#methylcyclohexanol
Explore tagged Tumblr posts
Text
Again, 2-methylcyclohexanol has two stereocentres and exists as 2² = 4 stereoisomers, with the cis isomer as one pair of enantiomers and the trans isomer as a second pair:

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
2 notes
·
View notes
Text

The bottle of conditioner that I was using ran out but there was another in the shower. It was a herbal conditioner with green tea and mint in it so it smelled like that when I put it on. But when I washed it off, dear my. I could feel the menthol in every single hair of mine, menthol dripping through my face, across my pores, it was like being combusted by a minty refreshing little hell. It was weird the feeling of such against the hot water. Everything smelled like ointment.
"You gotta try it." I told my sister as I narrated my experience, but no, she went to pick up the other conditioner bottle we still have in another part of our house.
Is menthol an alcohol cuz of that suffix at the end? Oh yeah, it is! It's 2-Isopropyl-5-methylcyclohexanol.
3 notes
·
View notes
Note
2 with beau? ❤️

Happy almost end of blurb weekend!
Tagging: @besthockeyfics @glassdanse @calgarycanuck @stlbluesbrat21 @dembenchboys @nhlboyshavemyhart88 @stars-canucks @gotpucks @beauvibaby
2.“I won’t let you do this alone.”
Warning: contains science
Word Count: 557
____________________________
You let out a groan so loud it was practically a scream. Nothing was more frustrating to you than studying for an exam, something that you told yourself you would never do again once you graduated college. Yet, there you were, sitting at the kitchen table, flashcards, blank pieces of computer paper, three open textbooks, and upwards of 100 colored pens scattered around you and your computer, studying for an exam.
“I like those groans better when they come from the bedroom,” Anthony says, poking his head into the kitchen. “They’re more fun when you make them because you’re happy.”
He comes up behind you, rubbing your shoulders in an attempt to relax you. “Why the fuck did I think it was a good idea to get a master’s degree?”
“Because you'll have more knowledge and make more money.”
“Ok, but why the fuck did I think it was a good idea to get one in chemistry of all subjects? Who likes chemistry?”
“You do. You have piles of notes next to our bed and you read them for fun before we go to sleep each night.”
“Fuck, I do, don’t I,” you groan, putting your head down on the table. “This is the worst idea I’ve ever had.”
“Hey, you’re going to be fine,” your boyfriend says, sitting down next to you and grabbing a stack of your flashcards. “I’m sure you know more than you think. I’ll quiz you.”
“Are you even going to know if I’m right?”
“If you made the flashcards right, then I’m sure I can figure it out,” he says, tapping them on the table to get them all even, flipping through the first few of them. “I’m not going to let you do this alone.”
“Fine,” you say begrudgingly, not sure this was even going to work.
“Alright, what are the two ways to prepare an alcohol from 1-methyl-1-cyclohexene?” he starts, struggling through the name of the compound. “This isn’t English.”
“You’re right, it’s organic chemistry. It’s a whole other language.”
“Well, what are they, though?”
You groan, knowing that he was going to have no idea what you were about to tell him. “The first is hydroboration, which is oxidation, and it’s syn-addition, and follows an anti-Markovnikov hydration. So the BH3 attacks the cyclohexene, and one hydrogen adds on to the same carbon that has the methyl group attached to it, while the BH2 attacks to the other carbon involved in the double bond, trans to the methyl, forming the intermediate. Then hydrogen peroxide attacks and the hydroxide replaces the boron group and yields trans-2-methylcyclohexanol,” you rant, Anthony nodding along as you explain to him the second one.
“Yes, fuck, babe, you got it perfectly!” Anthony yells, getting up to kiss you. “How about, every question you get right, you get a reward from me?”
“I like incentives, but it depends on what they are,” you tell him, giving him another kiss before he sits back down.
“Every question you get right, is one night where you have complete control in the bedroom. Anything you want, I will do, no questions asked,” he offers, taking one of the unused sheets of printer paper to keep tally.
“I like the sound of that,” you tell him. “What if I get it wrong?”
“I have control.”
“Deal. Ask me another question.”
#tito beauvillier#tito beauvillier imagines#anthony beauvillier#anthony beauvillier imagines#blurb weekend 521#new york islanders#new york islanders imagines
64 notes
·
View notes
Text
Cyclohexane For the following molecule: 3-bromo-5-methylcyclohexanol 1. Draw the ring structure. 2. Determine if it is an R- or a 5- structure
Cyclohexane For the following molecule: 3-bromo-5-methylcyclohexanol 1. Draw the ring structure. 2. Determine if it is an R- or a 5- structure
Orgnie Chemistry 23/ TR 2-320 Organic Chemistry Nomenclature Test Part 1: Name the following molecules: 3-methyi-1-propylnerane 1-propyl-cye lopropy oplopentane 2,5-dimethyl-3 propylhex-2-ene 1. 2. 3. 4-e thy-4propyl- 77-dichl oro-333- 4. ime thulhept-3-une 5. 6. Part6: Cyclohexane For the following molecule: 3-bromo-5-methylcyclohexanol 1. Draw the ring structure. 2. Determine if it is an R- or…

View On WordPress
0 notes
Text
REACTION PROCEDURE
REACTION PROCEDURE 5.0 ml of 2-methylcyclohexanol was placed in a 50 ml round bottom flask. Phosphoric acid (85%, 5 ml) was added cautiously, and the mixture swirled. Boiling stones were added to prevent bumping during heating. The mixture was heated to reflux temperature and refluxed for 15 minutes. The products were collected between 85-90 ˚C, in a small round bottom flask, cooled in ice water.…
View On WordPress
0 notes
Photo

TEST 5 - Please disregard. (Forwarding an existing G+ post, with text of mine) This reaction can be traced back to Debus’s transformation of hydrocyanic acid into methylamine over platinum in 1863, De Wilde’s hydrogenation of acetylene into ethylene and ethane in 1874,1 and Mond’s extensive work from 1890 to 1895.2 However, it was in 1899 that Sabatier and Senderens established nickel-based hydrogenation3 and converted the unsaturated organic molecules (e.g., ketones, aldehydes, alkenes and aromatics) into corresponding saturated compounds (i.e., alcohols, hydrocarbons) by passing the vapor of organic molecules and hydrogen over hot, finely divided nickel. This nickel-based vapor phase hydrogenation became one of the most practically useful reactions and won Sabatier the Nobel Prize in 1912.1 It is generally known as the Sabatier-Senderens reduction.4 This reaction is different from reductions using nascent hydrogen as the reducing agent, such as amalgam of sodium in alcohol (alkaline condition) and or zinc or tin with hydrochloric acid (acidic medium). In this reaction, the purity of nickel and reaction temperature are found to be critical for successful hydrogenation.1 For example, trace amounts of sulfur, bromine, or iodine will deactivate the nickel catalyst; in addition, it has been found that each hydrogenation process takes place only within a well-defined temperature range,1 as evidenced by the hydrogenation of benzene to cyclohexane at temperatures ranging from 70◦ to 190◦C, with an optimal temperature between 170◦ and 190◦C, and the further reduction of benzene to methane accompanied by the deposited carbon on nickel at temperatures > 300◦C.5 Under the correct hydrogenation conditions, Sabatier et al. successfully converted oleic acid into stearic acid, acetone to isopropanol, carbon monoxide into methane or a gaseous mixture rich in methane, phenol and p-cresol into cyclohexanol and p-methylcyclohexanol, benzene to cyclohexane, and naphthalene to tetralin, etc. (END) - How Do You Make Decisions, Exactly? The subtext of that question is "why is it so hard to make the right decision?". There is an answer to it. Dive in: https://goo.gl/gvCvSS. -- Carlos Alberto Teixeira (https://goo.gl/Kmpo9G) via David Amerland (https://goo.gl/GH9cwS)
0 notes
Text
Similarly, 3-methylcyclohexanol contains two stereocentres and exists as 2² = 4 stereoisomers, with the cis isomer as one pair of enantiomers and the trans isomer as a second pair:

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
2 notes
·
View notes
Text
Firstly, 4-methylcyclohexanol can exist as two stereoisomers – a pair of cis-trans isomers. Both the cis and trans isomers are meso compounds and are achiral.
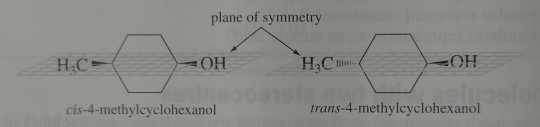
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#cis#trans#meso compound#chirality#achiral#isomers#methylcyclohexanol#symmetry
0 notes
Link
TEST 3 - Please disregard. (Link and text) This reaction can be traced back to Debus’s transformation of hydrocyanic acid into methylamine over platinum in 1863, De Wilde’s hydrogenation of acetylene into ethylene and ethane in 1874,1 and Mond’s extensive work from 1890 to 1895.2 However, it was in 1899 that Sabatier and Senderens established nickel-based hydrogenation3 and converted the unsaturated organic molecules (e.g., ketones, aldehydes, alkenes and aromatics) into corresponding saturated compounds (i.e., alcohols, hydrocarbons) by passing the vapor of organic molecules and hydrogen over hot, finely divided nickel. This nickel-based vapor phase hydrogenation became one of the most practically useful reactions and won Sabatier the Nobel Prize in 1912.1 It is generally known as the Sabatier-Senderens reduction.4 This reaction is different from reductions using nascent hydrogen as the reducing agent, such as amalgam of sodium in alcohol (alkaline condition) and or zinc or tin with hydrochloric acid (acidic medium). In this reaction, the purity of nickel and reaction temperature are found to be critical for successful hydrogenation.1 For example, trace amounts of sulfur, bromine, or iodine will deactivate the nickel catalyst; in addition, it has been found that each hydrogenation process takes place only within a well-defined temperature range,1 as evidenced by the hydrogenation of benzene to cyclohexane at temperatures ranging from 70◦ to 190◦C, with an optimal temperature between 170◦ and 190◦C, and the further reduction of benzene to methane accompanied by the deposited carbon on nickel at temperatures > 300◦C.5 Under the correct hydrogenation conditions, Sabatier et al. successfully converted oleic acid into stearic acid, acetone to isopropanol, carbon monoxide into methane or a gaseous mixture rich in methane, phenol and p-cresol into cyclohexanol and p-methylcyclohexanol, benzene to cyclohexane, and naphthalene to tetralin, etc. http://www.latimes.com/local/lanow/la-me-ln-bruce-paddock-20171025-htmlstory.html
0 notes