#intracellular tumour
Explore tagged Tumblr posts
Text
How does the Avastin injection (bevacizumab) suppress intracellular tumour growth?

Avastin, known scientifically as bevacizumab, is a pivotal drug in oncology. It is known for its efficacy in suppressing intracellular tumour growth through targeted inhibition of vascular endothelial growth factor (VEGF). This blog explores the profound impact of Avastin across various cancer types, detailing its mechanism of action, clinical applications, safety profile, and future directions in cancer treatment.
What does Avastin do to cancer patients?
Avastin works by specifically binding to vascular endothelial growth factor (VEGF), a protein that stimulates the growth of new blood vessels necessary for tumour progression. Here is everything you need to know:
Inhibits Tumor Growth: Avastin (bevacizumab) works by precisely targeting and inhibiting vascular endothelial growth factor (VEGF). This protein is crucial for the formation of new blood vessels that tumours need to grow. This precise mechanism of action is what makes Avastin so effective. By blocking VEGF, Avastin reduces the blood supply to tumours, thereby slowing down their growth and potentially shrinking them.
Enhances Treatment Effectiveness: When used in combination with chemotherapy or other cancer treatments, Avastin enhances their effectiveness. By reducing the blood flow to tumours, Avastin helps other treatments penetrate tumours more effectively, improving overall treatment outcomes.
Delays Disease Progression: Avastin is known to prolong the time before cancer progresses. In clinical trials, it has been shown to increase progression-free survival rates in various types of cancers, including colorectal, lung, breast, and kidney cancers, as well as glioblastoma.
Improves Quality of Life: For many cancer patients, Avastin not only slows disease progression but also improves the quality of life by reducing symptoms associated with advanced cancer, such as pain and discomfort caused by tumour growth.
Potential Side Effects: While generally well-tolerated, Avastin can cause side effects such as hypertension, proteinuria (excess protein in the urine), bleeding, gastrointestinal perforation, and impaired wound healing. Close monitoring by healthcare providers is essential to manage these risks effectively during treatment.
What types of cancer is Avastin used for?
In clinical settings, Avastin 100mg injection is prescribed to patients with advanced stages of cancer, including colorectal, lung, breast, and kidney cancers, among others. Its effectiveness lies in its ability to disrupt the tumour's blood supply, thereby shrinking tumours and preventing their progression.
Colorectal Cancer: Avastin is approved for use in combination with chemotherapy for metastatic colorectal cancer. It helps to slow down tumour growth and improve survival rates.
Lung Cancer: In non-small cell lung cancer (NSCLC), Avastin is used as a first-line treatment in combination with chemotherapy. It has been shown to extend survival and delay disease progression.
Breast Cancer: Avastin may be used in combination with chemotherapy for metastatic HER2-negative breast cancer. It helps to reduce blood flow to tumours, potentially shrinking them and improving treatment outcomes.
Kidney Cancer: Avastin is utilised for advanced renal cell carcinoma (kidney cancer), often in combination with other targeted therapies or immunotherapy agents. It targets VEGF, which is crucial for tumour blood vessel growth.
Does Avastin have side effects?
Avastin has demonstrated significant benefits for cancer patients, especially those in advanced stages of the disease. It notably improves both progression-free survival and overall survival rates by targeting vascular endothelial growth factor (VEGF), a protein crucial for tumour blood vessel formation.
While Avastin is generally well-tolerated, it can cause several potential side effects that require careful monitoring. Common side effects include
Hypertension (high blood pressure)
Proteinuria (excess protein in the urine)
Bleeding tendencies
Gastrointestinal perforation (a rare but serious complication)
Impaired wound healing
How do patients respond to Avastin treatment?
While Avastin is generally well-tolerated, it can cause several potential side effects that require careful monitoring. Common side effects include hypertension (high blood pressure), proteinuria (excess protein in the urine), bleeding tendencies, gastrointestinal perforation (a rare but serious complication), and impaired wound healing. Patients undergoing Avastin treatment should be closely monitored for these side effects, and healthcare providers may adjust treatment regimens as needed to manage these risks effectively.
#Avastin#Avastin injection#bevacizumab#Avastin treatment#oncology#intracellular tumour#Colorectal Cancer#Lung Cancer#Breast Cancer#Kidney Cancer#Avastin side effects#cancer patients#non-small cell lung cancer#cancer treatment
0 notes
Text
Abstract
Therapeutic applications of synthetic mRNA were proposed more than 30 years ago, and are currently the basis of one of the vaccine platforms used at a massive scale as part of the public health strategy to get COVID-19 under control. To date, there are no published studies on the biodistribution, cellular uptake, endosomal escape, translation rates, functional half-life and inactivation kinetics of synthetic mRNA, rates and duration of vaccine-induced antigen expression in different cell types. Furthermore, despite the assumption that there is no possibility of genomic integration of therapeutic synthetic mRNA, only one recent study has examined interactions between vaccine mRNA and the genome of transfected cells, and reported that an endogenous retrotransposon, LINE-1 is unsilenced following mRNA entry to the cell, leading to reverse transcription of full length vaccine mRNA sequences, and nuclear entry. This finding should be a major safety concern, given the possibility of synthetic mRNA-driven epigenetic and genomic modifications arising. We propose that in susceptible individuals, cytosolic clearance of nucleotide modified synthetic (nms-mRNAs) is impeded. Sustained presence of nms-mRNA in the cytoplasm deregulates and activates endogenous transposable elements (TEs), causing some of the mRNA copies to be reverse transcribed. The cytosolic accumulation of the nms-mRNA and the reverse transcribed cDNA molecules activates RNA and DNA sensory pathways. Their concurrent activation initiates a synchronized innate response against non-self nucleic acids, prompting type-I interferon and pro-inflammatory cytokine production which, if unregulated, leads to autoinflammatory and autoimmune conditions, while activated TEs increase the risk of insertional mutagenesis of the reverse transcribed molecules, which can disrupt coding regions, enhance the risk of mutations in tumour suppressor genes, and lead to sustained DNA damage. Susceptible individuals would then expectedly have an increased risk of DNA damage, chronic autoinflammation, autoimmunity and cancer. In light of the current mass administration of nms-mRNA vaccines, it is essential and urgent to fully understand the intracellular cascades initiated by cellular uptake of synthetic mRNA and the consequences of these molecular events.
4 notes
·
View notes
Text
Medical innovations and scientific advances at Harvard Medical School through the decades (Part 1 of 2)
1799 Smallpox vaccine
1843 Puerperal fever
1846 Anaesthesia
1886 Appendicitis
1890s–1910 Insect-borne disease transmission; scurvy; heat-killed vaccines
1914 Electrocardiograph
1922 Insulin; founding of Joslin Diabetes Center
1923 Heart valve surgery
1925 Three-flanged nail
1927 Iron lung; syphilis test
1929 First polio patient saved
1930s–1940s Overactive thyroid; rickets; osteoporosis
1933 Intracellular fluid and electrolytes
1938 Corrective heart surgery for children
1942 Burn treatment; blood bank; emergency-response plans
1945 Pap smear
1946 Rh disease
1947 Artificial kidney; pediatric remission of acute leukemia
1948 Stenotic mitral heart valves
1949 Vaccine culture technique; cortisone; White classification
1950 Wilms tumour
1951 Brain proteolipids
1952 Kidney transplant
1954 Oral contraceptives
1957 Brain structures
1960 Platelets; proton beam therapy; implantable cardiac pacemaker
1962 Human limb reattachment; heart rhythm restoration
1964 Human blood storage
1965 Pan-retinal coagulation
1968 Telemedicine
1969 Intra-aortic balloon catheter
1970 Human oncogene; positron emission tomography (PET) scan
1973 Non-invasive fetal heart monitoring
1974 Photochemotherapy
1976 Insulin resistance receptors
1977 Virus particle structure
1978 Prenatal DNA sequencing
1979 Magnetic resonance imaging (MRI)
1980s HIV/AIDS
1981 Artificial skin; premature puberty reversal
1982 Discovery of telomeres
1983 Congenital birthmark treatment; Huntington's disease
1984 Oncomouse; skin replacement; TIMI
1986 Kawasaki disease retrovirus; Alzheimer's disease
1987 Early-onset Alzheimer's gene; Duchenne muscular dystrophy gene
1988 Laser tattoo removal
1989 Tumour blood vessel growth compound
1990s Proteasome-inhibiting cancer therapy
1992 Diphtheria toxin structure; amyloid beta
1993 Neovascular macular degeneration; microRNAs; paralyzed vocal cord surgical method; colon cancer gene; VEGF molecule
1994 PR-39 molecule
1 note
·
View note
Text
mTOR
"Under nutrient-rich conditions mTORC1 promotes cell growth by stimulating biosynthetic pathways, including synthesis of proteins, lipids and nucleotides, and by inhibiting cellular catabolism through repression of the autophagic pathway.
"The convergence point of anabolic and catabolic processes is the mechanistic target of rapamycin (mTOR), which senses fluctuations in extracellular and intracellular nutrients to modulate cellular growth, metabolism and survival.
"Cellular demands for amino acids are cell- and tissue-specific; while glutamine is an important energy source feeding the tricarboxylic acid (TCA) cycle particularly in glycolysis-dependent tumour cells [41], arginine influx and arginase levels are tightly regulated in liver for the urea cycle [42].
"Leucine, glutamine and arginine have been widely shown as the main contributors to mTORC1 activation [43–47]. In particular, glutamine, in addition to serving as a key energy source, cooperates with leucine to activate mTORC1. Glutamine can be deaminated to produce α-ketoglutarate, which is both an intermediate in the TCA cycle and a regulator of mTORC1 activity and autophagy. However, this reaction requires the presence of leucine, which acts as a cofactor of the enzyme that catalyses the last step of glutaminolysis [48].
"It is now clear that cellular homoeostasis of metabolism and growth is exquisitely controlled by the coordination of AMPK and mTORC1. AMPK is crucial to the cellular response to low energy levels and, once active, drives processes which will replenish cellular energy stores, such as autophagy while inhibiting biogenic synthesis [116]. Opposite to AMPK, activated mTORC1 shifts the metabolic programme of the cell from catabolic to anabolic metabolism by up-regulating the synthesis of building blocks for cell proliferation, including proteins, lipids and nucleotides [117].
"Whereas the role of amino acids as key signal factors in the activation of mTORC1 has been accepted, to date, the inhibitory effect of amino acids on AMPK has been a debatable issue. Some studies have observed the inhibition of AMPK by amino acids [124–126], though this has not always been the case [127,128]. A recent work suggests amino acids as novel metabolic activators of AMPK. In this study, AMPK responded acutely and within 1 min to amino acid and its activation was independent of mTORC1 [129]. ... Furthermore, they demonstrate that AMPK activation occurs via the Ca2+/calmodulin-dependent kinase kinase-β (CaMKKβ) and that activation of the CaMKKβ–AMPK axis by amino acids does not inhibit mTORC1 but sustains autophagy."

0 notes
Text
THE EXCELLENT RANGE OF LECTIN CONJUGATES AND THEIR APPLICATION
WHAT ARE LECTIN CONJUGATES?
Proteins (building blocks) that attach to cells and specific carbohydrate groups on proteins or cell membrane proteins are known as lectin conjugates. They are further classified according to their amino acid sequences and biological characteristics. Lectins contain 120 amino acids that are involved in carbohydrate binding.
Because of its carbohydrate binding, lectin is employed in glycobiology to analyse cell surface glycoproteins. Lectins are synthesised in laboratories after being extracted from plant or animal components.
The capacity of Lectins to form precipitates with glycoconjugates is due to the fact that most lectin proteins are composed of non-covalently linked subunits. Agglutination of cells by Lectins is uncommon in nature and thus extremely difficult to detect.
Lectins enable scientists to investigate a wide range of biological structures and functions. Some Lectins bind to mannose or glucose residues, while others bind only to galactose residues due to their complex binding requirements. Some Lectins also require sugar-binding at specific oligosaccharide sites.
LECTINS APPLICATION
Lectins are being used in clinical laboratories to type blood cells. There is the extensive usage of Lectin in specialist applications such as-
• As chemotherapeutic agents
• In fractionation of animal cells as mitogens.
• While investigating cellular surfaces
• Lectins isolate specific cells or viruses with a mixture and study determined processes amongst several.
LECTIN IN ANIMALS
Regulate cell adhesion
• Glycoprotein synthesis is regulated by Lectins
• They can also regulate blood protein levels.
• Recognition of galactose residues on the surface of mammalian liver cells responds better to Lectins.
LECTIN IN PLANTS
Plants are naturally rich in lectins. Dietary lectins are found in protein sources such as beans and legumes, peanuts, lentils, wheat, uncooked kidney beans, fruits, and vegetables. Conversely, lectin-free diet items include pasture-raised meats, cooked sweet potatoes, cruciferous vegetables, asparagus, garlic, and onion.
Lectin activity and function in plants are both unknown. The content of Lectin in plant seeds decreases as they mature. Plant Lectin has the ability to recognise hydrophobic noncarbohydrate ligands.
Adenine, auxins, cytokinin, indole-3-acetic acid, and water-soluble porphyrins are examples. Because these compounds behave as phytohormones, their interactions may be psychologically significant.
Furthermore, the plasma membranes of human EL4 tumour cells are labelled with horseradish peroxidase-conjugated wheat-germ agglutinin. After the labelled intact cells are disrupted, plasma-membrane refinement is observed by ultrastructural examination of the various fractions for positive effect product on the membrane vesicles.
LECTINS AND OTHER CARBOHYDRATE-BINDING PROTEINS
Cellular proteoglycans, glycoproteins, and glycolipids include a wide range of oligosaccharides. Fluorescent carbohydrate-binding derivativesMicroscopy PROTEINS and flow cytometry both use proteins to identify intracellular glycoconjugates. This is done to isolate glycoproteins on protein blots and cause agglutination of specific cell types. Lectins can also be used to detect cancer since they have changed surface glycoproteins.
LECTINS INTERCONNECTING

Biotechnology has narrowed down biorecognition molecules with diagnostic potentials in light of the different diseases that impact the human species. Particular Lectin content binds with mono- or oligosaccharides with high affinity via no covalent connection via hydrogen bonds.
Lectins from viruses, bacteria, fungi, algae, mammals, and plants recognise carbohydrates in cells, tissue sections, and biological fluids. These are useful tools for diagnostic purposes. To find medicines and inhibitors, sialic acid-specific lectins such as Influenza Virus Hemagglutinin are being studied. These can remove or inhibit sialic acid in host cells, preventing it from binding.
Similarly, strong anti-HIV activity in vitro has been associated with bacterial Lectins. Large levels of algal Lectins are attracting interest for biomedical uses such as anti-HIV, anti-inflammatory, antibacterial, and antinociceptive properties. Animal Lectins are important in psychological processes such metastatic cancer, apoptotic pathways, and immunomodulation.
LECTIN INDUCED MECHANISMS OF INFLAMMATION RESPONSES
Immune systems act in two specific ways called; innate and adaptive responses. These responses are activated by a group of cells and molecules that promote the destruction of aggressive agents. Neutrophils, eosinophils, basophils, and mono/macrophages can generate and release molecules called cytokines.
These molecules modulate the activation of immune cells, inflammation, and humoral responses. Biomolecules like these are the answer for adjustment of immune conditions and therapeutic applications in regards to immune response-related diseases.
Lectins are thought to contribute to the development of diseases such as celiac disease, autoimmune diseases, rheumatoid arthritis, obesity, cardiovascular disease, and type 2 diabetes. This happens through translocation across the intestinal barrier and activation of the adaptive immune system. Common high-lectin foods include grains, legumes, and nightshades.
Lectins aren’t digestible. They bind to cell membranes lining the digestive tract, where they may disrupt metabolism and cause damage. Lectin sensitivity is the body’s delayed immune response that can occur some hours to even days after these foods are consumed.
Symptoms associated with lectin sensitivities include:
•Bloating and abdominal cramps
•Painful or swollen joints
•Tiredness
•Skin problems
•Hormonal fluctuations
•Nausea
•Allergies or allergy-like symptoms
•Neurological symptoms
The highest concentrations of lectins are found in healthy foods like legumes, grains, and nightshade vegetables. Fortunately, there are ways to reduce the lectin content of these healthy foods to make them safe to eat.
Research studies have shown that by cooking, sprouting, or fermenting foods high in lectins, their lectin content can easily be reduced to negligible amounts.
Foods That Are High in Lectins
1. Red kidney beans
Raw kidney beans contain high levels of a lectin called phytohaemagglutinin. Eating them raw or undercooked can cause severe nausea, vomiting, and diarrhea. As few as five beans can cause a response.
A hemagglutinating unit (hau) is a measure of lectin content. When in raw form, red kidney beans contain 20,000–70,000 hau. Once cooked, however, they contain only 200–400 hau, which is considered a safe level.
In cooked form, they are valuable and nutritious food.
2. Soybeans
Soybeans have several health benefits but are another food that also contains high levels of lectins.
As with red kidney beans, cooking soybeans almost eliminates their lectin content, provided they are cooked at high temperatures. Studies show that soybean lectins are almost completely deactivated when at 100°C for at least 10 minutes.
3. Wheat
Raw wheat, including wheat germ, is high in lectins, with around 300 mcg of wheat lectins per gram. (Whole-wheat flour has a much lower lectin content at about 30 mcg per gram). Lectins are almost completely eliminated by cooking and processing, and as most whole-wheat products consumed are cooked, it’s not likely that lectins pose a major risk to health.
4. Tomatoes
Tomatoes are part of the nightshade family. They are high in fiber, a good source of potassium and vitamin K1, and high in vitamin C. (One tomato provides about 20% of the daily recommended value.
Tomatoes also contain lectins, though there is little evidence that they have any adverse effects on humans. Some people have linked tomatoes and other nightshade vegetables to inflammation, such as arthritis. No formal research has supported this link.
5. Potatoes
Potatoes are also members of the nightshade family and a good source of vitamins and minerals. Potato skins are particularly high in antioxidants, such as chlorogenic acid, which has been linked to a reduced risk of heart disease and type 2 diabetes. As with tomatoes, adverse effects have been experienced by some when eating potatoes. Studies have shown that this could be linked to lectins.
6. Peanuts
Peanuts are an excellent source of protein, unsaturated fats, and many vitamins and minerals.
Peanuts do contain Lectin, and one study found that peanut lectins increased growth in cancer cells. With evidence that peanut lectins can enter the bloodstream, this has led some people to believe that lectins could increase the risk of cancer spreading in the body. However, the above study was carried out using very high doses of pure lectins placed directly onto cancer cells.
No studies have investigated their exact effects on humans. Evidence of their health benefits appears to be stronger than that of any risks.
DRUG DELIVERY USING LECTIN SOURCE OF PROTEIN
Chemical agent therapies often come across as barriers when the need for increasing dosages and action of metabolism reduces the effectiveness of treatments. Delivery of drugs to specific targets requires a new and an effective strategy to combat side effects and chemical reactions.
Lectin medicated bio adhesion constitutes specified interactions with receptor-like structures in the cell membrane, binding directly to targeted cells. Therefore Lectins can interact differently with distinct cells and act as drug carriers to desired tissue and cells. For it to be a tool in drug delivery Lectins, need to be avid binding, low toxicity, and site-specific molecules.
To conclude, Lectins from diversified sources with distinct carbohydrate recognition events play a vital role in many biotechnological applications/disease therapies. The uses in vitro and in vivo display Lectins with protective effects against viruses and microorganisms. Lectins are a highly potent modulator of an immune response, mitosis, proli9, healing, drug delivery therapies, and cancer regression.
Histochemistry, biosensors, detect diseases, and infections against glycans alterations on cells or tissue surfaces, and serum samples can be isolated using Lectin-based technology and techniques. There is potential to unravel new interpretations in the biological effects, pathways, and biotechnological potential of Lectins. They are focusing on their achievements in therapeutic applications and health effects.
Want to learn more about Lectin Conjugate, its usage, and health benefits? Contact our experts at Helvetica Health Care today!
1 note
·
View note
Text
The VEGF Molecular Signalling Pathway Mechanism Explained
By Amirali Banani
February 28, 2023
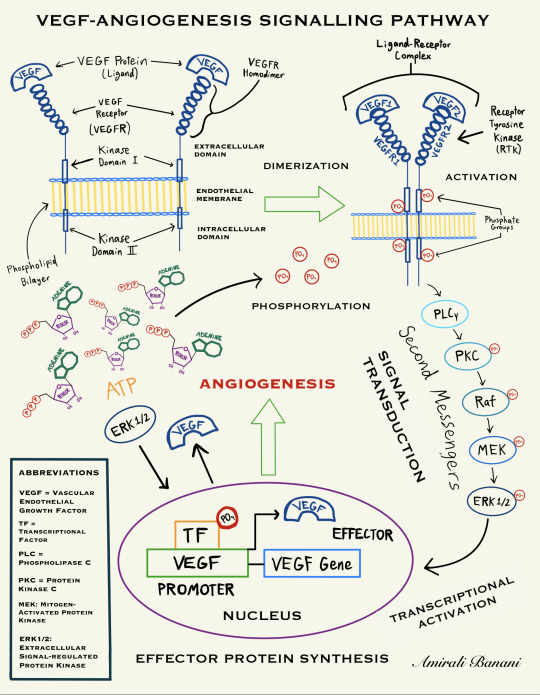
The PLC-γ-PKC-MAPK Pathway (simplified diagram sketched by Amirali Banani)
Vascular endothelial growth factor-A (VEGF-A) is essential for endothelial cell functions associated with angiogenesis (1). The signalling pathway begins with the binding of VEGF Receptors (VEGFR) to the VEGF cytokine to form a homodimer, a process known as Dimerization (2). The binding of a VEGF molecule to two VEGFR molecules induces the transphosphorylation of the intracellular domains of the receptors, leading to signal transduction (2, 3). In this section, we will be investigating the most prominent ligand-receptor complex in the VEGF System: VEGF-A/VEGFR2. VEGFR2, the major signal transducer for angiogenesis, is involved in the phospholipase C-γ-protein kinase C-mitogen-activated protein kinase (PLC-γ-PKC-MAPK) pathway, which is the pathway of interest that will be explained in this section (4). In this system, the VEGF-A molecule binds to and dimerizes VEGFR-1 and VEGFR-2, and signal transduction networks initiated by the VEGF-A/VEGFR2 complex promote angiogenesis, vascular permeability, as well as endothelial cell survival, migration, and proliferation (1,3,5). The dimerization of VEGFR-1 and VEGFR-2 as well as the transphosphorylation between the receptors’ intracellular kinase domains is followed by the activation of the VEGF Receptor Tyrosine Kinase (RTK) which induces the phosphorylation of an effector known as phospholipase C — gamma (PLC-γ) (5,6). After that, PLC-γ initiates a downstream intracellular signalling cascade that is facilitated by the sequential phosphorylation of a series of proteins called second messengers (6). The intracellular signal transduction process is regulated by several kinases of the MAPK pathway which phosphorylate and activate the downstream second messenger proteins, following the order: Ras-Raf-MEK-ERK (7,8). The Ras/Raf/MAPK (MEK)/ERK pathway is the most important signalling cascade among all MAPK signal transduction pathways and plays a paramount role in the survival and development of malignant tumour cells (7). In the last step of the PLC-γ-PKC-MAPK pathway, activated ERK 1/2 phosphorylates another kinase called RSK and both molecules travel to the nucleus of the vascular endothelial cell to activate multiple transcription factors which leads to effector protein synthesis, thus promoting cell proliferation and survival, and ultimately resulting in angiogenesis (9). Therefore, it can be observed through the PLC-γ-PKC-MAPK pathway that the hyperactivation of the expression of ERK 1/2 plays a crucial role in the development and progression of cancer through the promotion of rapid — and most of all aberrant — tumour neovascularization (10).
References
1. Abhinand C, Raju R, Soumya S, Arya P, Sudhakaran P. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. Journal of Cell Communication and Signaling. 2016;10(4):347–354.
2. Mac Gabhann F, Popel A. Dimerization of VEGF receptors and implications for signal transduction: A computational study. Biophysical Chemistry. 2007;128(2–3):125–139.
3. Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes & Cancer. 2011;2(12):1097–1105.
4. Shibuya M. VEGFR and Type-V RTK Activation and Signaling. Cold Spring Harbor Perspectives in Biology. 2013;5(10):a009092-a009092.
5. Koch S, Claesson-Welsh L. Signal Transduction by Vascular Endothelial Growth Factor Receptors. Cold Spring Harbor Perspectives in Medicine. 2012;2(7):a006502-a006502.
6. Lawson N. phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes & Development. 2003;17(>11):1346–1351.
7. Kowanetz M, Ferrara N. Vascular Endothelial Growth Factor Signaling Pathways: Therapeutic Perspective: Fig. 1. Clinical Cancer Research. 2006;12(17):5018–5022.
8. Li L, Zhao G, Shi Z, Qi L, Zhou L, Fu Z. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncology Letters. 2016;12(5):3045–3050.
9. Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer?. Cell Cycle. 2009;8(8):1168–1175.
10. Guo Y, Pan W, Liu S, Shen Z, Xu Y, Hu L. ERK/MAPK signalling pathway and tumorigenesis (Review). Experimental and Therapeutic Medicine. 2020;.
#molecular biology#oncology#research#medicine#cell signaling#clinical medicine#therapeutics#experimental#cytology
0 notes
Text
i think my intuition that blood cells are self is quite typical in that when people are cut they think "i'm bleeding!" and not "my pets are escaping!". i think if they have an autoimmune disease, they think "my body is turning against itself", not "i am actaeon, pursued by my own hounds".
i also think people are genepilled enough to believe in "my dna" and to think that if a cell has "my dna" then it is "me" -- and white blood cells certainly have this. i don't think people generally are aware of somatic recombination in B and T cells and to the extent they are, I don't think they think it results in non-self cells. certainly people seem to think gametes are self, and sperm are a much less central example of "me" cells.
i don't think white blood cells are particularly self-minded. once you account for the fact they exist loosely coupled in the tissue of the blood -- and that blood is a tissue and not empty space like in a a bad immunology cartoon -- rather than tightly connected in the intracellular matrix, white blood cells don't seem particularly independent. heart cells pulse all by themselves, all kinds of cells respond to hormones. all kinds of cells are presenting surface proteins and doing signalling. if white blood cells are spooky and independent, then i defy you to show me a cell that isn't spooky and independent.
the strongest example of a wilful bodily action i can think of is motor control of a limb, but even there muscle cells are largely doing their own thing -- contractile strength is built up in a muscle according to the degree that the motor units (i.e. a motor neuron and its fibres) are recruited into the action. And anyone who has done any kind of weightlifting knows that motor unit recruitment is not done by consciously asking each and every motor unit to please fire now. Instead, somehow it works out that when you begin a new training routine, your central nervous system takes a few weeks to adapt and learn to recruit the motor units better for the routine, resulting in the pleasing miracle of fast gains that happen all by themselves.
your heart beats by itself, and although it is certainly willing to take input from various sources, it very much beats by itself, and it beats by itself because the individual heart cells beat by themselves, and the individual heart cells beat synchronously not because of any decision you make, but because some of the heart cells in the sino-atrial node have taken it upon themselves to coordinate things. conscious control over these cells is a difficult thing to learn and people take drugs to help them.
i don't think they're very free either. they go where the blood goes, and where the blood goes is defined by the vascular infrastructure built at great expense and powered by the organ of organs, the heart. i think in this connection you're thinking especially of macrophages, but even then they operate pretty physiologically - they're created - mostly from more boring white blood cells - and destroyed in similar ways to other cells and their functions are clearly adapted to fit the context they find themselves in - it just so happens they sometimes range a little wider than most cells.
and of course they have "my dna". unlike tumours, macrophages aren't trying to reproduce at the expense of the tissue they find themselves in. like any other cell line, their growth is very regulated.
there are two other places where you also find cells doing amoeboid migration, and they are in metastases and embryos. while a metastatic neoplasm is more clearly parasitic and not-me, this is one of the ways in which metastases are neotenous -- amoeboid motion in embryology is important in, for example, the embryogenesis of nervous tissue and probably other places too.
but ignoring all this, and sticking with the mind's obviously unphysiologically based notion of "self", sure, you can freak yourself out and believe white blood cells aren't you. you can freak yourself out and think anything is you, or everything is you, or nothing is you. it doesn't mean anything. people don't normally do this to white blood cells, i think.
i think, choosing the word very carefully, it is sophomoric to do this.
I think white blood cells are fascinating because they're "part of your body", but they're not actually attached to you. And they're not in any way directly controlled by your brain. They live freely in your blood stream and move around totally autonomously. So they're really a lot like an independent organism that just lives inside your body.
529 notes
·
View notes
Text
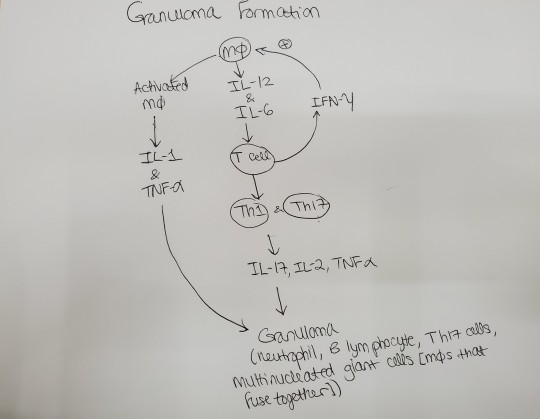
Reviewing questions:
Non-caseating granulomas with multinucleated giant cells (macrophages that fuse together and therefore the multinucleated giant cells have multiple nuclei) and increased serum calcium are seen in pts with sarcoidosis. You see bilateral hilar lymphadenopathy. Activated macrophages make 1,25-dihydroxyvitamin D-> increased Ca2+. I remember posting about why sarcoidosis causes increased calcium before. Here's what I said:
I wasn’t understanding how/why sarcoidosis causes hypercalcemia. It’s because the epithelioid histiocytes [macrophages in the epithelial tissue] of the granulomas have alpha 1 hydroxylase, which converts vitamin D to the active form, which makes you absorb more calcium. I know alpha 1 hydroxylase is in the kidney. Apparently, it’s also in the noncaseating granulomas you see in sarcoidosis.
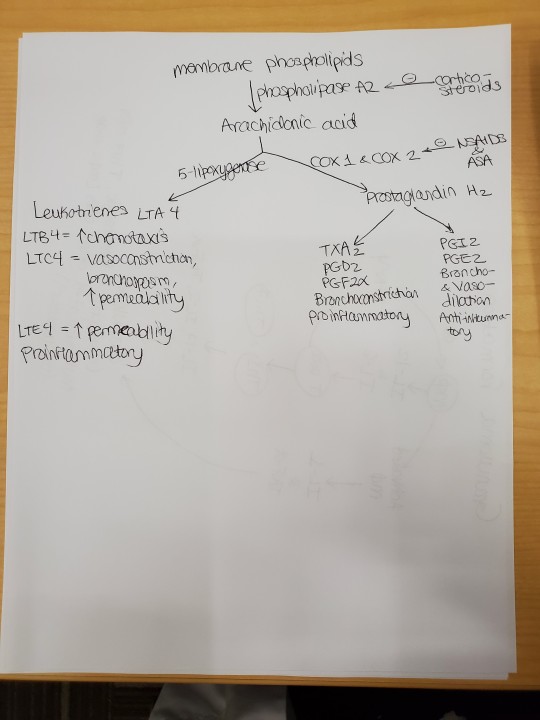
Glucocorticoids can be given to treat sarcoidosis in the short term. Glucocorticoids reduce inflammation by decreasing transcription of proinflammatory genes-> decreased macrophage and lymphocyte activation, so no inflammatory mediators (IL-1, IFN-gamma). Glucocorticoids also prevent endothelial cells from expressing selectins, which are necessary for neutrophils to leave the blood and go to sites of cytokine release. Glucocorticoids basically prevent margination and emigration of neutrophils-> increased neutrophil count in the blood. IL-8 = inflammatory cytokine that summons neutrophils; reduced by steroids. IL-10 = anti-inflammatory cytokine; increased by steroids. Steroids also cause apoptosis of eosinophils, monocytes, and lymphs-> susceptibility to infection. Steroids inhibit phospholipase A2-> decreased PGEs and leukotriene. Adverse effects of steroids include weight gain and glucose intolerance.
DiGeorge syndrome-> no thymus, so no T cells-> recurrent sinopulmonary infections. The structures that derive from the 3rd and 4th pharyngeal pouches (i.e., thymus) don't develop. Specifically, the ventral wings of the third pharyngeal pouch give rise to the thymus gland. In DiGeorge syndrome, this fails. Without a thymus, T cells can't mature and pts are susceptible to bacterial, viral, fungal, and protozoal pathogens. T cells are in the paracortex of lymph nodes (the cortex contains the naive B cells and the medulla contains the memory B cells). The paracortex of lymph node is undedeveloped in pts with DiGeorge syndrome (because there are no T cells to inhabit the paracortex).
Eosinophils respond to IgG and IgA on parasites by releasing their granules (e.g., major basic protein) and reactive oxygen species, which kill the parasites. This is antibody-dependent cell-mediated cytotoxicity. Th2 and mast cells make IL-5, which stimulates eosinophil activation and proliferation.
The Membrane Attack Complex (MAC) is made with complement factors C5b, C6, C7, C8, and C9. The MAC kills bacteria such as N. meningitidis. If you lack complement factors C5b through 9 (terminal complement deficiency), you can't make MAC and thus are susceptible to N. meningitidis meningitis. The petechial rash (on palms and soles, among other places) that you may see in N. meningitidis meningitis is due to vasculitis of small blood vessels due to N. meningitidis.
SCID = Sever Combined Immunodeficiency; can be X-linked recessive or AR; T cells don't develop; B cells also don't function normally; pts present with failure to thrive, recurrent viral, fungal, and opportunistic infections, and diarrhea. Pts will have hypogammaglobulinemia (not enough immunoglobulins) and lymphopenia (not enough B cells and T cells). When you do candidal antigen skin testing, these pts don't respond (no induration at site). In the candidal antigen skin test, you are trying to elicit the cell-mediated immune response of the type IV HSR, which involves not only CD8+ cytotoxic T cells, but also CD4+ helper T cells and macrophages. Macrophages are APCs, which present candida Ag to helper T cells, who then release cytokine to activate cytotoxic T cells, which destroy infected cells. IFN-gamma from both helper and cytotoxic T cells enhances macrophage phagocytosis of candida. Tx of SCID = stem cell transplant. Must r/o congenital HIV.
CD14 is the cell marker for macrophages. I didn't know that and that's what a question was asking. -_-
I answered a question where an old man got the flu even though he was vaccinated. The reason he got the flu was because of impaired naive B cell production. Vaccines expose you to antigens and your B cells should respond by making antibodies to the antigen. Vaccine failure will occur if the pt has atopy, uses steroids, or has impaired naive B cell production due to aging (called immunosenescence). So immunosenescence makes an older pt less likely to respond to a vaccine. Apparently, chronic inflammation with aging causes the B cells and T cells to respond to antigens the pt has already encountered and makes the pt less likely to respond to new Ags (e.g., those presented to the immune system in a flu vaccine).
From Wikipedia:
Immunosenescence refers to the gradual deterioration of the immune system brought on by natural age advancement. The adaptive immune system is affected more than the innate immune system.
Immunosenescence involves both the host's capacity to respond to infections and the development of long-term immune memory, especially by vaccination.[2] This age-associated immune deficiency is ubiquitous and found in both long- and short-living species as a function of their age relative to life expectancy rather than chronological time.[3] It is considered a major contributory factor to the increased frequency of morbidity and mortality among the elderly.
T cells are made in the bone marrow and mature in the thymus, hence T cells are also called "thymocytes." He explained T cell maturation with positive and negative selection really well in OnlineMedEd, so I won't go over it again here. I get it. Hopefully I will remember it.
Macrophages infected with intracellular pathogens (like mycobacterium tuberculosis) release IL-12-> T cells and NK cells release IFN-gamma-> macrophages become activated and increase phagocytosis; macrophages also release TNF-alpha, which leads to granuloma formation. IFN-gamma binds the IFN-gamma receoptor on macrophages-> receptor dimerization-> activation of Janus kinase 1 and 2-> STAT1 translocated to nucleus-> increased macrophage phagocytosis and killing of bacteria. Problems with the IFN-gamma pathway-> inability to kill M. tuberculosis-> disseminated infection.
From Wikipedia:
The JAK-STAT signalling pathway is a chain of interactions between proteins in a cell, and is involved in processes such as immunity, cell division, cell death and tumour formation. The pathway communicates information from chemical signals outside of a cell to the cell nucleus, resulting in the activation of genes through a process called transcription. There are three key parts of JAK-STAT signalling: Janus kinases (JAKs), signal transducer and activator of transcription proteins (STATs), and receptors (which bind the chemical signals).[1] Disrupted JAK-STAT signalling may lead to a variety of diseases, such as skin conditions, cancers, and disorders affecting the immune system.[1]
The JAK-STAT pathway in cytokine receptor signalling can activate STATs, which can bind to DNA and allow the transcription of genes involved in immune cell division, survival, activation and recruitment. For example, STAT1 can enable the transcription of genes which inhibit cell division and stimulate inflammation.
IFN-apha and beta are made by cells infected by viruses; the interferons prevent protein synthesis in virally infected cells. Specifically, IFN-alpha and beta cause cells to make enzymes that degrade viral proteins (e.g., RNAse L).
Neutrophils respond to IL-8, a pro-inflammatory cytokine. Pus = liquor puris + leukocytes (mostly neutrophils). So pus is basically dead neutrophils and a proteinaceous fluid called liquor puris. Chemotaxis = IL-8 released by macrophages at site of infection summons neutrophils. Other chemokines = leukotriene C4, C5a, 5-HETE.
TNF-alpha, IL-1, and IL-6 induce inflammation during sepsis. TNF-alpha from macrophages summons neutrophils and more macrophages.
In chronic lung transplant rejection, small airways become inflamed due to lymphocytes-> scar tissue blocks small airways (bronchiolitis obliterans).
Wiskott Aldrich syndrome = X-linked recessive mutation of genes coding for cell cytoskeleton proteins; pts present with thrombocytopenia (low platelets) + eczema + B cell and T cell deficiency (presents as recurrent infections). Platelets will be abnormal-> petechiae, epistaxis. No B cells-> pyogenic infections; no T cells-> opportunistic infections. Tx = bone marrow transplant.
HLA-DP, HLA-DQ, and HLA-DR are the genes that encode MHC Class II. In bare lymphocyte syndrome type II, you lack MHC Class II, so you can't activate B cells and T cells (normally, APCs present foreign antigens to naive B cells on their MHC-II receptors; this then causes B cells to activate T cells, which give the costimulatory signal to B cells, allowing the B cells to proliferate and class switch).
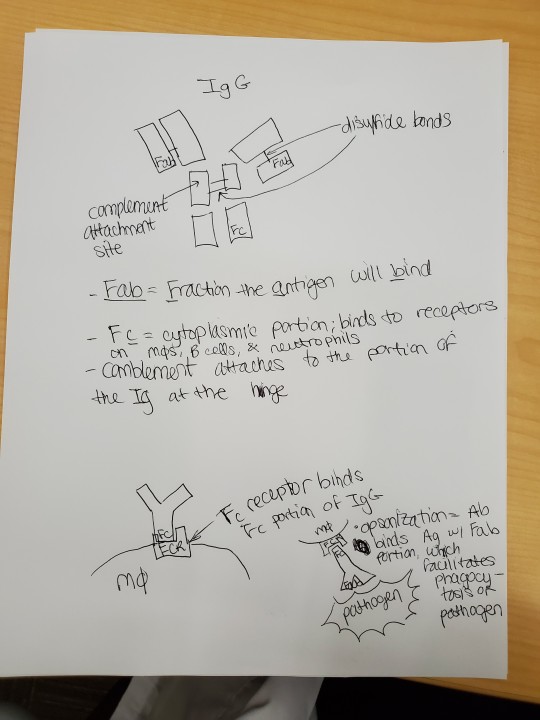
#sarcoidosis#hypercalcemia#interleukins#multinucleated giant cell#DiGeorge syndrome#steroids#major basic protein#meningitis#MAC#immunology#SCID#vaccines#immunosenescence#opsonization#Fab#antibody#cell markers#interferon gamma#IFN gamma#Janus kinase#JAK STAT#JAKSTAT#interferons#pus#transplant rejection#Wiskott Aldrich syndrome#MHC#HLA#IL12#chemotaxis
15 notes
·
View notes
Text
Squamous Cell Carcinoma (SCC)
Squamous cell carcinoma (SCC), the second most common form of skin cancer, is an uncontrolled growth of abnormal cells arising from the squamous cells in the epidermis, the skin’s outermost layer. It is sometimes called cutaneous squamous cell carcinoma (CSCC) to differentiate it from very different kinds of SCCs elsewhere in the body.
Scaly red patches, open sores, warts or elevated growths with a central depression; they may crust or bleed.
Can become disfiguring and sometimes deadly if allowed to grow.
Most located in the head-and-neck region.

Pathophysiology
Malignant transformation of normal epidermal keratinocytes is the hallmark of SCC.
One critical pathogenic event = development of apoptotic resistance through functional loss of TP53, (tumour suppressor gene).
UV radiation causes DNA damage through the creation of pyrimidine dimers, a process known to result in the genetic mutation of TP53.
Keratinocytes then undergo clonal expansion, acquiring further genetic defects, ultimately leading to invasive cSCC.
Many other genetic abnormalities are believed to contribute, including mutations of BCL2 and RAS. Likewise, alterations in intracellular signal transduction pathways, including the epidermal growth factor receptor (EGFR) and cyclo-oxygenase (COX), have been shown to play a role in the development of cSCC.

Squamous cell carcinoma in situ (CIS), aka Bowen disease, is a precursor to invasive cSCC. Characteristics of this lesion include nuclear atypia, frequent mitoses, cellular pleomorphism, and dyskeratosis, parakeratosis, and hyperkeratosis.
Histology
Conventional cSCC can be divided into the following four histologic grades, based the degree of nuclear atypia and keratinization found
Well differentiated - more normal-appearing nuclei with abundant cytoplasm and extracellular keratin pearls
Moderately differentiated - Exhibits features intermediate between well-differentiated and poorly differentiated lesions
Poorly differentiated - Shows a high degree of nuclear atypia with frequent mitoses, a greater nuclear-cytoplasmic ratio, and less keratinization
Highly undifferentiated - Shows epithelial cells that may be difficult to distinguish from mesenchymal, melanoma, or lymphoma cells

Other histologic variants include acantholytic (adenoid) SCC, which is characterized by a pseudoglandular appearance, and spindle cell SCC, which has atypical, spindle-shaped cells. Both of these variants exhibit a more aggressive clinical course.
#medicine#biomed#medblr#studyblr#notes#biomedicine#biomedical science#med#science#biology#human biology#medical biology#cancer#skin cancer#sciblr#premed#nursing#2#3#medschool#scc
188 notes
·
View notes
Text
What is Prostate Cancer,Professional advice

Etiology Etiology of prostate cancer development is not completely known. Factors that can influence the creation and development of this type of cancer include: genetic factors increase that is risk of falling ill among men with a positive family history regarding the prostate cancer. Mutations of suppressor genes are also taken into consideration (p53) dietetic factors – food rich in saturated fatty acids probably increases the risk of falling ill whereas the consumption of soya and rice may have a beneficial protective effect racial and geographical factors – Afro-Americans are 100% more likely to fall ill, whereas the lowest death rate is reported in Japan and in China occupational factors – cancerogenous influence of heavy metals and toxins infectious factors – viral infection may lead to/ be the cause of anaplasia of adenocyte cells of prostate Histopathologically, 95% prostate cancer cases occur in the form of adenocarcinoma. Other types (primary cancer that is intracellular squamous carcinoma, anaplastic carcinoma, and sarcoma) are rarely met. Adenocarcinoma usually develops in the peripheral area of the prostate (85%), in the transition area (25% ) and in the area that is central5%). Symptoms In symptomatology of the prostate cancer, 4 forms that are clinical distinguished: 1) visible form with distinct pathological symptoms 2) latent form (carcinoma latens) with no distinct pathological symptoms found 3) hidden form (ca occultum) which is detected in the case of distinct ailments caused by the existence of remote metastases, however changes in prostate are not found in the course of per rectum examination 4) accidentally detected form - based on histopathological test of the gland that was removed because of prostate overgrowth, or based on biochemical tests (PSA) During the development of prostate cancer, an induction phase that lasts about 30 years which is clinically invisible can be distinguished. During the stage that is next in situ phase (5-10 years) and invasive phase (1 year), ailments connected with the local growth of tumour start to appear. During this period, symptoms connected with sub bladder obstacle appear including mainly: - pallakiuria - nycturia - weak urine stream - painful vesical tenesmus - impression of incompletion of bladder emptying The above-mentioned symptoms are typical of cancer and in some cases they may suggest mild overgrowth of prostate, or neurogenic or athermatous bladder disorders. During the dissemination phase (about 5 years), prostate cancer develops continuously infiltrating surrounding organs, such as: urinary bladder, rectum, ureters, pelvic walls and leading to urinary retention in kidneys and to secondary failure of function. Ailments typical for this period include: - haematuria - dysuria - urinary incontinence - erection disorders - aches of perineum, lumbar area and anus - haematospermia Metastases spread through the lymphatic vessels and the vascular system. Symptoms caused by the existence of remote metastases are as follows: - osteodynia and pathological fractures - pressure symptoms and spinal paralysis - lymphadema of limbs - clotting disorders - cachexy - coma DIAGNOSTICS In order to diagnose the prostate cancer, patient should undergo per rectum tests (DRE), PSA concentration (prostate antigen that is specific in blood serum should be determined, ultrasonography per rectum examination (TRUS - transrectal ultrasound) should be done and if there is a suspicion of prostate cancer, histopathological test of the material obtained through a per rectum thick-needle biopsy done under the ultrasound control should take place. Histopathological test is the only test that confirms the presence of cancerous cells in the prostate gland area. DRE, which is an examination of sensitivity of 80% sensitivity and of specificity of 60%, enables to seize changes in the area of the prostate such as consistency change, palpable nodules and hardenings. It is the base for sending a patient to a biopsy that is diagnostic. At present, it is believed that cytological diagnosis achieved through a fine-needle biopsy is not sufficient to make a diagnosis that is right. It results from the fact that the assessment according to Gleason’s classification is an important prognostic factor for the prostate cancer (see: prognostic factors). That is why a biopsy that is thick-needle performed. Ultrasound use enables to take precise samples from suspicious foci. If there are no changes in TRUS picture, "sextant biopsy" is done (samples got for several places). Recommendations for the biopsy of prostate gland: 1) palpable suspicion of the prostate cancer 2) PSA value over 15ng/ml regardless of DRE or TRUS tests 3) PSA value between 4 and 15 ng/ml with abnormalities detected during DRE or TRUS tests 4) PSA value exceeds the norm for a given age in the case of a positive family history regarding the prostate cancer Recommendations for TRUS: 1) PSA between 4 and 12 ng/ml with abnormalities detected 2) questionable result of DRE test 3) necessity of a thick-needle biopsy Other diagnostic tests, such as CT and urography are not routinely performed because their value is questionable as far as the assessment of local stage and invasion of adjacent lymph nodes is concerned. Nowadays, magnetic resonance tomography done using transrectal coli (endorectal coil MRI - ERMR) to observe the prostate arouses interest that is great. Despite the increased sensitivity of the degree of the stage that is local costs of the test do not allow for its routine use in the prostate cancer diagnosis. Scintigraphy of the skeleton is the most test that is sensitive%) in bone metastases detection. It is assumed that a patient with PSA under 10 ng/ml does not undergo scintigraphy because the probability of metastases is low. Screening: Screening: It is recommended that patients aged over 50 should undergo per rectum tests and level that is PSA every year. PROGNOSTIC FACTORS: Three groups of prognostic factors can be distinguished in the case of the prostate cancer: 1) development stage according to TNM 2) differentiation degree of the cancer based on the classification of Gleason and Mostofi 3) PSA level (prostate-specific antigen) in serum TNM classification Preoperative assessment of the stage of the prostate cancer is made based on the tests that are above-mentioned. T-stage: primary tumour Tx - primary tumour cannot be assessed T0 - no evidence of primary tumour T1 - clinically unapparent tumour; not palpable or visible by per rectum imaging T1a - incidental tumour found in histopathological tests after transurethral resection of the prostate or after operational adenectomy: found in 5% or less resected tissue T1b - as above; found in more than 5% resected tissue T1c - tumour identified histopathologically by a needle biopsy (because of high PSA) T2 - tumour confined within the prostate gland T2a - tumour involves less than half of one lobe T2b - tumour involves more than half of one lobe only T2c - tumour involves both lobes T3 - tumour extends through the prostatic capsule T3a - extracapsular extensions (unilateral) T3b - extracapsular extensions (bilateral) T3c - tumour invades seminal vesicles T4 - tumour is fixed, invades adjacent structures other than seminal vesicles T4a - tumour invades bladder neck and/or external sphincter and/or rectum T4b - tumour invades levator muscles and/or pelvic wall N-stage: regional lymph nodes Nx - regional lymph nodes cannot be assessed N0 - no regional lymph node metastases N1 - metastasis to a single regional lymph node with the diameter under 2cm N2 - metastasis to a single regional lymph node with the diameter > 2cm but Mx - remote metastasis cannot be assessed M0 - no remote metastases M1 - remote metastases M1a - non-regional lymph nodes M1b - bones M1c - other sites According to Whitmor-Catalon classification, grades A, B, C, and D correspond to T1, T2, T3 and T4 of TNM classification respectively. Degree of cancer differentiation: Degree of differentiation is defined according to 2 classifications: by Mostofi and by Gleason. Mostofi’s classification uses a 3-grade assessment of differentiation dependent on the degree of cell anaplasia – grading (G1-G3). The higher grade, the lower differentiation of cancer tissue, the greater atypy and at the time that is same malignancy. In the case of a 10-grade Gleason system, the two extreme histological images in the preparation are assessed and then, added to produce a final grade. PSA is a proteolyctic enzyme responsible for sperm melting. It is mainly produced by glandular epithelium, it might be also produced in organs such as salivary glands, pancreas and mammary gland and by clear cell carcinoma. Commonly used norm is the following: 0-4 ng/ml. Such concentration of PSA is found among 97% of men over 40. The level over 12 ng/ml is always connected with pathology. Difficulties with diagnosis are found among patients who have this level between 5-10 ng/ml because it may both stem from the prostate cancer or a mild overgrowth of the prostate, which causes the necessity of diagnostic methods use, such as TRUS. This test makes it possible to determine PSA density (PSAD - PSA density) - PSA concentration converted to prostate volume unit. It should be under 0.15 ng/ml/g. In the case of prostate cancer differentiation and mild overgrowth of prostate, free to total PSA (PSA F/T) is used. If it is over 20%, one may assume the presence of cancerous cells in the gland. PSA level does not correlate well enough with the natural development of the prostate cancer. However, it is useful as a factor that is prognostic the treatment applied and in prognosis determination. However, high final levels indicate low survival rate. TREATMENT Proceeding strategy in patients with the prostate cancer depends on the degree of histological malignancy, the degree of local stage of development, coexisting diseases and age of a patient. There are many controversies as far as the choice of treatment is concerned. Radical treatment is possible in T1, T2 and N0 and Mo stages. The procedure is restricted to delay the cancer progression and mitigate its effects (palliative treatment) in advanced cases (T3, T4, N-+, M-+). Surgery treatment - radical prostatectomy The surgery consists in the prostate gland removal together with spermatic vesicles and tissues that are adjacent. Surgery is done through retropubic, transcoccgeal, perineal approach or through laparoscopy. Lymphadenectomy constitutes an part that is integral of surgery. If the approach makes it impossible to remove the gland and lymph nodes (perineal approach) at the same time, a separate surgery is carried out. It precedes the operation proper. It is believed that cancerous cells found in the removed lymph nodes are the good reason why prostatectomy cannot be performed. Invasion of lymph nodes to a extent that is certain PSA level over 40ng/ml together with grade >7 in Gleason’s scale. Recommendations for surgery: 1) cancer limited to the prostate gland (T1BN0M0Gx - T2N0M0Gx, T1AN0M0G3) 2) predictable life span over 10 years 3) consent of a patient If positive chirurgical margins, capsule infiltration or cancerous changes in the removed lymph nodes are found in postoperative microscopic assessment, the prognosis is worse – such patients are qualified for palliative treatment. The death rate in the postoperative period does not exceed 5%. Intraoperative complications first of all include: bleeding from Santorini’s plexus, damage of rectum wall, underpinning of ureter. Early complications after surgery: thrombotic and embolic complications (phlebothrombosis 3-12%, lung embolism 2-5%) and lymphocele. Late postoperative complications after prostatectomy include: urinary incontinence, erection disorders and narrowing of urethro-vesicular junction). Radiotherapy Apart from radical prostatectomy, radiotherapy is an effective method of treatment for patients with regional prostate cancer that is advanced. The most frequently done using radiation from external sources, the dose of 50-70 Gy in fractions continuing over 5-7 weeks are given in radical treatment. T1ABC - T2ABCG1 and T1ABCG2 stages require radiation limited to the prostate. In other cases, area that is radiated includes lymph that is adjacent as well. In recent years, multidimensional imaging with CT (3D conformal radiotherapy) is used in the treatment planning. Brachytherapy constitutes another method that is used. Recommendations for radical radiotherapy of the prostate: 1) prostate cancer confined with the organ 2) sufficiently long survival that is predictable 3) no disorders in lower urinary tract 4) no disorders in rectum and colon 5) consent of patient to carry out treatment 6) early complications of radiation energy treatment (30% of patients) include dysuria, haematuria, diarrhoea, rectal tenesmus, inflammation of large intestine and rectum. Among later complications (11% of patients) chronic diarrhea, ulceration of rectum, bladder neck stenosis and intestinal fistula stenosis are observed. Control of patients after radical prostatectomy and radiotherapy that is radical - per rectum test, PSA level in blood serum each 3 months. PSA level should be lower than 1 ng/ml (after radical prostatectomy it should be near to 0). Increase over 0.5 ng/ml within a means failure of radiotherapy year. Hormonotherapy Hormonal therapy is mainly used as palliative treatment in advanced prostate cancer. It makes it possible to stop symptoms of the disease for some time and then, further progression of the disease takes place. Nowadays, the use of therapy in pulsation system is considered as it delays the development of hormone-resistant cell clones. Ways of hormonal treatment include: 1) surgery castration (orchidectomy) 2) anti-androgens a) non-steroid b) steroid 3) analogues LH-RH 4) oestrogens, progestogens, inhibitors of androgens synthetase Hormonotherapy by analogues LH-RH is also recommended before planned radical radiotherapy. In the case of hormone-resistant cancer, treatment with combined cytoctatic and hormone (estramustine), however without significant effects. PROGNOSIS Prognosis depends on the development stage, degree of differentiation and PSA level (see: prognostic factors). In T1A, B stage prognosis is good. 10-years survival 35-80%, death rate of the cancer 7-30%. In T2 stage, overall survival equals 34-85%, death rate equals 8-26%. In T3 stage, among patients who undergo non-invasive treatment for 9 years, overall death rate equalled 63%, from cancer – 30%. Depending on the degree of cancer differentiation, 10-year survival of patients is the following: for cells well differentiated - 81%, for cells moderately differentiated - 58% and for cells poorly differentiated - 26%. Read the full article
0 notes
Text
Global Cell Counting Market To Be Driven By The Increasing Demand In Hospitals And Diagnostic Laboratories In The Forecast Period Of 2021-2026

The new report by Expert Market Research titled, ‘Global Cell Counting Market Size, Trend, Growth, Analysis, Report and Forecast 2021-2026’, gives an in-depth analysis of the global cell counting market, assessing the market based on its segments like product type, end use, and major regions. The report tracks the latest trends in the industry and studies their impact on the overall market. It also assesses the market dynamics, covering the key demand and price indicators, along with analysing the market based on the SWOT and Porter’s Five Forces models.
The key highlights of the report include:
Market Overview (2016-2026)
Historical Market Size (2020): USD 10 Billion
Forecast CAGR (2021-2026): 10%
Forecast Market Size (2026): USD 15.2 Billion
During the projection period, hospitals and diagnostic laboratories are estimated to observe significant growth. This can be attributed to an increase in capital expenditure for the development of cell culture-based vaccines and the rapid expansion of the pharmaceutical industry. The rising prevalence of various chronic ailments, such as cancer, HIV-AIDS, leukaemia, Alzheimer’s, etc., is propelling the demand for cell counting techniques. The growing adoption of cell counting instruments across diverse medical fields, such as molecular biology, immunology, pathology, etc., for developing next-generation therapeutics is also augmenting the market growth. In addition to this, the increasing utilisation of cell counting for the identification and determination of primary tumours, circulating tumours, and metastatic tumour is further bolstering the market growth. The widespread adoption of stem cell therapy is also propelling the demand for various cell counting instruments on a global level.
Cell Counting Industry Definition and Major Segments
Cell counting refers to a technique used to analyse the cells or micelles that are usually suspended in blood or other body fluids. Some of the standard instruments used for cell counting includes hemocytometers, spectrophotometers, flow cytometers, and automated cell counters, among others. Cell counting aids in classifying cell types and detecting disease through probes to develop the best treatment plan for the patient. It also helps in understanding the structure and composition of the cells for chromosome analysis, protein expression, cancer diagnosis, and haematological malignancies, among others.
Request a free sample copy in PDF or view the report summary@ https://www.expertmarketresearch.com/reports/cell-counting-market/requestsample
By product type, the industry is segmented into:
Instruments
Spectrophotometers
Flow Cytometers
Haematology Analysers
Cell Counters
Others
Consumables and Accessories
Media, Sera, and Reagents
Assay Kits
Others
The market can be broadly categorised on the basis of end use into:
Hospitals and Diagnostic Laboratories
Research and Academic Institutes
Pharmaceutical and Biotechnology Companies
Others
On the basis of region, the industry is divided into:
North America
Europe
Asia Pacific
Latin America
Middle East and Africa
Cell Counting Market Trends
The rising awareness of numerous benefits of cell counting techniques in immunophenotyping, cell sorting, cell proliferation assays, and intracellular calcium flux is further driving the market growth. The increasing investments by several government bodies in extensive research and development activities pertaining to the fields of biotechnology, oncology stem cell therapeutics, etc., are also bolstering the adoption of cell counting techniques. The presence of an array of medical research and biopharmaceutical businesses is anticipated to drive market growth in the coming years. With the rapid technological advancements, the market is predicted to be driven by innovations in existing products as well as the launch of new data visualisation and analysis tools. The market growth can also be associated with the increase in the number of proteomics and genomics researchers.
Key Market Players
The major players in the market are Thermo Fisher Scientific Inc., Becton, Dickinson and Company, Bio-Rad Laboratories, Inc., Beckman Coulter, Inc., and PerkinElmer Inc., among others. The report covers the market shares, capacities, plant turnarounds, expansions, investments and mergers and acquisitions, among other latest developments of these market players.
About Us:
Expert Market Research (EMR) is leading market research company with clients across the globe. Through comprehensive data collection and skilful analysis and interpretation of data, the company offers its clients extensive, latest, and actionable market intelligence which enables them to make informed and intelligent decisions and strengthen their position in the market. The clientele ranges from Fortune 1000 companies to small and medium scale enterprises.
EMR customises syndicated reports according to clients’ requirements and expectations. The company is active across over 15 prominent industry domains, including food and beverages, chemicals and materials, technology and media, consumer goods, packaging, agriculture, and pharmaceuticals, among others.
Over 3000 EMR consultants and more than 100 analysts work very hard to ensure that clients get only the most updated, relevant, accurate and actionable industry intelligence so that they may formulate informed, effective, and intelligent business strategies and ensure their leadership in the market.
Media Contact:
Company Name: Claight Corporation
Contact Person:- James Rowan, Business Consultant
Email: [email protected]
Toll Free Number: US +1-415-325-5166 | UK +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
#Cell Counting Market#Cell Counting Market Size#Cell Counting Market Report#Cell Counting Market Share
0 notes
Text
Oncogenes pt 2
check out the overview here
ACTIVATION OF PROTO-ONCOGENES
A proto-oncogene can become an oncogene by a relatively small modification:
an increase in protein activity
a loss of regulation of its activity
an increase in protein concentration, caused by an increase of protein expression
RAS
RAS is mutated/activated in up to 30% of all cancers. Ras point mutations are the single most common abnormality of human proto-oncogenes.
RAS proteins function as binary molecular switches that control intracellular signaling networks, controlling important processes such as cell proliferation, cell differentiation, cell adhesion, apoptosis, and cell migration.
Ras is it is a single-subunit small GTPase,
In the "off" state it is bound to the nucleotide guanosine diphosphate (GDP)
in the "on" state, Ras is bound to guanosine triphosphate (GTP),

MYC
encodes a DNA binding transcription factor
MYC binds to a DNA sequence in its target genes known as the E-box.
represses the expression of CDK inhibitors
activates the expression of cyclins
also downregulates apoptosis and upregulates protein synthesis

Tumour suppressor genes
either dampen regulation of the cell cycle, or promote apoptosis
Via: Repression of genes essential for the continuation of the cell cycle -inhibiting cell division
Coupling the cell cycle to DNA damage - repair or apoptosis
Some proteins involved in cell adhesion prevent tumour cells from dispersing - inhibit metastasis
Two Hit model
Unlike oncogenes, tumour suppressor genes generally follow the ‘two-hit model’ - both alleles must be affected before an effect is manifested.
if only one allele for the gene is damaged, the second can still produce the correct protein - recessive.
P53 - The guardian of the genome
Homozygous loss of p53 is found in 70% of colon cancers, 30–50% of breast cancers, and 50% of lung cancers.
Mutated p53 is also involved in the pathophysiology of leukemias, lymphomas, sarcomas, and neurogenic tumors.
Abnormalities of the TP53 gene can be inherited in Li-Fraumeni Syndrome, which increases the risk of developing various types of cancers.
Recognises DNA damage and can arrest growth by holding the cell cycle at the G1/S regulation point on DNA damage recognition (if it holds the cell here for long enough, the DNA repair proteins will have time to fix the damage and the cell will be allowed to continue the cell cycle).
It can initiate apoptosis if DNA damage proves to be irreparable.
It is essential for the senescence response to short telomeres. (hayflick limit)

It is obvious, therefore, that damage or inactivation will result in a much greater risk of cancer.
BRCA1
BRCA1 (breast cancer 1, early onset) mutations are associated with a significant increase in the risk of breast cancer .
BRCA1 is involved in DNA damage repair, especially error-free repair of DNA double strand breaks, ubiquitination, and transcription.
Women who have an abnormal BRCA1 gene have up to an 85% risk of developing breast cancer by age 70; and an increased risk of developing ovarian cancer 55%
(there are many more of both - these are just major examples)
#medicine#biomed#medblr#studyblr#notes#biomedicine#biomedical science#med#science#biology#human biology#medical biology#oncology#cancer#onco#tsg#oncogenes#med school#3#Physiology
68 notes
·
View notes
Text
Nanoparticles for Cancer Therapeutic
There is no scarier diagnosis in most peoples’ minds than that of cancer. Cancer is often thought of as an untreatable, unbearably painful disease with no cure besides surgery and chemotherapy. At the same time, Cancer is one of the critical public health problems globally and is the second leading cause of death. Traditional cancer therapies include surgery, chemotherapy, radiation, targeted therapy, and immunotherapy. However, those conventional therapies are associated with limitations such as lack of specificity, cytotoxicity, and multidrug resistance, which pose a massive challenge. Nanoparticles (NPs) have transformed the arena of cancer diagnosis and treatment. Nanoparticles not only solved the limitations of conventional cancer treatment but also overcame multidrug resistance.
Nanoparticle-based drug delivery systems have displayed benefits in cancer therapy and management by demonstrating good pharmacokinetics, accurate targeting, reduced side effects, and drug resistance. In addition, Nanotherapeutic drugs have shown promising results in the delivery system and anti-tumour multidrug resistance (MDR), providing an opportunity for drug recombination therapy and inhibition of resistance drug mechanism. Nanoparticles’ delivery systems are approved to increase drug stability by preventing the degradation of the encapsulated cargo. In addition, large volumes of drugs can be encapsulated without any chemical reactions. Another advantage is that nanoparticles have a high surface-to-volume ratio and intracellular uptake, allowing administered in many ways, including oral, nasal, parenteral, and intra-ocular. Nanoparticles should maintain specific essential characteristics such as the ability to remain stable in the vascular system until reaching the target (TME), escape the reticuloendothelial system (RES) clearance, escape mononuclear phagocyte system (MPS), accumulate in TME via tumour vasculature, high-pressure diffusion into the tumour fluid, and reach the target and only interact with tumour cells.
Nanoparticles can have different shapes, sizes, and structures. Therefore, the synthesis of nanoparticles can be characterized mainly into two main approaches: 1) bottom-up approach, building nanoparticles from atoms to clusters than to NPs, hence known as constructive method 2) top-down approach, also known as a destructive method which involves the breakdown or decomposes of large molecules into smaller units that are converted to NPs. These approaches can be classified into different subclasses based on reaction conditions and operation. Nanoparticles contain broadly classified two different targeting mechanisms: passive and active. The passive targeting mechanism mainly relies on the vascularity and leakiness of the tumour biology and carrier characteristics such as size and circulation time. This type of tumour-targeting does not require a specific ligand for certain types of tumour cells. The active targeting depends on specific ligands or molecules such as transferrin and folate, which bind to molecules or receptors mainly expressed or over-expressed on the targeted cells. Nanoparticles used in drug delivery systems include organic, inorganic, and hybrid NPs. The image below indicates different examples of nanoparticles used in the delivery systems.

Figure 1: Nanoparticles extensively used in drug delivery systems include organic, inorganic, and hybrid nanoparticles.
The amount of knowledge and research put into nanoparticles has steeply raised. But only a few of them make it up to clinical trials. This is because nanoparticles are associated with clinical challenges that can be divided into biological and technological and study-design-related challenges. The biological challenges include lack of routes of administration, tempering biodistribution, the channel of NPs across the biological barriers, NPs degradation, and NPs toxicity. Technological challenges involve scale-up synthesis, equal optimization, and performance predictions. The study-design challenges such as study size, intent, and timing of NP therapies during the therapy are affected significantly during clinical studies. Although nanoparticles showed a promising new era of cancer treatment, there are still many obstacles to overcome.
“Cancer is a journey, but you walk the road mostly alone. There are many places to stop along the way and get nourishment- you just have to be willing to take it.” In 2020, it was estimated that about 1.8 million people were diagnosed with cancer in the united state alone. COVID-19 pandemic stole the world’s attention for the past few years when cancer and other disease were nearly ignored. However, many studies showed promising results of using nanoparticles in cancer therapy as a delivery system. In addition, Nanoparticles were used in cancer therapy multidrug resistance due to the NP’s ability to co-encapsulate multiple therapeutic agents.
Gavas, Shreelaxmi et al. “Nanoparticles for Cancer Therapy: Current Progress and Challenges.” Nanoscale research letters vol. 16,1 173. 5 Dec. 2021, doi:10.1186/s11671-021-03628-6
1 note
·
View note
Text
Sweat and sweating.
Sweating glands are a part of mechanism, regulating metabolism. Sweating is a not specifical regulation, as a fever and inflammation. Sweating is a clinical symptom of abundant meaning.A sweat role is leading to removing substances, as filling in vessels and in intervention liquids in a big quantity as after muscle work, after abundant warm, after intoxication, sweating or after excitation.And patients with destroyed metabolism is on hyperthermic bath as for hour secreting are with sweat as more acidity substances, than kidneys within 24 hours.
And fever promotes for metabolites burning,and sweat theirs secreting. Fever and sweat are for the protection remedies for organism. Serious breath destroys as pneumothorax, pneumonia, agonic are having with alkaline sweat. After surgery sweat is sour/PH 3.0 till 6.0, in healthy humans Ph of sweat are 6.0-7.0, level of acidity for sweat are on the diseases' crisis having paramount meaning for prognosis. And if sweat is sour ,prognosis is favourable , if alkaline thus is very serious. Sticky alkaline sweat as on agonic time is a very dangerous destroy for acidity base balance, as on the cell's split. When skin, lungs, kidneys are not in ability regulating a acidity base balance,sweating is a keeping last chance for removing harmful substances. And constant sweating is a big relieving for a blood circulation organs/capillaries, arteries, heart.A sick skin is abundant with metabolites, she is fixing natrium chloride, urea acid, protein molecules.Need a clean for human in a common, dermatologists just cures a low cover.
And indications for a using hyperthermic baths.
1. poly neuritis.
2.iridocyclitis.
3.poliomyelitis/child s paralysis in pre- paralysis stage/in after paralysis period, a good effect giving are mixed turpentine baths.
4. Septicemia as with a hyperthermical bath lead effective result as on cases when are not affected antibiotics.
5.salpingitis.
6.otitis.
7.gonorrhoea arthritis.
8. tumours, rising resistance in patience, improving blood structure, effective lowering aches.
9.all children s infections are scarlet fever, dyphtery, measles.
10.whooping cough.
11. after dyphtery paralyses.
12. pleural exudates persistent .
13.Tertiary syphilis for inside organs. Progressive paralyses, dryness for spine brain.
And for us to say,that a how you dare to pretend on improving condition in patients and for a disease cure? And how you are dare to offer universal method cure, instead for remedies. Answer for you,that drama of an every sick attack due a filling for harmful substances, metabolites,which organism as attacked is not ability to burn,and decompose,and not oxidize .Artifical rising temperature decomposing toxical metabolites and transforms giant molecules in molecules with a less molecular mass, which easy secreting through kidneys , lungs, kidneys. As in gardens are dead leafs burning, in villages burning garbage. Hyperthermic baths burns organic dust, cleaning ways for messaging are capillaries, clean intracellular and space liquids. And here is secret for cure versatility of hyperthermic baths.Normal life,somatic and psychic is not unthinkable continuous, rhythmical, controlling burning.
via Blogger https://ift.tt/3iVWqGo
0 notes
Text
A new killer lymphocyte enters the ring
A new killer lymphocyte enters the ring
The fight between CD4 T-lymphocytes (in blue) against tumour cells (in orange). Credit: 2021 EPFL Hatice Altug Treatments for beating tumors are mainly based on CD8 T lymphocytes, which specialize in detecting and eliminating intracellular infections and in killing cancer cells. A large proportion of patients, however, do not respond to these treatments. This prompted a research team from the…

View On WordPress
0 notes
Text
Cancer: a new killer lymphocyte enters the ring
Cancer: a new killer lymphocyte enters the ring
A team from SCCL has discovered that CD4 T lymphocytes, which usually play a supporting role in fighting cancer cells, also have the power to destroy them. Credit: 2021 EPFL Hatice Altug Treatments for beating tumours are mainly based on CD8 T lymphocytes, which specialise in detecting and eliminating intracellular infections and in killing cancer cells. A large proportion of patients, however,…

View On WordPress
0 notes