#copper hydroxide
Explore tagged Tumblr posts
Text

#calamine#malachite#magnetite#cryolite#checmistry#solutions#zinc sulphate#copper carbonate#ferric oxide#aluminum fluoride#copper hydroxide#tri sodium aluminum fluoride#zinc carbonate
1 note
·
View note
Text
Significant features of the global cycle of copper are summarized in Fig. 13.7.

"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quotes#environmental chemistry#nonfiction#textbook#copper#atmosphere#lithosphere#hydrosphere#biosphere#aerosol#precipitation#industrial emissions#dust#salt spray#sea salt#sea water#fresh water#leaching#sedimentation#soil#oxide#hydroxide#sulfide#mining#tailings#plants#chalcopyrite
3 notes
·
View notes
Text

long story short we’re dealing with a copper (II) salt here
#why is psychic turning blue. why is he becoming one with the cations#copper (II) hydroxide more like copper (II) PSIdroxide amiright dudes#fnf mind games#friday night funkin#my artwork#doodle#psychic’s chemistry chronicles#fnf psychic#psychic fnf#fnf fanart#psychic daily
10 notes
·
View notes
Text

TSRNOSS, p 761.
#ice crystal#vapour pressure#colour of lakes#Antarctica#deep blue#handwriting#legibility#superoxide dismutase#copper#zinc#Antarctic summer#lake bottom#stratification of sediments#Carl Woese#nubigenous origin of life#clouds#Dead Sea#underground water#colloid#manganese hydroxide#tundra lakes#silicon
0 notes
Text
Writing Notes: Poison

References (Forms, Actions & Examples of Poison; Route of Administration; Some Symptoms; What to do if a Poisoning Happens)
400 years back, Paracelsus stated that, “All substances are poisons; there is none which is not a poison.”
If the right dose is taken, it could become a remedy, otherwise poisonous.
Poison - a substance which when administered, inhaled or swallowed by living organism causes ill effects on the body. It is defined also as a medicine in a toxic dose. Toxic substance may be solid, liquid, gas or any environmental agent.
Forms of Poison
Physical form: Gaseous/volatile/vaporous forms of poisons act faster than liquid poisons as they are quickly absorbed. Similarly, liquid poisons act faster than solid poisons. Gaseous or volatile > liquid > solid. For solid poisons, powdered poisons act quickly than the lumps. For example, there are certain seeds that escape the gastrointestinal tract as they are solid, but when crushed, they can be fatal. For solids: powdered > lumps
Chemical form: Few substances like mercury or arsenic are not poisonous as they are insoluble and cannot be absorbed when they are in combination with other substances like mercuric chloride, arsenic oxide, etc. In other cases, the action is vice versa. For example, there are some substances that become inert in combination with silver nitrate and hydrochloric acid and are deadly and poisonous when present in pure forms.
Mechanical combination: The effect of poisons is significantly altered when they are combined with inert substances.
Action of Poisons
Local action: Direct action on the affected site of the body. Examples include irritation and inflammation in strong mineral acids and alkalis, congestion and inflammation by irritants, the effect on motor and sensory nerves, etc.
Remote action: Affects the person due to absorption of that poison into the system of that person. For example, alcohol is absorbed in the system and then it affects the person.
Local and remote actions: Some poisons can affect both local and remote organs. Thus, they not only affect the area with contact to the poison but also cause toxic effect after absorption into the system.
General action: The absorbed poison affects more than one system of the body, for example, mercury, arsenic, etc.
Route of Administration
The route of administration is the path through which a drug, toxin, or poison is taken or administered into the body of a person which is distinguished by the location where any drug is applied. It is mostly classified on the basis of its target:
Topical—has a local effect
Enteral—has a wide effect, i.e., affect the whole system
Parental—follows a systemic action
Poisons are given or taken so that death can occur at once by shock due to stoppage of body’s vital systems.
Route of administration plays a very important role in determination of death by poison as time in which death occurs are fastest in inhaled poisons, relatively slow in injected and lastly when ingested orally.
Some Symptoms
Sore throat
Trouble breathing
Drowsiness, irritability, or jumpiness
Nausea, vomiting, or stomach pain without fever
Lip or mouth burns or blisters
Unusual drooling
Strange odors on breath
Unusual stains on clothing
Seizures or unconsciousness
Examples
Poisons Based on Mode of Action
1. Corrosive poisons
Strong Acid - sulfuric acid, nitric acid, hydrochloric acid
Strong Base - sodium hydroxide, potassium hydroxide, ammoniumhydroxide
2. Irritant poisons
(a) Inorganic:
Metallic - lead, arsenic, mercury, antimony, copper, zinc
Non-metallic - chlorine, bromine, iodine
(b) Organic:
Vegetable - croton oil, castor oil
Animal - snake venom, scorpion venom, spider venom
(c) Mechanical: powder glass, diamond dust
3. Neurotic poisons
Cerebral - alcohol, opium, barbiturates, benzodiazepines
Spinal - strychnine
Peripheral - curare
4. Cardiac poisons
5. Asphyxiants - CO2, CO
Poisons Based on Medicolegal Classification
Homicidal poisons - aconite, abrus precatorius, strychnos nux vomica
Suicidal poisons - opium, barbiturate, organophosphorous, organochloro compounds
Accidental poisons - snake bite, CO, dhatura's seeds as it resembles capsicum seeds
Abortifacient poisons - quinine, calotropis
Stupefying agents - dhatura, chloral hydrate
Agents used to cause body injury - corrosive acids
Cattle poison - abrus precatorius, calotropis
Used for malingering - semicarpus anacardium
Poisons Based on Toxico-analytical Classification
1. Gaseous poisons: methanol, ethanol, benzene, toluene, acetone
2. Volatile substances: ethane, butane
3. Organic Non-volatile substances:
Drugs - opiates and synthetic narcotics, sedatives and hypnotics, stimulants, depressants
Pesticides - insecticides, fungicides, herbicides, rodenticides, nematocides
4. Metallic poisons: arsenic, lead, mercury, antimony, zinc, copper
5. Anion poisons: bromide, cyanide, fluoride, hypochlorite, nitrate, phosphate, sulfide, sulfate
Poisons Based on Physical State
1. Solid: lead, arsenic, mercury
2. Liquid:
Organic - ethanol, methanol, chloroform, acetone
Inorganic - liquid ammonia, liquid sulfur dioxide
3. Gaseous: carbon dioxide, carbon monoxide
Poisonous Fumes or Gases
In the home, poisonous fumes can be emitted from the following sources:
A car running in a closed garage
Leaky gas vents
Wood, coal, or kerosene stoves that are not working properly
Mixing bleach and ammonia together while cleaning, which makes chloramine gas
Strong fumes from other cleaners and solvents
Common Household Products
Oily hydrocarbon products are thin and slippery and can easily suffocate if the substances are drawn into the lungs when ingested. The products can cause chemical pneumonia by coating the inside of the lungs. Products that are required to have a safety lid include:
Baby oils
Sunscreens
Nail enamel dryers
Hair oils
Bath, body, and massage oils
Makeup removers
Some automotive chemicals (gasoline additives, fuel injection cleaners, and carburetor cleaners)
Cleaning solvents (wood oil cleaners, metal cleaners, spot removers, and adhesive removers)
Some water repellents containing mineral spirits used for decks, shoes, and sports equipment
General-use household oil
Gun-cleaning solvents containing kerosene
Oil products that are thicker and more "syrupy" are not as problematic, since they are not as easily inhaled into the lungs.
What to do if a poisoning happens
Swallowed poisons
Stay calm, act quickly, and follow these guidelines:
Get the poison away
If the substance is still in the mouth, make them spit it out or remove it with your fingers (keep this along with any other evidence of what was swallowed)
Do not make them vomit
Do not follow instructions on packaging regarding poisoning because these are often outdated. Instead, call Poison Help to get connected to a local poison center.
Take or send the poison container with you to help the healthcare provider find out what was swallowed.
Poisons on the skin
If someone spills a chemical on his or her body, remove his or her clothes and rinse the skin with lukewarm—not hot—water.
If the area shows signs of being burned, continue rinsing for at least 15 minutes, no matter how much they may protest.
Then call the poison control center for further advice.
Do not use ointments or grease.
Poison in the eye
Flush the eye by holding the eyelid open and pouring a steady stream of lukewarm—not hot—water into the inner corner of the eye.
If this is a child, you may need help from another adult to hold the child while you rinse the eye.
Continue flushing the eye for 15 minutes, and call the poison control center for further instructions.
Do not use an eyecup, eyedrops, or ointment unless the poison center tells you to do so.
Poisonous fumes or gases
If someone breathes in fumes or gases, get him or her into fresh air right away.
If they are breathing without a problem, call the poison center for further instructions.
If they are having difficulty breathing, call 911 or your local emergency service (EMS).
If they have stopped breathing, start CPR and do not stop until they breathe on their own or someone else can take over.
If you can, have someone call 911 right away.
If you are alone, perform CPR for 2 minutes and then call 911.
Be prepared for a poisoning emergency by posting the poison center telephone number by every telephone in your home.
Sources: 1 2 3 ⚜ Writing Notes & References
Writing Notes: Fictional Poisons
#writing notes#poison#fiction#writing reference#writing inspiration#spilled ink#writeblr#creative writing#writers on tumblr#dark academia#literature#poets on tumblr#writing prompt#poetry#light academia#jean béraud#writing resources
212 notes
·
View notes
Text

Libethenite: -a rare copper phosphate hydroxide mineral © Stephan Wolfsried
#minerals#mineral#crystal#green aesthetic#green cyrstal#cristallo#minerali#mineralogia#scienza#nature#green nature
160 notes
·
View notes
Text
The Twelve Principles of Circular Hydrometallurgy, (Binneman & Jones, 2023) are:

The goal is, essentially, that if you have an "ore" of a laptop, you'd be able to 'extract' and separate the gold, cobalt, copper, thallium, zinc, etc by exploiting their physical and chemical properties, with minimal waste products and minimal harm. The process is continuous, and most of the reagents in the vats can be reused, or don't harm the system.
For copper, we separate sulfides from unwanted minerals by exploiting their hydrophobic surface. Then they're converted into a CuSO4 solution that is purified, and then we're able to add electricity to the system to get copper to drop out of solution in a usable form (native copper).
So I think for this essay/location, I'm going to pick Reduce Chemical Diversity, because according to the diagram here, they actually did a pretty good job of only using hydroxide additives? It looks very simple and interesting. I'll also do Use Benign Chemicals because the mill is right next to the Great Lakes and I'm curious if there are problems there. I'll also do Maximize Mass/Energy etc because that's easy fucking fruit. I don't know why that's in this circle. It bugs me.
Preventing Waste is also easy fruit, and combine circular hydrometallurgy with Zero Waste Mining which is an interesting topic, but I hate how the authors of this paper discussed it.
#I have a surprising amount of beef with this paper because the authors were chemists and picked one or two mining hydromet#examples and called it a day with 0 consideration for all the other shit in the ground that we have to consider for mining.#So for Zero Waste Mining what they mean is also extracting the silica and the aluminum and the Fe and the Mg from all the#random minerals that are just in the ground normally. Which is a great idea but really difficult when they're not in high concentrations#So you're essentially saying 'I have a lot of Fe as a side product in my system so I'm going to include 50 Ma worth of equipment to#save a little bit of this iron and pay for the cost to get it where it needs to go when it changes/damages my system overall.'#It's kind of like moon mining. It's a good idea in theory. In practice it's really difficult to design a system that checks that box becaus#all the elements need different solutions/conditions to separate.#I'm sorry if this is really boring and it's not cobalt processing ^^' I'll get to that next. I'm outlining and this is what's been hard.
8 notes
·
View notes
Text
DARK OXYGEN!?
Approximately half the oxygen we breathe comes from the ocean, however it may not all be from marine plants!

Polymetallic nodules or manganese nodules are mineral concretions (a hard mass formed by minerals between particles) on the sea floor formed by iron and manganese hydroxides.
Polymetallic nodules can be found in both shallow and deep waters (even some lakes!) And are thought to have been on the ocean floor since the deep oceans were oxygenated in the ediacaran period over 540 million years ago!
These nodules produce a gas known as ‘dark oxygen’ which is oxygen that DOESNT need light!
Unlike photosynthesis dark oxygen is produced in deep oceans by these polymetalic nodules!
Studies are showing that these naturally occurring lumps of metal 5km deep in the sea between hawaii and mexico are splitting seawater into hydrogen and oxygen!
The metal nodules are formed by dissolved metals collecting on fragments of shell or debris - this process can take millions of years!
Because the nodules contain metals like lithium, copper and cobalt (metals used in making betteries) They create a low voltage (around the same as a AA battery) which is what is splitting the H2O into just H and O (hydrogen and oxygen)
This discovery is creating new hypothesise left right and centre including the possibility of similar objects creating an oxygen rich atmosphere able to sustain life on other planets or even moons!
#science#geology#marine biology#marine bio#rocks#evolution#fun facts#environmental science#marine science
9 notes
·
View notes
Text

@khushallgems
Shattuckite, "a copper silicate hydroxide mineral with formula Cu₅(SiO₃)₄(OH)₂."
2 notes
·
View notes
Note
TOP SEVEN SEMI-PRECIOUS GEMSTONES GO
1. MALACHITE!!!! banded copper carbonate hydroxide!

2. AMETRINE!!!! silicon dioxide quartz—fused citrine and amethyst!

3. GREEN JADE!!!! of the jadeite variety—inosilicate pyroxene!

4. TURQUOISE!!!! copper-aluminum hydrous phosphate!

5. SEA JASPER!!!! microgranulate quartz and cryptocrystalline chalcedony aggregate!

6. OBSIDIAN!!!! volcanic glass produced from felsic lava!

7. LAPIS LAZULI!!! sulfate-sulfur-chloride tectosilicate!

31 notes
·
View notes
Text

#beakers#water#sodium hydroxide#copper sulfate#sodium chloride#temperature#chemistry#solutions#exothermic#endothermic#reactions
1 note
·
View note
Text
VSEPR predicts that a tetrahedral geometry is favoured for a coordination number of 4, but both tetrahedral and square planar geometries are found for 4-coordinate complexes, and these are illustrated in figures 13.14 and 13.15.
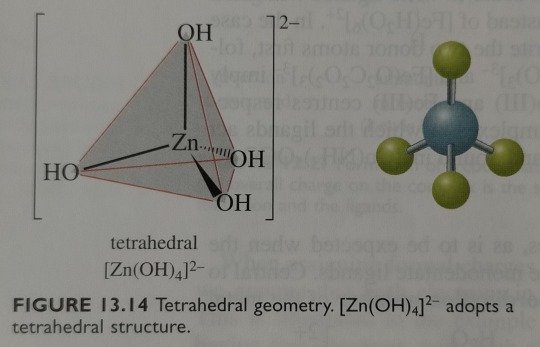

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#vsepr#prediction#tetrahedral#geometry#coordination complex#metal complex#tetrahedron#square planar#tetrahydroxozincate#tetraammine copper#hydroxide#zinc#copper#ammonia
0 notes
Text
Rosasite is a secondary mineral that normally forms in oxidized copper-zinc zones. It is chemically composed of copper zinc carbonate hydroxide, with the formula CuZnCO3(OH)2CuZnCO3(OH)2.
This mineral is recognized for its attractive greenish-blue color and its spherical, botryoidal or fibrous crystalline habit. Rosasite is closely related to malachite, aurichalcite and azurite, sharing similar formation environments and physical properties. It is relatively soft, with a Mohs hardness of about 4.5, and exhibits a glassy to silky sheen.Rosasite was first discovered in the early 20th century. The mineral is named after the Rosas mine in Sardinia, Italy, where it was initially found.
Learn More here 👇👇👇
https://www.supernovaminerales.com/minerals/mexican-rosasite/
#nature #singapore #travel #photography #photooftheday #naturephotography #instagood #beautiful #travelphotography #love #travelgram #gardensbythebay #asia #wanderlust #picoftheday #flowers #instatravel #landscape #naturelovers #garden #explore #green #summer #photo #art #traveling #instagram #trip #travelblogger #adventure
Hello guys! We have this amazing parcel of rosasite with malaquite a unique and beautiful combination.
This Is on sale
https://www.supernovaminerales.com/minerals/mexican-rosasite/
For only this week take 25 specimens for $7.5usd each plus shipping fee 😊
Also take a $25usd off If you WhatsApp us.
#nature #singapore #travel #photography #photooftheday #naturephotography #instagood #beautiful #travelphotography #love #travelgram #gardensbythebay #asia #wanderlust #picoftheday #flowers #instatravel #landscape #naturelovers #garden #explore #green #summer #photo #art #traveling #instagram #trip #travelblogger #adventure
2 notes
·
View notes
Text
What's your favorite rock?
Mine is malachite, for its awesome color and patterns. It makes sense that I like the color since my favorite color is Paris Green, or copper arsenate. Malachite is copper carbonate hydroxide, so they're both a similar green because of the copper.
4 notes
·
View notes
Note
Those melancholic days
when light from outside feels cold and static,
when silence is buzzing in my ears
telling me the gossip from friend's friend,
when the free and open world around me
is like a soft, white room with a door without a handle,
when earth stops her spinning
taking a breath;
are the days when my empty mind is filled with you.
A simple image of a lone person deep in their studies,
answering such trivial questions
like 'what colour are the contents of this vial'.
And I'm asking questions:
What colour is your soul?
Why is it blue?
And does it mean it's an acid?
And I'm asking the question:
What do I add so it's as warm and red with love as mine?
- 🥝
You should be castrated and hospitalised for the rest of your life
Anyways you say "blue" and then suggest low pH? my guy blue isn't the colour of pH indicators for acid but for alkali. Do you know how hard fucking pressed you'd be to find ANY blue acidiccompounds/solutions/elements?????????? BECAUSE I DO KNOW. 'CAUSE YOU KNOW WHAT? YEAH, FINE, I ACCEPTED THE CHALLENGE.
I couldn't find an element that'd make senseby itself - cobalt could work but that's not producing acidic pH by itself. Next idea I had was copper (II) hydroxide - I was even considering suggesting using some aldehydes in high temperature, which would produce hot, red residual (see it could've even worked out for yo!! Hot, red, but no, you're a fucking dumbarse who fucking decided to talk about BLUE FUCKING ACID), BUT ONCE AGAIN, Cu(OH)2 is NOT acidic.
So fine. have it your stupid, stupid way, dumbarse. Here's what i came up with in the end.
Copper Sulphate Pentahydrate. It's blue, it's acidic, idfk it's a crystal. Either way, good luck with turning CuSO4 into Cu2O IN ACIDIC PH. Fucking bitch ass loser smh.
Alright, next one. I GUESS you could go for Chromium(VI) peroxide, though I don't think that'd be acidic, but i guess you could argue CrO3 is?????? Either way, Chromium(VI) peroxide is a deep blue colour, and I suppose you could get it from CrO5 to CrO3 as I've mentioned earlier, which is indeed red, but you're probably gonna end up with green Cr3+ ions instead.
Ok last but not least - surprise, surprise!! It's the cobalt come back. Ions [CoCl4]2- and [Co(H2O)6]2+ stay in an equilibrium of sorts, having blue and pinkish-redish colours to them respectively. That'd mean you could throw that equilibrium off, and start off from blue, and go into this red-ish pink. Anyway to summarise I think on the second thought we should actually both be hospitalised.......... Hey wanna be roommates ;]
#🥝 anon#ask#asks#ask fern#oh god i am a nerd#fernless rants#emphasis on “rants”#tw chemistry#tw quackbur shipping#psycho competitive#this took me legitimately way longer than i'd like to admit#anyway fuck you you red phosphorus white-phosphorus-wanna-be bitch boy
11 notes
·
View notes