|⌬ Freeware for chemists, students, and teachers | Creat a chemistry workstation for scientists | Share daily-compound and molecules...
Don't wanna be here? Send us removal request.
Text
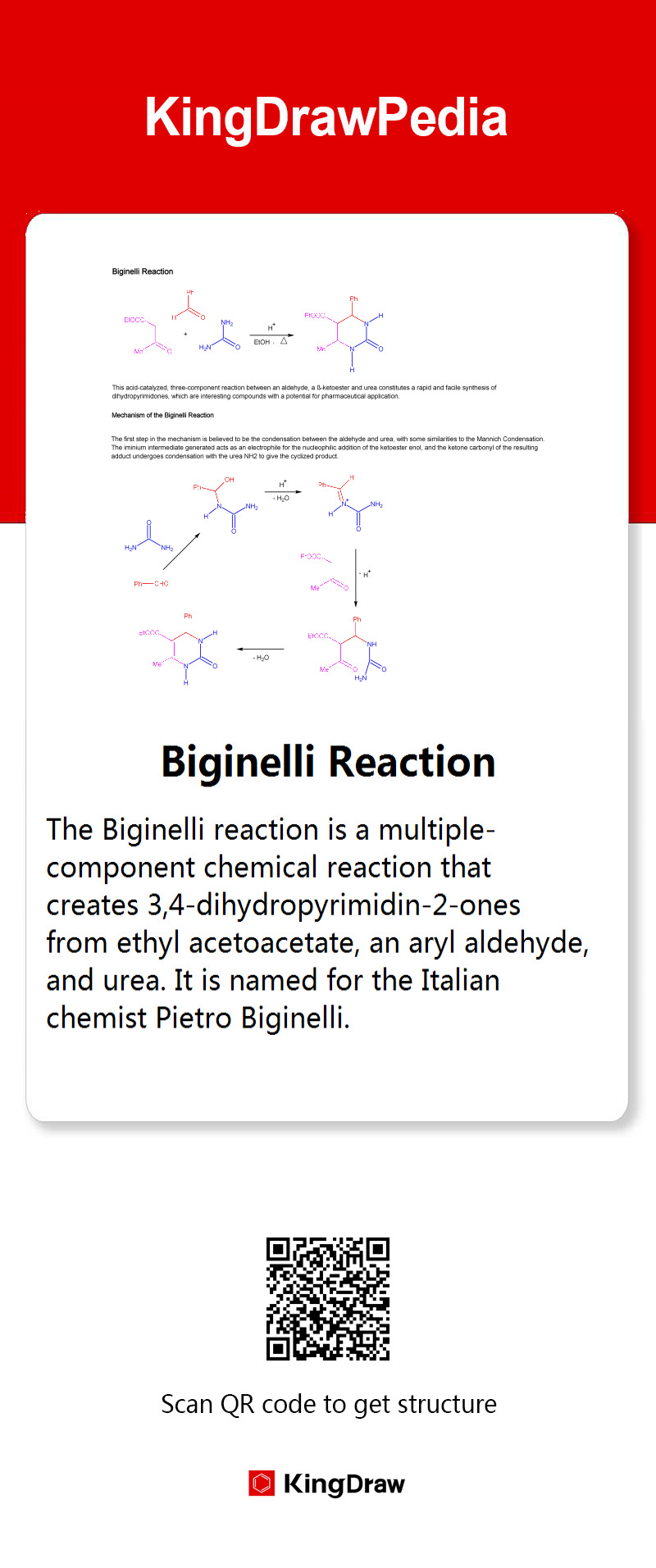
Bouveault-Blanc Reduction


2 notes
·
View notes
Text
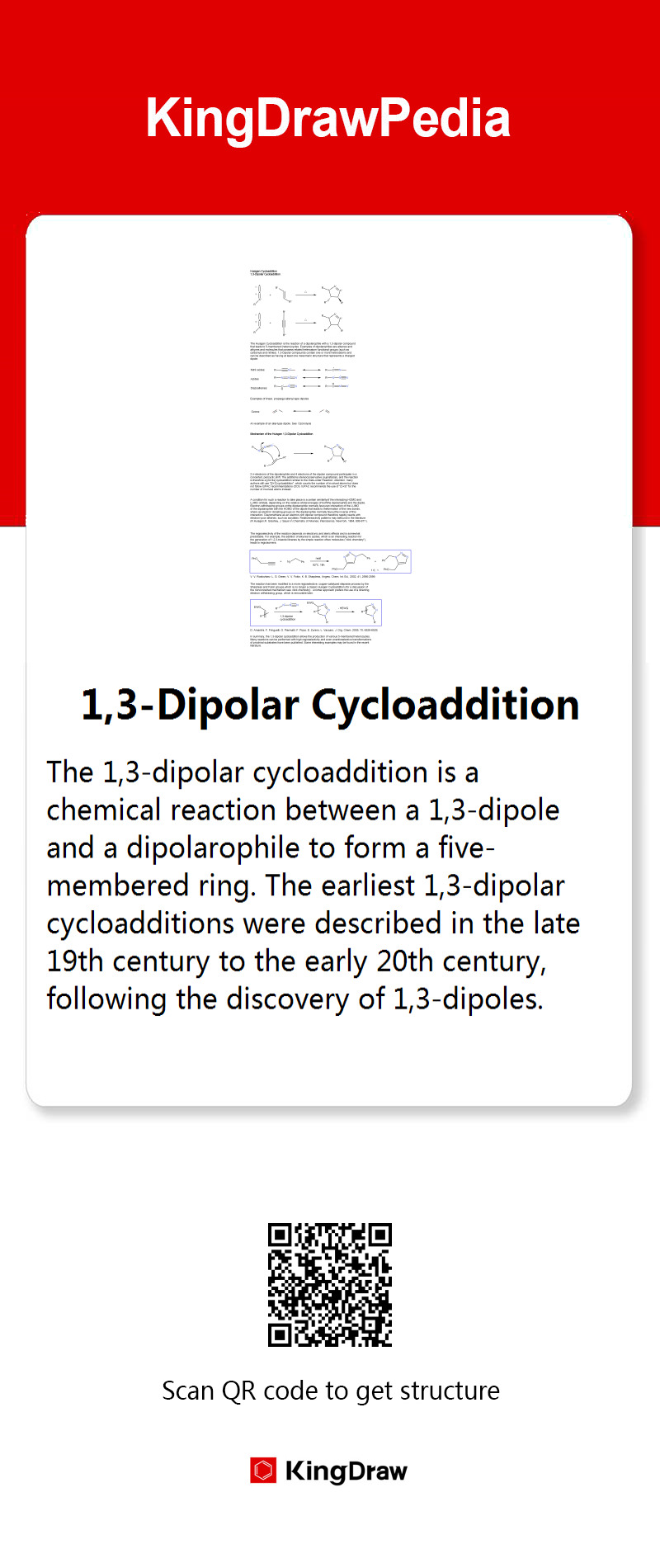
1,3-Dipolar Cycloaddition
#namereaction #KingDraw

2 notes
·
View notes
Text
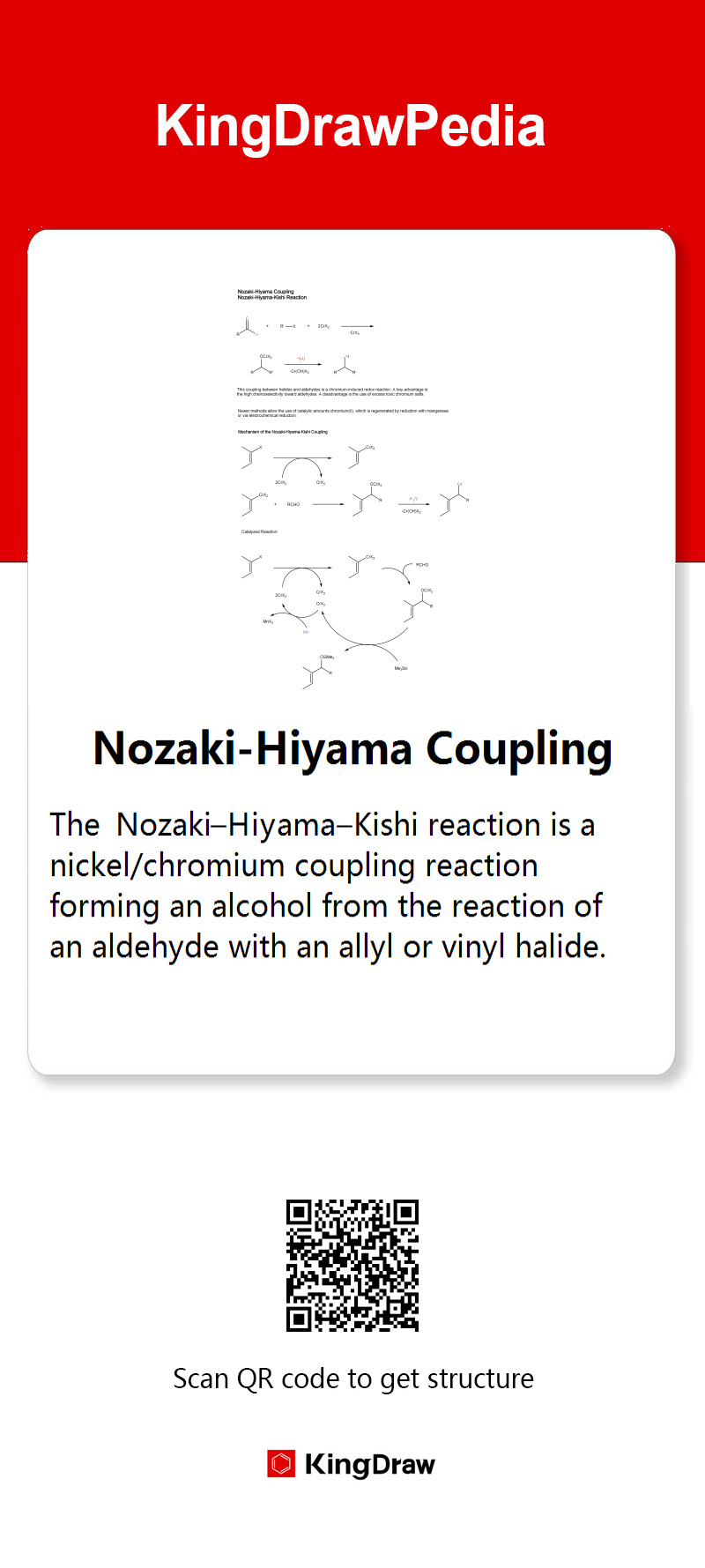
Nozaki-Hiyama Coupling

1 note
·
View note
Text
The chemistry behind starch paste

Paste (or starch paste) is a natural adhesive made from starch-based grains, with its earliest applications in China dating back to the Shang and Zhou dynasties, when people used rice soup to adhere bamboo slips. Later, during the Spring Festival, the tradition of making paste to attach Spring Festival couplets continued the warmth of agrarian civilization.
Traditional paste is made from wheat starch, and the secret to its stickiness lies in the "gelatinization reaction" of starch. When the water temperature rises above 60°C, the starch granules absorb water and swell. Amylose dissolves first, cross-linking through hydrogen bonds to form a three-dimensional network structure that encapsulates water molecules, establishing the basic viscosity. Amylopectin is released thereafter, filling the gaps in the network like a branched tree, further enhancing stickiness and structural strength. During this process, some starch hydrolyzes into shorter molecular chains known as dextrins, which retain the thickening properties of starch while enhancing colloidal stability and flowability. Ultimately, these three components work together to form a translucent colloid.



2 notes
·
View notes
Text
Kulinkovich-de Meijere Rreaction


3 notes
·
View notes
Text
Chemistry behind Stainless steel utensils

Stainless steel utensils, typically made from "austenitic stainless steel," feature a face-centered cubic (FCC) structure and are non-magnetic. They contain high levels of chromium, nickel, and other alloys that create a protective chromium oxide layer, offering excellent chemical corrosion resistance. Austenitic stainless steel, easy to shape and weld, is categorized into the "200" and "300" series, with 304 and 316 being common in the 300 series. Type 304 contains 18% chromium and 8% nickel, while type 316 includes 2% molybdenum for enhanced corrosion resistance, especially in chloride settings, ideal for marine, chemical, and medical applications. Heavy metals in stainless steel remain stable, and the oxide layer prevents ion migration, ensuring food-grade stainless steel is safe under normal conditions.
2 notes
·
View notes
Text
Chemistry behind the sun

The Sun, like a giant "fireball," releases vast amounts of light and heat through "nuclear fusion (proton-proton chain reaction)." The Sun's mass mainly comprises hydrogen (73%) and helium (25%). At its core, with a high temperature of 15 million degrees Celsius and extreme pressure 260 billion times that of Earth's atmospheric pressure, two hydrogen nuclei (protons) overcome electrostatic repulsion under such extreme conditions, forming a deuteron (containing one proton and one neutron), which releases a positron and a neutrino; subsequently, the deuteron collides with another proton to create a helium-3 nucleus (containing two protons and one neutron) and emits gamma rays. Finally, the two helium-3 nuclei merge to form a stable helium-4 nucleus (containing two protons and two neutrons), while releasing two additional protons that will again participate in the cyclic reaction. In the Sun's core, about 600 million tons of hydrogen are fused into helium every second, and approximately 4 million tons of matter are converted into energy in the process, which also serves as the basis for the existence and development of life on Earth.
6 notes
·
View notes
Text
🎨 Van Gogh’s Irises Weren’t Always Blue! Did You Know This Art Mystery? 🌈

In May 1889, Van Gogh voluntarily admitted himself to a mental hospital in Provence, France, a crucial period in his artistic career. The enclosed garden at the hospital connected him with nature and inspired his work, including "Irises." Although the flowers in the painting appear blue today, this was not their original color.
Originally, Van Gogh used the popular red pigment "Geranium lake" and blue pigment to create a vibrant violet color for the flowers. However, the main component of the Geranium lake, Eosin Y, is unstable and degrades when exposed to light and oxygen. As a result, over time, the red pigment gradually fades, causing the purple flowers in the painting to turn blue.

5 notes
·
View notes
Text
Chemistry behind table tennis ball


The material 'celluloid,' used in some table tennis balls, is a plastic material made from 'nitrocellulose' as the main ingredient, with 20% 'camphor' added as a plasticizer. It is the earliest thermoplastic resin in history and was once widely used. Nitrocellulose is also a raw material for explosives and can decompose and heat up at temperatures above 40°C and spontaneously ignite at 180°C. Camphor is also a flammable substance (with a flash point of 52°C and an ignition point of 60°C), which can act as a 'potent accelerant' when nitrocellulose decomposes and generates heat. Therefore, celluloid burns when exposed to an open flame, and friction, impact, and even improper storage can lead to spontaneous combustion. Early film stock was made from celluloid, and in 1936, a warehouse fire at Fox Film Corporation in the United States, triggered by consecutive days of high temperatures, led to the destruction of most of the precious film stock from the silent movie era. Due to its flammability and tendency to crack, celluloid has been replaced by safer synthetic materials in many areas.


3 notes
·
View notes
Text
Chemistry behind Tomatoes

Tomatoes are a popular fruit and vegetable, but did you know the best way to store them to preserve their flavor and texture? The correct answer is to keep them in a cool place at room temperature, which helps retain their aroma and taste, and keeping the stem on can extend their shelf life. While refrigerators can slow down the decay of fruits and vegetables, they are not the best place for storing tomatoes. Compounds such as (Z)-3-hexenal, which are six-carbon compounds, and other volatile compounds, are the main components that make up the unique flavor of tomatoes, and low-temperature storage can affect the release of these volatile compounds. A study in 2013 found that the total concentration of volatile compounds in tomatoes stored in a refrigerator at 4°C for 30 days decreased by 66%. Moreover, during the ripening process, tomatoes release pectinase to break down the cell wall structure, making the fruit softer and juicier. The low temperature of a refrigerator can inhibit the action of these enzymes and can even cause the tomatoes to freeze and rot; therefore, tomatoes are not suitable for storage in the fridge.

3 notes
·
View notes
Text
Chemistry behind spinach


Popeye, the famous American cartoon character, first appeared in the U.S. comic strip "Thimble Theatre" in 1929 and was adapted into an animated series in 1960. Popeye is known for gaining immense strength after eating canned spinach. But why spinach? This actually stems from a careless scientist and a well-intentioned writer. In 1870, German chemist Erich von Wolf was studying the iron content in spinach and other green vegetables, but due to a data processing error, he concluded that spinach had more than ten times the actual iron content, leading to the belief that "every 100g of spinach contains 35mg of iron." Moreover, the comic was created during the Great Depression in the United States, and the author may have wanted children to eat this cheap and nutritious food, so he chose spinach as Popeye's secret weapon. Popeye did not disappoint; spinach sales increased by 33% in the 1930s because of him. It wasn't until 1937 that Wolf's calculation error was discovered, but the notion that spinach could replenish iron had already taken root in people's minds.
3 notes
·
View notes
Text
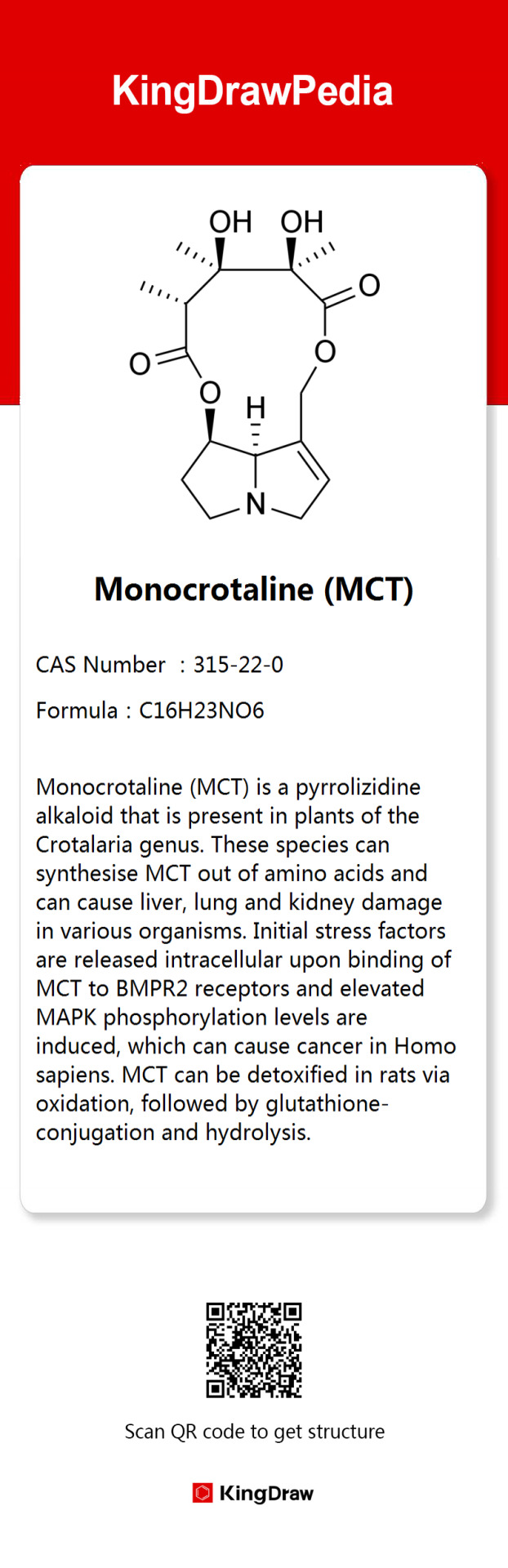
The chemistry behind The Greenbird Flower

The Greenbird Flower (Crotalaria cunninghamii), native to Australia, attracts pollinators with its bird-like green blooms. It contains toxic PAs—monocrotaline (MCT), Retrorsine, and Retronecine—which were once used medicinally but are now known for their hepatotoxic and genotoxic effects, prompting health and safety regulations.


#flower#chemistry#molecule#organicchemistry#dailychem#did u know#stemblr#amazing facts#australia#chemblr
2 notes
·
View notes
Text
The chemistry behind Greenland shark


The Greenland shark, found off Greenland and Iceland, is among the largest and longest-lived sharks, reaching over 7 meters and living 250-500 years. Its slow metabolism and growth—growing 1 cm annually and maturing at 150—contribute to its longevity. It's also highly toxic, with high urea and TMAO levels, making it unsafe to eat raw. Icelanders ferment it into "Hákarl," which has a strong, pungent odor due to ammonia and trimethylamine production.

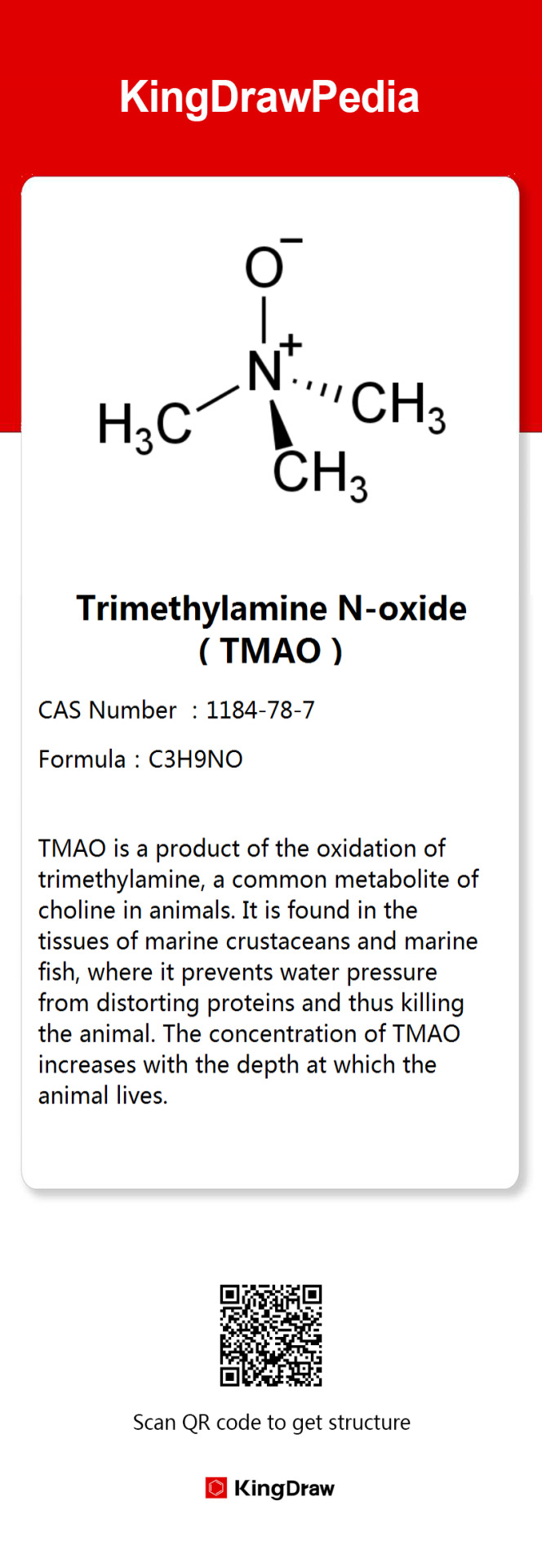
1 note
·
View note
Text
The chemistry behind the swimming pool

Swimming pools often use chlorinated disinfectants such as sodium hypochlorite for sanitation. The "chlorine" smell we detect near pools is commonly thought to be from the disinfectant itself, but it actually comes from "monochloramine." Chlorine disinfectants release free chlorine (hypochlorous acid, hypochlorite ions, elemental chlorine, etc.) in water, which reacts with organic matter from human urine and sweat to form volatile monochloramine with a slight chlorine odor. The higher the concentration of urine and sweat in the water, the stronger the smell. Thus, a strong chlorine odor in a swimming pool suggests someone has been adding "extras" to the pool. Monochloramine can be irritating, causing respiratory symptoms like coughing. Studies show that long-term exposure to monochloramine can lead to respiratory diseases like asthma among swimmers, which is one reason professional swimmers may develop asthma.

3 notes
·
View notes
Text
The chemistry behind Resurrection Plant

Selaginella lepidophylla, a small fern belonging to the family Selaginellaceae and genus Selaginella, is native to the Chihuahuan Desert in northern Mexico and southwestern United States. This unique plant is renowned for its survival strategy during extreme droughts—curling its fronds and forming a ball to enter a dormant state. It can survive for several years after losing up to 95% of its water content. Upon rehydration, the dried fronds reopen within hours and gradually regain their green color.

Selaginella lepidophylla is thus also known as the "resurrection plant" or "comeback plant." Research has found that synthesizing "D-trehalose" during dormancy is key to its revival. High salinity and dryness create a hyperosmotic environment, and trehalose acts as a compatible solute, replacing evaporated water to prevent excessive salt damage and maintain cell viability. When water returns to the plant tissues, the sugar crystals dissolve, and the plant's metabolism is reactivated.

6 notes
·
View notes