#Immunological diseases
Explore tagged Tumblr posts
Text
Application of Solar Cells in Three Dimensions to Generate Electrical Power – Solar Ball Column

Application of Solar Cells in Three Dimensions to Generate Electrical Power – Solar Ball Column in Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.006009.php
This manuscript is trying to bring more light on the importance of replacing the current source of energy. Till now, many parts of the world are generating electrical power using fossil source of energy (oil and charcoal), while the promising future is by generating electricity from solar energy. Instead of using renewable source of energy, counties still use fossil fuels which they import from other countries [1]. Climate extreme changes are only part of the evidence that centuries of burning fossil fuel led to highly destructive impacts on the planet. In this occasion flight emissions affecting the climate change needs to be controlled [2]. It is worth to mention here the heat strike that hit Canada just few days ago [3].
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01 Follow on Blogger :https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#journals on emergency medicine#regenerative medicine#immunological diseases#journal of biomedical research and reviews#journal of biomedical sciences research review
0 notes
Text
"Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis, or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today [May 17, 2024] in the scientific journal Cell.
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
-via WIRED, May 17, 2024. Article continues below.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”
Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
-via WIRED, May 17, 2024
#hiv#aids#aids crisis#virology#immunology#viruses#vaccines#infectious diseases#vaccination#immune system#public health#medicine#healthcare#hiv aids#hiv prevention#good news#hope#medical news
919 notes
·
View notes
Text
are we on the cusp of a breakthrough in endometriosis?
#tiktok#endometriosis#the guardian#health news#auto immune disease#autoimmine disease#auto immune disorder#immune system#immunology#science news#medical studies#medical news#medical history#misogny
3K notes
·
View notes
Text
For roughly one in every hundred people, food containing even the smallest amounts of gluten can deliver a gutful of hurt. While a domino effect of immunological reactions can be traced back to their genetic roots, a number of contributing factors are also involved, making it difficult to map the precise chain of events that causes an allergy to gluten to emerge. Using transgenic mice, an international team led by scientists from McMaster University in Canada has identified a crucial role played by the very cells making up the gut's lining, describing a major stepping stone that could lead to new therapies.
Continue Reading.
357 notes
·
View notes
Text

Vitiligo
#vitiligo#wikipedia#wikipedia pictures#skin#dermatology#melanin#immunology#autoimmune disorder#autoimmune disease#medicine
86 notes
·
View notes
Text

Disease-mimicking Mice
Mice with a mutation in the Linker for Activation of T cells (LAT) gene serve as a model for elucidating the molecular and cellular events underlying IgG4-related disease, a human autoimmune condition that can affect most organs causing fibrosis and inflammation with serious consequences
Read the published research article here
Image from work by Anais Joachim and colleagues
Aix Marseille Université, INSERM, CNRS, Centre d’Immunologie de Marseille-Luminy, Marseille, France
Video originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Journal of Experimental Medicine, August 2023
You can also follow BPoD on Instagram, Twitter and Facebook
7 notes
·
View notes
Text
Selectins
Molecule family
Cell-surface adhesion molecules
Found on leukocytes and endothelium
Bind to sugars on glycoproteins
.
Patreon
#studyblr#notes#my notes#medblr#medical notes#med notes#immunology#immunology notes#immune system#immune system notes#immune cells#immunity#pathology#pathophysiology#immune system cells#leukocytes#white blood cells#cell biology#cytology#cytology notes#cell biology notes#cellular immunology#cell immunology#diseases#diseases and disorders#science#scienceblr#life science#health science#note cards
2 notes
·
View notes
Text

The Antibody Odyssey
Have you ever wondered about the tiny superheroes zipping around your body, keeping you safe from invaders? No capes, no tights, but these incredible warriors are essential for our health: antibodies! These Y-shaped proteins play a crucial role in identifying and neutralizing these invaders, safeguarding our health. Produced by specialized white blood cells called B lymphocytes (B cells), antibodies are highly specific molecules, each designed to recognize a unique signature on a foreign substance, known as an antigen. This specificity, akin to a lock-and-key mechanism, ensures that antibodies only target the invading pathogens and not our own healthy tissues.
The earliest glimpse of antibodies came in 1890 when Emil von Behring and Shibasaburo Kitasato made a groundbreaking discovery. They observed that serum from animals immunized against diphtheria could protect other animals from the disease. This landmark finding laid the foundation for the concept of "antibodies" and paved the way for further exploration. In the early 20th century, Paul Ehrlich, renowned as the "father of immunology," proposed the "side-chain theory." This theory postulated the existence of specific receptors on cells that could bind to specific antigens (foreign molecules). This concept laid the groundwork for understanding the remarkable specificity of antibody-antigen interactions.
The 1960s witnessed a significant breakthrough with the work of Rodney Porter and Gerald Edelman. They elucidated the primary and secondary structure of antibodies, revealing their Y-shaped structure and intricate details of their amino acid sequences. This paved the way for a deeper understanding of their function and diversity. The process of antibody creation begins when a B cell encounters an antigen. This triggers the B cell to activate and divide rapidly, forming a clone of identical cells. These clones, called plasma cells, become antibody factories, churning out millions of antibodies specific to the encountered antigen. These antibodies then circulate throughout the bloodstream and lymphatic system, patrolling for their matching antigens.
Recognizing and Eliminating Threats: The Multifaceted Arsenal of Antibodies
Once an antibody encounters its specific antigen, it binds to it with remarkable precision. This binding initiates a multi-pronged attack on the pathogen:Neutralization: By binding to critical structures on the antigen, such as the viral envelope or bacterial toxins, antibodies can render them ineffective, preventing them from infecting cells or causing harm. Opsonization: Antibodies act as flags, coating the antigen with a special tag that attracts other immune cells, such as phagocytes (white blood cells that engulf and destroy foreign particles). This process, called opsonization, marks the antigen for destruction. Activation of the complement system: Antibodies can trigger a cascade of protein reactions called the complement system, which further aids in the destruction of the pathogen.
But, did you know that there's not just one type of antibody? These versatile molecules come in various forms, each with its unique structure and function. The type of antibody produced also plays a crucial role in the immune response. There are five main classes of antibodies (IgG, IgA, IgM, IgD, and IgE), each with distinct properties and functions:
Immunoglobulin G (IgG): The Mighty Defender - This is the most abundant antibody type, constituting around 70-80% of all antibodies in the bloodstream. IgG has four subclasses (IgG1-4) with subtle differences in function and lifespan. IgG is like a versatile soldier, capable of: Neutralizing toxins and viruses: By binding to pathogens, IgG prevents them from infecting cells. Triggering phagocytosis: It flags pathogens for specialized immune cells called phagocytes, which engulf and destroy them. Passing immunity to newborns: IgG antibodies can cross the placenta, offering newborns temporary protection against infections until their own immune system develops.
Immunoglobulin M (IgM): The First Responder - IgM is the first antibody produced by B cells in response to an infection. While less effective at neutralizing pathogens individually, it compensates through its: Pentameric structure: Five Y-shaped units join together, increasing the "avidity" or overall binding strength to pathogens. Complement activation: IgM can activate the complement system, a cascade of proteins that attracts immune cells and promotes pathogen destruction.
Immunoglobulin A (IgA): The Sentinel at the Gates - This antibody is primarily found in mucosal secretions like tears, saliva, and breast milk. IgA acts as the first line of defense against infections at these entry points by: Preventing pathogen attachment: It binds to pathogens, hindering their ability to adhere to and colonize mucosal surfaces. Neutralization and exclusion: IgA neutralizes pathogens and facilitates their removal through mucus flow.
Immunoglobulin D (IgD): The Enigmatic Player - IgD remains the least understood antibody type, making up only a tiny fraction (around 0.02%) of the total. While its exact function is still being unraveled, it's believed to be involved in: B cell activation: IgD might play a role in stimulating B cells to mature and produce other antibodies. Regulation of immune response: It's thought to be involved in fine-tuning the immune response by preventing B cells from overreacting.
Immunoglobulin E (IgE): The Double-Edged Sword - IgE is responsible for triggering allergic reactions. It binds to allergens (substances perceived as threats) on mast cells, which then release histamine and other chemicals. This leads to the characteristic symptoms of allergies like runny nose, itchy eyes, and wheezing. However, IgE also plays a role in expelling parasites, It can trigger the release of substances that help expel parasitic worms from the body.
The Building Blocks: Chains and Domains
An antibody is comprised of four polypeptide chains: two identical heavy chains and two identical light chains. Each chain folds into distinct regions called domains, which are responsible for specific functions.
Variable (V) domains: Located at the N-terminus (amino-terminal end) of both heavy and light chains, these domains boast highly diverse sequences. This variability allows the antibody to recognize a vast array of unique structures on antigens, the foreign molecules it targets.
Constant (C) domains: The C-terminus (carboxy-terminal end) of the heavy chains contains these domains. They determine the antibody's class (isotype), which influences its ability to interact with other components of the immune system and trigger specific effector functions.
The Architecture: Y-Shaped Majesty : The four chains assemble in a specific manner, forming the characteristic Y-shaped structure. The arms of the "Y" are formed by the Fab (fragment antigen-binding) fragments, each consisting of one light chain and one heavy chain linked together. These Fab fragments house the antigen-binding site, the crucial pocket where the antibody specifically recognizes and binds to its target antigen. The base of the "Y" is the Fc (fragment crystallizable) fragment, solely composed of the C domains of the heavy chains. This region interacts with immune cells and molecules, dictating the antibody's fate and activating various immune responses.
A Hinge for Flexibility and Diversity : Connecting the Fab and Fc fragments is a flexible hinge region. This hinge allows the Fab arms to have some degree of movement, enabling them to bind to antigens with different shapes and sizes. This flexibility also contributes to the remarkable diversity of antibody specificities, allowing the immune system to recognize and combat a wide range of pathogens.
When we encounter an antigen for the first time, our B-cells take a snapshot of its "fingerprint" and create a specific antibody to fight it. These "memory B-cells" then stick around, so if the same antigen tries to attack again, our bodies can respond quickly with a trained army of antibodies, preventing us from getting sick again. This is the genius behind vaccinations! Vaccines introduce weakened or inactive antigens, training our B-cells to create memory for specific villains, so we're prepared if they ever try to invade for real.
The knowledge gained from antibody research has revolutionized healthcare. Here are some notable examples:
Vaccines: By exposing the immune system to weakened or inactive forms of pathogens, vaccines stimulate the production of specific antibodies, providing long-term protection against diseases.
Diagnostic Tools: Antibody-based tests, like ELISA (Enzyme-Linked Immunosorbent Assay), are widely used to detect and diagnose various diseases, including viral infections and autoimmune disorders.
Therapeutic Antibodies: Monoclonal antibodies, produced in the lab to target specific antigens, have emerged as a powerful tool for treating various diseases, including cancer, autoimmune diseases, and infectious diseases.
Antibodies are a testament to the body's remarkable ability to defend itself. These meticulously designed proteins, constantly patrolling our systems, stand as a testament to the intricate and sophisticated nature of the immune system. By delving deeper into their diverse functions and potential applications, we gain a profound appreciation for the intricate dance of life and the ongoing battle against invading threats. As research continues to unveil the secrets of antibodies, we can anticipate even greater advancements in healthcare and disease prevention, all thanks to these extraordinary defenders within us.
#science sculpt#life science#molecular biology#science#biotechnology#artists on tumblr#immunology#scientific research#disease#antibody#antibodies#immunity#health and wellness#vaccinations#structure of antibody#immunoglobulin#illustrative art#illustration#biology#animal facts#zoology#human body
7 notes
·
View notes
Text
i know i left microbiology behind for neurosci but like. for as long as we have existed as Human Beings (or even before, when our ancestors were hominid primates developing some of that frontal lobe executive function shit) we have been haunted by these invisible forces, that seemingly randomly inhabit us. a ghost that is always looking for a new body, and depleting that body of its life force . and if you get too close to The Haunted Person you might be next but you need to get to that person to have any hope of saving them. and you don’t know how to treat it (and those plant bark teas and drinking animal bone broths or blood sometimes help but other times they still die…) . but like. for hundreds and hundreds of years poltergeists and shit are real and they possess people one by one and sometimes they travel in animal bodies . and then one day some guy invents The Microscope and turns out you can see the poltergeists and also they look pathetic as shit
#my posts#i do feel like modern cognitive neurosci and clinical psych has a lot of the same elements of Old Microbiology and Immunology#like Invisible Disease Fucks Things Up . Medications Etc Don’t Really Work#inspires a lot of like conceptions of it as hauntings or whatever#and gets even very materialist ppl acting like souls and shit are a thing.#(so much of modern psychiatry and psychology still describe things and u read and ur like. this is so dualist of u)#maybe that’s why i swapped from micro to neuro. i love ghost hunting
3 notes
·
View notes
Text
Impulsively completely rethought my entire future career
#I'm gonna be a fuckin immunologist or infectious disease doctor babyyyy#Honestly the only reason why I thought ��hell no” to a medical career is mostly I'm squeamish#But then I thought#After a month or so I'll probably stop being squeamish about it y'know#And honestly no matter what I'm gonna deal with high stress and burnout either way#(because I wanted to work in arts before)#(and lets be honest that not might bring in a lot of money and it'd cause so much burnout and shit)#(if I can barely make art consistently now I doubt I'd be able to do it better if my CAREER depended on it)#(and either way. I'd find time for art as a doc somehow)#(just find some time teehee)#like I've (since 2020) always had a major interest in medicine#(generally diseases and disorders hence why either immunology or infectious disease doc)#so it'd still be a dream job#OR I COULD BE A RESEARCHER!!#i dont know#Something to do with diseases or disorders or stuff like that!#Hehehe#I mean that's if highschool goes well#If it doesn't then I'm going back to my dream of a character designer#Design characters and see them brought to life by animators#That'd be so cool too#Ok ok sorry for ranting I wanted to get this out of my head
9 notes
·
View notes
Text
Non-Palpable Breast Lesions- Diagnosis and Treatment

Non-Palpable Breast Lesions- Diagnosis and Treatment in Biomedical Journal of Scientific & Technical Research
https://biomedres.us/fulltexts/BJSTR.MS.ID.005845.php
With the notion premalignant non-palpable lesions, we understand spectrum of morphological changes in the tissue of breast. These changes are risk factor for the formation of cancer. These changes are benign, but they are more associated with the cancer with the contrast of another clear benign lesions. We call them high res or precancer lesions. These findings are unclear biological behaviour, cells show malign architectural features and the proliferation is different than the normal regulation mechanism of organism. What is very important, they have no invasion ability or ability to create metastasis. These lesions threaten patients with formation of cancer, but the extent of risk is necessary to connect with pre-existing individual risk factors of every patient [1,2]. At the last decades, the incidence of non-palpable lesions of breast is increasing, because of mammography and another exact imaging methods [3-5]. As a result of this fact, there is the decrease of number of neoplasions and reduction of their spreading into the axillar lymphatic nodes [6,7]. Using of mammographic screening improvement of its sensitivity increase capture ratio of subclinical lesions. Ratio of non-palpable lesions in the time of diagnosis is 25-30% in countries with function screening programme [7]. With increasing diagnostic of non-palpable lesions at the early stadium, correct and complex treatment process is more important. Successful intraoperative localization of non-palpable lesion is necessary for surgeons because of complete excision during the one intervention without extensive excision of healthy tissue.
For more articles in Journals on Biomedical Sciences click here bjstr Follow on Twitter : https://twitter.com/Biomedres01 Follow on Blogger : https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#medical humanities#nano medicine#physical medicine and rehabilitation#immunological diseases#bio-psychological medicine
0 notes
Text
Though the change from 2013 to 2003 doesn’t make that much of a difference, I do like the weird meta it adds with the context of the opening scene taking place in 1968. Because by bumping the main storyline to 2023 (or the present for us as well), the theoretical discussion of a pandemic is very interesting in parallel to COVID.
Obviously, very different diseases, but a mysterious disease that seemingly comes from nowhere but also we’ve known about the potential of for literal decades, to the point where we have plans to handle outbreaks but implementation will still suffer and result in mass casualty and fear is an unfortunately good parallel to the real world that I sometimes don’t think we acknowledge enough. And to see it being so calmly discusses in the theoretical, especially the bit about there being no cure, is so ominous and sets a good tone for the series.
#the last of us#tlou#the last of us hbo#tlou hbo#tlou spoilers#disease and immunology#please tell me more
23 notes
·
View notes
Text
The Supernatural as Cancerous Beings
In biology, we learned a lot of cool stuff about cancer, and I think supernatural beings (vampires and shapeshifters) are easily reconstituted to represent walking tumours.
Vampires - easy, immortal unageing cell lines that require blood consumption to access nutrients, can survive anything except silver and wood (to be fair, you could technically kill cancers with these, some anticancer drugs are made of silver and compounds from trees). Cancers also quite literally parasitically steal nutrients, and can fool the immune system with their own version of a glamour.
Shapeshifters - cancers can reprogram their own genes, change conformation (tumours), expand, and proliferate rapidly, ergo, a shapeshifter explodes into a clump of cancer cells, that reprogram themselves into the new forms they desire.
I think cancers can very easily recapture elements of supernatural beings, and some of the traits of cancer fit in nicely with traits of supernatural creatures.
#biology#oncology#supernatural creatures#vampires#shape shifting#observe the product of my diseased mind#quite proud of this actually#cancer#cancer characteristics#immunology
12 notes
·
View notes
Text
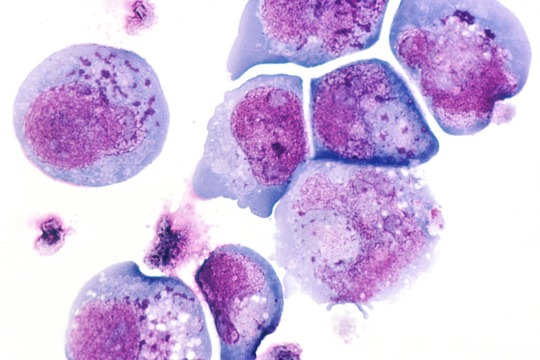
Human herpesvirus 6
“Light micrograph of infected T-lymphocytes with typical so-called inclusion bodies (HE staining). Inclusions in HHV-6 infection (dark blue dots).” - via Wikimedia Commons (original description translated from German using Google Translate)
#human betaherpesvirus 6a#herpesvirus#hhv#hhv-6#human herpesvirus 6#histopathology#micrograph#t lymphocytes#t cells#infectious diseases#immunology#fertility#infertility#wikipedia#wikipedia pictures#nature#wikimedia commons#medicine#medical#herpesviridae#medical microbiology#virology#virus#viruses#diseases#herpesvirales#roseolovirus#betaherpesvirus#orthoherpesviridae#he staining
19 notes
·
View notes
Text
its actually nuts how missing a single vaccination has shaped my entire fucking life. like not only would I not be deaf if I had gotten it on time but I probs wouldn't have adhd either 🫣
#like yeah I have a family history of adhd but im pretty sure the current model suggests u can be genetically *predisposed* but the actual-#development of adhd is thought to be closely linked to environmental 'triggers' like childhood stress or head injuries lol#or in my case brain trauma. fun fact: a suspected 62% of kids who survive hib meningitis later develop adhd symptoms#vs. 5% incidence in the general population.....#when I first heard that I was still in denial bc i thought of adhd as a 'natural' condition like ur just born that way#so if meningitis survivors displayed symptoms that didnt mean they were ACTUALLY adhd. except literally all adhd is-#is a collection of symptoms its not some tangible 'switch' thats flipped in some ppl and not others. maybe thats a rly obvious statement-#but I found it kinda hard to get my head around. i guess just bc of how a lot of psychology is viewed by the public innit#anyway being deaf + nd kinda fucking sucks yall better be jabbing ur babies with every vaccination possible or im coming for ur knees#its funny bc it sounds like im saying watch out !! vaccination may PREVENT neurodivergence NOT cause it !!#*andrew wakefield voice* u wouldnt want a child with autism#but thats not what i meaaaannn obvs ur kid not getting xyz disease that could kill them is the number 1 most important thing#its so cringe actually bc hib b incidence has been down to abt 2 in every 100 000 babies since the vax was introduced in 1985#so I was one of like. probably less than 10 babies to get it in the fucking country and they misdiagnosed me a bunch of times#bc it was so uncommon + I had some rarer symptoms plus the only way to actually CHECK is to test spinal fluid which is a faff#if theyd realised earlier then i also wouldnt be deaf bc it wouldnt have been as severe. just a series of unfortunate events i guess#anyway. immunology is so fascinating i wish id focused on it more in my degree tbh#over and OUT#.diaries
6 notes
·
View notes
Text
Adaptive Immune Response Phases - Diagram

Patreon
#studyblr#notes#my notes#medblr#medical notes#med notes#immunology#immunology notes#immune system#immune system notes#immune cells#immunity#pathology#pathophysiology#immune system cells#leukocytes#white blood cells#cell biology#cytology#cytology notes#cell biology notes#cellular immunology#cell immunology#diseases#diseases and disorders#science#scienceblr#life science#health science#note cards
4 notes
·
View notes