll try finishing up with the XI syllabus + XII syllabus (partially) if i can. (without compromising my understanding of important concepts)
🎧: futile devices by sufjan stevens.
#ri's studyblr#desi studyblr#realistic studyblr#study blog#studying#studyblr community#neet 2025#study inspiration#academic weapon#studyspo#study notes#study space#med studyblr#study aesthetic#studyblr#study goals#desi academia#stem academia
58 notes
·
View notes
Text

— Pinnacle [ tsukishima kei university au series ]
— so i pay the price of what i lost ; yes it is right that you can handle anything, but you can’t handle everything all at once
author’s notes : no mention of (y/n), written in second person pov, alternative universe, timeskip!tsukishima, college life, not proofread, english is not my first language, long written chapter
[ masterlist ] | [ ask daleelah go to box box 🐭 ]
Winter break felt like a blur of constant assignments, stress, and messages from your mother. You found yourself buried in work, avoiding the outside world—especially your phone, which you knew was filled only with your mom’s relentless reminders to study harder, do better, and aim higher. Tsukishima and Yamaguchi’s contacts had been pushed to the bottom of your recent conversations, untouched since that day in the gym.
You haven’t seen Yamaguchi or Tsukishima since that winter class you skipped to watch their game. That day feels like it happened in a different life—before the semester started to suffocate you, before your every waking moment was consumed by endless biochemistry coursework. You don’t have time to think about anything else anymore, not when every day feels like a battle to keep up with the expectations of your professors and the relentless academic pace.
Classes in the second semester are intense, perhaps even more than you expected. One of your courses, Organic Chemistry II, is particularly demanding. The subject matter dives deep into reaction mechanisms, synthesis pathways, and the stereochemistry of complex molecules. There’s also Molecular Biology, where you’re expected to learn and apply the intricate processes of DNA replication, transcription, and translation. Your third major course, Biophysical Chemistry, focuses on the thermodynamics of biological systems—another subject that stretches your mind to its limit.
It’s only the second week of your new semester in biochemistry, but it feels like you’ve been dragging yourself through months. Everything seems heavier this time—every lecture, every lab session, every assignment. The moment you open your textbooks and class notes, you can feel your brain protesting. There’s an exhaustion that hangs in the air, a feeling like you’re constantly one step behind even when you manage to complete your work on time.
Now, standing outside the lecture hall for Organic Chemistry II, you realized nothing much had changed. The same heavy textbooks, the same tight deadlines, the same competition between your classmates as they all tried to one-up each other. The new semester had brought a new intensity. You were still trying to keep up with your classmates—some of them seemed almost unnaturally gifted, answering the professors’ most complex questions with ease, while you constantly second-guessed yourself, even when you knew the answer.
Professor Saito, a man with a greying beard and an air of calm authority, strode into the room with his usual collected demeanor. His reputation preceded him—tough, no-nonsense, and known for pushing his students to think critically. Today was no different. He picked up a piece of chalk and began scribbling a chemical equation across the board.
Without glancing back, he posed his first question to the room. “Can anyone explain the significance of this reaction in the context of anaerobic respiration in yeast?”
The classroom, filled with second-year students, was eerily silent. Your eyes traced the chemical formula on the board—glucose breaking down into ethanol and carbon dioxide. The answer floated on the surface of your mind, but your heart pounded in your chest as self-doubt crept in. You scanned the room, hoping that one of the top students would break the silence and offer the answer instead. But they remained still, unfazed, as if this question was beneath them.
You bit your lip, feeling the weight of the quiet hanging over you. It was a simple question, one you knew the answer to, but something held you back. You hated this feeling—knowing, yet hesitating, paralyzed by the fear of saying something wrong. The silence stretched on, and finally, despite the knots of anxiety in your stomach, you slowly raised your hand.
Professor Saito turned to face you, his gaze resting on you with a slight lift of his eyebrows. “Yes?”
Your voice wavered as you spoke. “It’s… the fermentation of glucose into ethanol and carbon dioxide,” you said quietly, swallowing back the stammer in your throat. “Yeast uses this anaerobic process to generate energy in the form of ATP when oxygen isn’t available.”
Professor Saito nodded slightly, his expression unreadable. “Correct. And why is this process significant in industrial applications?”
You took a deep breath. “It’s used in brewing to produce alcohol and in baking for the carbon dioxide that helps dough rise.”
He considered your answer for a moment before nodding again. “Yes. Good. Remember, however, that the ATP yield here is significantly lower than in aerobic respiration. That’s the key difference.”
Relief washed over you, and you allowed yourself to relax—just a little. But before you could even savor that small victory, another voice broke the quiet.
“Professor, could you explain the exact mechanism for the stereoselective alkylation of an enolate in asymmetric synthesis?” The voice belonged to Renji, one of the top students in the class. His question was sharp and cutting, a deliberate challenge. “And maybe elaborate on the difference between kinetic and thermodynamic control in that context?”
A ripple of murmurs spread through the room, punctuated by a few suppressed giggles. You stiffened in your seat. The question was far beyond the scope of what you’d covered in class, meant to impress—or worse, embarrass—the professor. Renji’s tone dripped with arrogance, and the way he leaned back in his chair, arms crossed, told you he already knew the answer.
Professor Saito regarded him for a moment, his gaze steady. He began to respond calmly, “In asymmetric synthesis, the stereoselectivity of the alkylation depends on—”
Before he could finish, another voice interrupted. “What about stereoelectronic effects when using Evans' oxazolidinone in highly hindered substrates?” Yumi, another top-tier student, chimed in with a smirk playing at the corner of her lips. She leaned forward slightly, her question laden with the same smug intent—to derail the lesson, to show off her own knowledge.
The air in the room became stifling. You could feel it—the discomfort rippling through the other students, the growing tension as Renji and Yumi sought to outwit the professor rather than learn from him. They weren’t asking to deepen their understanding. No, they were playing a different game, one of one-upmanship and arrogance.
Your stomach twisted with unease as you watched the scene unfold. Professor Saito, usually unflappable, seemed to falter for just a moment. You caught a glimpse of weariness in his eyes as he straightened up, preparing to answer yet another convoluted question. He had always been patient with his students, no matter how difficult the questions, but there was something in the way his shoulders sagged ever so slightly that made your heart ache for him.
You glanced around the room. Some students were fidgeting uncomfortably, others quietly whispering to their neighbors. The whole room had been hijacked by these few who cared more about showing off than learning, and the rest of you were left feeling small, inconsequential. You clenched your fists under the desk, wishing you could say something, do something to stop it, but the words stayed lodged in your throat. What could you say? What could you do?
Professor Saito began explaining the stereoelectronic effects, his voice steady, but you could sense his weariness growing. The air felt oppressive, like the weight of these students’ arrogance had smothered any genuine learning atmosphere. You shifted in your seat, feeling anxiety gnawing at your insides, hating the smug smiles that played on Renji and Yumi’s lips.
Before you could think further, you raised your hand signaling to interrupt the class. Professor Saito caught your motion and stop his explanation. “I’m sorry, Professor, may i speak?” Your voice came out a little shaky but louder than you expected, you can’t stop yourself right now. Every eyes are on you when the professor nodded. You land your gaze to Yumi—her smug faltered as she turned toward your seat. “I don’t see any stereoselective alkylation of enolates in asymmetric synthesis in our syllabus for this entire semester. So, if you’re going to interrupt the class with questions, at least stick to the topic we’re actually supposed to be learning.”
And now you turned to Renji’s seat, his face hardening as the room went deathly quiet. You could feel the eyes of the other students on you, and though your heart pounded in your ears, you pressed on. “And if you’re feeling that generously smart, maybe you should come up there and be the professor yourself. But what do you actually get from trying to make others—let alone the professor—feel small by throwing out questions just to outsmart them?”
Yumi’s smirk vanished, replaced by a look of shock. Renji shifted in his seat, his face hardening, but he remained silent. You could feel the tension swirling in the room, but it wasn’t directed at you anymore—it was directed at the arrogance that had poisoned the air.
Professor Saito stood there for a moment, his expression unreadable. Then, slowly, a small smile tugged at the corners of his lips. He cleared his throat, and the room snapped back to attention.
The room goes quiet, tension crackling in the air. You don’t usually speak up like this, but something about the arrogance in the room pushed you past your breaking point. The student sneers at you, but you don’t flinch. You’ve had enough of people trying to make others feel small just to inflate their own egos.
Professor Saito gives you a small nod of appreciation before continuing his lecture, the class quiet now except for the sound of his chalk against the board.
That evening, you’re back at your desk, struggling to finish another assignment. The words blur together on the screen, and despite your best efforts, you keep having to re-read the same paragraph over and over. You’re exhausted. There’s no other word for it. Even though you’ve tried to catch up on sleep, it never feels like enough. And there’s always another deadline looming, another mountain of work to climb.
Your phone buzzes next to you, but you don’t pick it up. It’s probably your mom again, asking why you haven’t called or berating you for not keeping up with her expectations. You’ve been avoiding her texts and calls lately because you can’t deal with the added pressure. She doesn’t understand how hard this is, how much you’re trying to juggle. Or maybe she does, and just doesn’t care. Either way, you don’t have the energy to explain yourself to her right now.
By the time you finish the assignment and hit submit, it’s nearly 2 AM. You slump back in your chair, staring at the ceiling. Every muscle in your body aches, and there’s a tightness in your chest that hasn’t gone away for days. You feel like you’re sinking deeper into a hole you can’t climb out of.
The thought of opening your phone again fills you with dread, but you do it anyway, more out of habit than anything else. When you do, you see an email from Professor Saito.
Subject: Checking In
I hope this message finds you well. I noticed that you submitted your most recent assignment late last night. While I am aware of the pressures you and many other students are under, I wanted to reach out personally.
Over the past few weeks, I’ve noticed how diligently you’ve participated in my class. I’ve seen how you’ve quietly answered questions, even when you seemed uncertain of yourself. I also noticed how you stepped in during that difficult class discussion the other day and helped refocus the conversation. You have a sharp mind, and I hope you know that.
That said, I am concerned about you. I can tell that you’re pushing yourself hard, and while I appreciate your effort, I also want to remind you that your well-being comes first. I know what it’s like to feel the weight of academic pressure, and I want to encourage you to take care of yourself, too.
If you ever feel overwhelmed or need to talk, please know that my office door is always open to you. You are a valued member of my class, and I believe in your potential.
Take care of yourself, and don’t hesitate to reach out if you need anything.
Warm regards, Professor Saito
As you read the email, you feel a lump form in your throat. You hadn’t realized how much you needed to hear those words until now. For so long, you’ve felt like you were just going through the motions, never sure if you were really doing anything right. But here, someone was telling you that you mattered—that your efforts weren’t invisible.
You close the email and stare at the screen for a long moment. Then, without thinking, you bury your face in your hands. The tears come quickly, a mix of exhaustion, relief, and gratitude. You hadn’t expected this—this kindness, this small bit of recognition in a sea of doubt.
tagslist (free to mention) ; @theweirdfloatything @snowthatareblack @ilovemymomscooking @nayiiryun @knightofmidnight @kozumesphone @scxrcherr
sorry for posting this late, i’ve been super busy with karate practice all weekend—i’ve got a belt test coming up soon, so the training’s been extra intense. i’m exhausted, and my legs hurt so bad i can barely walk, but gotta stay strong and push through! 😣
#tsukishima kei x reader#daleelah writings 🐭#haikyuu x reader#haikyuu x you#haikyu x reader#kei tsukishima x reader#tsukishima x you#college au#haikyuu au#haikyuu fluff#haikyuu tsukishima#haikyuu!!#haikyuu fanfiction#haikyū!!#haikyuu#tsukishima fluff#hq tsukki#tsukishima x reader#tsukishima kei#hq smau#hq x you#hq x reader#hq fluff#hq fanfic#hq
96 notes
·
View notes
Text
MCSR As Chemical Compounds
idk either man. expect very little actual explanation and a lot of chemical yapping from a very big nerd
Silverr as Silver Nitrate:
AgNO3
the above is the crystal structure
appearance is just a white crystal kinda like sugar
it took everything in me to not just make silverr plain Ag
silver nitrate is the most common precursor for all other important silver salts
also an extremely important compound in the development of photography! (and iirc silverr is a film major)
Feinberg as Ozone:
O3

produced during lightning strikes
pale blue at high ppm
only leaves gas state at cryogenic temperatures
naturally occurring in the stratosphere and absorbs UV rays from the sun
Fruit as Nickel(II) Chloride Hexahydrate:
NiCl2•6H2O

green
the non-hydrate form is a sort of olive-y yellow color
used to absorb ammonia in gas masks
Raddles as Potassium Permanganate:
KMnO4

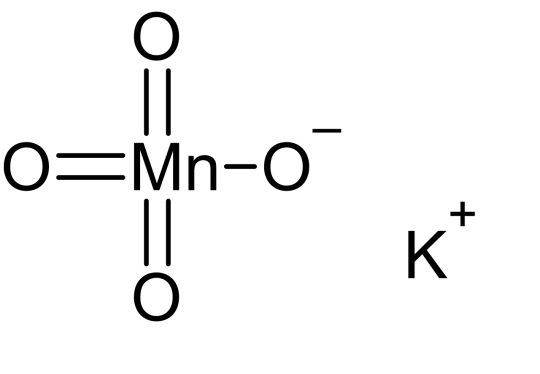
Sometimes referred to as Purple Potion Powder
goes CRAZY purple when dissolved and is lowkey my favorite chemical
very strong oxidizing agent
one time i stained my hand purple through my glove with this shit idk how it happened
if made in specific solvents can look extremely similar to dragon's breath in minecraft imo
K4 as Octathio[8]circulene:
C16S8
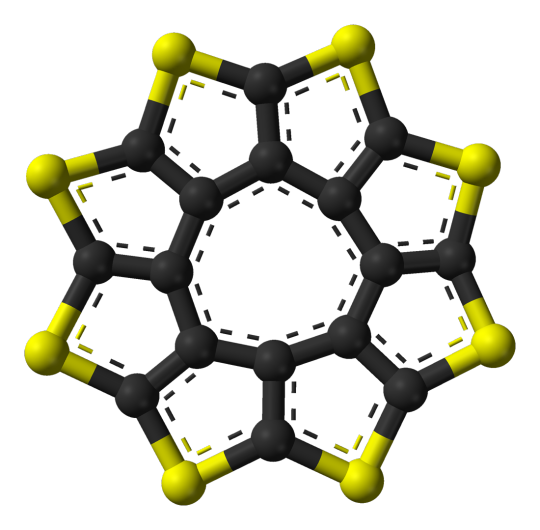
also referred to as Sulflower (like sulfur and sunflower haha get it)
planar which is fairly uncommon for molecules of this size
can be stacked together to make sheets of sulflowers
Cube as Cubane:
C8H8
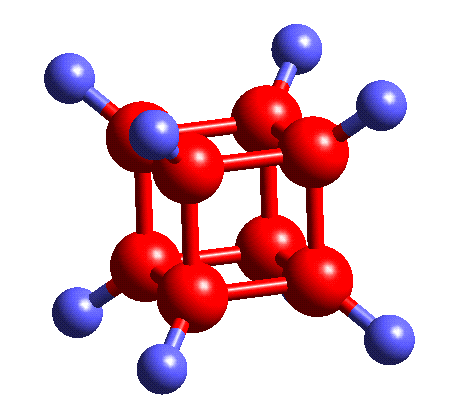
yeah this is self-explanatory
what is interesting though is that ring strain in 4 membered rings/squares is really high, so cubane existing is a bit of a chemical anomaly
i havent read into it enough to know for sure but i suspect that ring strain is why cubane is a precursor to a HELLA STRONG explosive compound
Reignex as PPTA:
Poly-p-paraphenylene terephthalamide
[-CO-C6H4-CO-NH-C6H4-NH-]n
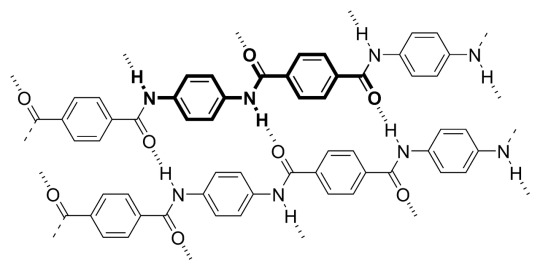
the name is complicated as shit but this is just kevlar!
aka bulletproof vest material
looks fluffy when not woven completely together
aligning of polymer chains w hydrogen bonds creates EXTREMELY high tensile strength
Mime as Phenylmagnesium Bromide:
C6H5MgBr

a common grignard reagent aka a compound that can be used in a grignard reaction, an extremely important reaction in organic synthesis as it creates new C-C bonds
another fun fact about grignard reagents is that if water is added to them- or even if they're handled in particularly moist air- they fucking explode
extremely strong nucleophile and base
Poundcake as Xenon Hexafluoride:
XeF6
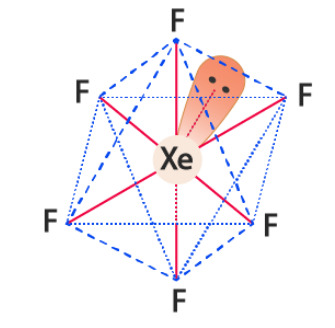
Noble gases don't react unless you REALLY make them
so a compound containing xenon is really interesting
colorless as a solid but sublimes (aka skips straight from solid to gas) into a bright yellow gas
fun fact a lot of instances where typical chemistry rules are broken (noble gases not reacting, octet rule in general, etc) involve fluorine to the point ive heard it referred to as a "batshit electron thief"
Fulham as Iron Hexacyanidoferrate:
C18Fe7N18


also known as prussian blue
extremely common pigment in paints and the first modern synthetic pigment
used extensively in The Great Wave
another one of my favorite molecules bc im biased and like inorganic chem aka things that contain metals
used as an antidote for heavy metal poisoning which is interesting bc it contains cyanide ligands!
Couriway as Bullvalene:
C10H10
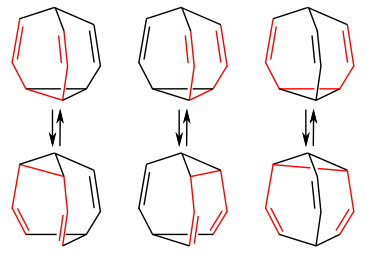
in a state of constant resonance
aka the double bonds are CONSTANTLY shifting and reforming bullvalene into... itself but moved around a little
the bonds fluctuate so rapidly that in nmr analysis each carbon and hydrogen in the entire molecule is read as equivalent (for my non-chem people that's very uncommon and very cool)
formed through photolysis (aka using light/photons to fuel a reaction)
#i made this for me and only me#chemistry is a disease and i will not be getting better anytime soon#90% of these picks are straight soul reads im gonna be so fr#mcsr#hbg#fruitberries#feinberg#couriway#fulham#president poundcake#raddles#silverrruns#reignex#talkingmime#cube1337x#k4yfour
86 notes
·
View notes
Text
Resources that have helped me in my classes!
(will add to this list as I find/remember more)
Classes I've used these in so far:
Honors Mechanics
Honors Thermodynamics & Optics
Relativity & Quantum Mechanics
Theoretical Mechanics
Calculus I, II, III
Differential Equations
Mathematical Techniques in Physics
Introduction to Astronomy
Fundamentals of Astronomy
Astronomy & Astrophysics
- - - - Youtube Channels - - - -
Physics
Michel van Biezen - oh my god I love him. I think he uses Sears and Zemansky's University Physics for example problems. His channel has 10k videos (!!!) and is very well-organized. He also lectures on math (from 5th grade to linear algebra), chem & organic chemistry, and astronomy.
Calculus
The Organic Chemistry Tutor - I mostly watch his videos on Calculus I-III and Differential Equations, but he also covers physics and chemistry.
Nancypi - Precalculus, Calculus I, and some of Calculus II. I barely showed up to calc lectures my first semester so she was a great help. also i have a crush on her
Michel van Biezen - I usually don't watch his math videos because his notation and techniques are different from what my prof makes us use
Astronomy
Urknall, Weltall, und das Leben - my literal dream channel. perfect levels of dryness, and videos are usually at least 45 minutes long. It's entirely in German though lmao
Michel van Biezen - covers important topics in introductory astronomy, and also does example problems (although I think they're all algebra-based)
- - - - Textbooks - - - -
Introduction to Cosmology - Barbara Ryden
Foundations of Astrophysics - Ryden & Peterson
University Physics - Sears and Zemansky
Calculus - James Stewart
Modern Physics - Kenneth Krane
Classical Mechanics - John Taylor
- - - - Workbooks - - - -
Essential Modern Physics - Chris McMullen, Ph.d. ----- LIFE CHANGING. BUY THIS FOR INTRO TO QUANTUM.
#astronomy#physics#mechanics#quantum physics#calculus#differential equations#academia#university#studyblr#undergraduate#astrophysics#college#student#undergrad#advice#studying#nasa#vector calculus#resource
240 notes
·
View notes
Text


🧸☃️Welcome ambitious students! 🧸☃️ I’m Lune, I am 20 years of age and my pronouns are she/her. I am a second year university student in North America studying Biochemistry; with an interest in research. I am also fluent in Spanish! My current subjects are: Physics I, Calculus II, Organic Chemistry II, Organic Lab, & Writing. I hope to motivate other students and be a source of guidance for all of us hard-working individuals — whether one is in secondary school or university. Additionally, I am aiming to live a stoic and disciplined life. I am not exactly sure how I will achieve this, but I hope to be successful in my attempt. I am also here to make friends! Please feel free to reach out, and let’s connect! Whether it’s on the tough courses we study, or simply to chat! My messages are always open ❤️🧸 My main account is: @beachflowerr Student Diaries: Achieving Academic Success Series My YouTube: Lune Study My only fans: jaja just kidding “The only way out, is through” ☆彡━━━━ ☆ ━━━━☆彡 ૮₍ ´ ꒳ `₎ა ☆彡━━━━
#study aesthetic#study inspiration#study blog#study notes#study motivation#artists on tumblr#studyblr#study buddy#studying#español#inspo
25 notes
·
View notes
Text


3.11.23 The semester started at full speed and hasn’t slowed down since! I’ve been taking Statistics and R Studio, Oral Communication, Black Queer Media and Literature, and Organic Chemistry II. Who knew that I would read a poc lesbian vampire historical-fiction novel for an academic class? It’s called the Gilda Stories by Jewelle Gomez and I’m definitely enjoying it so far.
🎼- Wildfire by Beach Weather
#mine#studyspo#studyblr#pre vet studyblr#stemblr#poc studyblr#black studyblr#black academia#dark academia#light academia#beige aesthetic
729 notes
·
View notes
Text
Subatomic particles from a chemist's point of view - part II: the proton
[part I: the electron]
Proton
In my subjective opinion, the runner-up in this informal ranking of subatomic particles that are important in chemistry. Protons may not form chemical bonds like electrons do, but they still play an important role in many chemical reactions, especially in organic chemistry. But their most meaningful task that places them right below the electron on my list is this: they quite literally define the elements.
Atomic number
Let’s put our Mendeleev hats on and have a look at the periodic table. Here, I’ll upload it for you so you don’t have to google it:

It doesn’t take a genius to realize the elements are compiled in an orderly fashion rather than a random one. What is the property that generates this order? You could say mass – that the elements are arranged by their increasing mass – but that’s not quite true. Sure, most of the time it is true, but there’s a handful of oddballs that refuse to fit this scheme. Argon and potassium, for example: argon has a mass of 39,948 u (units) while potassium has a slightly lower mass of 39,098 u. The difference isn’t big, but nevertheless if we want to arrange our elements by mass, we have to place potassium underneath neon and argon underneath sodium.
Obviously, we can’t do that. The cool thing about the periodic table is that there are several trends encoded in it, one of them being that the elements of any given group are usually fairly similar to each other. Group 18, where argon normally resides, is reserved for noble gases that are extremely chill and not eager to react (they might’ve taught you in school that noble gases never ever react with anything ever; THAT’S A LIE! But it is true that their chemistry is scant and their reactions rare). Potassium could never fit in with them. Fucker explodes in water the same way sodium does – which is yet another proof it belongs in the same group! Also, COOL EXPLOSION HERE!
This isn’t the only such strange pair in the periodic table: cobalt and nickel are like that too, and so are tellurium and iodine. It isn’t much – but it’s enough that we have to look for some other physical property to define the order of the elements. For some time, chemists and physicists had to accept this discrepancy (not that they were happy about it; I imagine they’d wake up at night drenched in sweat, screaming, “GODFORSAKEN ARGON!”). The atomic number, this sort of ordinal number that put every element in its place, was actually random, as in, not based on any known physical property. Yeah, potassium has an atomic number of 19, but why?
ENTER HENRY MOSELEY!
Henry Moseley conducted a series of experiments in which he zapped various elements with X-rays (I’m so jealous), then analyzed the resulting emission spectra. It turned out that the atomic number is proportional to the square root of the emitted radiation, which in turn depends on the proton count in the nucleus. This is what defines any given element: the number of protons it has. This is THE definition, the one you learn very early in your chemistry journey. The number of neutrons may vary among the atoms of the same element (because isotopes) and atoms can gain or lose electrons by becoming ions, but that doesn’t turn them into different elements. Only the number of protons is always constant for one and the same chemical element.
Organic chemists love protons too
And for more than one reason at that – because hoo boy, does a proton stir some shit in ochem!
My ochem lab instructor pointed to the mechanism I’d written on my lab report once and asked, “What does the acid do in this reaction?”. Very plainly I said, “It’s a source of protons which act as a catalyst,” to which he gave me his standard shit-eating grin and said, “They all are.”
And he wasn’t wrong! If you analyze a bunch of organic reaction mechanisms then you’ll see they very often begin with a proton (so H+) attaching itself to the substrate (or a lone electron pair on the substrate to be precise, because Coulomb force, right?) and thus initiating a chain reaction of sorts that leads, frequently through many infuriating steps, to the product. Take a look at the synthesis of aspirin, for example:
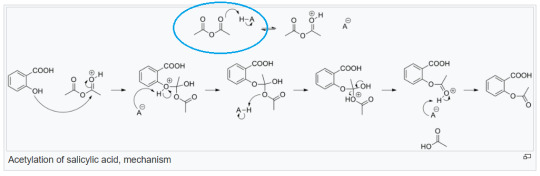
[via wikipedia]
You don’t need to understand everything that happens here. What matters is this first step I circled: a proton attaches itself to one of the substrates and starts the whole reaction.
The second reason I have in mind for why organic chemists love protons is NMR: nuclear magnetic resonance. NMR is a method of instrumental analysis and it’s cool as all fucks actually (as long as you don’t have to analyze the spectra because what the heck are those spikes), but this post is about protons, not NMR, so here’s the gist: you put your organic sample in the NMR spectrometer. The spectrometer drenches your sample in a magnetic field (which is probably why small dogs with metallic collars aren’t advised in an NMR lab). The spins of the protons in your sample (yes, protons have spin too!) go wooo! and align themselves in a specific manner. The computer connected to the spectrometer spits out a spectrum that tells you what your sample looks like.
Properties of the proton
Charge: positive one elementary electric charge, the exact opposite of an electron (how convenient!): +1.602×10^(−19) C
Mass: 1.673 × 10^(-27) kg – which is roughly 1837 times the mass of an electron. I want you to say, "Whoa, that's a lot!" right now because shit, it really is! And that's a great thing, because it gives us cool stuff like the Born-Oppenheimer approximation.
Radius: 0.841 fm (femtometers), but make no mistake: just like electrons, protons abide by the wave-particle duality, because they hate us all. I just remembered when my quantum chem professor told us during a lecture that even buckminsterfullerenes exhibit wave-particle duality. These are molecules made up of 60 carbon atoms. Sixty carbon atoms!! I almost cried, but I was sitting in the front, so I had to compose myself.
34 notes
·
View notes