#meso compound
Explore tagged Tumblr posts
Text
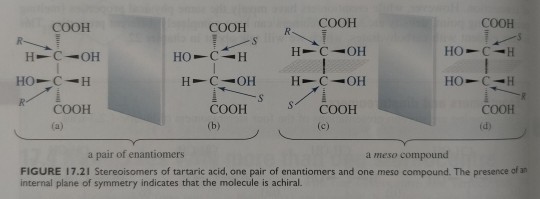
Because (c) and its mirror image (d) are superimposable, they are the same molecule and achiral.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
6 notes
·
View notes
Text
Figure 17.21 shows the two pairs of mirror images of this compound. Structures (a) and (b) are nonsuperimposable mirror images and, therefore, they are a pair of enantiomers. Structures (c) and (d) are also mirror images, but they are superimposable if they are rotated to the appropriate orientation. Therefore, (c) and (d) are not different molecules; they are the same molecule, just oriented differently. Because (c) and its mirror image (d) are superimposable, they are the same molecule and achiral.

Thus, even though (c) has two stereocentres, it is achiral. Its plane of symmetry is shown in figure 17.21. The stereoisomer of tartaric acid represented by (c) or (d) is called a meso compound, defined as an achiral compound with two or more stereocentres.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#tartaric acid#dihydroxybutanedioic acid#enantiomers#chirality#meso compound#mirror image
0 notes
Text
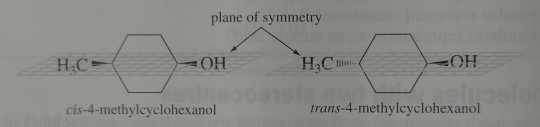
Firstly, 4-methylcyclohexanol can exist as two stereoisomers – a pair of cis-trans isomers. Both the cis and trans isomers are meso compounds and are achiral.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#cis#trans#meso compound#chirality#achiral#isomers#methylcyclohexanol#symmetry
0 notes
Text
Meso Compound [Ex. 1]



Patreon
#studyblr#notes#my notes#organic chemistry#ochem#orgo#orgo notes#organic chemistry notes#organic chem#orgo chem#study guides#mcat#mcat chemistry#mcat orgo#mcat ochem#mcat organic chemistry#mcat studyblr#premed studyblr#organic chemicals#organic reactions#chemical reactions#advanced chemistry#life science#meso compounds#chirality#chiral#achiral#achirality
6 notes
·
View notes
Note
Viv be a worm cop tell me more about nematodes
At the risk of sounding like a moron to ecologists, ecology is one of the great black boxes of the natural sciences. Instead of studying any specific element of the natural world in isolation, such as botany, geology, hydrology, and the half dozen other major disciplines, you're studying systems and dynamics, which presents inherent challenges that monodisciplines don't suffer. Compounding this, portions of ecological systems can remain cryptic even in the face of rigorous study, due to limitations in study design, inherent complexity of ecosystems, and the sheer noise and destruction that exists at the interfaces of the natural and constructed worlds.
Soil ecology compounds many of these challenges to the next level. Very literally, ecosystems move from the macro scale and the obvious to the meso to micro scale and the obscure. Soil ecology remains cryptic except to experts or whenever its effects translate to the macro scale, generally as harmful or helpful effects as they relate to other ecosystems. When they impact crop health or have impacts on plant growth or the geochemical behavior of certain compounds.
Long story short is I can't tell you any fun things about nematodes in soil because I don't KNOW NOTHING FUCK 12
3 notes
·
View notes
Text


[ aaron pierre, homosexual, cis male + he/him, florakinesis ] naji “shade” rosemonte is a neutral agent of pandora selected for their renown extensive knowledge and research in botany and toxicology and underwent the top-secret mutation process. to the rest of the world, the thirty-three year old originally from boston, massachusetts is deceased or missing. however, in atlantis, they are now known as nightshade of agent lust after developing the ability to communicate, control, create, manipulate, revive flora as wood, vines, plants, moss, and parts of the plants, such as leaves, seeds, roots, fruits and flowers, & may rearrange plant DNA structures. the agent has been with pandora for seven years and is trusted for being passionate & protective, but once reprimanded for being obsessive & obstinate.

Nightshade's prominence in the outside world barely scratches the surface of who he'd become under the ranks of PANDORA. He does not have the physical means of his accolades and acclaims but his extensive database proves to be the true reward. However, there's been a bit of a change. The mutation caused Naji to progressively develop the repressed expressions of his subconscious. Articulation and behaviors that he hadn't shown before. Though still involved greatly in his research, he's been noted to display quite carnal traits. He is quite versatile as an agent, toying with the interest's senses through his intricate compounds to make them highly susceptible to influence and suggestion. With one whisper or the stroke of his hand, he could have them slurping water from the palm of his hand. It should be advised to consider great caution while dealing with 'Shade' and his plethora of chloro-kind. The berry might be sweet but the suffering is quite bitter.

THE BREAKDOWN
NICKNAME: 'Shade CALLSIGN: Nightshade MORAL ALIGNMENT: Neutral DIVISION: Lust TENURE: 7yrs ALT GROUP: Botanist/Medicine FORMER OCCUPATION: Researcher & Scientist HOMETOWN: Boston, Massachusetts AGE: 33yrs GENDER: Cisgendered Male MARITAL STATUS: Single SEXUALITY: Homosexual ROMANTIC: Homoromantic HEIGHT: 6ft 5in (1.96m) ARCHETYPE: Meso-Endomorphic HAIR COLOR: Auburn HAIRSTYLE: Variable (example of his common style) IRIS COLOR: Green (various stages) FACIAL HAIR: Variable MORALITY: (Neutral) 'Shade decides which direction to swing and why. He considers the nature of the choice & all factors to be included, but ultimately he decides whether or not the lawfulness or the chaos should ensue.
WHY PANDORA: The years he'd spent devoting his life to progressing society felt as if they'd been wasted as government, politics, and agency all seemed to underline his procedures with "for profit" priorities. They offered him a chance to really make a difference on the global stage. How could he refuse that offer when your desire was and is to change the world?
MUTATION: Florakinesis (or Chlorokinesis)
DIVISION SKILLS: Culture & Politics and Seduction EXPERTISE IN: Biochemistry & Medicine PROFICIENT AT: Acrobatics & Evasion, Deception, Environmental Adaption, Perception, and Persuasion SUBSTANDARD AT: Engineering and Hacking & Cyber Warfare
3 notes
·
View notes
Text
Multiplex Assays Market Detailed in New Research Report By 2032
According to the research report, the global multiplex assays market was valued at USD 3,777.7 million in 2023 and is expected to reach USD 8,136.7 million by 2032, to grow at a CAGR of 8.81% during the forecast period.
Our newly published research report titled Multiplex Assays Market Insights offers a comprehensive analysis of the rapidly growing market. It highlights all the key factors anticipated to drive growth while shedding light on potential challenges and opportunities that could emerge in the market in the upcoming years. The market assessment includes a thorough analysis of Multiplex Assays market share, size, gross margin, and CAGR. The research report has been prepared using industry-standard methodologies to offer a thorough assessment of the major market participants and their market scope.
All the data and information provided in the study are curated and verified by expert analysts to provide a reliable and accurate market analysis. Also, pictorial representations such as tables, charts, and graphs have been used to enhance decision making and improve business strategy. The research report is a must-read for anyone involved or interested in the market in any form.
Key Report Features:
Comprehensive Market Data: Provides a thorough market examination of annual sales, current market size, and anticipated Multiplex Assays market growth rate during the forecast period.
Regional Analysis: Thorough analysis of all the major regions and sub-regions in the market.
Company Profiles: An in-depth assessment of all the leading market participants and emerging businesses.
Customization: Report customization as per your requirements with respect to countries, regions, and segmentation.
Major Market Participants:
The research report includes a comprehensive competitive landscape section that helps businesses understand their competitors and the market in which they operate. All the major Multiplex Assays market players have been covered in the report. By going through the competitive landscape, businesses can identify their competitors and understand their strengths and weaknesses. Also, businesses can better examine the products/services of their competitors and evaluate their offers and pricing. All the major competitive analysis frameworks, including SWOT analysis and PESTEL analysis, have been included in the research study to offer a thorough assessment of the market’s competitive scenario. Here are a few of the key players operating in the market:
Browse Full Insights
The top players operating in the market are:
Abcam plc
Agilent Technologies, Inc.
Antigenix America, Inc.
AYOXXA Biosystems GmbH
Bio-Rad Laboratories, Inc.
Bio-Techne Corporation
Becton, Dickinson and Company
Boster Biological Technology
Cayman Chemical Company
DiaSorin S.p.A.
Enzo Life Sciences, Inc.
Illumina, Inc.
Luminex Corporation
Merck KGaA
Meso Scale Diagnostics
Olink
PerkinElmer, Inc.
Promega Corporation
Qiagen N.V.
Randox Laboratories
Seegene
Shimadzu Biotech
Siemens Healthineers
Thermo Fisher Scientific Inc.
Market Dynamics:
Growth Drivers: The research report sheds light on all the major factors driving the robust growth of the market. Also, all the key trends and opportunities anticipated to have a favorable impact on market Multiplex Assays development have been covered in the study.
Technological Advancements: All the major advances in technology that can support market growth have been covered in the research report. Besides, the introduction of new products/services by major participants has been detailed.
Regulatory Policies: The research report examines the regulatory landscape of the constantly evolving market, shedding light on new market frameworks and policies projected to drive the market forward.
Segmental Overview:
This section of the research report categorizes the market into various segments, such as end use, product type, application, and region. Also, a thorough analysis of all the major sub-segments has been provided in the study. By going through the segmental analysis section, businesses and stakeholders can easily examine different Multiplex Assays market segments and identify consumer requirements within each of them. Besides, businesses can optimize their brand positioning and tailor their marketing efforts to specific segments. What’s more, companies can use market segmentation to identify gaps in their offerings that can developed up on.
Report Answers Questions Such As:
• What is the current market size and projected value? • What are the major factors driving Multiplex Assays market sales and demand? • What are the key developments and trends driving the market forward? • What are the key outcomes of the PESTEL analysis for the market? • Who are the major players offering their products/services in the market? • What are the major opportunities that market participants can capitalize on?
Report Summary:
The Multiplex Assays market research report is a reliable resource to understand the dynamic nature of the market. It covers several key market features, including capacity, revenue, price, consumption, production rate, and supply demand, to provide an in-depth market analysis. By going through the research study, readers can get a precise and reliable analysis of the rapidly evolving market.
More Trending Latest Reports By Polaris Market Research:
Risk Analytics Market
Liver Disease Diagnostics Market
Solar Panels Market
In-Building Wireless Market
Catalyst Carrier Market
Digital X-Ray Market
Medical Alert Systems Market
Hemato Oncology Testing Market
0 notes
Text
White Hat research - Anatomy and Physiology
so... for this topic, I wanted to continue with the sub-category of the Anatomy and Physiology of insects.
all insect species possess, (in the adult stage), three pairs of jointed legs and three main body parts - head, thorax, and Abdomen.



youtube
Overall Body Plan
Insect Body Regions: Head, Thorax, and Abdomen. See text for details. Modified from Packard 1890[1].
Insects have three major body regions: head, thorax, and abdomen (see Insect Body Regions, right).
The head is made of 5-7 fused segments and bears the eyes, antennae, and mouthparts.
The thorax consists of three segments called the pro-, meso-, and metathorax. Appendages used for movement are attached to the thorax. Each of the segments of the thorax bears one pair of legs and if wings are present they are found on the meso- and metathorax only. The top of the prothorax is called the pronotum.
An insect's abdomen consists of 11 or fewer segments that generally do not bear any appendages, except for segments near the rear which may have appendages associated with reproduction

Head and Mouthparts
The head can be divided into general regions (see General Insect Head Regions and Mouthparts, left): the top of the head is the vertex, the side or cheeks are gena, the front of the face is the front, and below the front is the clypeus. These regions may be highly modified or lost in some groups of insects. Adult insects may have two types of eyes, larger compound eyes that consist of many facets (ommatidia), and eyes that occur as a single facet, ocelli. The number and placement of ocelli can be important for identification.

The mouthparts of humans consist of five layers or horizons; upper lip, upper jaw, tongue, lower jaw, and lower lip. Insect mouthparts also consist of five horizons and are made of appendages modified for food handling (see General Mouthparts, right). The labrum is similar to an upper lip. It is not divided but may have a notch on the outer (distal) edge. Below the labrum are the mandibles, paired structures generally made of strong material (heavily sclerotized) and used for cutting or grinding. The specific shape and various features found on the mandibles may be essential for understanding what and how an organism eats. The hypopharynx is an internal structure located below the mandibles and has a tongue-like function. Below the mandibles (externally) are paired appendages called the maxillae. Generally, each maxilla bears an appendage, the maxillary palpus that is used for food handling and may contain taste or smell organs called sensillae. The bottom horizon of insect mouthparts is the labium which is made of two fused maxilla-like structures and bears labial palps.


All insect mouthparts are modifications of this basic plan. A mosquito's proboscis contains all five mouthpart types, see the cross section in Mosquito Mouthparts, B., right. In cases of extreme modification, some mouthparts may become fused, reduced, or lost. Mouthpart arrangement can be critical when studying an insect's potential to vector a disease, access a portion of a plant, etc.
Insect Legs
Insect Legs. All insect legs contain the same basic parts: coxa, trochanter, femur, tibia, and tarsus, the latter of which is armed with one or two claws. The color of each leg part is the same throughout all the anatomy figures.

Insects have three pairs of legs, one pair on each of the three segments of the thorax, and are generally called the fore-, mid-, and hind legs. Any of the pairs of legs may be heavily modified and are important for locomotion, prey capture, mating, etc. Thankfully, just like mouthparts, all insect legs contain the same basic parts. From proximal (toward or against the body) to distal (away from the body) the parts of an insect leg are the coxa,��trochanter, femur, tibia, and tarsus. The tarsus almost always has one or two claws at the type used to grasp the substrate. The figure Insect Legs, right, shows legs modified for numerous purposes: A, running; B, jumping; C, digging; D, grasping; E, catching; F, walking and digging; G, reduced leg used for walking and digging; H, male leg modified for grasping females during mating
Basic Internal Anatomy
The internal anatomy of insects is amazingly complex. A good-sized caterpillar has more muscles than a human. The internal anatomy of insects differs from vertebrates (including humans) in several major ways.
Digestive/excretory system: Insects have a complete digestive system just like vertebrates (tube from the mouth to the anus) but it differs in a very important way (see Digestive System, left). The insect digestive system has three major regions, foregut, midgut, and hindgut.[1] The foregut and the hindgut are lined with chitin, the same stuff that makes up much of the exoskeleton of the insect. When an insect molts (sheds it's "skin", see below) it also sheds the internal lining of the fore- and hindguts. Loss of the gut contents is a problem if the insect relies on gut microorganisms (gut fauna) to help with digestion. The gut fauna often lives in the hind gut (termites, for example). Suddenly the gut fauna is lost and must be replenished with every molt.
Insects do not have kidneys. Instead, metabolic wastes are removed with the Malpighian tubules[2].
Respiratory (ventilation) system: Insects don't have lungs. They obtain oxygen and dispel carbon dioxide through a series of tubes called tracheae (see Respiratory System, right). The tracheae are attached to openings on the body called spiracles. The number and placement of spiracles varies and smaller insects may not have any. Traditionally, the view has been held that respiration in insects is passive, but recent evidence has demonstrated that some insects actively expand and contract trachea to ventilate their bodies.
Circulatory system: Insects do not have blood, or blood vessels that are part of a closed circulatory system. Instead, insects have an open circulatory system where a substance called hemolymph bathes the organs directly. Some insects have a long heart-like organ along the dorsal side of the internal organs that helps circulate the hemolymph through the body. It comprises a single sheath of tissue and a series of muscles, and in many insects includes a tubular portion that functions as a dorsal aorta. Hemolymph also circulates through the legs, wings, and antennae via a series of simple one-way valves.


info from https://wiki.bugwood.org/
0 notes
Text
The Strength of Resilience: How to Find Strength in Difficult Times
What are the differences between training for size and strength? Are there any? Yes!
Although very similar, training for size is different than strength training. The result is very different. Although, when training very hard, you will get bigger and stronger, you can focus on one rather than the other, depending on your goals. And that is what Mike Israetel is here to elucidate.
Dr. Mike Israetel has a PhD in sports physiology and co-founded Renaissance Periodization, a YouTube channel focused on hypertrophy. He is the person many bodybuilders turn to when building muscle.
He shared a few key differences between training for size and strength. Check it out.
Differences Between Training for Size and Strength
Here are the differences between training for size and strength, depending on each category.
Loading
3-6 rep range for strength
5-30 rep range for hypertrophy
Volume
Regarding volume, what are the differences between training for size and strength?
> Strength training is more fatiguing per set. Hypertrophy training does not require as high preparedness, meaning just grinding through is fine.
Training for size sees higher stimuli with higher volumes than strength training does.
In reality, you cannot do both training for strength and size optimally simultaneously.
Progression
This is where the differences between training for size and strength are more significant.
When training for strength, progression in load is everything, while volume is not a must-do.
However, load progression works very well for hypertrophy purposes, but the other shouldn’t be discarded either.
In simpler terms, if you ask yourself if you should add 15lbs to the barbell and no extra sets or add 5 pounds to the bar and 1 set next week, the answer is:
For strength, add 15 pounds
For stability, add an extra set and increase the weight load slightly
Frequency
Israetel explains that you primarily need local muscles to heal between sessions for hypertrophy gains. For strength purposes, stimulative sessions need fuller recovery.
Exercise Selection
What about exercise selection? What are the differences between training for size and strength?
Strength training is defined by exercises, usually training the movement or similar ones and other activities. Think of “increasing my squat” instead of “stronger legs.” Training is not ideal for single-joint and machine movements.
Training for size thrives on intra-week exercise variation. Barbell squats alone will not get you more giant legs; you must add variation. In the same way, walking lunges won’t give you a strength boost but will help you get more giant legs.
How to Get The Best of Both
Is it possible to try and reap the benefits of training for size and strength? You definitely can.
Choose more compound movements, free-weight exercises
Perform 3-6 rep range strength-focused exercises at the beginning of your sessions
Do accessories that are still as beneficial as possible in the 6–12 rep range later in the session
Begin at your hypertrophy’s minimum practical volume and stay close to it
Progress is mostly in load week, rarely on set numbers
Ideally, you should phase-potentially do 2-3 mesos of hypertrophy training (6–15 rep range), 2-3 mesos of strength training (3-6 rep range), and take a 1-3 week active recovery phase after the phase. Then repeat.
And that was Dr. Mike Israetel’s explanation about the differences between training for size and strength. For more in-depth clarification, click on the video below.
#Strength#muscle and strength#muscle and strength workouts#muscle and fitness hers#muscle strength examples#muscle and strength coupons#muscle and fitness hers cover contest 2023#muscle and fitness vince McMahon#muscle and strength supplements#muscle strength is#muscle and strength dumbbell workout#muscle and strength full body workout#muscle and strength pyramid#muscle strength exercises examples#muscle and strength ab workout#muscle strength exercises at home#muscle and strength promo code#muscle and strength app
0 notes
Text
May 3 Reflection
Oh man.
This is late because I spent the hours after working out to study with Daddy Brandon Pham last night for our stats midterm at 8:00am.
Yesterday, things started looking up. I was no longer in over my own head so I was only 83.33% depressed. I started the day with some The Study. Got a pretty mid latte yet again (I guess the study just doesn't have good drinks) but I walked to Kerckhoff with Trinity and sat in the patio with her and Jake until 10:00. I went to ochem (ugh) and learned about diastereomers and meso compounds. Then I got 12/16 on an ONLINE TEST because I told the group I was fine with being the guinea pig for to check our score. I shouldn't have done that because it ruined the rest of my day. But then I went to stats and chem discussion and learned a lot. Then I went to the Study again to meet up with big boy Jayden Arevalo. Then I ate with Karis. For two hours. It was fun.
Then I worked out and saw friends and that brightened my day even more. Then I stayed up till 3 and almost missed my alarm this morning so that was great. But we're still alive so it's cool.
1 note
·
View note
Text
Preprints | Hegemonic Design Bias in Massive Open Online Courses (MOOCs): A Conceptual Framework for Why MOOCs Struggle to Democratise Learning
See on Scoop.it - Education 2.0 & 3.0
Hegemonic design bias describes a series of processes, constraints, and biases that optimise MOOC production toward the already well-educated. At the macro level, the relative importance of knowledge production compared to knowledge dissemination among elite institutions of higher education, the tendency for this focus to produce extremely exclusionary admissions standards, and elitist mimicry resulting in institutional isomorphism all influence the design of MOOCs. At the meso level, a process termed ‘early-adopter iteration bias’ skews this design further; through this process, well-educated users make up most MOOC participants, producing the data that researchers and practitioners analyse to iterate and improve MOOCs. A separate but related process, termed ‘research-praxis bias,’ further prevents MOOC development from meeting the needs of underserved learners. At the micro level, a series of pedagogical, curricular, and technological design processes compound these issues further. This theory-building research exercise yields a conceptual framework for how to consider the socio-technical ecosystems producing MOOCs, which can be further refined and tested.
0 notes
Text
just astral projected to 2012 and made a post “why isnt the alina/darkling shipname SunnyD lol”
#the d is for darkling NOT dick#what possessed me to think this i hate my brain#i cant figure out how tf a meso compound works for my exam but my brain does this
16 notes
·
View notes
Text

I’ve been on spring break recently, so there wasn’t much to post.
Studying for my second exam, and I’m really struggling with enantiomers and meso compounds. Any tips? I feel like I’ve watched every video on the subject.
#organic chemistry#study aesthetic#studyspo#studywithme#aesthetic notes#gel pen#notes#study#study hard#study motivation#studyblr#studyspiration#academia#homework#isomers#enantiomer#diastereoisomer
20 notes
·
View notes
Text
MCAT notes (3)
organic chemistry
isomerism: a relationship between two molecules, in which both have the same molecular formula but differ structurally in some way as denoted below.
isomers may differ in physical and/or chemical properties due to structural disparity
physical property = unrelated to molecular composition; chemical property = alterable aspect of molecular composition/reactivity
structural (constitutional) isomers – differ in intramolecular atomic connectivity (i.e, different bond types and/or numbered order of substituents)
stereoisomers – same intramolecular atomic connectivity but different configuration (i.e., spatial position)
1. conformers – essentially 1 compound in 2+ different, rotational states
antiperiplanar / synperiplanar – denote that two groups of any type are in opposite ( ‘anti’ ) and the same ( ‘syn’ ) regions, respectively, of 1 plane ( ‘periplanar’ )
linear conformers – different rotational states of 1 single linear molecule, all of which 1) have different energy costs to remain in and 2) can interrotate to any of the others given enough energy
depicted using Newman projection between a designated viewpoint with 1 front and 1 back carbon
the more space between largest substituents on F and B, the lower energy cost, in descending order as follows:
» anti – 2 largest substituents on F and B carbon are 180º apart in Newman
» gauche – 2 largest substituents on F and B carbon are 60º apart in Newman
» eclipsed – 2 largest substituents on F and B carbon are 120º apart, meaning that they overlap with smaller substituents on respective C’s
» total eclipsed – 2 largest substituents on F and B carbon are 0º apart, meaning that they overlap with each other
cyclic conformers – different positional orientations of 1 single cyclic molecule, all of which 1) have different energy costs and 2) can flip into any one of the others given enough energy
substituents and H’s are alternatively axial (±y) and equatorial (±x)
cyclic conformers lower energy costs by reducing 1 of 3 types of strain:
» angle (distorted bond angles between bonded C’s)
» torsional (syn/eclipsed substituents on bonded C’s)
» van der Waals non-bonding (e- repulsion between proximal large substituents on non-bonded C’s)
chair = 2 almost identical and most low-energy positions
more favorable chair is that which orients larger substituents in equatorial position to avoid non-bonding strain
2. configurational isomers – in order to convert 1 isomeric molecule to the other, bonds would need to be broken
chirality: at one carbon center, two molecules have an external symmetry to reflect shared connectivity but no internal planes of symmetry across which to interrotate
optical activity: light that is filtered to go in one direction – polarized light – is rotated by a chiral molecule to some degree | standardized to 1g/mL substance in 1dm-long tube
enantiomers – two molecules are completely chiral to one another across all carbon chiral centers
racemic mixture – equal volumes of an enantiomeric pair that cancel each other’s optical activity out
diastereomers – two molecules are chiral to one another across 1+, but not all, carbon chiral centers
cis-trans isomers – two molecules with a single non-rotatable double bond have the same substituents on different sides of C=C
meso compounds – two molecules have chirality but the overall molecule exhibits an internal plane of symmetry and destroys optical activity
intramolecular substituent relationships – describing substituent positions in relation to one another
levels of configuration – spatial arrangement of atoms/substituents at a particular isomeric chiral carbon center
relative configuration: chirality relationship between two molecules, described via isomerism
absolute configuration: spatial arrangement of intramolecular components with regards to one another, described below
Cahn-Ingold-Prelog (CIP) order – determine substituent positional precedence via upward atomic number comparison, carbon atom by carbon atom extending out from chiral center or double bond
E-Z forms – CIP-determined largest substituents = on same side (Z) or opposite side (E) of a double bond
E-Z naming: place letter in parentheses and separate from everything else via hyphen, i.e., ‘(Z)-2-chloro-2-pentene’
R-S forms – CIP-determined descending substituent order goes clockwise to the right (R) or counterclockwise to the left (S) when the least-priority group is at the back (dash |||||) position
R-S formatting: to get least-priority group to the back, either visually re-configure the chiral center – or switch least-priority group with back group and reverse the apparent R/S designation
R-S naming: place letter(s) in parentheses, separating by comma and ordering by carbon number of chiral center if multiple, and hyphenate to separate from everything else
Fischer projection – drawing method in which vertical lines form carbon chain going into the page (dash |||||) and horizontal lines form wedge substituents
Fischer configuration to obtain R-S designation: determine apparent order as is and reverse apparent R/S designation
or switch least group once to front or back vertical position and reverse apparent R/S designation
or switch entire molecule by 180º until least group is at top or bottom
to move substituents in general without changing chirality, hold one group and rotate the other three
1 note
·
View note
Text
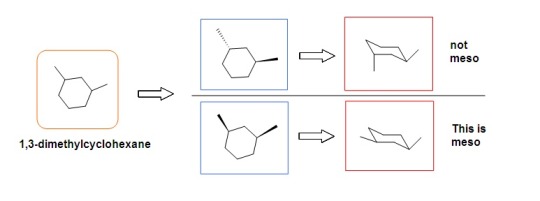
Meso compounds
Compounds that have a chiral center but are nevertheless considered achiral because they contain a symmetry plane.
1 note
·
View note
Text
How To Make Drug Discovery Successful With In Vitro Toxicity Testing Methods?

Serious concerns about animal testing for drug discovery and testing have opened the way for the reduction or replacement of animal testing with an effective in vitro toxicity testing.
What is In Vitro Toxicity Testing?
Regulatory agencies around the globe are accepting in vitro data as a replacement for animal testing studies. To predict the toxic properties of compounds and mixtures, In Vitro Toxicity Testing uses cells or tissues maintained or grown in controlled laboratory conditions.
With cost-effective as well as time saving benefits, In Vitro Toxicity Testing offers you a quick assessment about your drug safety. For these reasons, drug developers nowadays are more inclined towards In Vitro Toxicology screening of their drug.
The crucial part that plays a major role in drug safety profiles is – toxicity assessment. Yes, the assessment of your drug toxicity is the main reason for drug development termination and withdraw from the market. Thus, an early stage assessment of the toxicity level of your candidate drug is vital to save valuable time and cost.
Nowadays, there are many fast, affordable and highly efficient In Vitro assays for assessing drug safety risks recommended by most regulatory authorities.
Selecting a Contract Research Organization (CRO) to perform In Vitro Toxicity Testing
In vitro toxicity testing assays bring a number of technical benefits to the traditional method of testing substances on animal models. These benefits include: the capacity to clear cellular-response networks and toxicity pathways; the utilization of concentrations relative to human exposure; and enabling high-throughput studies. So, choose world-class In Vitro Toxicity Testing with a well-established CRO – IONTOX.
IONTOX is highly specialized in vitro methods following both GCCP GIVIMP guidelines. IONTOX in vitro toxicity testing service includes cytotoxicity screens, organ specific toxicity assessments and 3D tissue models for OECD compliant irritation studies. To assess systemic toxicity, a meso-scale, human based, flexible HuDMOP platform is used.
Cytotoxicity screening is done to predict compound viability early in the preclinical stage of drug development or new product discovery.
Cytotoxicity screens range from multi-endpoint models to standard cell viability screens (ATP) to order compounds or mitochondrial toxicity predictions for companies wanting to comprehend how their compound affects mitochondrial health.
All 3D testing-ocular irritation, dermal irritation and dermal corrosion assays are run in accordance with OECD Guidelines.
To know how a compound may affect the health of a particular organ, organ specific toxicity testing is performed. Pharmaceutical, chemical, and cosmetic businesses who manufacture products for topical use not only have to ensure the safety of a product, but they are also often needed to do these safety studies without animal testing. Organ specific toxicity testing is highly predictive of the real effects of these products on human skin.
For more information regarding innovative In Vitro Toxicity Testing, visit https://www.iontox.com/in-vitro-toxicity-testing/ now.
1 note
·
View note