#autoimmune disorders
Explore tagged Tumblr posts
Text
If you have celiac and buy the brand Van’s for their waffles, please know that 9 days ago there was a recall because some of the packages of the gluten free waffles may contain “undeclared” wheat. And if you have celiac, you know “may” might as well mean “does”.
This recall only applies to boxes with the matching lot codes and numbers, and do not pertain to other products that Van’s has to offer. These boxes were distributed in AZ, CA, FL, GA, IL, NC, & WA. Please check your boxes immediately to ensure your own safety and save yourself the painful reactions to gluten. It’s advised the purchased packages be either thrown out (or given to someone who can eat wheat so as not to waste it) or return the product to where you’ve purchased it from.

“The U.S. Food & Drug Administration website published the recall July 3. It applies to certain packs of Van's Gluten Free Original Waffles with lot code UW40193L, expiration date Jan. 19, 2024, and UPC 0 89947 30206 4. According to the Van's recall, some of the packs of waffles may contain undeclared wheat.”
#gluten free#celiac disease#coeliac disease#gluten intolerance#gluten allergy#food allergies#chronic illness#autoimmune disorders#celiac#important
10K notes
·
View notes
Text

I WILL have this….
241 notes
·
View notes
Text
.
Went to a new doctor today for my neuropathy and paresthesia. He seemed smart and dedicated, so I'm pleased. He ordered a cervical MRI to see if something in my neck or whatnot could be causing my symptoms. TBH from what he said I think he's worried I might have MS (multiple sclerosis).
Apparently you can have both MS and sarc/oidosis, which is frankly some shit. Like, talk about winning the worst luck in the world award. There's such a high overlap in symptoms between neuro-sa/rc and MS that it can cause one to be missed because all the symptoms get attributed to whichever one was diagnosed first.
I'm not super worried about it though because I feel like if it was MS surely one of the many neurologists or neurosurgeons I've had scanning and analyzing my brain in the past decade would've tested for that? MS is, afterall, a lot more common than the disease I've since been diagnosed with.
Actually the more I read about MS the more worried I'm getting. So, I should just stop with that for now XD. Haven't told my fam about the possibility yet cuz I don't want to worry them needlessly if it's nothing. It will be good to rule it though because if I did have it, I need to get off Humira ASAP because you (afaik) should not be on it if you have MS. It in fact, can actually cause MS in people.
Anyway that was my day. Went to a cafe for lunch, went to the health food store to grab a few things (and I had $5 off in rewards, which I attribute to the many many people that will not sign up for rewards cards there and instead generously give out my number and help get me points >:)), caught the bus and then went downtown for a coffee date at a different cafe for a couple hours (chatted about side-hustles and goals and God and books and WTF is cishet men's deal?--among other things).
And now I'm back home. Here's hoping I didn't catch Co*vid or some other rotten disease with my unusual amount of not-being-in-my-house-ness I did today.
#chronic illness#chronic disability#autoimmune disease#autoimmune disorders#healthcare#health talk#daily blog#mundane shit#a day in the life of me#long post
4 notes
·
View notes
Text
Esoteric take, but I will say, the existence of autoimmune disorders pisses me off on an existential level.
Not just because of the people I've known who's lives they've fucked up but also because the idea of the bodily system meant to protect you randomly deciding "Now and forever, we have decided to cause problems on purpose" is just cosmically-enraging on a level that's hard to describe.
21 notes
·
View notes
Text
youtube
Sodium Effects on Inflammation, Migraines & Acid Reflux AMA #104
Hey all! Buckle up because episode #104 of our AMA series is about to blow your mind. We're talking about something you might not expect: sodium! We all know salt adds flavor and keeps us hydrated, but there's a whole hidden world to this stuff.
This week, we're joined by Justin Nault, a super cool Certified Nutritional Therapist who specializes in keeping athletes and fitness enthusiasts fueled. He's here to spill the tea (or maybe the electrolytes?) on how sodium can actually help with some surprising things, like fighting inflammation, preventing those awful migraines, and even easing acid reflux.
Justin's gonna break down the science behind how sodium works its magic in our bodies and explain why getting the right amount is key to feeling fantastic. So whether you've been a Clovis Culture devotee for ages or are just joining the party, this episode is packed with info to help you level up your health game.
Want to geek out further? Clovis.show has all the resources, detailed notes, and maybe even some secret bonus content (wink wink). Plus, if your brain is overflowing with questions about sodium or anything health-related, join our Clovis Academy Facebook group! It's a fantastic community where you can connect with like-minded folks and even submit questions for future AMAs.
So grab your favorite snack, salty or sweet, tune in, and let's unlock the hidden power of sodium together! This is gonna be epic!
#sodium effects on inflammation#sodium effects#inflammation#migraines#reflux#sodium intake#acid reflux#chronic inflammation#migraine relief#clovis culture#sodium deficiency#low sodium#nutrition#sodium deficiency symptoms#low salt diet#low sodium symptoms#gut health#sodium#nutritional sciences#chronic pain#wellness#leaky gut#health qu0026a#autoimmune disorders#sodium side effects#nutritional therapy#nutritional therapist#nutritional therapists#health#Youtube
2 notes
·
View notes
Text
me: has a small infection, nbd
my immune system:

#i love getting a small cold and then having flare ups from two different autoimmune disorders immediately after#this is fine#chronic illness#autoimmune disorders#chronic pain
2 notes
·
View notes
Text
A Journey of Healing: Autoimmune Disorder
Witnessing Miracles with Autoimmune Disorders Hello, beautiful souls! Today, I want to share a heartwarming story that reminds us of the incredible power of healing and the miracles that can unfold when we connect with the Creator’s divine energy. Recently, a client came to me with a remarkable update that filled both of us with gratitude and awe. This client had been battling an autoimmune…
#autoimmune disorders#healing#Mystic Pam Jackson#personal development#Spiritual Coaching#spirituality
0 notes
Text
Mitochondrial Dysfunction in Autoimmune Disorders

Mitochondria, the energy-producing organelles of eukaryotic cells, are involved in a myriad of cellular functions beyond ATP production, including calcium homeostasis, reactive oxygen species (ROS) generation, apoptosis regulation, and the modulation of immune responses. In autoimmune diseases, mitochondrial dysfunction is increasingly recognized as a key factor in disease pathogenesis. These disorders, characterized by the immune system's aberrant recognition and attack on self-antigens, often manifest in conditions such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS). This article delves into the mechanisms linking mitochondrial dysfunction to autoimmune disorders, highlighting cellular changes, signaling pathways, and potential therapeutic approaches.
Mitochondrial Function in Immune Cells
Mitochondria are critical for cellular energy metabolism, primarily through oxidative phosphorylation, which occurs in the inner mitochondrial membrane and generates ATP. However, their roles extend far beyond energy production. Mitochondria are involved in immune cell activation, differentiation, and the regulation of apoptosis. Immune cells, including T lymphocytes, B lymphocytes, dendritic cells, and macrophages, rely on mitochondrial function for their metabolic demands during immune responses. These cells undergo rapid changes in mitochondrial dynamics and energy production during immune activation.
Mitochondria also produce ROS as byproducts of oxidative phosphorylation, and these ROS, under normal conditions, function as signaling molecules that regulate immune responses. However, excessive ROS generation, stemming from mitochondrial dysfunction, can lead to oxidative stress, damage to cellular components, and dysregulated immune responses that are characteristic of autoimmune diseases.
Mitochondrial Dysfunction and Autoimmune Pathogenesis
In autoimmune diseases, mitochondrial dysfunction contributes to disease progression through multiple interconnected mechanisms, including dysregulated immune responses, enhanced oxidative stress, impaired mitochondrial dynamics, and defective apoptosis. The following section outlines key mechanisms linking mitochondrial dysfunction to autoimmune pathogenesis.
1. Oxidative Stress and Immune Dysregulation
Mitochondria are a major source of ROS, which are produced as intermediates during oxidative phosphorylation in the electron transport chain (ETC). Under normal conditions, ROS are tightly regulated by antioxidant systems, including superoxide dismutase (SOD), catalase, and glutathione peroxidase. However, in autoimmune diseases, mitochondrial dysfunction often results in increased ROS production, overwhelming the antioxidant capacity of the cell and resulting in oxidative stress.
Oxidative stress can damage cellular macromolecules such as lipids, proteins, and nucleic acids. In immune cells, excessive ROS lead to the activation of several signaling pathways, including the nuclear factor-kappa B (NF-κB) pathway, which promotes the expression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β. In systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS), ROS-induced activation of these inflammatory pathways amplifies the autoimmune response, driving tissue damage and inflammation.
Moreover, oxidative stress exacerbates the production of autoantibodies, particularly in diseases like SLE, where ROS contribute to the formation of immune complexes that target self-antigens, including nuclear components like double-stranded DNA (dsDNA) and mitochondrial antigens.
2. Mitochondrial Dynamics in Autoimmune Diseases
Mitochondrial dynamics, encompassing processes such as mitochondrial fission, fusion, and mitophagy, are critical for maintaining mitochondrial function and cellular homeostasis. Mitochondrial fission, mediated by proteins such as dynamin-related protein 1 (Drp1), leads to the division of mitochondria, while fusion, regulated by mitofusins (Mfn1/2) and optic atrophy 1 (OPA1), facilitates the merging of mitochondria to restore mitochondrial function and DNA integrity. Mitochondrial fusion is crucial for maintaining the respiratory capacity and metabolic flexibility of immune cells.
In autoimmune disorders, an imbalance in mitochondrial dynamics is often observed. For example, in rheumatoid arthritis (RA), T cells exhibit excessive mitochondrial fission and impaired fusion, leading to mitochondrial fragmentation, reduced mitochondrial membrane potential, and diminished oxidative phosphorylation capacity. This dysfunction contributes to the failure of immune cells to produce sufficient ATP during activation, resulting in altered cytokine production and a hyperactive immune response.
Similarly, in multiple sclerosis (MS), aberrant mitochondrial dynamics in oligodendrocytes and T cells contribute to neuroinflammation and demyelination. The dysregulated fission and fusion processes impair mitochondrial function, leading to increased oxidative stress, mitochondrial damage, and exacerbation of inflammatory cascades that damage myelin.
3. Mitochondrial-Derived Damage-Associated Molecular Patterns (DAMPs)
Mitochondria are also sources of damage-associated molecular patterns (DAMPs), which are intracellular molecules released upon cellular injury or stress. DAMPs, such as mitochondrial DNA (mtDNA), cardiolipin, and mitochondrial N-formyl peptides, serve as danger signals recognized by pattern recognition receptors (PRRs) on immune cells. These include toll-like receptors (TLRs), particularly TLR9, which recognizes mtDNA, and TLR2/4, which are involved in the recognition of mitochondrial lipids.
In autoimmune diseases, mitochondrial dysfunction leads to the release of mtDNA and other mitochondrial components into the extracellular space, where they act as endogenous ligands for PRRs. The activation of these receptors triggers inflammatory responses, including the activation of NF-κB, interferon pathways, and the inflammasome, all of which amplify immune responses and contribute to chronic inflammation and tissue damage.
In SLE, circulating mtDNA fragments have been detected in the serum, correlating with disease activity. The presence of mtDNA in the bloodstream is thought to exacerbate the activation of autoreactive immune cells and the production of autoantibodies, particularly those targeting nuclear antigens.
4. Impaired Apoptosis and Autoimmunity
Mitochondria are key regulators of apoptosis, particularly the intrinsic or mitochondrial pathway of cell death. The mitochondrial pathway is initiated by the release of pro-apoptotic factors such as cytochrome c and apoptosis-inducing factor (AIF) from the mitochondrial intermembrane space into the cytosol. This release activates caspases, leading to cell death. In autoimmune diseases, mitochondrial dysfunction often leads to defective apoptosis, which prevents the elimination of autoreactive immune cells.
In diseases like systemic lupus erythematosus (SLE), defective apoptosis of T cells and B cells contributes to the survival of autoreactive lymphocytes that would normally be eliminated through programmed cell death. The persistence of these cells results in the production of autoantibodies, including anti-dsDNA and anti-mitochondrial antibodies, which target self-tissues and exacerbate inflammation.
Moreover, impaired mitophagy, the process by which damaged mitochondria are selectively degraded by autophagy, further contributes to the accumulation of dysfunctional mitochondria, promoting sustained oxidative stress and inflammation.
Mitochondrial Dysfunction in Specific Autoimmune Disorders
1. Systemic Lupus Erythematosus (SLE)
In SLE, mitochondrial dysfunction plays a pivotal role in disease pathogenesis. Increased ROS production, defective mitophagy, and impaired apoptosis contribute to the activation of autoreactive T cells and B cells, leading to the production of autoantibodies. The release of mitochondrial DAMPs, such as mtDNA, amplifies the inflammatory response, and mitochondrial damage is directly linked to the severity of disease activity in SLE.
2. Rheumatoid Arthritis (RA)
In RA, mitochondrial dysfunction in immune cells, particularly in synovial T cells and macrophages, is associated with increased mitochondrial fragmentation, oxidative stress, and dysregulated immune activation. ROS and mitochondrial-derived DAMPs contribute to the chronic inflammatory environment in the synovium, driving the production of pro-inflammatory cytokines and promoting joint destruction.
3. Multiple Sclerosis (MS)
Mitochondrial dysfunction in MS primarily affects oligodendrocytes, the myelinating cells in the central nervous system. Impaired mitochondrial function in these cells contributes to neuroinflammation and demyelination. Additionally, T cells with dysfunctional mitochondria play a role in the perpetuation of inflammation, leading to the destruction of myelin and axonal injury in the CNS.
Therapeutic Implications
Targeting mitochondrial dysfunction presents a promising therapeutic strategy for autoimmune diseases. Potential approaches include:
Mitochondrial Antioxidants: Compounds like MitoQ, MitoTEMPO, and other mitochondrial-targeted antioxidants can help reduce oxidative stress, protect mitochondrial function, and alleviate inflammation.
Modulating Mitochondrial Dynamics: Agents that promote mitochondrial fusion or inhibit excessive fission, such as Mfn2 activators, could restore mitochondrial integrity in immune cells, reducing inflammation and improving immune regulation.
Enhancing Mitophagy: Stimulating mitophagy through compounds such as spermidine or activation of the PINK1/PARK2 pathway may help remove damaged mitochondria and reduce the accumulation of mitochondrial DAMPs.
Targeting Mitochondrial DAMPs: Inhibiting the release of mtDNA and other mitochondrial DAMPs using TLR inhibitors or blocking downstream signaling pathways may reduce the inflammatory response in autoimmune diseases.
Conclusion
Mitochondrial dysfunction is a key factor in the pathogenesis of autoimmune diseases. Through mechanisms such as increased oxidative stress, impaired mitochondrial dynamics, and dysregulated apoptosis, mitochondrial dysfunction exacerbates immune activation and inflammation. The accumulation of mitochondrial-derived DAMPs further amplifies the autoimmune response. Targeting mitochondrial health offers a promising therapeutic strategy to mitigate the effects of mitochondrial dysfunction and improve disease outcomes in autoimmune disorders.
#Mitochondrial dysfunction#Autoimmune disorders#Oxidative stress#Reactive oxygen species (ROS)#Mitochondrial dynamics#Mitochondrial fission#Mitochondrial fusion#Mitophagy#Mitochondrial DNA (mtDNA)
0 notes
Text
instagram
1 note
·
View note
Text
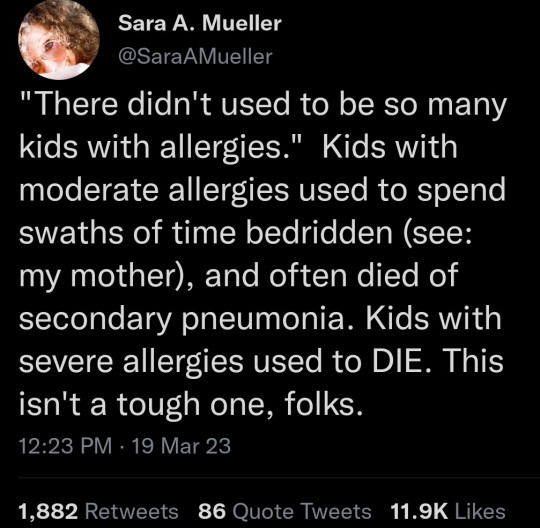
Every novel I’ve read written from, like, 1750 onward mentions at least one character who is just Sickly. Of Poor Constitution. Generally Unwell. Delicate. Usually women. I’d bet they were suffering from an allergy or a chronic illness that we didn’t have a diagnosis for until recently. (Funnily enough, women are also more likely to have autoimmune disorders.) If your family was wealthy enough back then, you were just weak until you died earlier than your hearty and hale relatives. If your family wasn’t wealthy, you died much faster.
45K notes
·
View notes
Text
Shamis Tate Explains The Connection Between Neuropathy and Autoimmune Disorders
Neuropathy, a condition marked by nerve damage, often intersects with autoimmune disorders, leading to a complex interplay of symptoms and challenges. In this video, Shamis Tate delves into the relationship between neuropathy and autoimmune disorders. With five key subheadings, we will explore the mechanisms linking these conditions, common autoimmune diseases associated with neuropathy, diagnostic approaches, treatment options, and lifestyle adjustments for managing symptoms.
0 notes
Text

#chronic illness#chronically ill#chronic illness memes#autoimmune#autoimmune disorder#autism#mental health#mental illness
5K notes
·
View notes
Text
Over 50+ hrs streamed so far despite illness! A win is a win.

#autism#actually autistic#disabled#disability#autistic#twitch#live streaming#twitch streaming#hypermobile spectrum disorder#autoimmune disorders#MCAS#POTS#achievement unlocked
1 note
·
View note
Text
Autoimmune Hepatitis Market: Transformative Treatments Await
Global Autoimmune Hepatitis Market, By Type (Type 1, Type 2), Treatment (Medications, Liver Transplant, Others), Diagnosis (Liver Biopsy, Blood Tests, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2030
Market Overview
In recent years, the autoimmune hepatitis market is anticipated to grow rapidly during the forecast period. According to the 2019 study "Burden of Liver Diseases in the World," liver illness causes roughly 2 million fatalities worldwide, with 1 million deaths due to cirrhosis complications and 1 million deaths due to viral hepatitis and hepatocellular cancer. Cirrhosis is the 11th most prevalent cause of mortality globally, while liver cancer is the 16th most common cause of death. They are responsible for 3.5 percent of all deaths worldwide.
According to Pharmanucleus, the autoimmune hepatitis market was valued at USD 156.76 million in 2021 and is predicted to reach USD 210.61 million by 2030, showing a CAGR of 3.70% from 2023 to 2030. The Pharmanucleus team curated the market study, which contains extensive expert analysis, patient epidemiology, pipeline analysis, price analysis, and regulatory framework.
Please click here:
Market Definition
Autoimmune hepatitis is an uncommon but fatal liver disease. When the body mistakes healthy tissue and cells for infectious tissue and cells, antibodies are created to target the healthy liver cells. Autoimmune hepatitis can develop quickly or gradually. The condition's aetiology is unknown, however it may be related to other systemic disorders or medication exposure in some situations. Autoimmune Hepatitis Market Dynamics
Drivers
Increased incidence of autoimmune hepatitis
The rising prevalence of autoimmune hepatitis is a major contributor to the market's rapid expansion. Complications of such illnesses include swollen veins in the oesophagus, fluid accumulation in the abdomen, liver failure, and liver cancer.
Increasing healthcare infrastructure investment
Growing healthcare spending, which aids in infrastructure improvement, is another important aspect driving the autoimmune hepatitis market's growth rate.
The greater governmental and private-sector efforts to promote awareness of the would boost the autoimmune hepatitis market. Furthermore, people's changing lifestyles and high disposable income will drive the autoimmune hepatitis market upward. Furthermore, the expanding older population and an increase in medical tourism will accelerate the market's development pace.
Opportunities
Increase in the number of R&D activities
Moreover, when R&D activity increases, so does the market. This will create new prospects for the autoimmune hepatitis industry to expand. Additionally, higher medication approvals and launches will drive market growth.
Furthermore, rising investments in the development of innovative technologies, as well as an increase in the number of emerging markets, will create further chances for the autoimmune hepatitis market to expand throughout the projected period..
Restraints/Challenges
On the other hand, the high cost of treatment will hamper the growth rate of the market. Lack of healthcare infrastructure in developing economies and low awareness of autoimmune hepatitis will pose major challenges to the market growth rate. Additionally, shortage of skilled professionals and missed diagnoses will further limit and hamper the growth rate of the market over the forecast period 2023-2030.
This report on the Autoimmune Hepatitis Market discusses recent new developments, trade regulations, import-export analysis, production analysis, value chain optimisation, share market analysis, the impact of national and localised market players, analyses opportunities in terms of emerging revenue pockets, market changes regulations, strategic analysis of market growth, market size, category market growth, niches and dominance of applications, product approvals, and p Contact Pharmanucleus for an Analyst Brief for more information on the Autoimmune Hepatitis industry; our experts will assist you in making an informed market choice to achieve market growth.
Please click here for full report:
https://www.pharmanucleus.com/reports/autoimmune-hepatitis-market
Patient Epidemiology Analysis
Autoimmune hepatitis is an uncommon condition that affects four times as many women as males. Type 1 diabetes is the most common and affects most individuals. Type 2 diabetes is more frequent in young individuals and progresses faster. Every year, 1 to 2 new cases per 100,000 people are expected, for a total of around 24 cases per 100,000 people.
Furthermore, the Autoimmune Hepatitis Market offers in-depth market data for patient analysis, prognosis, and therapy. Prevalence, incidence, mortality, and adherence rates are among the statistical aspects evaluated in the study. Analyses of the direct or indirect influence of epidemiology on market growth are performed in order to develop a more robust cohort multivariate statistical model to forecast market growth during the boom era.
Click here for Request free sample:
Post COVID Impact
Since its emergence in December 2019, the COVID-19 virus has indeed had a profound impact on healthcare systems worldwide, which has affected various medical conditions, including autoimmune hepatitis. The declaration of the virus as a public health emergency by the World Health Organization (WHO) prompted healthcare systems to prioritize COVID-19-related treatments and control measures. As a result, specialist healthcare services for other conditions, including autoimmune hepatitis, have faced delays and disruptions.
The financial crisis caused by the pandemic has further compounded the challenges in healthcare systems, leading to resource constraints and reduced access to medical services. Patients with autoimmune hepatitis have faced difficulties in seeing their healthcare providers for various reasons. Some individuals have struggled to access doctors due to overwhelmed healthcare facilities or limited availability of appointments. Fear of contracting the virus has also deterred patients from seeking in-person consultations. Moreover, pandemic-related restrictions, such as lockdowns and travel limitations, have hindered the continuity of essential therapies and procedures for autoimmune hepatitis patients.
These circumstances have the potential to negatively impact the autoimmune hepatitis market in recent months. Reduced access to care, delayed diagnoses, and interruptions in treatment may result in suboptimal disease management, increased disease progression, and worsened patient outcomes. Additionally, the economic consequences of the pandemic may limit patients' ability to afford necessary medications and therapies, affecting market demand.
However, as the global healthcare system adapts and recovers from the pandemic, efforts are being made to address these challenges. Telemedicine and remote healthcare services have gained prominence, allowing patients to connect with their healthcare providers virtually. Gradual easing of pandemic restrictions and resumption of regular healthcare services are expected to alleviate some of the barriers faced by autoimmune hepatitis patients, helping to stabilize the market over time.
Global Autoimmune Hepatitis Market Scope
The market for autoimmune hepatitis is classified by type, therapy, diagnosis, method of administration, end-users, and distribution channel. The growth in these segments will assist you in analysing the growth sectors in industries and providing users with a beneficial market overview and industry insights to assist them in making strategic decisions for finding key market applications.
#autoimmune hepatitis#transformative treatments#liver health#autoimmune disorders#disease management options7
0 notes
Text
The Connection Between Damaged Mitochondria and Arthritis

Mitochondria are integral organelles responsible for various critical cellular functions, primarily energy production through oxidative phosphorylation. They are involved in maintaining cellular homeostasis, regulating metabolism, modulating calcium levels, and controlling apoptosis. Emerging evidence has highlighted mitochondrial dysfunction as a key contributor to a variety of diseases, including arthritis. This formal overview aims to explore the complex relationship between damaged mitochondria and arthritis, focusing on the molecular mechanisms that link mitochondrial dysfunction to the pathogenesis of inflammatory joint diseases, particularly rheumatoid arthritis (RA) and osteoarthritis (OA).
Mitochondrial Structure and Function
Mitochondria are double-membraned organelles found in eukaryotic cells, and they are crucial for cellular energy metabolism. Their primary role is the production of adenosine triphosphate (ATP) via oxidative phosphorylation, a process that takes place in the inner mitochondrial membrane. During this process, the electron transport chain (ETC) generates a proton gradient across the inner membrane, which drives ATP synthesis through ATP synthase. However, this process also generates reactive oxygen species (ROS) as byproducts, primarily from complexes I and III of the ETC. Under normal physiological conditions, ROS are neutralized by antioxidants, including superoxide dismutase (SOD), catalase, and glutathione. However, under pathological conditions, excessive ROS production can lead to oxidative stress, contributing to cellular damage and dysfunction.
In addition to ATP production, mitochondria have essential roles in calcium buffering, apoptosis regulation, and the maintenance of cellular integrity. Damage to these organelles disrupts these functions, contributing to various diseases, including arthritis.
Mitochondrial Dysfunction in Arthritis
Arthritis is a group of diseases characterized by inflammation and degeneration of the joints. It includes conditions like rheumatoid arthritis (RA), an autoimmune disease, and osteoarthritis (OA), a degenerative disease. In both types of arthritis, mitochondrial dysfunction has been identified as a critical factor that exacerbates disease progression through several mechanisms, including increased oxidative stress, immune activation, and tissue damage.
1. Oxidative Stress and Mitochondrial Damage
Oxidative stress is a hallmark of both RA and OA, and mitochondria are central to its production. In these conditions, mitochondrial dysfunction results in an increase in ROS production, overwhelming the cell’s antioxidant defenses. This oxidative stress leads to the modification of cellular structures, including proteins, lipids, and DNA, causing further mitochondrial damage. In RA, pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) stimulate immune cells like macrophages and neutrophils to release large amounts of ROS. These ROS contribute to the local inflammatory environment and accelerate joint destruction by damaging mitochondria and amplifying oxidative stress.
Mitochondrial damage results in a feedback loop where impaired mitochondrial function generates more ROS, further promoting inflammation. For instance, in RA, markers of oxidative damage such as 8-hydroxy-2'-deoxyguanosine (8-OHdG) and malondialdehyde (MDA) have been found to correlate with disease activity, suggesting a direct relationship between mitochondrial dysfunction and disease severity.
2. Mitochondrial DNA Damage and Inflammatory Signaling
Mitochondrial DNA (mtDNA) is particularly vulnerable to oxidative damage due to its proximity to the ETC, where ROS are produced during ATP synthesis. Unlike nuclear DNA, mtDNA is not protected by histones and has limited repair mechanisms, making it prone to mutations. Damage to mtDNA impairs mitochondrial function and can lead to the release of mtDNA fragments into the cytoplasm or extracellular space.
In the context of arthritis, mtDNA damage has been implicated in immune activation. When damaged mtDNA is released into the cytoplasm, it is recognized by pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), on immune cells. TLRs, particularly TLR9, activate downstream inflammatory signaling pathways that lead to the production of pro-inflammatory cytokines such as TNF-α and IL-6. This further exacerbates the inflammatory response in joints and contributes to the progression of arthritis. Studies have shown that the presence of mtDNA fragments in the serum of RA patients correlates with disease activity, indicating the role of mtDNA in driving inflammation.
3. Mitochondrial Dynamics and Arthritis Pathogenesis
Mitochondrial dynamics refer to the continuous processes of mitochondrial fission (division) and fusion (joining), which maintain mitochondrial function and integrity. Fission allows for the removal of damaged mitochondria, while fusion helps to integrate mitochondrial contents and maintain a healthy mitochondrial pool. Imbalance between fission and fusion is associated with several diseases, including arthritis.
In the case of RA, excessive mitochondrial fission and reduced fusion have been observed. This imbalance results in mitochondrial fragmentation, which impairs mitochondrial function, increases ROS production, and contributes to cellular stress. Fission is regulated by proteins such as dynamin-related protein 1 (Drp1) and fission 1 protein (Fis1), while fusion is controlled by mitofusins (Mfn1 and Mfn2) and optic atrophy 1 (OPA1). Dysregulation of these proteins in RA leads to a fragmented mitochondrial network, which exacerbates oxidative stress and inflammation in synovial tissues.
4. Mitochondrial-Dependent Cell Death
Mitochondria are also central regulators of programmed cell death, particularly apoptosis. In the pathogenesis of arthritis, excessive or dysregulated apoptosis contributes to joint destruction. Mitochondrial dysfunction plays a critical role in the intrinsic apoptotic pathway by releasing pro-apoptotic factors such as cytochrome c and apoptosis-inducing factor (AIF). These factors activate caspase-dependent and caspase-independent pathways, leading to the death of synovial cells and cartilage cells, which contributes to the progressive tissue damage observed in both RA and OA.
Furthermore, mitochondrial permeability transition pore (mPTP) opening, which is induced by oxidative stress, can lead to necrosis, a form of uncontrolled cell death. Necrotic cell death in the joints increases inflammation and tissue degradation, particularly in OA, where cartilage breakdown is a hallmark feature.
Therapeutic Approaches Targeting Mitochondrial Dysfunction in Arthritis
Given the significant role of mitochondrial dysfunction in the pathogenesis of arthritis, various therapeutic strategies aimed at improving mitochondrial function are under investigation.
1. Mitochondrial Antioxidants
Mitochondrial-targeted antioxidants, such as MitoQ and MitoTEMPO, have been developed to selectively accumulate in mitochondria, where they can neutralize ROS and reduce oxidative stress. These compounds have shown promise in preclinical models of arthritis, where they help to reduce inflammation, protect mitochondrial function, and limit joint damage. The use of mitochondrial antioxidants could be an effective strategy to mitigate oxidative stress in arthritic conditions.
2. Mitochondrial Biogenesis Enhancement
Another potential therapeutic approach is the activation of mitochondrial biogenesis, the process by which new mitochondria are formed to compensate for damaged mitochondria. Agents that activate peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a key regulator of mitochondrial biogenesis, could help restore mitochondrial function in arthritic tissues. Compounds such as resveratrol and NAD+ precursors are under investigation for their ability to promote mitochondrial biogenesis and improve cellular metabolism in arthritis.
3. Mitochondrial Dynamics Modulation
Restoring the balance between mitochondrial fission and fusion is another therapeutic strategy. Inhibiting excessive mitochondrial fission or promoting mitochondrial fusion may help maintain mitochondrial integrity and reduce inflammation in arthritis. Drugs targeting Drp1 or enhancing Mfn1/Mfn2 activity are potential candidates for modulating mitochondrial dynamics in arthritic diseases.
4. Mitophagy Enhancement
Mitophagy, the selective autophagic degradation of damaged mitochondria, is essential for maintaining mitochondrial quality. Enhancing mitophagy through the use of compounds like spermidine or activators of the PINK1/PARK2 pathway could help eliminate dysfunctional mitochondria and reduce inflammation, making it a promising therapeutic approach in arthritis.
Conclusion
Mitochondrial dysfunction plays a critical role in the pathogenesis of arthritis, contributing to oxidative stress, inflammation, and joint damage. The intricate relationship between damaged mitochondria and immune activation highlights the importance of targeting mitochondrial health in the treatment of arthritis. Emerging therapeutic strategies aimed at restoring mitochondrial function, reducing oxidative stress, and modulating mitochondrial dynamics hold promise for improving the management of arthritis and preventing joint destruction. Further research into mitochondrial biology and its role in arthritis is essential for the development of more effective, targeted therapies for these debilitating conditions.
#Mitochondrial dysfunction#Autoimmune disorders#Oxidative stress#Reactive oxygen species (ROS)#Mitochondrial dynamics#Mitochondrial fission#Mitochondrial fusion#Mitophagy#Apoptosis#Mitochondrial DNA (mtDNA)#Damage-associated molecular patterns (DAMPs)#Immune cell activation#Systemic lupus erythematosus (SLE)#Rheumatoid arthritis (RA)#Multiple sclerosis (MS)#Pattern recognition receptors (PRRs)#Toll-like receptors (TLRs)#Pro-inflammatory cytokines#Cytochrome c#NF-κB signaling#MitoQ#MitoTEMPO#Spermidine#PINK1/PARK2 pathway#Mitochondrial-targeted antioxidants#Immune dysregulation#Chronic inflammation#Mitochondrial fragmentation#Mitochondrial permeability transition pore (mPTP)#Autoantibodies
0 notes