Science is a celebration of curiosity—a relentless pursuit of understanding the world around us. It's a journey of asking questions, seeking answers, and being in awe of the intricate mechanisms that govern our existence. Whether you're a seasoned scientist or just someone with a curious mind, this blog is for you.
Don't wanna be here? Send us removal request.
Text
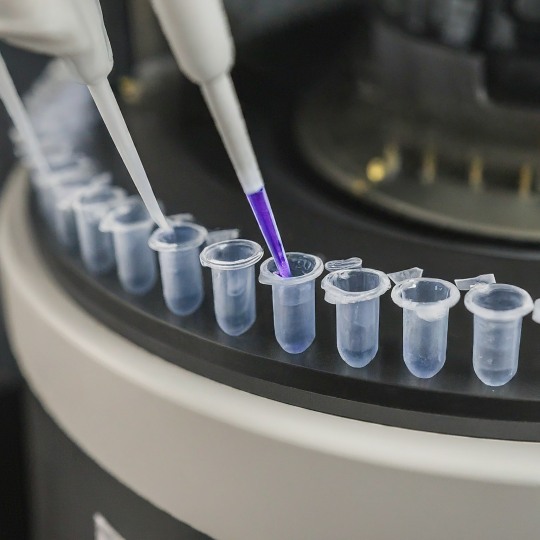

Amplifying Revolution: The Polymerase Chain Reaction (PCR)
Imagine a scenario where you have a crucial document, but there's only one fragile copy. You need numerous duplicates to analyze and share. This is exactly the challenge faced by scientists dealing with DNA. Thankfully, a revolutionary technique called Polymerase Chain Reaction (PCR) comes to the rescue. PCR, often referred to as molecular photocopying, is a fundamental tool in molecular biology. It allows scientists to exponentially amplify a specific DNA segment, creating millions of copies from a minuscule sample. This has revolutionized various fields, from diagnosing diseases to unraveling genetic mysteries.
The credit for inventing PCR is widely attributed to Kary Mullis, a biochemist working at Cetus Corporation in the early 1980s. Inspired by his nighttime drives through California, Mullis envisioned a method for exponentially copying DNA segments through repeated cycles of heating, annealing (primer attachment), and extension (polymerase-mediated DNA synthesis). This elegant concept became the foundation of PCR. Mullis's concept was brilliant, but a crucial hurdle remained. The process required a DNA polymerase enzyme that could withstand repeated heating and cooling cycles. The solution came from an unexpected source: hot springs. In 1976, researchers discovered Taq polymerase, a heat-stable enzyme isolated from the thermophilic bacterium Thermus aquaticus. This discovery was a game-changer, as Taq polymerase could function optimally during the high-temperature steps of PCR. In recognition of its transformative impact on science, Kary Mullis was awarded the Nobel Prize in Chemistry in 1993, alongside Michael Smith, who pioneered site-directed mutagenesis.
While the core concept of PCR was established, the technique required further refinement. Pioneering researchers like Henry Erlich at Cetus played a vital role in optimizing reaction conditions, automating the process, and developing the now-ubiquitous thermal cyclers that precisely control the temperature changes needed for PCR. The 1980s and 1990s witnessed a surge in PCR applications. In 1985, PCR was used for the first time to analyze sickle cell anemia, demonstrating its potential for clinical diagnostics. Forensic science embraced PCR in 1987, with the successful amplification of DNA from a single human hair. By 1989, highly sensitive DNA fingerprinting techniques based on PCR became a game-changer in criminal investigations.
At the heart of PCR lies a clever exploitation of the natural process of DNA replication. The key players in this drama are:
Template DNA: The DNA sequence that contains the target region to be amplified
Primers: Short sequences of nucleotides that flank the target DNA region and serve as starting points for DNA synthesis.
DNA Polymerase: Enzyme responsible for synthesizing new DNA strands by extending the primers using nucleotides.
Nucleotides: The building blocks of DNA, including adenine (A), thymine (T), cytosine (C), and guanine (G).
Buffer Solution: Provides optimal conditions for the enzymatic reactions to occur.
Thermal Cycler: Instrumentation used to automate the PCR process by cycling through different temperatures.
At its core, PCR mimics the natural process of DNA replication within an organism. However, PCR condenses this complex process into a series of controlled steps carried out within a test tube. Here's a breakdown of the PCR cycle:
Denaturation: The first step involves heating the reaction mixture to a high temperature (usually around 95°C), causing the double-stranded DNA to separate into two single strands. This process is known as denaturation.
Annealing: The temperature is then lowered to allow the primers to bind (anneal) to their complementary sequences on the single-stranded DNA. This typically occurs around 50-65°C, depending on the primer sequences.
Extension: With the primers bound, the temperature is raised again, and DNA polymerase synthesizes new DNA strands by extending from the primers using the nucleotides present in the reaction mixture. This step occurs at a temperature optimal for the DNA polymerase enzyme, typically around 72°C.
Cycle Repetition: These three steps—denaturation, annealing, and extension—are repeated multiple times (usually 20-40 cycles), resulting in an exponential increase in the number of DNA copies. Each cycle doubles the amount of DNA, leading to millions of copies of the target sequence after just a few cycles.
The beauty of PCR lies in its repetitive nature. With each cycle, the number of copies of the target DNA segment doubles. After 30 cycles, for example, you can have billions of copies of the specific DNA region, enough for further analysis.
This versatile technique has spawned numerous variations, each tailored for a specific purpose. Let's delve into some of the most common types of PCR:
Real-Time PCR (qPCR): Real-Time PCR, or quantitative PCR (qPCR), revolutionized nucleic acid quantification by enabling the real-time monitoring of DNA amplification. This technique utilizes fluorescent reporter molecules to measure the accumulation of PCR products during each cycle. qPCR is invaluable in gene expression analysis, microbial quantification, and diagnostic assays due to its high sensitivity and quantitative capabilities.
Reverse Transcription PCR (RT-PCR): Reverse Transcription PCR combines PCR with reverse transcription to amplify RNA sequences. This technique converts RNA into complementary DNA (cDNA) using reverse transcriptase enzyme before proceeding with PCR amplification. RT-PCR is pivotal in gene expression studies, viral load quantification, and the detection of RNA viruses such as HIV and SARS-CoV-2.
Nested PCR: Nested PCR involves two rounds of amplification, with the second round using a set of nested primers that bind within the product of the first round. This nested approach increases specificity and reduces nonspecific amplification, making it ideal for detecting low-abundance targets and minimizing contamination. Nested PCR is commonly used in forensic analysis, pathogen detection, and rare allele identification.
Multiplex PCR: Multiplex PCR allows simultaneous amplification of multiple target sequences within a single reaction. This technique employs multiple primer sets, each specific to a distinct target region, enabling the detection of multiple targets in a single assay. Multiplex PCR is valuable in microbial typing, genetic screening, and detection of pathogens with complex genetic profiles.
Digital PCR (dPCR): Digital PCR partitions the PCR reaction into thousands of individual micro-reactions, each containing a single DNA template molecule or none at all. By counting the number of positive and negative partitions, dPCR accurately quantifies target DNA molecules without the need for standard curves or reference samples. This technique is useful for absolute quantification of rare targets, allelic discrimination, and copy number variation analysis.
Allele-Specific PCR: Allele-Specific PCR selectively amplifies alleles containing specific nucleotide variations, enabling the detection of single nucleotide polymorphisms (SNPs) or mutations. This technique utilizes primers designed to match the target sequence with single-base mismatches at their 3' end, allowing discrimination between different alleles. Allele-Specific PCR finds applications in genetic testing, pharmacogenomics, and population studies.
PCR's ability to amplify DNA has made it an indispensable tool in various fields. Here are a few examples of its diverse applications:
Disease Diagnosis and Surveillance: PCR plays a pivotal role in the rapid and accurate diagnosis of infectious diseases. By amplifying specific nucleic acid sequences, PCR enables the detection of pathogens with high sensitivity and specificity. PCR-based tests have become indispensable in diagnosing viral infections such as HIV, hepatitis, influenza, and COVID-19. Additionally, PCR facilitates the surveillance of disease outbreaks and the monitoring of antimicrobial resistance.
Genetic Testing and Personalized Medicine: PCR empowers genetic testing by enabling the detection of genetic mutations, polymorphisms, and variations associated with inherited diseases, cancer, and pharmacogenomics. Through techniques like allele-specific PCR and real-time PCR, researchers can identify disease-causing mutations, assess drug efficacy, and tailor treatments to individual patients. PCR-based genetic tests have transformed healthcare by enabling early disease detection, risk assessment, and personalized therapeutic interventions.
Forensic Analysis and DNA Profiling: PCR has revolutionized forensic science by enabling the analysis of minute DNA samples collected from crime scenes. Techniques like short tandem repeat (STR) analysis and multiplex PCR allow forensic experts to generate DNA profiles with high resolution and accuracy. PCR-based DNA profiling is used in criminal investigations, paternity testing, disaster victim identification, and wildlife forensics, contributing to the administration of justice and conservation efforts worldwide.
Environmental Monitoring and Microbial Ecology: PCR facilitates the study of microbial communities in diverse environments, including soil, water, air, and the human microbiome. Environmental DNA (eDNA) analysis using PCR-based methods enables the detection and characterization of microbial species, including bacteria, fungi, and archaea. PCR-based assays are employed in environmental monitoring, food safety testing, and microbial source tracking, aiding in the preservation of ecosystems and public health.
Agricultural Biotechnology and Food Safety: PCR plays a vital role in agricultural biotechnology by enabling the detection of genetically modified organisms (GMOs), plant pathogens, and foodborne pathogens. PCR-based assays are used to verify the authenticity and safety of food products, detect allergens, and monitor the presence of contaminants such as pesticides and toxins. PCR-based technologies contribute to ensuring food security, quality control, and regulatory compliance in the food industry.
Evolutionary Biology and Phylogenetics: PCR-based methods are indispensable tools for studying evolutionary relationships and biodiversity. Techniques like DNA barcoding and metagenomics employ PCR to amplify and analyze DNA sequences from diverse organisms, elucidating their evolutionary history and ecological interactions. PCR facilitates the identification of new species, the study of population genetics, and the conservation of endangered species, enriching our understanding of the natural world.
PCR's versatility and precision make it indispensable in unlocking the secrets of genetics and unraveling complex biological mysteries. Its ability to amplify minute DNA samples with remarkable speed and accuracy has opened doors to countless possibilities in research and diagnostics. s we delve deeper into the intricacies of the genetic world, PCR will undoubtedly remain a powerful tool for unlocking the secrets of life itself.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#double helix#genetics#dna#polymerase chain reaction#medical science#the more you know#scientific research#scifiart#scientific advancements#scientific illustration#scientific instruments#scientific discovery
7 notes
·
View notes
Text

A snip, a splice : Power of rDNA Technology
Deoxyribonucleic acid (DNA), the blueprint of life, holds the secrets to the intricate workings of every living organism. But what if we could manipulate this blueprint, adding, removing, or tweaking its code? This revolutionary concept forms the core of recombinant DNA (rDNA) technology, a powerful tool that has transformed biology and medicine.
The story starts in the early 1970s with two brilliant scientists; Stanley Cohen at Stanford University and Herbert Boyer at the University of California, San Francisco. Cohen, a microbiologist, had been studying plasmids – small circular DNA molecules found in bacteria. Boyer, a biochemist, was an expert on restriction enzymes – molecular scissors that could cut DNA at specific sequences. Their collaboration proved groundbreaking. They envisioned combining these tools to create the first ever recombinant DNA molecule. Cohen provided the plasmids, which would act as vectors to carry foreign DNA into host cells. Boyer, on the other hand, used restriction enzymes to cut both the plasmid and the desired foreign DNA, allowing them to be pieced together. Through meticulous experimentation, they successfully created the first recombinant DNA molecule, forever altering the course of biology.
Cohen and Boyer's work wouldn't have been possible without the earlier discoveries of restriction enzymes. These "molecular scissors" were independently identified by three separate research groups in the 1960s. Werner Arber in Switzerland, along with Hamilton Smith and Daniel Nathans in the US, unraveled the role of restriction enzymes in bacterial defense mechanisms. These enzymes helped bacteria defend against invading viruses by cutting up their foreign DNA. Recognizing the potential of these "genetic scalpels," the groundwork was laid for their application in rDNA technology.
Here's a simplified breakdown of the rDNA process:
Isolation of DNA: The journey starts with isolating DNA from a donor organism.
Cleavage with Restriction Enzymes: Specific enzymes cut the DNA at defined sequences.
Selection of Vector: A carrier molecule (often a plasmid) is chosen to transport the recombinant DNA.
Ligation: The DNA fragments and vector are stitched together using DNA ligase, an enzyme.
Transformation: The recombinant DNA enters a host cell (usually bacteria or yeast).
Selection and Expression: The transformed cells are selected, and the gene of interest is expressed, leading to the desired protein production.
Since its inception, rDNA technology has played a pivotal role in several groundbreaking advancements. Let's take a whirlwind tour through some of the most significant moments in R-DNA history:
1978: Birth of Insulin on the Factory Floor: Scientists achieved a feat of genetic engineering by using R-DNA to produce human insulin in bacteria. This marked a turning point for diabetics, offering a readily available and more consistent source of this life-saving hormone.
1980s: Gene Wars and the Rise of GMOs: The 1980s saw the development of genetically modified organisms (GMOs). Plants were engineered with genes for insect resistance or herbicide tolerance, sparking debates about the safety and ethics of this technology. R-DNA research continues to be at the forefront of discussions regarding genetically modified foods.
1990s: The Human Genome Project Sets Sail: This ambitious international project aimed to sequence the entire human genome. R-DNA techniques played a crucial role in deciphering the 3 billion letters of our genetic code, opening doors for personalized medicine and a deeper understanding of human health and disease.
2000s: Gene Therapy Takes Center Stage: The first successful gene therapy trials for inherited diseases like severe combined immunodeficiency (SCID) took place. R-DNA technology offered a glimmer of hope for treating genetic disorders by introducing healthy genes to replace defective ones.
2010s and Beyond: CRISPR Takes Over: The emergence of CRISPR-Cas9, a revolutionary gene editing tool based on R-DNA principles, has ushered in a new era of genetic manipulation. With unprecedented precision, scientists can now edit genes in various organisms, holding immense potential for gene therapy, crop improvement, and even the eradication of diseases.
But with great power comes great responsibility, and R-DNA raises a host of ethical concerns.Tinkering with the building blocks of life carries the risk of unintended consequences. Engineered genes could escape and disrupt ecosystems, or modified organisms could have unforeseen health effects. The ability to edit human genes opens the door to designer babies, raising questions about social equity and the potential misuse of the technology for eugenics.
Who Controls the Tools? Access to R-DNA technology could be restricted to wealthy nations or corporations, exacerbating existing inequalities. Biosecurity is also a concern, as the technology could be misused for bioterrorism. Creating entirely new organisms forces us to confront what it means to be "natural." Should we modify plants and animals for human benefit, or preserve their original forms? R-DNA technology is a powerful tool, and we must have open discussions about its ethical implications. Scientists, policymakers, and the public all need to be involved in shaping the future of this technology. As we move forward, open dialogue and collaboration between scientists, policymakers, and the public are crucial to ensure the safe and ethical application of this powerful technology.
The journey of rDNA technology is a testament to human ingenuity and its potential to reshape our world. From decoding the secrets of life to creating solutions for healthcare, agriculture, and beyond, rDNA technology continues to evolve, promising a future filled with exciting possibilities.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#dna#double helix#genetics#recombinant#genetic engineering#insulin#research#education#learning#academics#scientific research#scientific illustration#medical science#scifi#daily dose of science#scientific advancements#scientific tools#medical school
17 notes
·
View notes
Text

A Journey into the World of Microscopy: From Humble Beginnings to High-Tech Magnification
The science of looking into the hidden invisible Microscopy has transformed our understanding of the world around us. It can explore the universe beyond the reach of our naked eyes, with complex cellular structures, red blood cells, viruses and other viruses and microorganisms taking on amazing perspectives
The history of the microscope is a fascinating story of human curiosity, scientific genius, and relentless exploration. From the humble beginnings of simple magnifying glasses to the sophistication of modern electronic microscopes, the invention of microscopes has shaped our understanding of the microscopic world
In the 1600s, Dutch opticians such as Hans and Zachary Janssen are credited with inventing the first microscope. Known for this hybrid microscope, many lenses were used to magnify objects up to 30 times.At the end of the 17th century, Antony van Leeuwenhoek, Dutch draper some changed our perception of thumbnails. Armed with a well-made single-lens microscope, and explored the hidden reaches of nature. In 1674, Leeuwenhoek discovered microorganisms in lake water, which he aptly named “animalcules”. His discovery laid the foundations of biology and inspired generations of scientists. This incredible feat allowed him to uncover a hidden universe – the first sightings of bacteria, red blood cells, and other microorganisms.
Formation of the scientific environment (17th-19th centuries): Leeuwenhoek’s discoveries boosted scientific research. Robert Hooke, an English scientist, established these developments. In 1665, his book "Micrographia" recorded his observations with a compound microscope. Notably, the term "cell" was coined by Hooke when he examined cork tissue, laying the foundation for cell biology.Microscope systems flourished throughout the 18th and 19th centuries Joseph Lister and other scientists addressed the limitations of the early lenses, introducing improvements that reduced image distortion.
Beyond the Limits of Light: The Beginning of the New Age (19th-20th century): As the 19th century progressed, the limitations of optical microscopy became apparent and scientists yearned for a tool which can go deeper into cells. This research culminated in the development of the electron microscope in the 1930s. The 20th century was revolutionary with the invention of the electron microscope. Unlike light microscopes, which use visible light, electron microscopes use electron beams to achieve much higher magnification.Formation of the scientific environment (17th-19th centuries): Leeuwenhoek’s discoveries boosted scientific research. Robert Hooke, an English scientist, established these developments. In 1665, his book "Micrographia" recorded his observations with a compound microscope. Notably, the term "cell" was coined by Hooke when he examined cork tissue, laying the foundation for cell biology.Microscope systems flourished throughout the 18th and 19th centuries Joseph Lister and other scientists addressed the limitations of the early lenses, introducing improvements that reduced image distortion.
Beyond the Limits of Light: The Beginning of the New Age (19th-20th century): As the 19th century progressed, the limitations of optical microscopy became apparent and scientists yearned for a tool which can go deeper into cells. This research culminated in the development of the electron microscope in the 1930s. The 20th century was revolutionary with the invention of the electron microscope. Unlike light microscopes, which use visible light, electron microscopes use electron beams to achieve much higher magnification.
In the 1930s, German experts Max Knoll and Ernst Ruska made the first electron microscope. This tool let us see tiny things like cells and even atoms by using electron beams, not light, getting images many times bigger. This cool invention showed us the tiny parts inside cells, viruses, and stuff too small to see before. The 1900s brought even more cool microscopes. New kinds like phase-contrast and confocal microscopy let scientists look at live cells without using stuff that could hurt them. Now, the world of looking at tiny things is getting even better. Today, we have high-tech microscopes that use computers and lasers. These let us see and even change tiny things in ways we never could before.
Modern Microscopy's Diverse Arsenal - Today, the field of microscopy boasts a diverse range of specialized instruments, each tailored to address specific scientific needs. Here's a glimpse into some remarkable examples:
Scanning Electron Microscope (SEM): Imagine a high-tech camera that captures images using a beam of electrons instead of light. That's the essence of a SEM. By scanning the surface of a sample with a focused electron beam, SEMs generate detailed information about its topography and composition. This makes them ideal for studying the intricate structures of materials like insect wings, microchips, and even pollen grains.
Transmission Electron Microscope (TEM): While SEMs provide exceptional surface detail, TEMs take us a step further. They function by transmitting a beam of electrons through a very thin sample, allowing us to observe its internal structure. TEMs are the go-to instruments for visualizing the intricate world of viruses, organelles within cells, and macromolecules like proteins.
Confocal Microscopy: Ever wished to focus on a specific layer within a thick biological sample and blur out the rest? Confocal microscopy makes this possible. It utilizes a laser beam to precisely illuminate a chosen plane within the sample, effectively eliminating information from out-of-focus regions. This allows researchers to create sharp, three-dimensional images of cells, tissues, and even small organisms.
Atomic Force Microscopy (AFM): This technique takes a completely different approach, venturing into the realm of physical interaction. AFM employs a tiny cantilever, akin to a microscopic feeler, to physically scan the surface of a sample. By measuring the minute forces between the cantilever and the sample's surface, AFM can map its topography at an atomic level. This provides invaluable insights into the properties of materials at an unimaginable scale, making it crucial for research in fields like nanotechnology and surface science.
Fluorescence Microscopy: Imagine illuminating a sample with specific wavelengths of light and observing it glowing in response. That's the essence of fluorescence microscopy. This technique utilizes fluorescent molecules or tags that bind to specific structures within a cell or tissue. When excited by light, these tags emit their own light, highlighting the target structures with remarkable clarity. This allows researchers to visualize specific proteins, DNA, or even pathogens within biological samples.
Super-resolution Microscopy (SRM): Overcoming the limitations imposed by the wavelength of light, SRM techniques like STED (Stimulated Emission Depletion) and PALM (Photoactivated Localization Microscopy) achieve resolutions surpassing the diffraction limit. This allows researchers to visualize structures as small as 20 nanometers, enabling the observation of intricate cellular machinery and the dynamics of individual molecules within living cells.
Cryo-Electron Microscopy (Cryo-EM): This powerful technique takes a snapshot of biological samples in their near-life state. Samples are rapidly frozen at ultra-low temperatures, preserving their native structure and minimizing damage caused by traditional fixation methods. Cryo-EM has been instrumental in determining the three-dimensional structures of complex molecules like proteins and viruses, providing crucial insights into their function and potential drug targets.
Correlative Microscopy: Combining the strengths of multiple microscopy techniques, correlative microscopy offers a comprehensive view of biological samples. For instance, researchers can utilize fluorescence microscopy to identify specific structures within a cell and then switch to electron microscopy to examine those structures in high detail. This integrated approach provides a deeper understanding of cellular processes and their underlying mechanisms.
Light Sheet Microscopy (LSM): Imagine illuminating a thin slice of a sample within a living organism. LSM achieves this feat by focusing a laser beam into a thin sheet of light, minimizing photobleaching and phototoxicity – damaging effects caused by prolonged exposure to light. This allows researchers to observe dynamic processes within living organisms over extended periods, providing valuable insights into cellular behavior and development.
Expansion Microscopy (ExM): This innovative technique physically expands biological samples by several folds while preserving their structural integrity. This expansion allows for better resolution and visualization of intricate cellular structures that would otherwise be difficult to distinguish using traditional microscopy methods. ExM holds immense potential for studying the organization and function of organelles within cells.
Scanning Near-Field Optical Microscopy (SNOM): This innovative technique pushes the boundaries of resolution by utilizing a tiny probe that interacts with the sample at an extremely close range. SNOM can not only image the surface features of a sample with exceptional detail but also probe its optical properties at the nanoscale. This opens doors for research in areas like material science and photonics, allowing scientists to study the behavior of light at the interface between materials.
X-ray Microscopy: Stepping outside the realm of light and electrons, X-ray microscopy offers unique capabilities. By utilizing high-energy X-rays, this technique can penetrate deep into samples, making it ideal for studying the internal structure of dense materials like bones and minerals. Additionally, it allows for the visualization of elements within a sample, providing valuable information about their distribution and composition.
From revealing the building blocks of life to aiding in the development of new medicines, the microscope has played an undeniable role in shaping our scientific understanding. As technology continues to evolve, one can only imagine the future breakthroughs this remarkable invention holds in unveiling the secrets of our universe, both seen and unseen. These advancements hold the potential to revolutionize our understanding of biological processes, develop new materials with extraordinary properties, and ultimately pave the way for breakthroughs in medicine, nanotechnology, and countless other fields. As we continue to refine and develop novel microscopy techniques and the future holds immense promise for further groundbreaking discoveries that will undoubtedly revolutionize our perception of the world around us.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#microscopy#microscope#Scanning Electron Microscope#Transmission Electron Microscope#Confocal Microscopy#Atomic Force Microscopy#Fluorescence Microscopy#Expansion Microscopy#X-ray Microscopy#Super-resolution Microscopy#Light Sheet Microscopy#illustration#illustrator#illustrative art#education#educate yourself#techniques in biotechnology#scientific research#the glass scientists#scientific illustration#scientific advancements
7 notes
·
View notes
Text
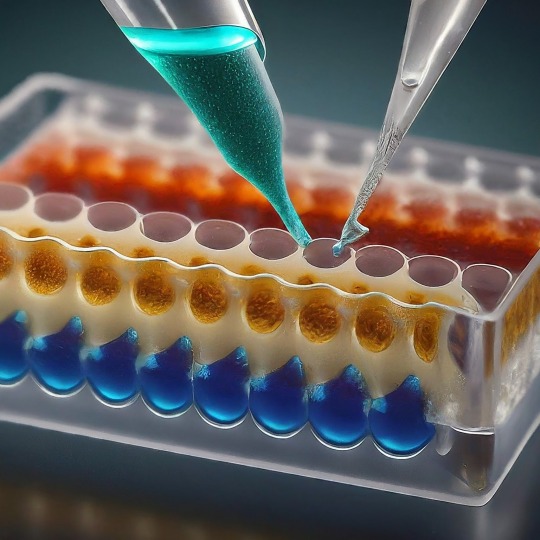
ELISA: A Powerful Tool for Detecting the Invisible
ELISA, or Enzyme-Linked Immunosorbent Assay, has become a cornerstone of medical diagnostics and biological research. This versatile technique allows scientists to detect and quantify minute amounts of target molecules, such as proteins, antibodies, and even viruses, with remarkable accuracy. In this blog, we'll delve into the world of ELISA, exploring its various types, its applications, and the exciting future directions this technology holds.
At its core, ELISA relies on the exquisite specificity of antibodies. Antibodies are highly specialized proteins produced by the immune system in response to foreign invaders. Each antibody can bind to a unique structure, called an antigen, on a specific molecule. In an ELISA, scientists leverage this binding property to create a sensitive detection system.
The 1960s witnessed a surge in interest in immunoassays, techniques that utilize the specificity of antibodies to detect target molecules. One such technique, radioimmunoassay (RIA), developed by Rosalyn Yalow and Solomon Berson, revolutionized medical diagnostics. RIA used radioactively labeled antibodies to detect antigens, offering high sensitivity. However, concerns regarding the safety of radioactive materials fueled the search for a safer alternative. The year 1971 marked a turning point. Independently, Eva Engvall and Peter Perlmann published their work on a novel technique – the enzyme-linked immunosorbent assay (ELISA). ELISA replaced radioactive labels with enzymes, eliminating the safety concerns associated with RIA. Like RIA, ELISA harnessed the specific binding between antibodies and antigens. However, it employed enzymes that could generate a detectable signal, such as a color change, upon interacting with a substrate. This innovation paved the way for a safer and more user-friendly diagnostic tool.
The basic ELISA protocol involves immobilizing the target antigen on a solid surface like a plate well. Then, a sample containing the molecule of interest (e.g., a suspected virus) is introduced. If the target molecule is present, it will bind to the immobilized antigen. Next, an antibody specific to the target molecule, linked to an enzyme, is introduced. This "detection antibody" binds to the target molecule already attached to the antigen. Finally, a substrate specific to the enzyme is added. This antigen-antibody binding is visualized using an enzyme linked to a reporter molecule. When the enzyme encounters its substrate, a detectable signal is produced, such as a color change or luminescence. The intensity of this signal is directly proportional to the amount of antigen present in the sample, allowing for quantification. The beauty of ELISA lies in its adaptability. Several variations exist, each tailored for specific detection needs.
The Four Main ELISA Formats are:
Direct ELISA: Simplicity at its finest. In this format, the antigen is directly coated onto the ELISA plate. A labeled antibody specific to the antigen is then introduced, binding directly to its target. After washing away unbound molecules, the enzyme linked to the antibody generates a signal upon addition of the substrate. Direct ELISA offers a rapid and straightforward approach, but sensitivity can be lower compared to other formats due to the lack of amplification.
Indirect ELISA: Unveiling the Power of Amplification. Similar to the direct ELISA, the antigen is first coated onto the plate. However, instead of a labeled primary antibody, an unlabeled one specific to the antigen is used. This is followed by the introduction of a labeled secondary antibody that recognizes the species (e.g., mouse, rabbit) of the primary antibody. This two-step approach acts as an amplification strategy, significantly enhancing the signal compared to the direct ELISA. However, the presence of an extra incubation step and the potential for cross-reactivity with the secondary antibody add complexity.
Sandwich ELISA: Capturing the Antigen Between Two Antibodies. Here, the capture antibody, specific for one region of the antigen, is pre-coated onto the ELISA plate. The sample containing the antigen is then introduced, allowing it to be "sandwiched" between the capture antibody and a detection antibody specific for a different region of the same antigen. A labeled secondary antibody or a labeled detection antibody itself can then be used to generate the signal. Sandwich ELISA boasts high sensitivity due to the double-antibody recognition and is often the preferred format for quantifying analytes.
Competitive ELISA: A Race for Binding Sites. In this format, the antigen competes with a labeled antigen (usually a known amount) for binding sites on a capture antibody pre-coated onto the plate. The more antigen present in the sample, the less labeled antigen can bind to the capture antibody. Following a washing step, the amount of bound labeled antigen is measured, providing an inverse relationship between the signal and the concentration of antigen in the sample. Competitive ELISA is particularly useful for studying small molecules that may be difficult to directly conjugate to an enzyme.
ELISA's Reach: From Diagnostics to Research. The applications of ELISA are as vast as they are impressive. Let's delve into some key areas where ELISA plays a vital role:
Unveiling the Mysteries of Disease: Diagnostics: ELISA is a cornerstone of diagnosing infectious diseases like HIV, Hepatitis, and Lyme disease. It detects antibodies produced by the body in response to the invading pathogen, providing valuable information for early detection and treatment. Monitoring Autoimmune Diseases: ELISA helps monitor autoimmune diseases like rheumatoid arthritis and lupus by measuring specific antibodies associated with these conditions. Cancer Screening: Certain cancers can be detected by identifying tumor markers, proteins elevated in the blood of cancer patients. ELISA assays are being developed to detect these markers for early cancer screening.
Safeguarding Food Quality: Allergen Detection: Food allergies can be life-threatening. ELISA ensures food safety by enabling the detection of allergens like peanuts, gluten, and milk in food products, protecting consumers with allergies. Monitoring Foodborne Pathogens: ELISA can identify harmful bacteria, viruses, and toxins in food, preventing outbreaks of foodborne illnesses.
Environmental Monitoring: Pollutant Detection: ELISA can detect pollutants like pesticides and herbicides in water and soil samples, contributing to environmental protection efforts. Microbial Analysis: This technique can be used to identify and quantify specific microbes in environmental samples, providing insights into ecosystem health.
Research and Development: ELISA plays a crucial role in various research fields: Drug Discovery: It helps researchers assess the effectiveness of new drugs by measuring drug-target interactions and monitoring drug levels in the body. Vaccine Development: ELISA is instrumental in developing vaccines by evaluating immune responses to vaccine candidates. Basic Research: Scientists use ELISA to study various biological processes by detecting and quantifying specific molecules involved in these processes.
Despite its established role, ELISA is evolving alongside technological advancements. New multiplex platforms allow for the simultaneous detection of various targets in a single sample, boosting efficiency in biomarker discovery and disease analysis. Automation streamlines workflows minimizes errors, and increases throughput, making high-throughput screening feasible in drug development and clinical settings. Miniaturization and portable devices enable rapid on-site diagnostics, providing healthcare professionals with real-time data for quicker interventions. Additionally, ongoing research is improving assay sensitivity, reducing background noise, and expanding detection limits, allowing for the identification of trace analytes and early disease biomarkers with greater accuracy than ever before. Integration of ELISA with emerging technologies such as microfluidics, nanotechnology, and artificial intelligence holds promise for enhancing assay performance, scalability, and data analysis capabilities.
These advancements hold promise for even wider applications of ELISA in the future. ELISA has revolutionized our ability to detect and quantify biological molecules. Its versatility, accuracy, and adaptability make it an invaluable tool across various scientific disciplines. As research continues to refine and innovate ELISA techniques, we can expect even more exciting possibilities to emerge in the years to come. ELISA's future is bright, promising to play a pivotal role in unraveling the mysteries of the biological world and improving human health.
#science sculpt#life science#molecular biology#science#biology#artists on tumblr#ELISA#immunology#immunotherapy#diagnostic management software#diagnosticimaging#history of immunology#scientific advancements#biotechnology#scientific research#scientific equipment#scientific instruments#techniques in biotechnology#scientific illustration#lab equipment#sciencenature#laboratory#lab skills#molecular diagnostics market
10 notes
·
View notes
Text
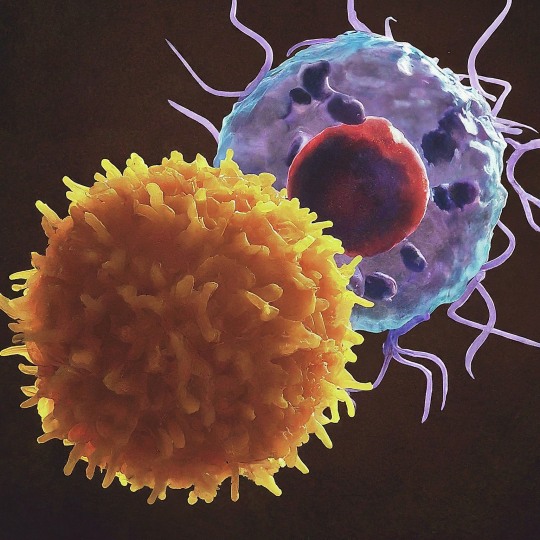
The T Cell Landscape
T cells, a critical component of the adaptive immune system, stand as the body's elite force in combatting infections and diseases. These specialized lymphocytes boast remarkable diversity, each type playing a distinct role in orchestrating a targeted and effective immune response.
T cells, like all blood cells, originate from hematopoietic stem cells residing in the bone marrow. However, their training ground lies within the thymus, a specialized organ located in the chest. Here, they undergo a rigorous selection process known as thymocyte education. During this process, immature T cells, called thymocytes, are presented with self-antigens (molecules unique to the body) by special cells. Thymocytes that bind too strongly to these self-antigens are eliminated, preventing them from attacking healthy tissues later. Only thymocytes that demonstrate the ability to recognize foreign invaders while exhibiting tolerance to self are released into the bloodstream as mature T cells.
Following this rigorous training, mature T cells exit the thymus and embark on their patrol, circulating throughout the bloodstream and lymphatic system. They remain vigilant, constantly scanning for their specific targets – antigens. Antigens are foreign molecules, such as fragments of viruses, bacteria, or even cancerous cells, that trigger the immune response.
The hallmark of a T cell is its T cell receptor (TCR), a highly specialized protein complex embedded on its surface. This receptor acts like a lock, uniquely shaped to fit a specific antigen, the "key." Each T cell develops a unique TCR capable of recognizing only a single antigen, enabling a highly specific immune response.
But how do T cells encounter these hidden antigens lurking within infected or cancerous cells? This critical role is played by antigen-presenting cells (APCs). APCs, such as macrophages and dendritic cells, engulf pathogens or abnormal cells, break them down into smaller fragments (peptides), and present them on their surface complexed with major histocompatibility complex (MHC) molecules. MHC molecules act as identification tags, allowing T cells to distinguish between "self" and "non-self." When a T cell's TCR encounters its specific antigen bound to an MHC molecule on an APC, a dance of activation begins. The T cell becomes stimulated, and a cascade of signaling events is triggered. This leads to the T cell's proliferation, producing an army of clones specifically tailored to combat the recognized threat.
T cells are not a single, monolithic entity. They comprise a diverse population, each type with a specialized function:
Helper T Cells (Th Cells):
Helper T cells, often abbreviated as Th cells, play a central role in coordinating immune responses. They express the CD4 surface marker and can recognize antigens presented by major histocompatibility complex class II (MHC-II) molecules. Subtypes of helper T cells include Th1, Th2, Th17, and regulatory T cells (Tregs), each with distinct functions and cytokine profiles.
Th1 cells mediate cellular immunity by activating macrophages and cytotoxic T cells, crucial for defense against intracellular pathogens.
Th2 cells are involved in humoral immunity, promoting B cell activation and antibody production, thus aiding in defense against extracellular parasites.
Th17 cells contribute to the immune response against extracellular bacteria and fungi, producing pro-inflammatory cytokines. Regulatory T cells (Tregs) maintain immune tolerance and prevent autoimmunity by suppressing excessive immune responses.
Cytotoxic T Cells (Tc Cells):
Cytotoxic T cells, also known as Tc cells or CD8+ T cells, are effector cells responsible for directly killing infected or aberrant cells. They recognize antigens presented by MHC class I molecules on the surface of target cells. Upon activation, cytotoxic T cells release perforin and granzymes, inducing apoptosis in target cells and eliminating the threat.
Memory T Cells:
Memory T cells are a long-lived subset of T cells that persist after the clearance of an infection. They provide rapid and enhanced immune responses upon re-exposure to the same antigen, conferring immunological memory. Memory T cells can be either central memory T cells (TCM), residing in lymphoid organs, or effector memory T cells (TEM), circulating in peripheral tissues.
γδ T Cells:
Unlike conventional αβ T cells, γδ T cells express a distinct T cell receptor (TCR) composed of γ and δ chains. They recognize non-peptide antigens, such as lipids and metabolites, and are involved in immune surveillance at epithelial barriers and responses to stress signals.
Beyond the Battlefield: The Expanding Roles of T Cells: The remarkable capabilities of T cells have opened doors for several groundbreaking applications in medicine:
Vaccines: By presenting weakened or inactivated forms of pathogens, vaccines "train" the immune system to generate memory T cells. This prepares the body to recognize and rapidly eliminate the real pathogen upon future exposure, preventing disease.
Cancer immunotherapy: CAR T-cell therapy, a revolutionary approach, genetically engineers a patient's own T cells to express chimeric antigen receptors (CARs) that recognize and target specific cancer cells. These "supercharged" T cells are then reintroduced into the patient, unleashing a potent attack against the tumor.
Autoimmune disease treatment: Researchers are exploring ways to manipulate T cells to suppress harmful immune responses that underlie autoimmune diseases like rheumatoid arthritis and multiple sclerosis.
The diverse array of T cells underscores the immune system's complexity and adaptability in mounting tailored responses against a myriad of threats. From orchestrating immune reactions to maintaining tolerance and establishing long-term immunity, T cells play multifaceted roles in safeguarding the body's health. Understanding the intricacies of T cell biology not only sheds light on immune-mediated diseases but also paves the way for developing novel therapeutic strategies harnessing the power of the immune system.
T cells represent a fascinating aspect of immunology, with their diversity and specificity driving the complexity of immune responses. As research advances, further insights into T cell biology promise to revolutionize immunotherapy and enhance our ability to combat diseases ranging from infections to cancer. By understanding and harnessing their power, we can unlock new avenues for protecting and improving human health.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#t cells#T helper cells#autoimmune#autoimmunity#helathcare#immunology#immunotherapy#medical care#cancer#human health#research#scientific research#the glass scientists#scientific illustration#research scientist
11 notes
·
View notes
Text

The Antibody Odyssey
Have you ever wondered about the tiny superheroes zipping around your body, keeping you safe from invaders? No capes, no tights, but these incredible warriors are essential for our health: antibodies! These Y-shaped proteins play a crucial role in identifying and neutralizing these invaders, safeguarding our health. Produced by specialized white blood cells called B lymphocytes (B cells), antibodies are highly specific molecules, each designed to recognize a unique signature on a foreign substance, known as an antigen. This specificity, akin to a lock-and-key mechanism, ensures that antibodies only target the invading pathogens and not our own healthy tissues.
The earliest glimpse of antibodies came in 1890 when Emil von Behring and Shibasaburo Kitasato made a groundbreaking discovery. They observed that serum from animals immunized against diphtheria could protect other animals from the disease. This landmark finding laid the foundation for the concept of "antibodies" and paved the way for further exploration. In the early 20th century, Paul Ehrlich, renowned as the "father of immunology," proposed the "side-chain theory." This theory postulated the existence of specific receptors on cells that could bind to specific antigens (foreign molecules). This concept laid the groundwork for understanding the remarkable specificity of antibody-antigen interactions.
The 1960s witnessed a significant breakthrough with the work of Rodney Porter and Gerald Edelman. They elucidated the primary and secondary structure of antibodies, revealing their Y-shaped structure and intricate details of their amino acid sequences. This paved the way for a deeper understanding of their function and diversity. The process of antibody creation begins when a B cell encounters an antigen. This triggers the B cell to activate and divide rapidly, forming a clone of identical cells. These clones, called plasma cells, become antibody factories, churning out millions of antibodies specific to the encountered antigen. These antibodies then circulate throughout the bloodstream and lymphatic system, patrolling for their matching antigens.
Recognizing and Eliminating Threats: The Multifaceted Arsenal of Antibodies
Once an antibody encounters its specific antigen, it binds to it with remarkable precision. This binding initiates a multi-pronged attack on the pathogen:Neutralization: By binding to critical structures on the antigen, such as the viral envelope or bacterial toxins, antibodies can render them ineffective, preventing them from infecting cells or causing harm. Opsonization: Antibodies act as flags, coating the antigen with a special tag that attracts other immune cells, such as phagocytes (white blood cells that engulf and destroy foreign particles). This process, called opsonization, marks the antigen for destruction. Activation of the complement system: Antibodies can trigger a cascade of protein reactions called the complement system, which further aids in the destruction of the pathogen.
But, did you know that there's not just one type of antibody? These versatile molecules come in various forms, each with its unique structure and function. The type of antibody produced also plays a crucial role in the immune response. There are five main classes of antibodies (IgG, IgA, IgM, IgD, and IgE), each with distinct properties and functions:
Immunoglobulin G (IgG): The Mighty Defender - This is the most abundant antibody type, constituting around 70-80% of all antibodies in the bloodstream. IgG has four subclasses (IgG1-4) with subtle differences in function and lifespan. IgG is like a versatile soldier, capable of: Neutralizing toxins and viruses: By binding to pathogens, IgG prevents them from infecting cells. Triggering phagocytosis: It flags pathogens for specialized immune cells called phagocytes, which engulf and destroy them. Passing immunity to newborns: IgG antibodies can cross the placenta, offering newborns temporary protection against infections until their own immune system develops.
Immunoglobulin M (IgM): The First Responder - IgM is the first antibody produced by B cells in response to an infection. While less effective at neutralizing pathogens individually, it compensates through its: Pentameric structure: Five Y-shaped units join together, increasing the "avidity" or overall binding strength to pathogens. Complement activation: IgM can activate the complement system, a cascade of proteins that attracts immune cells and promotes pathogen destruction.
Immunoglobulin A (IgA): The Sentinel at the Gates - This antibody is primarily found in mucosal secretions like tears, saliva, and breast milk. IgA acts as the first line of defense against infections at these entry points by: Preventing pathogen attachment: It binds to pathogens, hindering their ability to adhere to and colonize mucosal surfaces. Neutralization and exclusion: IgA neutralizes pathogens and facilitates their removal through mucus flow.
Immunoglobulin D (IgD): The Enigmatic Player - IgD remains the least understood antibody type, making up only a tiny fraction (around 0.02%) of the total. While its exact function is still being unraveled, it's believed to be involved in: B cell activation: IgD might play a role in stimulating B cells to mature and produce other antibodies. Regulation of immune response: It's thought to be involved in fine-tuning the immune response by preventing B cells from overreacting.
Immunoglobulin E (IgE): The Double-Edged Sword - IgE is responsible for triggering allergic reactions. It binds to allergens (substances perceived as threats) on mast cells, which then release histamine and other chemicals. This leads to the characteristic symptoms of allergies like runny nose, itchy eyes, and wheezing. However, IgE also plays a role in expelling parasites, It can trigger the release of substances that help expel parasitic worms from the body.
The Building Blocks: Chains and Domains
An antibody is comprised of four polypeptide chains: two identical heavy chains and two identical light chains. Each chain folds into distinct regions called domains, which are responsible for specific functions.
Variable (V) domains: Located at the N-terminus (amino-terminal end) of both heavy and light chains, these domains boast highly diverse sequences. This variability allows the antibody to recognize a vast array of unique structures on antigens, the foreign molecules it targets.
Constant (C) domains: The C-terminus (carboxy-terminal end) of the heavy chains contains these domains. They determine the antibody's class (isotype), which influences its ability to interact with other components of the immune system and trigger specific effector functions.
The Architecture: Y-Shaped Majesty : The four chains assemble in a specific manner, forming the characteristic Y-shaped structure. The arms of the "Y" are formed by the Fab (fragment antigen-binding) fragments, each consisting of one light chain and one heavy chain linked together. These Fab fragments house the antigen-binding site, the crucial pocket where the antibody specifically recognizes and binds to its target antigen. The base of the "Y" is the Fc (fragment crystallizable) fragment, solely composed of the C domains of the heavy chains. This region interacts with immune cells and molecules, dictating the antibody's fate and activating various immune responses.
A Hinge for Flexibility and Diversity : Connecting the Fab and Fc fragments is a flexible hinge region. This hinge allows the Fab arms to have some degree of movement, enabling them to bind to antigens with different shapes and sizes. This flexibility also contributes to the remarkable diversity of antibody specificities, allowing the immune system to recognize and combat a wide range of pathogens.
When we encounter an antigen for the first time, our B-cells take a snapshot of its "fingerprint" and create a specific antibody to fight it. These "memory B-cells" then stick around, so if the same antigen tries to attack again, our bodies can respond quickly with a trained army of antibodies, preventing us from getting sick again. This is the genius behind vaccinations! Vaccines introduce weakened or inactive antigens, training our B-cells to create memory for specific villains, so we're prepared if they ever try to invade for real.
The knowledge gained from antibody research has revolutionized healthcare. Here are some notable examples:
Vaccines: By exposing the immune system to weakened or inactive forms of pathogens, vaccines stimulate the production of specific antibodies, providing long-term protection against diseases.
Diagnostic Tools: Antibody-based tests, like ELISA (Enzyme-Linked Immunosorbent Assay), are widely used to detect and diagnose various diseases, including viral infections and autoimmune disorders.
Therapeutic Antibodies: Monoclonal antibodies, produced in the lab to target specific antigens, have emerged as a powerful tool for treating various diseases, including cancer, autoimmune diseases, and infectious diseases.
Antibodies are a testament to the body's remarkable ability to defend itself. These meticulously designed proteins, constantly patrolling our systems, stand as a testament to the intricate and sophisticated nature of the immune system. By delving deeper into their diverse functions and potential applications, we gain a profound appreciation for the intricate dance of life and the ongoing battle against invading threats. As research continues to unveil the secrets of antibodies, we can anticipate even greater advancements in healthcare and disease prevention, all thanks to these extraordinary defenders within us.
#science sculpt#life science#molecular biology#science#biotechnology#artists on tumblr#immunology#scientific research#disease#antibody#antibodies#immunity#health and wellness#vaccinations#structure of antibody#immunoglobulin#illustrative art#illustration#biology#animal facts#zoology#human body
8 notes
·
View notes
Text
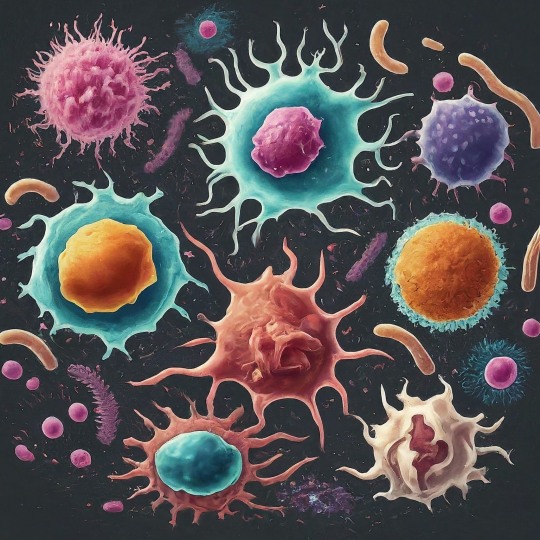
The Immune System's All-Star Team: The Mighty Cells That Protect You
Your body is like a bustling city, constantly facing threats from outside invaders like viruses and bacteria. Thankfully, you have a team of dedicated defenders keeping you safe: your immune cells! Our immune system is a marvel of biological defense, tirelessly safeguarding our bodies from harmful invaders like bacteria, viruses, and parasites. At the forefront of this defense are numerous types of immune cells, each with its unique functions and capabilities. Did you know the average adult has about 2 trillion white blood cells, which contain most immune cells? That's more people than live in China! These tiny warriors come in different shapes and sizes, each with unique superpowers to protect you. Let's meet some of the key players:
The Innate Force: First up, we have the innate immune system. This frontline defense acts fast and nonspecifically, providing immediate protection against any threat. The key players: 1. Neutrophils: Think of these guys as the city's SWAT team. They're the first responders, rushing to attack invaders with toxic chemicals and swallowing them whole with their arsenal of enzymes! These are the most abundant immune cells, are short-lived but highly effective. Unfortunately, they die in the fight, leaving behind a green gooey mess (pus) that signals infection.
2. Macrophages: These are the veterans, the wise generals of the immune system. They go beyond mere engulfing, processing antigens (foreign molecules) and presenting them to other immune cells for recognition and attack. They also act as scavengers, cleaning up debris and orchestrating healing. These are the cleaners and recyclers. They gobble up dead neutrophils, debris, and even worn-out cells, keeping your city sparkling clean.
3. Natural Killer (NK) Cells: These are the ninjas of the immune system. They silently patrol, sniffing out suspicious cells infected with viruses or even cancer and eliminating them with a swift punch. The Adaptive Arsenal:
The Adaptive Arsenal: If the innate system fails, the adaptive immune system steps in. This highly specific defense remembers past encounters and tailors its response to each unique threat. The cells of adaptive immune system are:
B Cells: These are the antibody factories, producing highly specific proteins called antibodies that neutralize pathogens and toxins. Each B cell produces a unique antibody, like a lock and key, targeting specific invaders. They whip up special proteins called antibodies that lock onto specific invaders, like sticky notes, marking them for destruction.
T Cells: These are the generals, coordinating the entire defense. There are different types of T cells:
Helper T Cells: These are the commanders, directing and coordinating the immune response through chemical signals. They activate B cells, macrophages, and other immune cells, orchestrating a multi-pronged attack.
Cytotoxic T Cells: These are the elite soldiers, directly targeting and eliminating infected cells or cancer cells. They recognize and bind to specific enemy markers, unleashing a lethal attack.
Memory T Cells: These are the veterans, remembering past encounters with invaders and helping the immune system respond faster next time.
The Unsung Heroes: Beyond these main players, numerous other immune cells contribute to our defense. These include:
Dendritic cells: These antigen-presenting cells capture and process pathogens, presenting their fragments to T cells for activation. They're like the scouts, gathering enemy intel and relaying it to the command center.
Mast cells: These cells reside in tissues and release inflammatory chemicals in response to allergens or parasites. They're like the alarm system, alerting the immune system to local threats.
Eosinophils: These specialize in fighting parasitic infections, releasing toxic chemicals to neutralize them.
Basophils: These are involved in allergic reactions and contribute to wound healing.
The beauty of the immune system lies in its intricate collaboration. These diverse cell types work together in a complex and beautiful dance, each playing a specific role to achieve a common goal: protecting our health. They communicate extensively through chemical signals, creating a complex network of interactions. Imagine B cells producing antibodies that bind to a pathogen, flagging it for destruction. Macrophages engulf and eliminate the tagged pathogen, while T cells coordinate the attack and eliminate any infected cells. Dendritic cells present captured fragments to T cells, priming them for future encounters. This seamless cooperation ensures a swift and effective response to any threat.
Your immune system is constantly learning. Each time you get a vaccine or fight off an infection, your immune cells create memory T cells, making you more resistant to future attacks. Understanding these cellular heroes can help us appreciate the incredible machinery that keeps us healthy and appreciate the importance of maintaining a strong immune system. It also allows us to make informed decisions about supporting our immune system. Maintaining a healthy lifestyle, getting adequate sleep, managing stress, and consuming a balanced diet can all contribute to a robust immune response.
#science sculpt#life science#science#biology#biotechnology#artists on tumblr#immunology#immunotherapy#immune cells#b cells#t cells#neutrophil#macrophages#immunity#health and wellness#medicine#healthcare#illustration#illustrative art
9 notes
·
View notes
Text

A Journey Through Our Defenses: A Dive into the History of Immunology
The human body possesses an extraordinary ability to resist disease, a complex system known as the immune system. Our understanding of this intricate network, however, has evolved significantly over time, shaped by both ancient observations and groundbreaking scientific discoveries. From the ancient Greeks observing immunity after the plague to the cutting-edge research on immunotherapy, the history of immunology is a captivating saga of human curiosity, scientific breakthroughs, and the relentless pursuit of understanding our body's remarkable defense system.
The earliest observations of immunity date back to ancient civilizations. The Chinese practiced variolation, a process where dried smallpox scabs were blown into the nose to induce mild illness and subsequent immunity. Similar practices existed in India and Africa, demonstrating an intuitive understanding of acquired immunity. In 430 BC, Thucydides, a historian of the Athenian plague, noted that recovered individuals could safely care for the sick, suggesting an early recognition of disease resistance. The turning point in immunology arrived in 1796 with Edward Jenner, a British physician. Observing that milkmaids who had contracted cowpox were resistant to smallpox, he conducted a daring experiment, inoculating a young boy with cowpox pus. The boy developed a mild illness but remained protected from the deadly smallpox, marking the birth of vaccination. Jenner's work paved the way for further research into understanding immunity and developing vaccines against other diseases.
The 19th century saw significant progress in understanding the immune system's mechanisms. Louis Pasteur, a French microbiologist, developed vaccines against cholera and rabies, further solidifying the role of vaccination in disease prevention. In 1880, Ilya Mechnikov, a Russian biologist, discovered phagocytes, white blood cells that engulf and destroy pathogens, earning him a Nobel Prize in 1908. Emil von Behring and Shibasaburo Kitasato identified antibodies in the blood of vaccinated individuals, revealing the humoral immune response, where specific molecules target and neutralize pathogens.
The 20th Century: A Golden Age of Immunology: The 20th century witnessed a revolution in immunology. Karl Landsteiner discovered blood groups, leading to safer blood transfusions. Peter Medawar elucidated the concept of self-tolerance, explaining how the immune system distinguishes self from non-self. The discovery of T cells and B cells by Jean Dausset, Peter Doherty, and Rolf Zinkernagel unraveled the intricacies of the adaptive immune system, where lymphocytes tailor their response to specific pathogens. These breakthroughs paved the way for organ transplantation, autoimmune disease therapies, and the development of new vaccines.
21st Century: Expanding Horizons: The 21st century continues to push the boundaries of immunology. Monoclonal antibodies, engineered to target specific molecules, revolutionized cancer treatment and immune-based therapies. The Human Genome Project provided invaluable insights into the genetic underpinnings of the immune system, opening doors for personalized medicine. Research on immunotherapies harnessing the body's own immune cells to fight cancer and other diseases is rapidly evolving.
The history of immunology is a testament to human curiosity and ingenuity. From ancient observations to modern marvels, our understanding of this intricate system has transformed healthcare and continues to evolve. As we delve deeper into the mysteries of the immune system, we unlock the potential for even more effective treatments and preventive measures, paving the way for a healthier future for all. The history of immunology is a wild ride, filled with brilliant minds, bizarre experiments, and discoveries that continue to shape our world. And the best part? This story is still being written, with each new chapter promising even more amazing advancements in the fight to keep us healthy.
#science sculpt#life science#science#molecular biology#biotechnology#immunology#artists on tumblr#immunotherapy#scientific illustration#scifiart#curiosity#narrative#create#concepts#history of immunology#medical school#medical science
4 notes
·
View notes
Text

Exploring RNA Interference
Imagine a molecular switch within your cells, one that can selectively turn off the production of specific proteins. This isn't science fiction; it's the power of RNA interference (RNAi), a groundbreaking biological process that has revolutionized our understanding of gene expression and holds immense potential for medicine and beyond.
The discovery of RNAi, like many scientific breakthroughs, was serendipitous. In the 1990s, Andrew Fire and Craig Mello were studying gene expression in the humble roundworm, Caenorhabditis elegans (a tiny worm). While injecting worms with DNA to study a specific gene, they observed an unexpected silencing effect - not just in the injected cells, but throughout the organism. This puzzling phenomenon, initially named "co-suppression," was later recognized as a universal mechanism: RNAi.
Their groundbreaking work, awarded the Nobel Prize in 2006, sparked a scientific revolution. Researchers delved deeper, unveiling the intricate choreography of RNAi. Double-stranded RNA molecules, the key players, bind to a protein complex called RISC (RNA-induced silencing complex). RISC, equipped with an "Argonaut" enzyme, acts as a molecular matchmaker, pairing the incoming RNA with its target messenger RNA (mRNA) - the blueprint for protein production. This recognition triggers the cleavage of the target mRNA, effectively silencing the corresponding gene.
So, how exactly does RNAi silence genes? Imagine a bustling factory where DNA blueprints are used to build protein machines. RNAi acts like a tiny conductor, wielding double-stranded RNA molecules as batons. These batons bind to specific messenger RNA (mRNA) molecules, the blueprints for proteins. Now comes the clever part: with the mRNA "marked," special molecular machines chop it up, effectively preventing protein production. This targeted silencing allows scientists to turn down the volume of specific genes, observing the resulting effects and understanding their roles in health and disease.
The intricate dance of RNAi involves several key players:dsRNA: The conductor, a long molecule with two complementary strands. Dicer: The technician, an enzyme that chops dsRNA into small interfering RNAs (siRNAs), about 20-25 nucleotides long. RNA-induced silencing complex (RISC): The ensemble, containing Argonaute proteins and the siRNA. Target mRNA: The specific "instrument" to be silenced, carrying the genetic instructions for protein synthesis.
The siRNA within RISC identifies and binds to the complementary sequence on the target mRNA. This binding triggers either:Direct cleavage: Argonaute acts like a molecular scissors, severing the mRNA, preventing protein production. Translation inhibition: RISC recruits other proteins that block ribosomes from translating the mRNA into a protein.
From Labs to Life: The Diverse Applications of RNAi
The ability to silence genes with high specificity unlocks various applications across different fields:
Unlocking Gene Function: Researchers use RNAi to study gene function in various organisms, from model systems like fruit flies to complex human cells. Silencing specific genes reveals their roles in development, disease, and other biological processes.
Therapeutic Potential: RNAi holds immense promise for treating various diseases. siRNA-based drugs are being developed to target genes involved in cancer, viral infections, neurodegenerative diseases, and more. Several clinical trials are underway, showcasing the potential for personalized medicine.
Crop Improvement: In agriculture, RNAi offers sustainable solutions for pest control and crop development. Silencing genes in insects can create pest-resistant crops, while altering plant genes can improve yield, nutritional value, and stress tolerance.
Beyond the Obvious: RNAi applications extend beyond these core areas. It's being explored for gene therapy, stem cell research, and functional genomics, pushing the boundaries of scientific exploration.
Despite its exciting potential, RNAi raises ethical concerns. Off-target effects, unintended silencing of non-target genes, and potential environmental risks need careful consideration. Open and responsible research, coupled with public discourse, is crucial to ensure we harness this powerful tool for good.
RNAi, a testament to biological elegance, has revolutionized our understanding of gene regulation and holds immense potential for transforming various fields. As advancements continue, the future of RNAi seems bright, promising to silence not just genes, but also diseases, food insecurity, and limitations in scientific exploration. The symphony of life, once thought unchangeable, now echoes with the possibility of fine-tuning its notes, thanks to the power of RNA interference.
#science sculpt#life science#science#molecular biology#biology#biotechnology#dna#double helix#genetics#artists on tumblr#rna#rna sequencing#RNA interference#cell biology#cells#biomolecules#illustrates#scientific illustration#illustration#illustrative art#scientific research
12 notes
·
View notes
Text

Evolution of Gene Therapy: A Journey Through History
Gene therapy stands as one of the most promising frontiers in modern medicine, offering potential solutions to a myriad of genetic disorders and diseases. Its journey through history is both fascinating and complex, marked by remarkable breakthroughs, challenges, and ethical considerations.
The concept of manipulating genetic material to treat diseases dates back to the mid-20th century. In 1953, the discovery of the DNA double helix structure by Watson and Crick ignited the imagination of scientists worldwide, laying the foundation for genetic research. It wasn't until the 1970s that the term "gene therapy" emerged, coined by researchers Richard Mulligan and Theodore Friedmann. The 1980s marked the first foray of gene therapy into the clinical realm. The 1970s witnessed the first milestone in gene therapy with the successful introduction of foreign DNA into mammalian cells. This breakthrough, accomplished by Paul Berg in 1972, laid the groundwork for subsequent research endeavors. In 1980, Martin Cline performed the first gene therapy trial on a patient with beta-thalassemia, though ethical concerns arose due to the lack of proper patient consent and scientific rigor. Despite setbacks, the 1990s saw a surge of research, with gene therapy trials targeting various conditions like cystic fibrosis and severe combined immunodeficiency. However, tragic events, such as the tragic death of a young teenager in 1999, a young participant in a 1999 gene therapy trial, highlighted the need for stringent safety measures.
One of the landmark achievements in gene therapy occurred in 1990 when the first successful gene therapy trial took place. Researchers corrected a rare genetic disorder called Adenosine deaminase (ADA) deficiency in two young girls. This groundbreaking feat marked a crucial turning point, demonstrating the potential of gene therapy to treat genetic diseases.
Luxturna became the first gene therapy approved by the U.S. Food and Drug Administration (FDA) in 2017 for the treatment of an inherited retinal disease called Leber congenital amaurosis. This milestone underscored the therapeutic potential of gene therapy and paved the way for future advancements. The development of CRISPR-Cas9 revolutionized the field of gene editing, offering a versatile and precise tool for modifying DNA. This breakthrough has accelerated research in gene therapy and holds immense promise for the treatment of genetic diseases.
Gene therapy isn't a monolith; it dons various hats depending on the target and approach. Here are the major types:
Somatic vs. Germline: Somatic gene therapy: This targets non-reproductive (somatic) cells, impacting only the treated individual's lifespan and not passing changes onto offspring. This is the more prevalent and ethically accepted approach. Germline gene therapy: This modifies genes in reproductive cells, potentially impacting future generations. Ethical and safety concerns surround this approach, and it is not currently used in humans.
Ex Vivo vs. In Vivo: Ex Vivo gene therapy: Cells are extracted from the patient, modified in a laboratory, and then reintroduced. This allows for precise targeting but involves complex procedures. In Vivo gene therapy: The therapeutic gene is delivered directly to the target cells within the body. This offers minimally invasive approaches but poses challenges in targeting specific cells.
Gene Editing vs. Gene Replacement: Gene editing: Utilizes tools like CRISPR to modify existing genes, correcting mutations or fine-tuning their expression. This offers unparalleled precision but raises concerns about unintended consequences. Gene replacement: Introduces a functional copy of a missing or defective gene into the cells, restoring their normal function. This approach is well-established but may require permanent expression of the new gene.
The journey of gene therapy from its conceptual origins to clinical reality is a testament to human ingenuity and perseverance. With each passing year, advancements in technology and scientific understanding propel this field forward, offering hope to millions affected by genetic disorders. The evolution of gene therapy is a testament to human ingenuity and perseverance in the quest to conquer genetic diseases. From humble beginnings to cutting-edge innovations, the journey of gene therapy has been marked by triumphs and challenges alike. As researchers continue to unravel the complexities of the genome and refine therapeutic approaches, the future of gene therapy shines brighter than ever, holding the promise of transformative treatments and cures for diseases once deemed incurable.
#science sculpt#life science#science#molecular biology#biology#biotechnology#dna#double helix#genetics#gene therapy#genetic engineering#daily dose of science#scifiart#scientific advancements#scientific research#artists on tumblr#illustration
8 notes
·
View notes
Text
RNA: The Dynamic Molecule Driving Life's Diversity
DNA, the blueprint of life, often steals the spotlight when it comes to genetics. But lurking in its shadow is another crucial molecule, RNA (Ribonucleic Acid), playing a pivotal role in the symphony of life. More than just a passive messenger, RNA boasts a vibrant history and holds exciting potential for the future. Let's embark on a journey to unveil the world of RNA, exploring its captivating story and why it deserves your attention.
The story of RNA's discovery began in 1860 when Friedrich Miescher isolated a mysterious "nuclein" from white blood cells. However, it wasn't until the 1950s that James Watson and Francis Crick, alongside Rosalind Franklin (whose contributions were initially overlooked), unraveled the structure of DNA, relegating RNA to a supporting role as a mere messenger molecule. But the plot thickened in the 1960s when researchers like Howard Temin and David Baltimore stumbled upon reverse transcriptase, an enzyme that could convert RNA into DNA, challenging the long-held "central dogma" of DNA being the sole source of genetic information. This discovery opened the door to a whole new understanding of RNA's diverse capabilities.
The Many Faces of RNA
But RNA isn't just a protein puppet master. There are different types of RNA, each with unique jobs:
Messenger RNA (mRNA): Delivers the protein-making message. Transfer RNA (tRNA): Brings the amino acids, the building blocks of proteins, to the party. Ribosomal RNA (rRNA): The foreman of the ribosome factory, making sure everything runs smoothly. Non-coding RNA (ncRNA): A diverse bunch with various roles, from regulating genes to fighting viruses.
The true game-changer came in the early 2000s. Scientists stumbled upon a vast class of non-coding RNAs that don't code for proteins but have diverse and crucial functions. microRNAs (miRNAs), for example, regulate gene expression by silencing specific genes, while long non-coding RNAs (lncRNAs) control various cellular processes like development and disease. This discovery shattered the dogma that only protein-coding genes mattered, highlighting the crucial roles played by non-coding RNAs.
This newfound understanding of RNA's potential has ignited a revolution in medicine. Researchers are exploring RNA-based therapies for various diseases, from cancer and neurodegenerative disorders to viral infections. mRNA vaccines, like the ones used against COVID-19, harness the power of messenger RNA to deliver genetic instructions directly to cells, triggering immune responses. The future holds even more promise, with scientists exploring techniques like CRISPR-Cas9 to edit RNA and potentially treat genetic diseases.
New discoveries are constantly rewriting our understanding of this versatile molecule. Its adaptability and diverse roles make it a powerful tool for exploring the very essence of life, from evolution and development to disease and therapy. So, the next time you hear about genes, remember that RNA, the often-overlooked player, is just as crucial in shaping the tapestry of life. It's a story of constant evolution, unexpected discoveries, and immense potential, making RNA a molecule brimming with fascination and promise for the future.
#science sculpt#life science#science#molecular biology#biotechnology#biology#genetics#RNA#daily dose of science#dailyprompt#meaningful#scientific illustration#the glass scientists#microscopic world#microscopy#artists on tumblr#digital artist
12 notes
·
View notes
Text
A Journey Through the History of Cells
A world smaller than a raindrop, yet bustling with activity like a miniature city. Welcome to the incredible universe of the cell, the fundamental unit of life! The cell might seem microscopic, but its history stretches back eons and holds stories as vast as the galaxies. Today, we embark on a voyage through time, delving into the fascinating discoveries that shaped our understanding of these minuscule marvels.
The First Glimpse: Unveiling the "Cells", Our journey begins in 1665, where Robert Hooke, wielding an early compound microscope, peered into the intricate world of cork. He observed box-like structures, resembling the rooms monks inhabited, and christened them "cells." While he couldn't fathom their true nature, Hooke had opened a window into a hidden universe.
From Cork to Critters: Expanding the Cellular Realm: Another pioneer, Antonie van Leeuwenhoek, soon pushed the boundaries further. His improved microscopes unveiled a teeming microcosm of single-celled organisms, like the "animalcules" (protozoa) he described in 1674. This marked the realization that life existed beyond visible forms, its very foundation laid in these intricate units.
The Cell Theory Takes Shape: Unifying Principles Emerge: Fast forward to the 19th century, where the stage was set for a defining moment. Matthias Schleiden and Theodor Schwann, inspired by countless observations, proposed the cell theory in 1839. This groundbreaking concept established three fundamental principles:All living organisms are composed of one or more cells. The cell is the fundamental unit of structure and function in living organisms. All cells arise from pre-existing cells.
This unifying theory laid the cornerstone for modern cell biology, revolutionizing our understanding of life.
With the cell theory established, curiosity shifted inwards. The nucleus, discovered by Robert Brown in 1833, became the focus. Further advancements in microscopy revealed a bustling metropolis within each cell, teeming with specialized structures called organelles, each with its unique function. The 20th century saw a whirlwind of discoveries. Rudolf Virchow solidified the third tenet of the cell theory with his famous dictum, "omnis cellula e cellula" (all cells come from cells), while Louis Pasteur challenged spontaneous generation, further solidifying the cell's central role.
The human body has trillions of cells, more than all the stars in our Milky Way galaxy and you're constantly replacing cells shedding about 30,000 skin cells every minute!! You're a bustling metropolis of trillions of tiny workers, all working together to keep you going. The knowledge gained from countless studies of cells propelled medical breakthroughs. The identification of chromosomes and genes within cells paved the way for genetics, opening doors to understanding diseases and developing treatments. Cell therapies and regenerative medicine offer glimpses into a future where damaged cells can be repaired or replaced. As we stand at the threshold of the 21st century, the exploration of cells continues. Advanced microscopes like electron microscopes and confocal laser scanning microscopes offer unprecedented views into the cellular world. CRISPR technology allows us to edit genes within cells, holding immense potential for treating genetic diseases.
As we delve deeper, the mysteries of the cell unfold, revealing its adaptability, complexity, and beauty. This tiny titan, once just a glimpse through a primitive lens, holds the key to understanding life itself. The journey through the history of cells is a testament to human curiosity and ingenuity, and it's a journey that continues, offering endless possibilities for the future.
#biology#science sculpt#life science#science#molecular biology#biotechnology#cell#cells at work#cell biology#unit of life#mitochondria#nucleus#artists on tumblr#daily dose of science#feb 2024
6 notes
·
View notes
Text

Within the cell, a symphony untold,
A dance of molecules, secrets unfold.
DNA's twisted ladder, a spiral divine,
Holds blueprints of life, in each intricate line.
From single-celled whispers to towering trees,
A tapestry woven on life's swirling seas.
Bacteria's whispers, fungi's hidden might,
Insects on wings, taking flight at twilight.
The beating of hearts, a rhythmic command,
Blood's crimson river, coursing through the land.
Lungs inflate, exhale, a symphony's breath,
A delicate balance, defying death.
Brains ablaze, firing synapses' spark,
Memories flicker, emotions leave their mark.
Eyes that behold, a kaleidoscope's view,
From vibrant landscapes to skies bathed in blue.
Flowers unfurl, their petals ablaze,
Nature's grand canvas, painted in sunlit daze.
Evolution's whisper, through eons it weaves,
A tapestry vast, where life truly believes.
But mysteries linger, in shadows they hide,
From consciousness' depths to diseases that ride.
Cancer's dark secret, locked in a twisted cell,
And the origin of life, a story to tell.
With microscopes' eyes, we delve into the small,
Unraveling mysteries, standing tall.
Genetics unlocks, the code life bestows,
A future unfolding, like a blooming rose.
So join the adventure, explore and ignite,
The spark of curiosity, burning ever bright.
For biology's secrets, like stars in the night,
Beckon us onward, with wonder and light.
#science sculpt#life science#science#molecular biology#biology#double helix#biotechnlogy#microbiology#artists on tumblr#writers on tumblr#writerscommunity#science poem#environment#enviroment art#environmetalists#illustration#daily dose of science#scifiart
8 notes
·
View notes
Text
DNA Mishaps: When the Script Gets Flipped!
DNA, the molecule that holds the blueprint of life, isn't always static. It's like a library of instructions, constantly copied and passed on. But sometimes, errors creep in, leading to changes in the genetic code known as mutations. These alterations can be small and subtle, or large and dramatic, impacting the organism in various ways.
Imagine you're writing a super important essay, and accidentally mix up the letters. Instead of "the quick brown fox jumps over the lazy dog," you end up with "the qick brown foz jmups ovetr te laxy dog." Oops! This, my friends, is kind of what happens in DNA mutations. But instead of an essay, it's the blueprint of life getting a little jumbled. Understanding these changes is crucial, as they hold the key to understanding evolution, genetic diseases, and even the potential for future therapies. Sometimes, due to mistakes during copying or exposure to things like radiation, chemicals, or even sunlight, those A, T, C, and G chemicals get swapped, added, or deleted. It's like the gremlin wrote "foz" instead of "fox."
Let's dive into the wacky world of DNA mutations
Mutations come in all shapes and sizes, classified based on the extent of the change:
Point Mutations: These are the most common, involving a single nucleotide (the building block of DNA) being substituted, deleted, or inserted. Think of these as single typos. One little DNA letter gets swapped for another. Sometimes it's harmless, like mistaking "flour" for "flower" (just add more water!). But other times, like switching "sugar" for "salt," it can completely change the outcome Point mutations can be: 1. Silent: No change in the encoded protein, like a synonym in language. 2. Missense: A different amino acid is incorporated, potentially impacting protein function. 3. Nonsense: The mutation creates a "stop codon," prematurely terminating protein production.
Insertions & Deletions: It's like adding or removing words from a sentence. These larger mutations involve adding or removing nucleotides, disrupting the reading frame and potentially causing significant functional changes.
Chromosomal Mutations: When entire segments of chromosomes are duplicated, deleted, inverted, or translocated (swapped between chromosomes), the impact can be far-reaching, affecting multiple genes and potentially leading to developmental disorders.
More Than Just a Glitch: Mutations can be beneficial, neutral, or detrimental. Some mutations are neutral, like a typo you don't even notice. But others can be like changing "hilarious" to "hairless" – they might have a big impact. Beneficial mutations, like the one enabling lactose tolerance in some humans, drive evolution. Neutral mutations have no impact, while detrimental ones can cause genetic diseases like cystic fibrosis or sickle cell anemia.
Where Do Mutations Occur? Mutations can happen in two types of cells: Germline Mutations: These occur in egg or sperm cells, meaning they get passed on to offspring, potentially impacting future generations. Somatic Mutations: These occur in body cells after conception and don't get passed on, but can contribute to diseases like cancer.
Scientists use various techniques to study mutations, from analyzing individual DNA sequences to tracking mutations across populations. This research helps us understand the causes and consequences of mutations, potentially leading to therapies for genetic diseases and even the development of new drugs.
Mutations are not errors, but rather the dynamic fuel of evolution. Thankfully, our cells have built-in proofreaders who try to catch and fix these typos. But sometimes, mutations slip through. By understanding their types, impact, and study, we gain a deeper appreciation for the intricate dance of life, where change and adaptation intertwine to create the diverse tapestry of the living world.
#life science#science#science sculpt#molecular biology#dna#mutations#genetics#somatic mutation#germline mutation#double helix#biotechnology
5 notes
·
View notes
Text

The Nucleosome: DNA's Fancy Packaging and Party Trick!
Imagine cramming two meters of yarn into a pea-sized box. Sounds impossible, right? Well, that's the impressive feat that cells pull off every single day with DNA! They use a clever structure called the nucleosome to pack this massive genetic blueprint into the tiny nucleus.
The journey began in 1974 when Don and Ada Olins, peering through an electron microscope, spotted repeating beads – the first glimpse of nucleosomes. Roger Kornberg, building upon this observation, proposed the now-iconic "subunit theory," envisioning DNA wrapped around histone protein cores. This theory, later solidified by Pierre Oudet's term "nucleosome," laid the groundwork for further exploration. The 1980s witnessed a flurry of activity, with Aaron Klug's group using X-ray crystallography to reveal the left-handed superhelical twist of DNA around the histone octamer. But the true masterpiece arrived in 1997 when the Richmond group, armed with advanced techniques, unveiled the first near-atomic resolution crystal structure of the nucleosome. This intricate map, showcasing the precise interactions between DNA and histones, remains a cornerstone of our understanding.
The Players:
DNA: The star of the show, carrying our genetic code in the form of a double helix.
Histones: Protein spools around which DNA tightly winds. Imagine eight of them forming a core, like a mini-protein drum set.
Linker DNA: Short stretches of DNA connecting the spools, like the spaces between beads on a necklace.
The Steps:
Wrap and Roll: Picture DNA gracefully wrapping around the histone core, like thread around a spool. Each nucleosome holds about 146 base pairs of DNA, making about 1.67 turns.
Connect and Repeat: Linker DNA bridges the gap between nucleosomes, forming a "beads-on-a-string" structure. Think of it as pearls strung between the spools.
Compact and Condense: This repetitive unit folds further, creating intricate 30-nanometer fibers. Imagine these as twisted strands of pearls!
Here's the coolest part: histones aren't static. They can be chemically modified, like adding or removing phosphate groups. These modifications act like tiny flags that tell the cell how tightly to wrap the DNA, essentially throwing a "party" for specific genes by making them more accessible. This fine-tuning allows cells to respond to their environment and express the right genes at the right time. Understanding the nucleosome model is crucial for unraveling the mysteries of gene regulation and diseases like cancer. By studying how modifications affect nucleosome structure and gene access, scientists can develop new therapies to target specific genes and potentially treat diseases at the root cause.
While the nucleosome model is the foundation, the story gets even more intriguing. Different histone types and modifications create variations, influencing chromatin structure and function. Think of it as different music genres influencing the dance moves! Additionally, other proteins interact with the nucleosome, adding another layer of complexity to this fascinating choreography.
The nucleosome model is more than just a neat way to package DNA. It's a testament to the intricate dance between molecules that orchestrates life's processes. By understanding this fundamental structure, we gain deeper insights into cellular function, paving the way for advancements in medicine and beyond.
Remember, this is just the beginning! The world of nucleosomes and chromatin is vast and ever-evolving. So, keep exploring, keep questioning, and keep dancing to the rhythm of DNA!
#molecular biology#biology#science sculpt#life science#science#dna#biotechnology#genetics#Histone#Nucleosome#chromatin
10 notes
·
View notes
Text

Epigenetics: A Journey Through Inheritance Beyond Genes
For centuries, scientists have been fascinated by the mysteries of heredity and how traits are passed down from generation to generation. DNA, the molecule that stores our genetic code, was once thought to be the sole determinant of our characteristics. However, a new frontier in biology, revealing a captivating layer of complexity beyond the DNA sequence itself: Epigenetics.
What is Epigenetics?
The term "epigenetics" was first coined in the 1940s by British biologist Conrad Waddington, but it wasn't until the late 20th century that its significance truly blossomed. Epigenetics, literally meaning "above genetics," refers to the study of heritable changes in gene expression that occur without alterations to the DNA sequence itself. Imagine DNA as the musical score, but epigenetics are the conductor and musicians who determine how the music is played. Through chemical modifications and adjustments to the proteins around DNA, epigenetics dictates which genes are turned on or off, influencing how cells function and ultimately shaping our health, development, and even behavior. Think of your DNA as the hardware: it contains the basic instructions for building and running your body. But epigenetics acts like the software, fine-tuning those instructions and determining which genes get turned on or off at specific times and in specific cells. These modifications, like chemical tags or changes in the packaging of DNA, don't alter the underlying code itself, but they can have a profound impact on how it's read and interpreted.
The Key Players:
DNA methylation: This process involves adding a methyl group to DNA, essentially silencing the gene it's attached to. Imagine it like putting a dimmer switch on a light bulb.
Histone modifications: Histones are proteins that package DNA, and changes in their structure can make genes more or less accessible to the cellular machinery needed for expression. Think of it like adjusting the curtains around a window - open wide for full light, slightly closed for filtered light.
Non-coding RNAs: These are molecules that don't code for proteins but can regulate gene expression in various ways. They're like the backstage crew in a play, ensuring everything runs smoothly.
The Power of Epigenetic Regulation
Epigenetic regulation plays a crucial role in various biological processes, including:
Development: During embryonic development, different cell types emerge from the same DNA blueprint by activating or silencing specific gene sets through epigenetic modifications.
Cellular differentiation: Specialized cells like muscle or nerve cells have unique functions due to differences in their active genes, controlled by epigenetic mechanisms.
Learning and memory: Epigenetic changes in brain cells are thought to be essential for learning and forming memories.
Aging: As we age, our epigenome accumulates changes that can contribute to age-related decline and disease.
Environmental influences: Diet, exercise, stress, and exposure to toxins can leave epigenetic marks on our genes, potentially impacting our health and even the health of future generations.
Epigenetics reminds us that we are not simply products of our genes. Our environment, choices, and experiences leave their mark, shaping who we are and potentially influencing our children's health. This deeper understanding of ourselves opens doors for self-awareness, empowerment, and potentially reshaping our narratives – not just as individuals, but as a species with the potential to leave a healthier legacy for generations to come.
#life science#biology#science sculpt#molecular biology#biotechnology#epigenetics#daily dose of science#dna#genetic inheritance#genetics#decoding dna#genetic code#science#double helix
126 notes
·
View notes
Text
A Dive into CAR-T Cell Therapy
Imagine training your own soldiers to fight cancer. Not just any soldiers, but elite warriors genetically modified to recognize and demolish the enemy with laser-like precision. That's the essence of CAR-T cell therapy, a revolutionary approach turning heads in the medical world. In the fight against cancer, CAR-T cell therapy embodies this very concept, harnessing the body's own immune system to wage war against malignant cells. T cells are the special forces of your immune system, constantly scanning for and eliminating threats. But sometimes, cancer cells outsmart them, hiding in plain sight. CAR-T cell therapy steps in, equipping these T-cells with a unique weapon: a Chimeric Antigen Receptor (CAR). Think of it as a GPS that locks onto a specific protein on cancer cells, guiding the T-cells straight to their target.
How does it work? Here's the simplified version:
Recruitment: First, T cells are extracted from your blood. Modification Station: In the lab, scientists use a virus or other tools to insert the CAR gene into the T cells' DNA. This equips them with the cancer-targeting GPS. Bootcamp Boost: The modified T cells are grown in a special environment, multiplying into a powerful army. Redeployment: The CAR-T cell troops are infused back into your bloodstream, ready to seek and destroy.
Sounds amazing, right? But like any powerful technology, CAR-T comes with its own set of challenges. The treatment process is complex and expensive, and there can be serious side effects like cytokine release syndrome, where the immune system goes into overdrive. So, is CAR-T a miracle cure? Not yet. But for some patients with aggressive blood cancers like leukemia and lymphoma, it has shown remarkable results, offering hope where other treatments have failed. Researchers are constantly working to improve the safety and efficacy of CAR-T, making it a potential game-changer for even more cancers in the future. CAR-T cell therapy has demonstrated remarkable efficacy in the treatment of certain types of hematologic malignancies, including acute lymphoblastic leukemia (ALL) and certain subtypes of non-Hodgkin lymphoma. Clinical trials have shown unprecedented response rates and durable remissions in patients who have exhausted all other treatment options. Furthermore, ongoing research is exploring the potential of CAR-T cell therapy in treating solid tumors, extending its therapeutic reach to a broader spectrum of cancers. The future is bright for CAR-T. It's a testament to the power of human ingenuity and our ongoing quest to conquer one of humanity's greatest foes. While there's still a way to go, this groundbreaking therapy is a beacon of hope, reminding us that even the seemingly impossible can become reality.
3 notes
·
View notes