#carbonyl group
Explore tagged Tumblr posts
Text
Polarization of the Carbonyl Group
-- Carbonyl group:
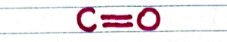
-- The oxygen has two lone pairs
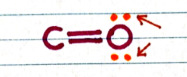
-- The oxygen is more electronegative than the carbon
-- The polarization arrow will show electrons being pulled toward the oxygen

-- The carbon is partially positive
-- Partially positive = δ+
-- The oxygen is partially negative
-- Partially negative = δ-
-- These symbols go by the arrow
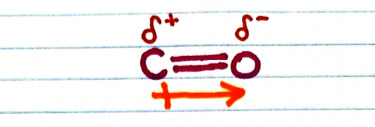
-- The polarization makes the boiling points of aldehydes and ketones higher than hydrocarbons
-- The larger the carbonyl group, the less soluble it is in water
-- If it has more than six carbons, it is insoluble
.
Patreon
#studyblr#notes#my notes#functional groups#polarization#electronegativity#carbonyl group#aldehydes#ketones#polarity#organic chemistry#ochem#orgo#orgo notes#organic chemistry notes#organic chem#orgo chem#study guides#mcat#mcat chemistry#mcat orgo#mcat ochem#mcat organic chemistry#mcat studyblr#premed studyblr#organic chemicals#organic reactions#chemical reactions#advanced chemistry#life science
9 notes
·
View notes
Text

The Science Notebooks of S. Sunkavally. Page 138.
#polarization#colour discrimination#rhodopsin#evolution#ultraviolet#B-carotene#carbonyl group#chlorophyll#tryptophan#cancer#aromatic ring#carotene#conjugated bonds#theoretical biology#manuscript#notebooks#diaries#handwriting#calligraphy
0 notes
Text
OH before I like, fully lose my mind and forget: organic chemistry as a field has the stupidest fucking terms and I’m in love with it. did you know that today I got to watch a 50 year old man with a PhD and a research lab be forced to explain why he just called a mechanism an ‘umpolung’ (pronounced in a way reminiscent of Oompa Loompa in the emphasis, for those who aren’t good with Germanic pronunciations). he literally had to stop and be like yeah idk a lot of organic chemistry happens in Germany and I. Have to assume it sounds more reasonable to them.
#for those who are wondering - it describes the reversing of a specific functional groups reactivity#if you turn your carbonyl from a nucleophile to an electrophile. reverse the polarity of that region. you get the vibe
5 notes
·
View notes
Text
If the carbonyl group of an aldehyde or a ketone is lower in precedence than other functional groups in the molecule, it is indicated by the infix, -oxo-.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#carbonyl#aldehyde#ketone#precedence#priority#functional group#infix#oxo
0 notes
Text
But first, the common functional groups that you will encounter are outlined in table 2.7.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#functional group#hydroxyl#alcohol#carbonyl#aldehyde#ketone#carboxyl#carboxylic acid
0 notes
Text

Can you pass the nackle?
Chemical Formulas [Explained]
Transcript Under the Cut
[Cueball is holding a pointer and gesturing towards a whiteboard that shows the chemical formulas HCOOH and CH₃COOH. Below these, respectively, are classic diagramatic representations of formic/methanoic acid [with an apparently accidental doubled bond between the carbon and the hydroxy group] and acetic/ethanoic acid; being, in turn, a single- and double-carbon chain molecule with a double-bonded oxygen (carbonyl group) plus an oxygen-hydrogen (hydroxy) upon one carbon of each, to form the full carboxyl grouping, and hydrogens completing all other expected bonds.] Cueball: The two simplest carboxylic acids are hakoo and chuckoo. Emphatic off-panel voice: No!! [Caption below the panel:] How to annoy chemists
1K notes
·
View notes
Text
When sodium hypochlorite (bleach) solution is added to luminol, a chemical reaction occurs that releases energy in the form of light. This is called chemiluminescence. The bleach solution acts as an oxidizing agent, which means it takes electrons away from the luminol molecule. This causes the luminol molecule to become excited, and it releases the energy as light.
🎥 Courtesy: Kendra Frederick
The luminol molecule is made up of two amino groups, a carbonyl group, and an azo group. The amino groups are electron-rich, while the carbonyl group is electron-poor. The azo group is a conjugated system, which means that the electrons in the double bonds can move freely from one atom to another.
When sodium hypochlorite (bleach) solution is added to luminol, the bleach molecules react with the amino groups of the luminol molecule. This reaction takes electrons away from the luminol molecule, which causes the luminol molecule to become oxidized. The oxidized luminol molecule is in an excited state, which means that it has more energy than it normally does.
The excited luminol molecule then releases the extra energy as light. This light is called chemiluminescence. The light emitted by the chemiluminescence reaction is blue because the luminol molecule has a blue fluorescence.
The chemiluminescence reaction between luminol and sodium hypochlorite is catalyzed by the presence of a metal ion, such as iron or copper. The metal ion helps to stabilize the excited state of the luminol molecule, which makes it more likely to release the extra energy as light.
The chemiluminescence reaction is very sensitive to impurities, so it is important to use pure chemicals. The reaction can also be affected by the pH of the solution. The optimal pH for the reaction is around 9.
The chemiluminescence reaction between luminol and sodium hypochlorite can be used to detect blood, as the iron in hemoglobin can catalyze the reaction. The reaction is also used in some commercial products, such as glow sticks and emergency lights.
I hope you enjoyed learning about this. ❤️🙏
#chemiluminescence#luminol#bleach#science#chemistry#scienceexperiment#glowing#light#excitedstate#oxidation#chemicalreact
5K notes
·
View notes
Text
New research has uncovered the potentially harmful substances that are produced when e-liquids in vaping devices are heated for inhalation. The study, published in Scientific Reports, highlights the urgent need for public health policies concerning flavored vapes. The research team at RCSI University of Medicine and Health Sciences, Dublin, used artificial intelligence (AI) to simulate the effects of heating e-liquid flavor chemicals found in nicotine vapes. They included all 180 known e-liquid flavor chemicals, predicting the new compounds formed when these substances are heated within a vaping device immediately prior to inhalation. The analysis revealed the formation of many hazardous chemicals including 127 which are classified as "Acute Toxic," 153 as "Health Hazards" and 225 as "Irritants." Notably, these included a group of chemicals called volatile carbonyls (VCs) which are known to pose health risks. Sources for VCs were predicted to be the most popular fruit, candy and dessert-flavored products.
Continue Reading.
#Science#Chemistry#Analytical Chemistry#AI#Artifical Intelligence#Machine Learning#Vapes#Nicotine Vapes#E-liquids
129 notes
·
View notes
Text
How do you make a whore moan?
Extract sapogenins from a Mexican yam and employ Marker degradation to degrade the sapogenin side chain while leaving similar functional groups on the steroid nucleus (relatively) unaffected. Use acetic anhydride to block the hydroxyl group formed by opening the six-membered pyran ring. Then oxidatively open the five-membered furan ring with chromic acid. This forms the acetyl side chain of progesterone and an esterified hydroxyl group on the steroid nucleus. The ester is then hydrolyzed under strongly basic conditions. The use of acetic acid leads to the production of 16-dehydropregnenolone acetate (16-DPA). 16-DP can be converted into progesterone in two steps. Firstly, the double bond in ring D is hydrogenated, followed by Oppenauer oxidation of the hydroxyl group and the concurrent migration of the remaining olefin from ring B to ring A so that it is in conjugation with the ketone carbonyl group at position 3. Alternatively, a three-step procedure involving Br2, CrO3, and Zn/HOAc can be used. 16-DP can also be converted into testosterone and the downstream products estrone and estradiol. 👍
#jokes#funny#lmao#lol#monday giggles#teehee moment#humour#comedic#thoughtful humour#joke of the day#classic laughs#silly#goofs#entertaining#lighthearted#share if you chuckled#laughter provoking#witty#the lighter side of life#guffaw daily#hilarious#amusing stuff#rofl#lmbo#droll#merriments#jestercore#weekday smiles#laughter is the best medicine#grin and the world grins back
19 notes
·
View notes
Text
if I were a weak little ketone and you were a strapping borohydride would you hold me down by my r chains and reduce my carbonyl groups and make me a secondary alcohol or do you hate me
77 notes
·
View notes
Text
Carbonyl Group Formulas

Patreon
#studyblr#notes#carbonyl groups#functional groups#my notes#organic chemistry#ochem#orgo#orgo notes#organic chemistry notes#organic chem#orgo chem#study guides#mcat#mcat chemistry#mcat orgo#mcat ochem#mcat organic chemistry#mcat studyblr#premed studyblr#organic chemicals#organic reactions#chemical reactions#advanced chemistry#life science#note cards#flashcards#flash cards
1 note
·
View note
Photo

Degradable polyethylene plastics from the nonalternating terpolymerization of ethylene, CO, and polar monomers
In a study published in the journal National Science Review and led by Dr. Zhongbao Jian (State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, CAS), E/CO/PM terpolymerizations were carried out with seven palladium catalysts, which were strictly non-alternating (>99%) with Pd4.
Polar monomers included acrylates, acrylic acid, vinyl ethers, vinyl acetate and acrylonitrile. High molecular weight linear polyethylene with the low content of isolated carbonyl group (selectivity > 99%) and polar functional group was developed.
The molecular structure of the resulting polymer was analyzed in detail, and the microstructure of the E/CO/PM copolymer was clearly characterized by IR, nuclear magnetic resonance spectroscopy (1H/13C/2-D NMR) and 13CO labeling technology.
Read more.
#Materials Science#Science#Polyethylene#Catalysts#Palladium#Polymers#Plastics#Materials Characterization
14 notes
·
View notes
Note
for the ask game!! 🔭 🪐 and 🔬 :)
🔬 a science class you loved, and why
i am currently loving my organic chemistry class, my prof is amazing! he explains everything on such a fundamental level, and brings in applications in other fields such as biochemistry :) yesterday he explained how poison ivy works on a molecular level just because it involves one of the concepts from lecture (α,β-unsaturated carbonyl compounds)
🪐 something you learned this week
in the same class, we also learned how benzene rings can act as nucleophiles when attacking a sufficiently activated electrophile! we then learned the effects of different (de)activating groups on reactions of substituted arenes and how to, given a disubstituted arene, decide on the mechanism of reaction :)
🔭 what sparked your interest in science/your field?
i've been interested in the human body basically forever, but i took a molecular neuroscience class the summer before uni and really loved it, so i'm a neuro major now with minors in chemistry and art! i looooove biochem and want a PhD in cancer immunology
thank you so much for the ask <333
4 notes
·
View notes
Text
Because several different functional groups contain a carbonyl group, it is often not possible to tell from signals in this region alone whether the carbonyl-containing compound is an aldehyde, a ketone, a carboxylic acid or an ester.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#functional group#carbonyl#aldehyde#ketone#carboxylic acid#ester
0 notes
Text
something I really love about when a professor asks a question on the first day of class is the way that all the kids in the class will like, mutter the answer under their breath instead of actually just saying it? bc it happens every year and the effect of like, disembodied whispers telling you what kind of carbonyl group a certain carbon is is very funny to me
4 notes
·
View notes
Text
Aldehydes and ketones are very reactive due to the polarity and structure of the carbonyl group (figure 21.3).

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#aldehyde#ketone#reactive#carbonyl#polarity#electron density
3 notes
·
View notes