#vsepr
Explore tagged Tumblr posts
Text
girl you're looking so. tetrahedral today :3
#VSEPR#chemistry#my chem teacher explicitly told me not to say this to someone#im doing it anwyay#molecular geometry
38 notes
·
View notes
Text
Learning about chemical bonding in intro chemistry class is so funny because it’s like “so this is how atoms form bonds. Yep, that’s pretty much what happens. Actually, that model is a little oversimplified, this is how atoms form bonds. Actually, that’s still an oversimplification, this is how atoms really form bonds. Okay, that’s also an oversimplified model, this is the actual way that atoms form bonds.” I’m learning molecular orbital theory right now; I think that’s the last bonding model this class is going to cover. I don’t know if there are others lurking in the murky depths somewhere.
#chemistry#college#school#chemistry class#chemical bonding#electrons#lewis dot structures#vsepr#hybrid orbitals#molecular orbitals#studyblr
3 notes
·
View notes
Text

1 note
·
View note
Text
VSEPR predicts that a tetrahedral geometry is favoured for a coordination number of 4, but both tetrahedral and square planar geometries are found for 4-coordinate complexes, and these are illustrated in figures 13.14 and 13.15.
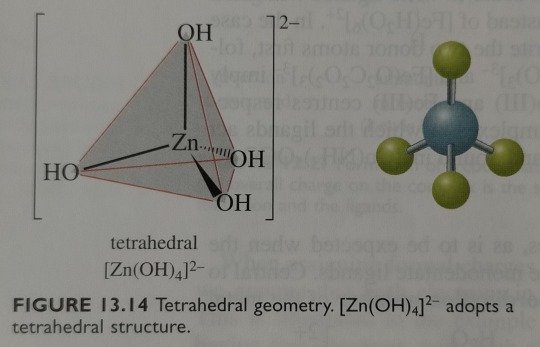

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#vsepr#prediction#tetrahedral#geometry#coordination complex#metal complex#tetrahedron#square planar#tetrahydroxozincate#tetraammine copper#hydroxide#zinc#copper#ammonia
0 notes
Text
Why we need VSEPR
VSEPR stands for valence shell electron pair repulsion theory. The initial idea was that electron pair(s) in the valence shell repel each other, affecting the molecule's shape. But we now know this doesn't apply just to electron pairs. You could also be talking about a single electron if you have an odd number of electrons, double bonds where the bond = 2 pairs of electrons, or triple bonds where the bond = 3 pairs of electrons. This region of space occupied by the electrons causing repulsion = the domain, which is not necessarily the same thing as orbitals. Either way, we live in a 3D world, so VSEPR theory giving us 3D molecular shapes gives us more info than Lewis structures can.
What VSEPR tells us that Lewis structures cannot:
If two structures superimposable, they're the same molecule. Lewis structures don't show this (you may have different Lewis diagrams for the same molecule). Say you have this compound CH2ClF and you move the Cl and F in the Lewis structure. It's still the same molecule when you look at it in 3D because you can rotate the molecule in a certain way and see that the molecules represented by the two Lewis structures superimpose.

If two structures are NOT superimposable, they're not the same molecule. Lewis structures don't show this either (you may have 1 Lewis diagram for different molecules). Say you have the compound CHBrClF. From the Lewis structure, you can't tell that there are different ways you can structure this molecule in 3D space. If you were to look at it in 3D, you'll see there's no way to rotate the molecules and have the two structures be the same. (This is why VSEPR is really useful for organic chemistry because you have lots of isomers - stuff with the same chemical formula but different structure like this. This kind of isomer in particular is called an enantiomer.)

0 notes
Text
electron pairs are so relatable, i too try to stay away as far as possible from everyone like me
2 notes
·
View notes
Text
boy i love getting tipsy and then drunk and gushing about my special interest to people who cannot possibly care about this even half as much as i do and being deeply annoying and embarrassing myself and wanting to crawl into a hole once the harsh light of sobriety hits
#like i cannot stress enough that i want to die right now#it's not a physical hangover it's a mental hangover. a ''why am i incapable of shutting the fuck up'' hangover.#i become so deeply annoying when drunk that i should not be allowed to use my phone#i turn into the goddamned boom de yada commercial and inflict it on everyone in range#like i go off about the discworld series a LOT#one time at a party i cornered two guys who had no science background and tried to explain how avogadro's number was found#i gush about fullmetal alchemist or the story structure of everything everywhere all at once#i cry over interstellar or the cosmos series#my friends and family back home all already know this and give me their ''sure thing sarah now let's get you to bed'' looks#too few people here have been exposed to this to yet know how to stop it#eta: i should also stress that when i discovered that said guys did not know what vsepr theory was my reaction was not to stop#it was to get a piece of paper and start explaining lewis dot structures#eta again: you know after considering this long-standing history of doing this i feel paradoxically less embarrassed#like it will be very funny to explain the avogadro's number story and all the things i have done this about#like look i'm sorry i hit you with my special interest gushing but i have done this many times before to many people#the ''drunk!sarah highbeams of random essays and lectures'' is well-established and tbh kind of a rite of passage at this point
14 notes
·
View notes
Text
I was made to babygirlify fictional men and read my sapphic books all day, instead I have to study vsepr and molecular symmetry operations :((
#the woes of being a woman in stem#chemistry#chemical engineering#orgo exam and then inorganic right after is hellish#vsepr is chill though
7 notes
·
View notes
Text
DOES ANYBODY HERE KNOW FUCKING CHEMISTRY
#im dead#idk anything#im going to be jointly murdered by my oarents and chemistry teacher#what. is vsepr theorey#rena.posts
3 notes
·
View notes
Text
All of these compounds contain 4 sets of electron pairs and therefore, according to VSEPR theory (p. 176), their structures are based on the tetrahedron, with 0, 1, 2 or 3 lone pairs.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#methane#silane#ammonia#phosphine#water#hydrogen sulfide#hydrogen fluoride#hydrogen chloride#neon#argon#vsepr theory#lone pairs#electrons
0 notes
Text
guys will see this and just think "hell yeah"

1 note
·
View note
Text
To determine the shape of a molecule using VSEPR theory, we use the following procedure:
Determine and draw the Lewis structure of the molecule.
Count the number of sets of bonding pairs and lone pairs of electrons around any inner atom, and use table 5.2 to determine the optimum geometry of the sets. Note that VSEPR theory makes no distinction between electron pairs in single, double and triple bonds. Each is treated as being one set.
Modify the geometry, if necessary, to take account of the fact that the magnitudes of repulsions between sets of electron pairs depend on whether the electron pairs involved are bonding pairs (BP) or lone pairs (LP). The repulsions are in the order: LP-LP > BP-LP > BP-BP

This is satisfied by placing the two sets in a linear arrangement (table 5.2) and results in a linear shape for this molecule, with a H-Be-H bond angle of 180°. (...) The optimal geometry for three sets of electron pairs is trigonal planar (table 5.2). (...) The optimal geometry for four sets of electron pairs is tetrahedral (table 5.2). (...) The geometry which places these as far apart as possible is trigonal bipyramidal (table 5.2). (...) An octahedral geometry of the six sets (table 5.2) places them as far apart as possible, as shown in figure 5.17b.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#electrons#election pairs#valence shell electron pair repulsion#vsepr#geometry#linear#trigonal planar#tetrahedral#tetrahedron#trigonal bipyramidal#trigonal bipyramid#octahedral#octahedron#optimization#sulfur hexafluoride
0 notes
Text
Even I don't want to 😭😭
It's not even like I CAN'T do it. I basically don't WANT TO
#ye chemistry itni boring kyu haii#why i have to learn all those rangoliss *VSEPR THEORY wale diagrams *#what will i do by finding bond angle ;it's length: it's strength; bond order; it's enthalpy#nahi bhaii nahii i can't handle it now#hey bhgwan uthaaa le (meko nahi chemistry wali teacher ko)
21 notes
·
View notes
Text
Molecular and ionic compound structure and properties Pt. 2
Part 1 here
Lewis diagrams/structures
Lewis diagrams show how valence electrons = distributed around atoms in a molecule; thus, in general, follow the octet rule
Shared pairs of electrons are drawn as lines b/t atoms
Lone pairs of electrons are drawn as dots next to atoms
Goals for every Lewis diagram:
Have the correct number of total valence electrons (v.e.)
Each atom has the correct number of valence electrons for that atom
Ensure the way electrons are shared fulfills the octet rule for every atom unless it's an exception: For H, instead of octet rule, it's just 2 valence electrons (duet rule). Boron and Al can form stable molecules where they only have 6 v.e., not 8. 3rd period and beyond: atoms can form stable molecules where they have more than 8 v.e. (this may happen because these atoms can put electrons in their empty valence d orbitals? but this is a subject of debate)
To make a Lewis diagram:
Count total number of valence electrons in compound. If compound has a negative charge, add an electron to the total for every negative charge. If compound has a positive charge, subtract an electron from the total for every positive charge.
In general, put the least electronegative atom that is NOT hydrogen in the center of the diagram - more electronegative atoms or H are terminal atoms (atoms on the outside).
First, assume single bonds. Subtract the electrons used from the total.
Assign leftover electrons to the terminal atoms. Subtract the electrons used from the current total number of unused electrons.
If needed, assign any leftover electrons to the central atom. If the central atom has an octet or exceeds an octet, you're usually done. If the central atom does NOT have an octet, create multiple bonds until you do.









Resonance and formal charge
Resonance occurs when a molecule or ion has more than 1 valid Lewis structure, e.g. nitrate - with the 1st 3 possible Lewis structures, you'd think 1 of the bonds (the double bond) is shorter and has a higher bond energy than the others, but in reality, all 3 bonds have the same length and energy.

Each molecule or ion that experiences resonance truly exists as a resonance hybrid - the weighted average of the possible Lewis structures ("resonance structures"), a hybrid of the possible Lewis structures (not alternating b/t the different structures). E.g. in nitrate, each bond is someplace in b/t a single and double bond b/c the molecule's electrons are delocalized throughout. It's a weighted average because for some molecules/ions, some resonance structures contribute more to the resonance hybrid than others, aka the molecule/ion has nonequivalent resonance structures.
Formal charge = the charge of an atom in a molecule if all the bonding electrons were shared equally. It helps us determine w/c resonance structures contribute most to a resonance hybrid.
The closer a resonance structure's formal charges is to 0, the more likely it is to contribute more to the resonance hybrid compared to other resonance structures.







#14 days of chem#my unsolicited notes#studyblr#chemistry#forgot to post this#original plan was to also include notes for vsepr and bond hybridization but... i didn't make any notes for those...
2 notes
·
View notes
Note
Something that has so much potential but I don't think ive seen anyone talk about is scientist!MC. Like yeah it arguably doesn't match the game thematically but since when does obey me have cohesive themes to begin with. Idk where im going with this I just needed to yell.
But like since I imagine the science curriculum at RAD is rather lacking, I'm cackling imagining that MC running around like "what do you MEAN you people have had millions of years to study and still don't know VSEPR theory???"
It is SUPER fun thinking about the intersection between science and magic, in my opinion. I feel like that magic should work better if you understand the scientific theories behind how things work. If you know why ice is formed, you can be more specific in what you make water do to magically create it.
Also I feel like some of the boys would find it SO HOT to list to you go off on tangents about things they don't know anything about (aka Mams, Levi and Asmo especially). Satan I feel like would get a little competitive in a playful way, wanting to be able to understand what you talk about.
IN OTHER NEWS
MC introduces the boys to Bill Nye the Science Guy and blows their damn minds.
#Magic School Bus also becomes a quick fave and regular group watch#obey me headcanons#obey me hcs#blithe asks
24 notes
·
View notes
Note
Very well then, we shall start with the VSEPR theory
The Valence Shell Electron Pair Repulsion theory is a theory used to predict the geometry of molecules based on the number of electron pairs. It is assumed that the electron pairs sall be in an orientations such that replusion between electron pairs is minimum.
sick

my cat yawned
11 notes
·
View notes