#electronegative
Explore tagged Tumblr posts
Text
A hydrogen bond occurs between a small, highly electronegative atom with a lone pair of electrons and a positively polarised hydrogen atom.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#hydrogen#hydrogen bond#electronegative#lone pair#electrons#polarized
0 notes
Text
Very important addition to my chemistry notes

63 notes
·
View notes
Text
Polarization of the Carbonyl Group
-- Carbonyl group:
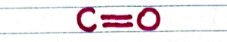
-- The oxygen has two lone pairs
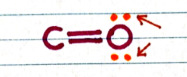
-- The oxygen is more electronegative than the carbon
-- The polarization arrow will show electrons being pulled toward the oxygen

-- The carbon is partially positive
-- Partially positive = δ+
-- The oxygen is partially negative
-- Partially negative = δ-
-- These symbols go by the arrow
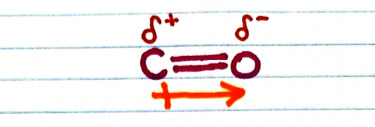
-- The polarization makes the boiling points of aldehydes and ketones higher than hydrocarbons
-- The larger the carbonyl group, the less soluble it is in water
-- If it has more than six carbons, it is insoluble
.
Patreon
#studyblr#notes#my notes#functional groups#polarization#electronegativity#carbonyl group#aldehydes#ketones#polarity#organic chemistry#ochem#orgo#orgo notes#organic chemistry notes#organic chem#orgo chem#study guides#mcat#mcat chemistry#mcat orgo#mcat ochem#mcat organic chemistry#mcat studyblr#premed studyblr#organic chemicals#organic reactions#chemical reactions#advanced chemistry#life science
9 notes
·
View notes
Text
god i love the idea that bill and the other weirdo monsters couldnt leave gravity falls is because theyre TOO weird yk? like gravity falls attracts weird and theyre so weird that they physically cant leave? love it. eating that shit up
#gravity falls#headcanon#side note:#also reminds me of atomic radius and how how electronegative an atom is affects how close electrons are pulled to the nucleus#can you tell i love chemistry#btw!!! in university!!?? shits crazy man
4 notes
·
View notes
Text
For example, the electron density around the hydrogen atoms in fluoromethane (figure 20.23) is less than that around the hydrogen atoms in chloromethane (figure 20.24), due to the greater electronegativity of fluorine relative to chlorine.


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#electron density#hydrogen#fluoromethane#fluorine#chloromethane#chlorine#methane#electronegativity
2 notes
·
View notes
Text
Okay but it's obviously fluorine, right? Most electronegative element. Fluorine's out there getting slutty, serving cunt. Fluorine's reactive, it fucks.
Tumblr staff: ten options is enough for polls, right? No one needs more than that on a regular basis. The average tumblr user: Hey guys which element of the periodic table do you think is the most fuckable?
#I'm ace so I guess what do I know about fuckability#but y'all see my vison right?#right?#Fluorine#electronegativity
274K notes
·
View notes
Text
ptable.com save me save me ptable.com
#rare woman in stem post????#lignin nmr is killing me rn#just nmr spectra in general#theyre very useful but GOD#why is it NOBODY CAN TEACH THEM PROPERLY?????#electronegativity table seems to haunt me
0 notes
Text
VSEPR and Molecular Geometry Explained!
youtube
Being able to draw Lewis Structures with the correct geometry is the first step in chemistry to being able to understand and explain different properties and behaviors of various molecules. In this video we will cover Valence Shell Electron Pair Repulsion, also known as VSEPR, and how that affects the shapes of different molecules!
Molecular Geometry Chart: Email us and we will send you a FREE copy!
Tadashi Science
https://www.youtube.com/channel/UCXrKyd6XS4oyhjKppE4ZZvw/videos
https://www.youtube.com/channel/UCXrKyd6XS4oyhjKppE4ZZvw/about
#tadashiscience#chemistry#science#physics#physicalscience#tutoring#bonddipoles#atoms#molecules#polarity#cations#anions#electronegativity#Youtube
1 note
·
View note
Text
What is Electron Affinity?
Electron affinity refers to the amount of energy released or absorbed when an electron is added to a neutral atom in the gas phase to form a negative ion. Essentially, it measures the tendency of an atom to gain an electron. This property is critical for understanding how atoms become ions and participate in ionic bonding. Enroll now at Tutoroot.
0 notes
Text
me trying to tell if a molecule is polar or non polar based on vibes alone
#chem class did not make it explicitly clear how to tell the difference without using electronegativity#not allowed periodic tables in the quiz#so i must use the vibe check#my posts
0 notes
Text
Substitution at the α-carbon with an atom or a group of atoms of higher electronegativity than carbon increases the acidity of carboxylic acids, often by several orders of magnitude.
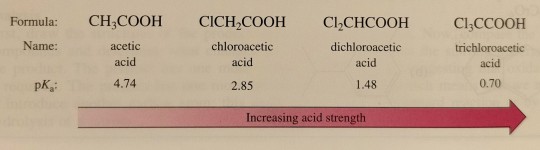
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#substitution#alpha#electronegativity#carbon#acidity#carboxylic acid#acetic acid#chloroacetic acid#dichloroacetic acid#trichloroacetic acid
1 note
·
View note
Photo

#halogen#fluorine#bromine#iodine#chlorine#oxidation state#oxidising agent#oxyacid#mono basic#electronegativity#chemistry#solutions
1 note
·
View note
Text
247 notes
·
View notes
Text



found out noble gases can sometimes bond with halogens if the noble gas's pull on their own valence electrons is weak enough and if the halogen's electronegativity is strong enough. That is what resulted in me making this
#the reaction requires microwaves to even work at all but let me be funny ok#i do a drawings#elementcattos#chemistry
77 notes
·
View notes
Text
Hydrogen bonding (Fig. 12.5). A variety of hydrogen-bonding reactions are possible, involving oxygen- and nitrogen-containing functional groups of both the HM and the organic pesticide molecule. In the example shown, the insecticide carbaryl is hydrogen-bonded to HM using the two electronegative atoms, nitrogen and oxygen, in the carbamate structure.

"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#hydrogen#chemical bonding#chemical reactions#oxygen#nitrogen#humic matetial#pesticide#insecticide#carbaryl#electronegativity#carbamate
0 notes
Text
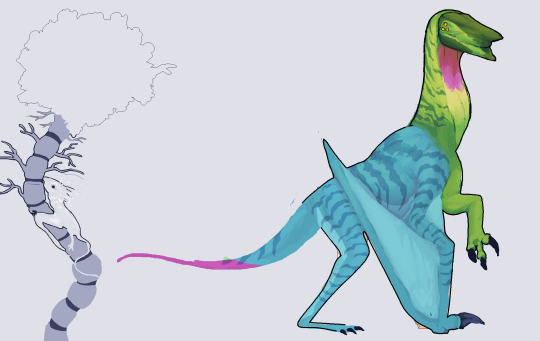


Finished this guy's lil redesign!
My ocs aren't quite spec bio, I don't consider myself smart enough to write the same level everyone in the spec bio community does but alot of people tag my ocs as such, so I decided to entertain a bit and write abit about Q'wilqilth's biology :3
They are basically an overgrown giant bacteria, often referred as "plague angels", the angel part comes from the fact these critters live in a colossal interplanetary organism that many human cultures refer it as the one and true God.
read-
Q'wilqilth-óó is a Neobacteria, often referred generally as 'angels' (though angels include many other non-Neobacteria), Neobacteria are a domain of organisms whose bodies are made up of bacterial colonies acting in tandem as organs, like organs and cells - each colony houses a bacteria with different physiologies to fit a certain role.
And for the same reason, Neobacteria may be composed of species that are pathogenic to humans (hence the 'plague' angel name), they also take on similar mechanisms and ecological roles to their single celled/simple counterparts.
Q'wilqilth in this case (often just shortened to Q'wi) is a Neomyco leprae, derived from the bacteria that causes leprosy in humans, this species is sophont and is often wearing clothing but I decided to add it in there just to see their markings better, they don't have common names and are mostly just referred to as leprosy plague angels, but some species of Neobacteria refer to them as 'Jade emperors' or simply 'Leviathans'
Like many plague angels, this species is a parasitoid/facultative parasite and relies on deploying their genetic material on a host to reproduce, N. leprae prefers doing this to Neural coils, a species derived from Schawnn cells who produce a mucus that stimulates the production of electricity from Neuron trees.
When bursting out of a Neural coil, most will spend years attached to the Neuron trees their former host was attached to, this phase is important to the development of the 'Ouabain whip', a small organ in their tail that helps them regulate their electronegativity and is later used as a weapon, disrupting Na+/K+ ATP-ase in any predators towards them.
They are mostly nomadic, save for a few small tribes/clans, they can travel long distances thanks to their lipid-rich diet, this species has taken lipid/fat as their primary energy source and carbohydrate last, it may not look like but they have extremely mobile/flexible lips and strong toungues/jaws to suck vegetable oil and starch off Neuron trees, unlike their pulmonary counterparts (Tuberculosis), they are facultative aerobics and can use electricity/eletrons provided by the Neuron trees as a substitute for oxygen in ATP production. When migrating out of the Neural forest, they are equipped with mostly molars, strong jaws, and specific digestory enzymes in their mouths to digest tough animal (?) fat. This gives them a much slower metabolism, allowing them to travel without eating for large amounts of time.
Last but not least, though they are gliders, they have evolved their mycolic acid to be hydrophobic in nature, keeping their skin from getting wet/humid, they are great swimmers and their facultative aerobic nature allows them to survive underwater without oxygen, they can live in a variety of biomes within the Super-organism; squashed between arteries close to the red rivers, in the high lands, golden hills or even the filter. They will however, avoid warmer temperatures and prefer sticking to the poles.
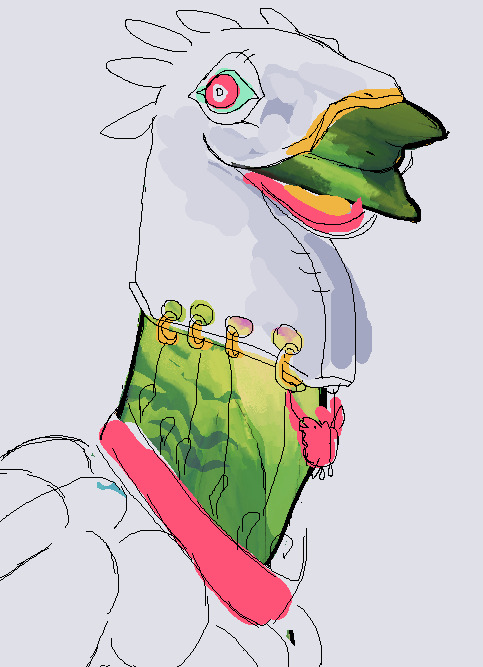
lil sketch of their clothing, representing a epitheloid cell, the body is usually covered in bone sculptures of phagossomes and liver-otters
108 notes
·
View notes