#Lectin proteins
Explore tagged Tumblr posts
Text
THE EXCELLENT RANGE OF LECTIN CONJUGATES AND THEIR APPLICATION
WHAT ARE LECTIN CONJUGATES?
Proteins (building blocks) that attach to cells and specific carbohydrate groups on proteins or cell membrane proteins are known as lectin conjugates. They are further classified according to their amino acid sequences and biological characteristics. Lectins contain 120 amino acids that are involved in carbohydrate binding.
Because of its carbohydrate binding, lectin is employed in glycobiology to analyse cell surface glycoproteins. Lectins are synthesised in laboratories after being extracted from plant or animal components.
The capacity of Lectins to form precipitates with glycoconjugates is due to the fact that most lectin proteins are composed of non-covalently linked subunits. Agglutination of cells by Lectins is uncommon in nature and thus extremely difficult to detect.
Lectins enable scientists to investigate a wide range of biological structures and functions. Some Lectins bind to mannose or glucose residues, while others bind only to galactose residues due to their complex binding requirements. Some Lectins also require sugar-binding at specific oligosaccharide sites.
LECTINS APPLICATION
Lectins are being used in clinical laboratories to type blood cells. There is the extensive usage of Lectin in specialist applications such as-
• As chemotherapeutic agents
• In fractionation of animal cells as mitogens.
• While investigating cellular surfaces
• Lectins isolate specific cells or viruses with a mixture and study determined processes amongst several.
LECTIN IN ANIMALS
Regulate cell adhesion
• Glycoprotein synthesis is regulated by Lectins
• They can also regulate blood protein levels.
• Recognition of galactose residues on the surface of mammalian liver cells responds better to Lectins.
LECTIN IN PLANTS
Plants are naturally rich in lectins. Dietary lectins are found in protein sources such as beans and legumes, peanuts, lentils, wheat, uncooked kidney beans, fruits, and vegetables. Conversely, lectin-free diet items include pasture-raised meats, cooked sweet potatoes, cruciferous vegetables, asparagus, garlic, and onion.
Lectin activity and function in plants are both unknown. The content of Lectin in plant seeds decreases as they mature. Plant Lectin has the ability to recognise hydrophobic noncarbohydrate ligands.
Adenine, auxins, cytokinin, indole-3-acetic acid, and water-soluble porphyrins are examples. Because these compounds behave as phytohormones, their interactions may be psychologically significant.
Furthermore, the plasma membranes of human EL4 tumour cells are labelled with horseradish peroxidase-conjugated wheat-germ agglutinin. After the labelled intact cells are disrupted, plasma-membrane refinement is observed by ultrastructural examination of the various fractions for positive effect product on the membrane vesicles.
LECTINS AND OTHER CARBOHYDRATE-BINDING PROTEINS
Cellular proteoglycans, glycoproteins, and glycolipids include a wide range of oligosaccharides. Fluorescent carbohydrate-binding derivativesMicroscopy PROTEINS and flow cytometry both use proteins to identify intracellular glycoconjugates. This is done to isolate glycoproteins on protein blots and cause agglutination of specific cell types. Lectins can also be used to detect cancer since they have changed surface glycoproteins.
LECTINS INTERCONNECTING

Biotechnology has narrowed down biorecognition molecules with diagnostic potentials in light of the different diseases that impact the human species. Particular Lectin content binds with mono- or oligosaccharides with high affinity via no covalent connection via hydrogen bonds.
Lectins from viruses, bacteria, fungi, algae, mammals, and plants recognise carbohydrates in cells, tissue sections, and biological fluids. These are useful tools for diagnostic purposes. To find medicines and inhibitors, sialic acid-specific lectins such as Influenza Virus Hemagglutinin are being studied. These can remove or inhibit sialic acid in host cells, preventing it from binding.
Similarly, strong anti-HIV activity in vitro has been associated with bacterial Lectins. Large levels of algal Lectins are attracting interest for biomedical uses such as anti-HIV, anti-inflammatory, antibacterial, and antinociceptive properties. Animal Lectins are important in psychological processes such metastatic cancer, apoptotic pathways, and immunomodulation.
LECTIN INDUCED MECHANISMS OF INFLAMMATION RESPONSES
Immune systems act in two specific ways called; innate and adaptive responses. These responses are activated by a group of cells and molecules that promote the destruction of aggressive agents. Neutrophils, eosinophils, basophils, and mono/macrophages can generate and release molecules called cytokines.
These molecules modulate the activation of immune cells, inflammation, and humoral responses. Biomolecules like these are the answer for adjustment of immune conditions and therapeutic applications in regards to immune response-related diseases.
Lectins are thought to contribute to the development of diseases such as celiac disease, autoimmune diseases, rheumatoid arthritis, obesity, cardiovascular disease, and type 2 diabetes. This happens through translocation across the intestinal barrier and activation of the adaptive immune system. Common high-lectin foods include grains, legumes, and nightshades.
Lectins aren’t digestible. They bind to cell membranes lining the digestive tract, where they may disrupt metabolism and cause damage. Lectin sensitivity is the body’s delayed immune response that can occur some hours to even days after these foods are consumed.
Symptoms associated with lectin sensitivities include:
•Bloating and abdominal cramps
•Painful or swollen joints
•Tiredness
•Skin problems
•Hormonal fluctuations
•Nausea
•Allergies or allergy-like symptoms
•Neurological symptoms
The highest concentrations of lectins are found in healthy foods like legumes, grains, and nightshade vegetables. Fortunately, there are ways to reduce the lectin content of these healthy foods to make them safe to eat.
Research studies have shown that by cooking, sprouting, or fermenting foods high in lectins, their lectin content can easily be reduced to negligible amounts.
Foods That Are High in Lectins
1. Red kidney beans
Raw kidney beans contain high levels of a lectin called phytohaemagglutinin. Eating them raw or undercooked can cause severe nausea, vomiting, and diarrhea. As few as five beans can cause a response.
A hemagglutinating unit (hau) is a measure of lectin content. When in raw form, red kidney beans contain 20,000–70,000 hau. Once cooked, however, they contain only 200–400 hau, which is considered a safe level.
In cooked form, they are valuable and nutritious food.
2. Soybeans
Soybeans have several health benefits but are another food that also contains high levels of lectins.
As with red kidney beans, cooking soybeans almost eliminates their lectin content, provided they are cooked at high temperatures. Studies show that soybean lectins are almost completely deactivated when at 100°C for at least 10 minutes.
3. Wheat
Raw wheat, including wheat germ, is high in lectins, with around 300 mcg of wheat lectins per gram. (Whole-wheat flour has a much lower lectin content at about 30 mcg per gram). Lectins are almost completely eliminated by cooking and processing, and as most whole-wheat products consumed are cooked, it’s not likely that lectins pose a major risk to health.
4. Tomatoes
Tomatoes are part of the nightshade family. They are high in fiber, a good source of potassium and vitamin K1, and high in vitamin C. (One tomato provides about 20% of the daily recommended value.
Tomatoes also contain lectins, though there is little evidence that they have any adverse effects on humans. Some people have linked tomatoes and other nightshade vegetables to inflammation, such as arthritis. No formal research has supported this link.
5. Potatoes
Potatoes are also members of the nightshade family and a good source of vitamins and minerals. Potato skins are particularly high in antioxidants, such as chlorogenic acid, which has been linked to a reduced risk of heart disease and type 2 diabetes. As with tomatoes, adverse effects have been experienced by some when eating potatoes. Studies have shown that this could be linked to lectins.
6. Peanuts
Peanuts are an excellent source of protein, unsaturated fats, and many vitamins and minerals.
Peanuts do contain Lectin, and one study found that peanut lectins increased growth in cancer cells. With evidence that peanut lectins can enter the bloodstream, this has led some people to believe that lectins could increase the risk of cancer spreading in the body. However, the above study was carried out using very high doses of pure lectins placed directly onto cancer cells.
No studies have investigated their exact effects on humans. Evidence of their health benefits appears to be stronger than that of any risks.
DRUG DELIVERY USING LECTIN SOURCE OF PROTEIN
Chemical agent therapies often come across as barriers when the need for increasing dosages and action of metabolism reduces the effectiveness of treatments. Delivery of drugs to specific targets requires a new and an effective strategy to combat side effects and chemical reactions.
Lectin medicated bio adhesion constitutes specified interactions with receptor-like structures in the cell membrane, binding directly to targeted cells. Therefore Lectins can interact differently with distinct cells and act as drug carriers to desired tissue and cells. For it to be a tool in drug delivery Lectins, need to be avid binding, low toxicity, and site-specific molecules.
To conclude, Lectins from diversified sources with distinct carbohydrate recognition events play a vital role in many biotechnological applications/disease therapies. The uses in vitro and in vivo display Lectins with protective effects against viruses and microorganisms. Lectins are a highly potent modulator of an immune response, mitosis, proli9, healing, drug delivery therapies, and cancer regression.
Histochemistry, biosensors, detect diseases, and infections against glycans alterations on cells or tissue surfaces, and serum samples can be isolated using Lectin-based technology and techniques. There is potential to unravel new interpretations in the biological effects, pathways, and biotechnological potential of Lectins. They are focusing on their achievements in therapeutic applications and health effects.
Want to learn more about Lectin Conjugate, its usage, and health benefits? Contact our experts at Helvetica Health Care today!
1 note
·
View note
Note
are pokebeans edible? they are good for a pokemon's health but can humans eat them? they look sooooo good
as with many legumes, it's not safe to eat them raw. they're packed full of proteins called lectins that cause digestive upset in humans. unfortunately, cooking them tends to make them taste fairly unappealing- they get bland when cooked.
that being said, i've heard of malasada shops in alola that mash up cooked pokebeans with fruit to make sweet, colorful malasada filling for malasadas that are safe for both humans and pokemon!
62 notes
·
View notes
Note
Hi!! I tried the kalmbach feed with my chickens once so far, and they loved it, but I was wondering what was up with the lentils in there. I know its for protein, iron etc thats in them, but Im under the impression theyre raw. That made me nervous because I know raw beans are unsafe to feed to chickens, and I've asked around and researched to find an explanation but haven't found anything, so I was wondering if you're able to explain maybe? Like maybe they're actually cooked and dried and the bag just doesn't say that. It's ok if you don't know either. Thanks!!
Raw beans are bad due to the high amount of lectins in them. Raw lentils do still have some lectins in them so they are unsafe for us to eat but the amount isn't very dangerous for birds and it's very good feed ingredient since it's high in protein and fiber but low in fat. So the nutritional benefits outweigh the risk tbh and i have never heard of anyone's bird dying due to lentils being a part of their diet. In most high quality poultry feeds dried peas and lentils are a large part of the protein source and I prefer that to stuff like fish meal which can have a high bacterial load which can lead to certain intestinal diseases in chickens. Ideally you want your chickens main protein source in their feed to come from seeds,insects, and legumes.
You will see a lot of forum post from people who aren't very well versed in poultry nutrition panicking about lentils in their birds feed due to the phytohaemagglutinin but lentils are very good for chickens (and pigeons!) And there is plenty of research on the topic.
Here is this one for example (but there are a lot more)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7070479/
Like any feed ingredient they shouldn't be the bulk of a birds diet forever, barley for example can be very problematic in high amounts but its my favorite feed ingredient. Flax seed can cause sudden liver hemorrhage in high amounts (yikes!) but its still a great source of fats. So even the tiniest wee pigeon fledgling that only wants to eat lentils while weaning isn't going to keel over and die due to the lectins in the lentils.
Mung beans are also a safe chicken and pigeon feed ingredient and aren't like other beans just wanted to say that. Stay away from raw beans but mung beans are good 👍
32 notes
·
View notes
Text
CDC calls itself the nation’s “leading science-based, data-driven, service organization.” So, one might assume that the statements on its website are carefully vetted by at least one of its 1,700 scientists. Unfortunately, CDC is unable to provide ICAN with any records relied upon to support its statements on its COVID-19 vaccine “Myths and Facts” webpage.
The CDC.gov website is supposed to be one of the most authoritative public health websites in the world. Perplexed by many of the statements made on its (ironically-titled) webpage “Bust Myths and Learn the Facts about COVID-19 Vaccines,” ICAN’s legal team submitted numerous FOIA requests asking CDC for the evidence it relied upon to make those statements. We’ll discuss each of these “facts” in our new series: No Records Found.

“Nearly all the ingredients in COVID-19 vaccines are also ingredients in many foods – fats, sugars, and salts.” This was certainly news to us, especially since ICAN has closely studied the ingredients listed in each COVID-19 vaccine insert. In order to get to the bottom of this, ICAN’s attorneys sent multiple FOIA requests, phrased in different ways, asking for all records showing:
The foods that contain the same ingredients as those found in the COVID-19 vaccines.
The foods that contain recombinant spike protein from the SARS-CoV-2 virus.
The foods that contain nucleoside-modified messenger RNA.
The foods that contain extracts from the soapbark tree.
The foods that contain baculovirus and cellular DNA, lentil lectin, methyl-α-D-mannopyranoside, simethicone, pluronic F-68, Triton X-100 and/or Tergitol.
Eating an ingredient found in food has the same effect on the body as injecting that same ingredient into the body.
A rational person would assume that these requests would be answered with records, studies, or at least emails between CDC scientists that support CDC’s claimed “fact.” In fact, CDC is required to have this support pursuant to the Information Quality Act, which requires that the agency be able to “substantiate the quality of the information it has disseminated.”
It will come as no shock to ICAN supporters to learn that CDC was not able to find ANY records whatsoever in response to any of our requests about this claim. Not a single study, white paper, or even an email from the “leading” public health agency on the planet to support a statement it calls “fact” on a page about “busting myths.” Truly incredible, ironic, and disturbing.
Stay tuned for Segment 2 to learn about other CDC claims that are completely unsupported by any evidence. Until then, ICAN will continue to work to ensure that this agency starts serving the public with real, verifiable science—not myths.
6 notes
·
View notes
Text
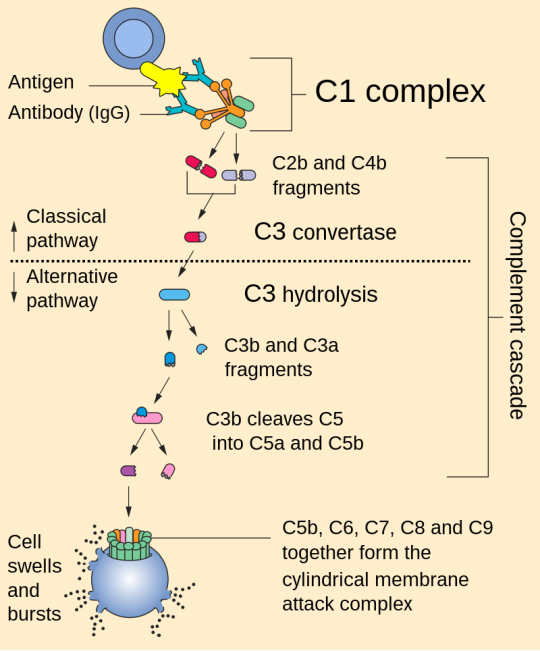
Complement system
1. Activation: The complement system can be activated through three main pathways: the classical pathway, the alternative pathway, and the lectin pathway. Each pathway involves different initiating events but converges on a common cascade of reactions.
2. Cascade of Reactions: Once activated, the complement system triggers a cascade of enzymatic reactions that result in the cleavage of complement proteins. This cascade ultimately leads to the formation of several key components, including C3b, C4b, and C5b.
3. Opsonization: C3b and C4b are opsonins, which means they can bind to pathogens and label them for phagocytosis by immune cells like macrophages and neutrophils. This enhances the removal of pathogens from the body.
4. Inflammation: Complement activation also results in the release of small peptides called anaphylatoxins, such as C3a and C5a. These peptides promote inflammation by increasing blood vessel permeability and attracting immune cells to the site of infection.
5. Membrane Attack Complex (MAC): The final step of complement activation involves the assembly of the membrane attack complex (MAC). C5b, C6, C7, C8, and multiple C9 molecules come together to form the MAC, which can create pores in the membranes of target cells, leading to cell lysis and destruction of pathogens.
References:
1. Walport, M. J. (2001). Complement. First of two parts. New England Journal of Medicine, 344(14), 1058-1066.
2. Ricklin, D., Hajishengallis, G., Yang, K., & Lambris, J. D. (2010). Complement: a key system for immune surveillance and homeostasis. Nature Immunology, 11(9), 785-797.
3. Merle, N. S., Church, S. E., Fremeaux-Bacchi, V., & Roumenina, L. T. (2015). Complement system part I – molecular mechanisms of activation and regulation. Frontiers in Immunology, 6, 262.
Please note that for the most current and detailed medical information on the complement system, I recommend consulting recent textbooks or academic journals in immunology and microbiology.
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#immunology#immune system#complement system
50 notes
·
View notes
Text
More Nightmaren Diet Headcanons
Another Diet headcanon post based off of @redrockbluerock's headcanons! Hope you all enjoy.
Despite having a love for Nightopian meat, Reala can only eat it up to 1-2 times a day due to a variety of reasons; one of them being that Nightopian meat contains LOTS of protein. Should he eat more than required, Reala will be prone to getting sick.
Nightmarens are allergic to legumes (i.e., beans, lentils, peas, chickpeas, and peanuts) as they all contain a chemical called lectin, which will cause nausea and an upset stomach. Reala happens to have this allergy a lot worse than the other Nightmarens as his skin even gets hives too.
If given the chance, Second-levels will eat up to 75 pounds of food due to the fact that they are larger than both First-levels and Third-levels. That's roughly 19 pounds more than how much a human stomach can take.
As hatchlings, NiGHTS and Reala could not eat solid foods until they started growing teeth. So during their baby days, they had to drink warm milk that was mixed with a certain type of nectar that can only be found in the Night Dimension.
Desserts for Nightmarens include, but are not limited to, sugared fruit, cookies made with honey, and custard. NiGHTS, since their rebellion, can always be seen eating these. Reala's favorite dessert, aside from cake, happens to be custard as well.
11 notes
·
View notes
Text
Shedding new light on sugars, the “dark matter” of cellular biology - Technology Org
New Post has been published on https://thedigitalinsider.com/shedding-new-light-on-sugars-the-dark-matter-of-cellular-biology-technology-org/
Shedding new light on sugars, the “dark matter” of cellular biology - Technology Org
Scientists at Université de Montréal’s Department of Chemistry have developed a new fluorogenic probe that can be used to detect and study interactions between two families of biomolecules essential to life: sugars and proteins.
Our idea was to label sugar molecules with a chromophore, a chemical that gives a molecule its colour,” explained Cecioni. “The chromophore is actually fluorogenic, which means that it can become fluorescent if the binding of sugar with the lectin is efficiently captured. Image credit: Cecioni Lab
The findings by professor Samy Cecioni and his students, which open the door to a wide range of applications, were published in mid-October in the prestigious European journal Angewandte Chemie.
Found in all living cells
Sugar is omnipresent in our lives, present in almost all the foods we eat. But the importance of these simple carbohydrates extends far beyond tasty desserts. Sugars are vital to virtually all biological processes in living organisms and there is a vast diversity of naturally occurring sugar molecules.
“All of the cells that make up living organisms are covered in a layer of sugar-based molecules known as glycans,” said Cecioni. “Sugars are therefore on the front line of almost all physiological processes and play a fundamental role in maintaining health and preventing disease.”
“For a long time,” he added, “scientists believed that the complex sugars found on the surface of cells were simply decorative. But we now know that these sugars interact with many other types of molecules, particularly lectins, a large family of proteins.”
Driving disease, from flu to cancer
Like sugars, lectins are found in all living organisms. These proteins have the unique ability to recognize and temporarily attach themselves to sugars. Such interactions occur in many biological processes, such as during the immune response triggered by an infection.
Lectins are attracting a lot of attention these days. This is because scientists have discovered that the phenomenon of lectins “sticking” to sugars plays a key role in the appearance of numerous diseases.
“The more we study the interactions between sugars and lectins, the more we realize how important they are in disease processes,” said Cecioni. “Studies have shown how such interactions are involved in bacteria colonizing our lungs, viruses invading our cells, even cancer cells tricking our immune system into thinking they’re healthy cells.”
Difficult to detect…until now
There are still many missing pieces in the puzzle of how interactions between sugars and lectins unfold because they are so difficult to study. This is because these interactions are transient and weak, making detection a real challenge.
Two of Cecioni’s students, master’s candidate Cécile Bousch and Ph.D. candidate Brandon Vreulz, had the idea of using light to detect these interactions. The three researchers set to work to create a sort of chemical probe capable of “freezing” the meeting between sugar and lectin and making it visible through fluorescence.
The interaction between sugar and lectin can be described using a “lock and key” relationship, where the “key” is the sugar and the “lock” is the lectin. Chemists have already created molecules capable of blocking this lock-and-key interaction, and can now to identify exactly what sugars are binding to lectins of high interest to human health.
“Our idea was to label sugar molecules with a chromophore, a chemical that gives a molecule its colour,” explained Cecioni. “The chromophore is actually fluorogenic, which means that it can become fluorescent if the binding of sugar with the lectin is efficiently captured. Scientists can then study the mechanisms underlying these interactions and the disturbances that can arise.”
Cecioni and his students are confident their technique can be used with other types of molecules. It may even be possible to control the colour of new fluorescently labelled probes that are created.
By making it possible to visualize interactions between molecules, this discovery is giving researchers a valuable new tool for studying biological interactions, many of which are critical to human health.
Source: University of Montreal
You can offer your link to a page which is relevant to the topic of this post.
#applications#Bacteria#Biology#Biomolecules#Cancer#cancer cells#Cells#cellular structures#challenge#chemical#chemistry#Chemistry & materials science news#Dark#dark matter#detection#Disease#Diseases#diversity#Explained#Fundamental#Giving#Health#how#human#Human health#immune response#immune system#infection#interaction#it
2 notes
·
View notes
Text

"Headcanons I Have of Saitama"
I'm going to do a daily post blog of headcanons i have of Saitama based either on canon, cai interactions, or just some i made up off the top of my head.
I'm doing daily post of each headcanon because i'll have more time to go a bit in depth about it and i wont run out of posting ideas. Enjoy!
"He's Vegan!"
It has been stated that he prefers cooking and eating vegetables. It's also ironic that this guy doesnt eat a lot of protein to help him bulk up on his body and strength.
But like Popeye, he gets his strength from good ol' spinach! Or whatever leafy greens he fancies in his occassional stir fry dishes he makes at home.
He wont admit it. He's clearly a vegan. He gets most of his protein from nuts and lectins he obtains locally. They dont get transferred often, but he does occassionally eat avocados for his fat intake.
It's crazy how he gets so strong on vegetables alone, but it seems to have the Popeye effect on him regardless. Though, he enjoys adding variety every time he stir fries tempura.
He doesnt mention that he's vegan to his friends unless the topic is brought up. And from time to time he shows slight enthusiasm when he shares recipes with people who are curious about the lifestyle.
He's not shy about it, but he also isn't crazy or obsessed about it either. Though, if he ever puts chicken or beef in his dishes.
It's most likely artificially delicious pieces of tofu substitute. You cant tell the difference though.
It took him time to perfect it but he finally was able to make the tofu substitute convincing enough to taste like meat.
He also likes tofu as is. Though, if you're like me and hate tofu, dont tell him how gross it is. He'll probably ignore you anyway.
He only makes the meat substitute for his meat eaters in the friend group. No complaints yet though.

(I'll probably revise this later with more knowledge on Japanese marketfood. I only know about leeks and honestly i didnt want him being a leek lover to be the talking point of my headcanon. At least i remember tempura being one. i still hope you liked it.)
like, reblog, whatev ✩

#saitama headcanons#headcanon#my headcanons#opm saitama#saitama opm#saitama one punch man#saitama#headcanons blog#my blog
3 notes
·
View notes
Text

Kochō Shinobu / Wisteria Poisoning

For the past couple of years, Shinobu has been dosing herself with wisteria extract in an effort to poison her body. This was done in anticipation of her eventual fight with Douma, believing that if she couldn't beat him in life, she could at least poison him in death if he were to eat her. However, wisteria isn't just poisonous to demons, it's also toxic to humans if ingested. This made her efforts that much more dangerous.
It took Shinobu several rounds of testing to come up with the appropriate dosage to use when poisoning herself. When ingested, wisteria is known to cause dizziness, confusion, oral burning and varying digestive upsets. Wisteria, in particular the seeds and seed pods, contain the compounds lectin and wisterin, both of which are toxic to humans.
Lectin itself is a protein that binds to carbohydrates and they can resist being broken down by the digestive symptom. Studies have found that lectins can interfere with the absorption of some minerals and are theorized that over a long period of time, lectins can lead to an autoimmune response, possibly resulting in inflammatory conditions.
Shinobu injects herself with a specially brewed wisteria solution in order to avoid most issues with her digestive system. Though she's found that her stomach is sensitive and most days you'll find her drinking ginger tea to sooth the issue. She believes that while she's always been small and physically weaker, it's possible that the wisteria poisoning contributed to her stunted growth. She's closely documented any changes in her body since she's begun taking the wisteria.
Overall, she has some mild rheumatoid arthritis symptoms (tenderness and swelling of the joints) and has to take extra vitamins in order to better support her body. She keeps all of this to herself, performing regular checkups after everyone else has gone to sleep. She just has to hope that she'll meet Douma before her secret weapon becomes too much for her to handle.
#been trying to do my own research so i hope this makes sense#∞࿚ kochō shinobu ( it would hit you like poison if you knew what i knew. )
1 note
·
View note
Text
LECTIN CONJUGATES' APPLICATIONS IN MOLECULAR AND CELLULAR BIOLOGY

Lectins are carbohydrate-binding proteins found in almost all plants and a few mammals. The term Lectins is derived from the Latin word 'Legree,' which meaning to choose or select. Lectins do not cause antigenic activation inside the immune system, but they do have the ability to bind analogously to an antibody.
LECTIN CONJUGATES
Lectin Conjugates are proteins (building blocks) that bind to cells and specific lectin carbohydrate groups on proteins or cell membrane proteins. They are further classified based on their amino acid sequences and biological characteristics. Lectins contain 120 amino acids that are responsible for carbohydrate binding.
Lectins proteins with a high degree of stereo specificity that recognise diverse sugar structures and generate reversible connections when they engage with glycoconjugate complexes. These are abundant in plant lectins, animal lectins, and many other species known to agglutinate numerous erythrocyte blood types.
Because of its carbohydrate binding, lectin is employed in glycobiology to analyse cell surfaces glycoproteins. Lectins are synthesised in laboratories after being extracted from either plant or animal components.
Lectins enable researchers to explore a wide range of biological activities and processes. Because of their complex binding requirements, certain Lectins bind to mannose or glucose residues, whilst others bind only to galactose residues. Some lectins also require sugar-binding in specific locations in oligosaccharides.
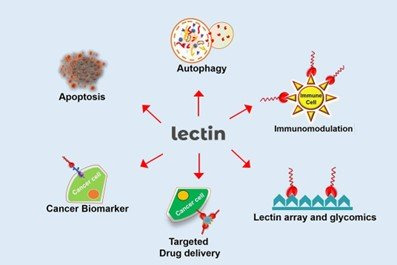
Lectin proteins are widely employed in biomedical applications due to their ability to recognise carbohydrates via carbohydrate-binding sites, which identify glycans linked to cell surfaces, glycoconjugates, or free sugars, detecting aberrant cells and disease biomarkers.
A variety of infectious agents and diseases afflict the human species, causing a chain reaction of repercussions. The biotechnological area has looked for biorecognition molecules with diagnostic and therapeutic potential from natural or recombinant sources. Numerous algal lectins have also received significant interest for biological uses such as antinociceptive, anti-HIV, antitumoral, anti-inflammatory, and antibacterial properties.
MOLECULAR AND CELLULAR BIOLOGY APPLICATIONS
1. Lactin-Induced Immunological and Inflammatory Responses: Immunological and inflammatory responses are important in protecting the organism from invading agents and altered cells. The immune system functions in two ways: innate immunity and adaptive immunological responses, which are activated by a variety of cells and molecules and promote the destruction or inactivation of a hostile agent.
Basophils, neutrophils, eosinophils, and monocytes/macrophages all have specialised tasks and the ability to release and create chemicals known as cytokines. Cytokines can influence immune cell inflammation, humoral response, and activation. Lectins are also being studied for their ability to protect the host and vectors against parasites and viruses.
2. Many medical researchers have observed therapeutic benefits and effects promoted by lectins. Healing is the process of repairing tissue following harm. A monitored group of cells and molecules initiates ordered phases that result in morphological and functional healing of wounded tissues.
The importance of lectins as a healing agent is not totally understood; nonetheless, lectins may influence the immune response, inflammatory response, cytokine generation, and cell antiproliferative action throughout the healing process. Lectins have stimulated wound healing and scarring modification, with outstanding results and therapeutic potential.
3. Lectins for Drug Delivery: Therapies based on chemical agents face some challenges, primarily in terms of rising dosages and metabolic activity, which reduces treatment effectiveness. Systems for delivering medications to a specified target may be interesting and practical solutions for troubleshooting these issues and minimising undesirable side effects. To be a promising drug delivery method, lectins must be avid mannose-binding lectins with low toxicity and site-specific molecules.
4. Histochemical Markers of Lectins: Glycan moieties on cell surfaces play a role in a variety of physiological and pathological processes. Disruptions in the cell environment caused by illnesses typically cause changes in glycans]. This technique has been used in the study, diagnosis, and prognosis of human disorders characterised by changed cells in tissues, such as cancer.
Lectin histochemistry is an appealing method for identifying altered tissues and clinical processes such as metastasis. Differential lectin binding patterns may discriminate between normal, benign, and malignant tumours of varying degrees. Lectins used to study the glycan profile in altered tissues are valuable tools for cancer detection and prognosis.
5. Lectin-Based Biosensors for Disease Detection: Glycans can be identified and quantified using lectin-based biosensors. These systems work by translating lectin-carbohydrate interactions into a quantifiable signal on a surface, allowing biomarkers to be measured.
Biosensing and transduction methods can be electrochemical, optical, mass, and thermal in accordance with a single kind. However, the electrochemical biosensor is more appealing since it is a practical, quick, user-friendly, and simple-to-use assay with different designs and analytical performance. Electrochemical lectin-positioned biosensors are more appealing as analytical tools for glycans and their application in detecting pathogens and diagnosing diseases via biomarker identification.
6. Lectins as Anticancer Agents: Lectins from many sources have cytotoxic effects on cancer cells, such as activation of cell death pathways and inhibition of proliferation. Furthermore, several anticancer lectins have poor cytotoxicity against nontransformed cells. This is most likely due to the differential expression of glycans on the surfaces of cancer and normal cells, which allows lectins to recognise malignant cells uniquely.
CONCLUSIONS
Many biotechnological applications and medical therapeutics rely on lectins from various sources with varying carbohydrate recognition events. Lectins do have antimicrobial and antiviral properties; it is a powerful modulator of mitosis, immune response, proliferation, drug-delivery therapies, cancer regression, and healing.
Using lectin-based techniques such as biosensors and histochemistry, which can detect illnesses and infectious agents, changed glycans on tissue or cell surfaces and serum samples can be identified. As a result, the accomplishments attributed to lectins are focused on biotechnological/pharmacological and therapeutic applications, serving as a significant resource for future research into the biological effects, routes, and biotechnological potential of lectins.
Contact the experts at Helvetica Health Care for more information on the molecular and cellular application of lectins conjugates.
0 notes
Text
From a link off the Toxicity section of the kidney bean’s Wikipedia page:
“Phytohaemagglutinin (PHA, or phytohemagglutinin) is a lectin found in plants, especially certain legumes. PHA actually consists of two closely related proteins, called leucoagglutinin (PHA-L) and PHA-E. These proteins cause blood cells to clump together. PHA-E cause erythrocytes (red blood cells) to clump. PHA-L causes leukocytes (white blood cells) to clump. Phytohaemagglutinin has carbohydrate-binding specificity for a complex oligosaccharide containing galactose, N-acetylglucosamine, and mannose.[2]”
And now I want to make a t-shirt with “Galactose The Intolerant, Devourer of Worlds” on it.
And maybe a crest or coat of arms of some kind with a kidney bean and cleansing fire motif that reads “Stay calm or perish” above and “Rage powers my survival” below.
In Latin.
nothing in the world makes me more evil than just being kind of annoyed
207K notes
·
View notes
Text

CDC calls itself the nation’s “leading science-based, data-driven, service organization.” So, one might assume that the statements on its website are carefully vetted by at least one of its 1,700 scientists. Unfortunately, CDC is unable to provide ICAN with any records relied upon to support its statements on its COVID-19 vaccine “Myths and Facts” webpage.
The CDC.gov website is supposed to be one of the most authoritative public health websites in the world. Perplexed by many of the statements made on its (ironically-titled) webpage “Bust Myths and Learn the Facts about COVID-19 Vaccines,” ICAN’s legal team submitted numerous FOIA requests asking CDC for the evidence it relied upon to make those statements. We’ll discuss each of these “facts” in our new series: No Records Found.
In this first segment, we’ll tackle perhaps the most surprising of all the statements on the page:

“Nearly all the ingredients in COVID-19 vaccines are also ingredients in many foods – fats, sugars, and salts.” This was certainly news to us, especially since ICAN has closely studied the ingredients listed in each COVID-19 vaccine insert. In order to get to the bottom of this, ICAN’s attorneys sent multiple FOIA requests, phrased in different ways, asking for all records showing:
The foods that contain the same ingredients as those found in the COVID-19 vaccines.
The foods that contain recombinant spike protein from the SARS-CoV-2 virus.
The foods that contain nucleoside-modified messenger RNA.
The foods that contain extracts from the soapbark tree.
The foods that contain baculovirus and cellular DNA, lentil lectin, methyl-α-D-mannopyranoside, simethicone, pluronic F-68, Triton X-100 and/or Tergitol.
Eating an ingredient found in food has the same effect on the body as injecting that same ingredient into the body.
A rational person would assume that these requests would be answered with records, studies, or at least emails between CDC scientists that support CDC’s claimed “fact.” In fact, CDC is required to have this support pursuant to the Information Quality Act, which requires that the agency be able to “substantiate the quality of the information it has disseminated.”
It will come as no shock to ICAN supporters to learn that CDC was not able to find ANY records whatsoever in response to any of our requests about this claim. Not a single study, white paper, or even an email from the “leading” public health agency on the planet to support a statement it calls “fact” on a page about “busting myths.” Truly incredible, ironic, and disturbing.
1 note
·
View note
Text
What’s Next for Plant-based and Alternative Proteins?

The Business of Food Trends
The plant-based and alternative protein industry has seen explosive growth in recent years, driven by increasing consumer demand for more sustainable, ethical and healthier food options. As we look to the future, this sector is poised for continued innovation and expansion. From novel ingredients to advanced manufacturing techniques, the next wave of alternative proteins promises to revolutionize our food systems and eating habits.
Emerging Protein Sources
While soy, wheat and pea proteins have dominated the plant-based market, companies are now exploring a wider array of protein sources to improve taste, texture and nutritional profiles. Emerging options include:
Algae: Rich in protein and omega-3 fatty acids, algae is a highly sustainable option with a small environmental footprint.
Mycoprotein: Derived from fungi, mycoprotein offers a meat-like texture and complete protein profile.
Duckweed: This aquatic plant is protein-dense and grows rapidly, making it a promising sustainable protein source.
Insects: Though facing cultural barriers in some markets, insect protein is highly nutritious and environmentally friendly.
Cultured Meat: Lab-grown meat from animal cells could provide a cruelty-free alternative to traditional animal agriculture.
These novel protein sources will likely play an increasing role in future alternative protein products, offering consumers more variety and potentially superior nutritional profiles.
Advanced Manufacturing Techniques
The next generation of alternative proteins will benefit from cutting-edge production methods that enhance texture, flavor and overall quality:
3D Printing: This technology allows for the creation of complex, meat-like structures with precise control over texture and composition.
Shear Cell Technology: This method produces fibrous, meat-like textures through controlled application of heat and mechanical shear forces.
High-moisture Extrusion: An improved version of traditional extrusion techniques, this process creates more realistic meat analogues.
Fermentation: Both traditional and precision fermentation techniques are being leveraged to produce proteins, fats and flavor compounds.
These advanced manufacturing methods will enable the creation of more sophisticated plant-based products that better mimic the sensory experience of animal-based foods.
Improved Nutritional Profiles
As the alternative protein market matures, there’s an increasing focus on nutritional optimization. Future products are likely to feature:
Complete Amino Acid Profiles: By combining complementary plant proteins or through precision fermentation, products will offer more balanced protein nutrition.
Enhanced Bioavailability: New processing techniques and ingredient combinations will improve the body’s ability to absorb and utilize plant-based nutrients.
Fortification with Micronutrients: Products will increasingly be fortified with vitamins and minerals typically found in animal products such as vitamin B12, iron and zinc.
Reduced Antinutrients: Innovative processing methods will help minimize compounds that can interfere with nutrient absorption, such as phytates and lectins.
These nutritional improvements will help address concerns about the healthfulness of plant-based diets and make alternative proteins a more viable option for a wider range of consumers.
Expanding Product Categories
While plant-based burgers and milk alternatives have led the way, the future will see alternative proteins expanding into new product categories:
Whole-cut Meats: Technologies like 3D printing and mycelium growth will enable the creation of plant-based steaks, chicken breasts and other whole-cut meat analogues.
Seafood Alternatives: As overfishing concerns grow, expect to see more convincing plant-based and cell-cultured seafood options.
Egg Replacements: Beyond simple substitutes, we’ll see more versatile egg alternatives suitable for a wide range of culinary applications.
Cheese Alternatives: Improved fermentation techniques will yield plant-based cheeses with more authentic flavors and melting properties.
Infant Formula: Plant-based and cell-cultured breast milk alternatives could offer new options for parents and caregivers.
This diversification will make it easier for consumers to adopt plant-based diets across all aspects of their food consumption.
Regulatory Landscape and Labeling
As the alternative protein industry grows, it will face increasing regulatory scrutiny and potential labeling changes:
Standardized Terminology: Expect more clearly defined guidelines for terms like “plant-based,” “cell-cultured,” and “animal-free.”
Nutritional Equivalence Standards: Regulators may require alternative proteins to meet certain nutritional benchmarks to be marketed as replacements for animal products.
Safety Assessments: Novel ingredients and production methods will undergo rigorous safety evaluations before market approval.
Sustainability Claims: There may be increased regulation around environmental impact claims to prevent greenwashing.
These regulatory developments will help ensure consumer safety and trust in alternative protein products.
Sustainability and Environmental Impact
The next phase of alternative protein development will place an even greater emphasis on sustainability:
Circular Economy Approaches: Companies will increasingly use byproducts and waste streams as inputs for alternative protein production.
Localized Production: Smaller-scale, regional production facilities will help reduce transportation emissions and support local economies.
Water-efficient Crops: Drought-resistant and low-water input protein crops will become more prominent in response to climate change concerns.
Biodiversity Promotion: Alternative protein producers will explore a wider variety of crop sources to support agricultural biodiversity.
These sustainability initiatives will further cement the environmental advantages of alternative proteins over traditional animal agriculture.
Read More: https://foodnbeverageinsights.com/whats-next-for-plant-based-and-alternative-proteins/
1 note
·
View note
Text
Boost Insulin Sensitivity Naturally

Improving insulin sensitivity helps control blood sugar and reduces the risk of metabolic disorders.
Regular physical activity enhances how cells respond to insulin.
Sleep and stress management play a role in insulin sensitivity.
Nutrient-rich, bioavailable foods are key for supporting insulin function.
Prioritizing healthy fats supports stable blood sugar levels.
What is Insulin Sensitivity?
Insulin is a hormone produced by the pancreas that helps regulate blood sugar by allowing glucose to enter cells for energy. Insulin sensitivity is how well your cells respond to this process.
Higher sensitivity means that less insulin is required to lower blood sugar levels, while low sensitivity (insulin resistance) means that more insulin is needed to achieve the same effect.
Over time, insulin resistance can lead to consistently high blood sugar and an increased risk of chronic health conditions.
Factors Affecting Insulin Sensitivity
Several factors influence insulin sensitivity, including diet, physical activity, sleep, and stress levels.
Diets high in processed carbohydrates, lack of exercise, and poor sleep habits can reduce insulin sensitivity.
On the other hand, a nutrient-dense, animal-based diet combined with an active lifestyle can greatly improve how your body uses insulin.
Diet & Insulin Sensitivity

The foundation of a healthy diet for improving insulin sensitivity lies in prioritizing nutrient-dense, bioavailable foods.
These foods provide the necessary nutrients to support insulin function without causing spikes in blood sugar.
Prioritizing Nutritious Foods
Bioavailable foods, especially grass-fed red meat and organ meats, are packed with essential amino acids, vitamins and minerals that help regulate blood sugar and insulin levels.
Pasture-raised eggs, rich in choline, high-quality protein and healthy fats, further support metabolic health.
Wild-caught seafood offers omega-3 fatty acids, which have been shown to improve insulin sensitivity and reduce inflammation.
Foods That Improve Insulin Sensitivity
Grass-fed red meat: Nutrient-dense, high in healthy fats, and supports blood sugar balance.
Pasture-raised eggs: High in protein and fat, eggs help stabilize insulin and glucose levels.
Wild-caught seafood: Rich in omega-3 fats, seafood helps reduce inflammation and improve insulin response.
Healthy fats: Ghee, butter, and tallow provide long-lasting energy and help maintain stable blood sugar.
Limiting Plant-Based Foods
While certain vegetables can complement an animal-based diet, they should not exceed 10% of total food intake.
Lettuce, cabbage, cucumbers, watercress, and carrots are low-carb options that can be included without disrupting insulin function.
Avoid high-oxalate and high-lectin foods, as they can interfere with nutrient absorption and negatively impact insulin sensitivity.
Exercise and Insulin Sensitivity

Regular physical activity is one of the most effective ways to boost insulin sensitivity. Exercise helps your muscles absorb more glucose, lowering blood sugar and improving how your body uses insulin.
Both resistance training and aerobic exercises contribute to better insulin response.
Recommended Physical Activities
Strength training: Building muscle through weightlifting or resistance exercises increases glucose uptake and improves insulin sensitivity.
Aerobic exercise: Activities like walking, swimming, and cycling help lower blood sugar levels and improve metabolic health.
High-intensity interval training (HIIT): Short bursts of intense activity followed by rest periods can significantly enhance insulin sensitivity in a short amount of time.
Sleep and Stress Management
Poor sleep and high stress levels can negatively affect insulin sensitivity. Sleep deprivation disrupts blood sugar control, leading to increased insulin resistance.
Chronic stress triggers the release of cortisol, a hormone that raises blood sugar and impairs insulin function.
Strategies for Improving Sleep and Reducing Stress
Prioritize 7-9 hours of sleep: Consistent, quality sleep is essential for maintaining healthy insulin levels.
Manage stress through relaxation techniques: Practices like meditation, deep breathing, and mindfulness can help reduce cortisol levels and support insulin function.
Avoiding Ultra-Processed Foods

To naturally improve insulin sensitivity, it is essential to eliminate ultra-processed foods and refined carbohydrates from the diet.
These foods cause rapid spikes in blood sugar, leading to higher insulin levels and worsening insulin resistance. Instead, focus on whole, nutrient-rich animal foods that provide sustained energy without blood sugar fluctuations.
Foods to Avoid
Refined carbohydrates: Bread, pasta, and sugary snacks should be avoided to prevent insulin spikes.
Processed and synthetic foods: Artificial ingredients and additives can disrupt blood sugar balance and insulin response.
Conclusion
Boosting insulin sensitivity is achievable through a natural, animal-based diet and an active lifestyle. Grass-fed meats, eggs, and healthy animal fats provide the nutrients needed to support insulin function and maintain stable blood sugar levels. Incorporating regular physical activity, managing stress, and getting quality sleep further enhances insulin sensitivity. By focusing on these natural methods, you can improve your overall health and reduce the risk of developing insulin resistance or related conditions.
FAQs
What are the best foods for improving insulin sensitivity?
Grass-fed red meat, wild-caught seafood, pasture-raised eggs, and healthy animal fats like ghee, butter, and tallow are excellent for supporting insulin sensitivity.
How does exercise affect insulin sensitivity?
Exercise increases glucose uptake by the muscles, which lowers blood sugar and improves how effectively the body uses insulin.
Can poor sleep lead to insulin resistance?
Yes, poor sleep disrupts blood sugar control and raises the risk of insulin resistance by affecting hormone balance and insulin function.
What lifestyle changes can help boost insulin sensitivity?
Eating a nutrient-dense animal-based diet, engaging in regular physical activity, managing stress, and improving sleep quality can all help improve insulin sensitivity.
How does stress impact blood sugar and insulin sensitivity?
Chronic stress raises cortisol levels, which can lead to higher blood sugar and reduced insulin sensitivity. Managing stress is important for maintaining healthy insulin levels.
Research
Arumugam, S. and Suyambulingam, A., 2024. Association Between Serum Ferritin and the Duration of Type 2 Diabetes Mellitus in a Tertiary Care Hospital in Chennai. Cureus. [online] https://doi.org/10.7759/cureus.53117.
Chitturi, S. and George, J., 2003. Interaction of iron, insulin resistance, and nonalcoholic steatohepatitis. Curr Gastroenterol Rep, [online] 5(1), pp.18-25. https://doi.org/10.1007/s11894-003-0005-y.
DiNicolantonio, J.J., Mangan, D. and O’Keefe, J.H., 2018. Copperdeficiency may be a leading cause of ischaemic heart disease. Open Heart, [online] 5(2), p.e000784. https://doi.org/10.1136/openhrt-2018-000784.
DiNicolantonio, J.J., Mangan, D. and O’Keefe, J.H., 2018. The fructose–copper connection: Added sugars induce fatty liver and insulin resistance via copper deficiency. Journal of Metabolic Health, [online] 3(1). https://doi.org/10.4102/jir.v3i1.43.
Dubey, P., Thakur, V. and Chattopadhyay, M., 2020. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients, [online] 12(6), p.1864. https://doi.org/10.3390/nu12061864.
Ferrannini, E., Vichi, S., Beck-Nielsen, H., Laakso, M., Paolisso, L. and Smith, G., 1996. Insulin Action and Age: European Group for the Study of Insulin Resistance (EGIR). Diabetes, [online] 45(7), pp.947–953. https://doi.org/10.2337/diab.45.7.947.
Freeman, A.M., Acevedo, L.A. and Pennings, N., 2023. Insulin Resistance. In: StatPearls. StatPearls Publishing, Treasure Island (FL). PMID: 29939616.
Jiang, X., Hu, R., Huang, Y., Xu, Y., Zheng, Z., Shi, Y., Miao, J. and Liu, Y., 2023. Fructose aggravates copper-deficiency-induced non-alcoholic fatty liver disease. The Journal of Nutritional Biochemistry, [online] 119, p.109402. https://doi.org/10.1016/j.jnutbio.2023.109402.
Kahn, B.B. and Flier, J.S., 2000. Obesity and insulin resistance. Journal of Clinical Investigation, [online] 106(4), pp.473–481. https://doi.org/10.1172/jci10842.
Kant, R., Verma, V., Patel, S., Chandra, R., Chaudhary, R., Shuldiner, A.R. and Munir, K.M., 2021. Effect of serum zinc and copper levels on insulin secretion, insulin resistance and pancreatic β cell dysfunction in US adults: Findings from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Diabetes Research and Clinical Practice, [online] 172, p.108627. https://doi.org/10.1016/j.diabres.2020.108627.
Kaye, T.B., Guay, A.T. and Simonson, D.C., 1993. Non-insulin-dependent diabetes mellitus and elevated serum ferritin level. Journal of Diabetes and its Complications, [online] 7(4), pp.245–249. https://doi.org/10.1016/s0002-9610(05)80252-1.
Kohgo, Y., Ikuta, K., Ohtake, T., Torimoto, Y., & Kato, J. (2007). Iron overload and cofactors with special reference to alcohol, hepatitis C virus infection and steatosis/insulin resistance. World Journal of Gastroenterology : WJG, 13(35), 4699-4706. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4611191/
Laakso, M., 1993. How Good a Marker Is Insulin Level for Insulin Resistance? American Journal of Epidemiology, [online] 137(9), pp.959–965. https://doi.org/10.1093/oxfordjournals.aje.a116768.
Lee, S.-H., Park, S.-Y. and Choi, C.S., 2022. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes & Metabolism Journal, [online] 46(1), pp.15–37. https://doi.org/10.4093/dmj.2021.0280.
Mendler, M.H., Turlin, B., Moirand, R., Jouanolle, A.M., Sapey, T., Guyader, D., Le Gall, J.Y., Brissot, P., David, V. and Deugnier, Y., 1999. Insulin resistance-associated hepatic iron overload. Gastroenterology, [online] 117(5), pp.1155-63. https://doi.org/10.1016/s0016-5085(99)70401-4.
Menezes-Santos, M., Santos, B. da C., Santos, R.K.F., da Costa, S.S.L., dos Santos, S.H., e Silva, A.M. de O., Rocha, V. de S. and Pires, L.V., 2024. Copper Deficiency Associated with Glycemic Control in Individuals with Type 2 Diabetes Mellitus. Biological Trace Element Research. [online] https://doi.org/10.1007/s12011-024-04185-6.
Petersen, K.F. and Shulman, G.I., 2006. Etiology of Insulin Resistance. The American Journal of Medicine, [online] 119(5), pp.S10–S16. https://doi.org/10.1016/j.amjmed.2006.01.009.
Petersen, M.C. and Shulman, G.I., 2018. Mechanisms of Insulin Action and Insulin Resistance. Physiological Reviews, [online] 98(4), pp.2133–2223. https://doi.org/10.1152/physrev.00063.2017.
Reaven, G.M., 1988. Role of Insulin Resistance in Human Disease. Diabetes, [online] 37(12), pp.1595–1607. https://doi.org/10.2337/diab.37.12.1595.
Reaven, G.M., 2003. The insulin resistance syndrome. Current Atherosclerosis Reports, [online] 5(5), pp.364–371. https://doi.org/10.1007/s11883-003-0007-0.
Schinner, S., Scherbaum, W.A., Bornstein, S.R. and Barthel, A., 2005. Molecular mechanisms of insulin resistance. Diabetic Medicine, [online] 22(6), pp.674–682. https://doi.org/10.1111/j.1464-5491.2005.01566.x.
Shoelson, S.E., 2006. Inflammation and insulin resistance. Journal of Clinical Investigation, [online] 116(7), pp.1793–1801. https://doi.org/10.1172/jci29069.
Sesti, G., 2006. Pathophysiology of insulin resistance. Best Practice & Research Clinical Endocrinology & Metabolism, [online] 20(4), pp.665–679. https://doi.org/10.1016/j.beem.2006.09.007.
Shulman, G.I., 2000. Cellular mechanisms of insulin resistance. Journal of Clinical Investigation, [online] 106(2), pp.171–176. https://doi.org/10.1172/jci10583.
Song, M., Vos, M. B., & McClain, C. J. (2018). Copper-Fructose Interactions: A Novel Mechanism in the Pathogenesis of NAFLD. Nutrients, 10(11). https://doi.org/10.3390/nu10111815
Tan, P.Y. and Soma Roy, M., 2021. Dietary copper and selenium are associated with insulin resistance in overweight and obese Malaysian adults. Nutrition Research, [online] 93, pp.38–47. https://doi.org/10.1016/j.nutres.2021.06.008.
Tuomainen, T.-P., Nyyssönen, K., Salonen, R., Tervahauta, A., Korpela, H., Lakka, T., Kaplan, G.A. and Salonen, J.T., 1997. Body Iron Stores Are Associated With Serum Insulin and Blood Glucose Concentrations: Population study in 1,013 eastern Finnish men. Diabetes Care, [online] 20(3), pp.426–428. https://doi.org/10.2337/diacare.20.3.426.
Wallace, T.M. and Matthews, D.R., 2002. The assessment of insulin resistance in man. Diabetic Medicine, [online] 19(7), pp.527–534. https://doi.org/10.1046/j.1464-5491.2002.00745.x.
Wilcox, G., 2005. Insulin and insulin resistance. Clin Biochem Rev, [online] 26(2), pp.19-39. PMID: 16278749; PMCID: PMC1204764.
Zhang, M., Zhang, C., Zhang, X., Li, J., Zhao, J., Xu, X., Liu, Y. and Chen, J., 2020. Relationship between serum iron levels and insulin resistance in type 2 diabetes patients. Diabetes Research and Clinical Practice, [online] 165, p.108231. https://doi.org/10.1016/j.diabres.2020.108231
0 notes