#endothermic
Explore tagged Tumblr posts
Text

#reaction#reactant#product#molecular#energy#entropy#chemistry#solutions#exothermic#endothermic#transition state#equilibrium
1 note
·
View note
Text
An endothermic reaction can not become exothermic by using a catalyst.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Text
A potential energy diagram for an endothermic reaction, where the potential energy of the products is higher than that of the reactants (∆H > 0), is shown in figure 15.16.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Note

There is a happiness no-one else knows: the feeling of mud between fully webbed toes; the caress of a breeze on your moist shiny skin; the warmth of the sunlight that slowly soaks in; the gentlest hum of a thought far away, as you sit and you soak and let time tick away.
#poetry#herpetology#frogs#meme#you cannot win in a game of patience against any amphibian or reptile#to me nothing signifies the difference in our minds and bodies more than that simple fact#endotherms are so bloody impatient#because we feel ourselves ticking faster than everything around us#ectotherms literally chill to the tempo of the moment#it's a marvellous and challenging thing to behold#answers by mark#princezilla
2K notes
·
View notes
Text
Having a normal time (debating on whether or not time lords can be classified as mammals)
#I am doing this instead of working on my actual mammology homework#like they obviously didn’t evolve on earth but they do have a lot of traits that mammals do#idk if it’s explicitly stated anywhere but they seem to be endothermic#also they have hair although I wonder if it’s made of something other than keratin#or is there like some sort of universal convergent evolution with that#actually it would be interesting if time lord hair wasn’t made of keratin because then what would it be made of#and if it wasn’t would that mean they also had non keratin nails#this is not the point but I got off track#it’s also not that they give live birth but that’s like because of a curse they used to do that#also like when they did have actual children did they produce milk to feed them or was there some other way#so like they might be able to be classified as mammals because they fit some of those things and it’s a unsure on the others#I should really be doing my homework or like studying#I have an exam tomorrow#this is fine#time lord speculative biology is more fun#doctor who#time lords
94 notes
·
View notes
Text
i want a cat :/
#'i want a cat' you DON'T youre just COLD#it's morally wrong to get an animal just so you can hold something endothermic when your buildings heat isnt back on!#box opener#also an argument against getting a girlfriend. but the ship may have sailed
14 notes
·
View notes
Text
actually doesnt blaze use a blanket in book 3 because she's cold. The dragons are warm-blooded ?
#for context i mentioned icewings being coldblooded in the icewing post a few hours ago#cold-blooded means exothermic which means they get heat from external sources#blankets work by trapping the heat produced by ur body because humans are endothermic and warmblooded#so reptiles and stuff cant actually use blankets or sweaters to keep warm!#wings of fire#wof#talking
98 notes
·
View notes
Text
I'm cold, let me use your warmth, it's the nectar I feed on, don't deny my life from me, let me get inside you
#it would fix me for like 5 minutes until I need you again#I never get enough of you#I am too awful even for my own taste#I need pussy. It's for me. You are there to serve my needs#my body doesn't produce the warmth it needs to live on its own. you need to share yours with me. i am not endothermic#you need to give me life. over and over again. i'll use you to regenerate my hp
3 notes
·
View notes
Text
There is this whole idea that flipping a two sided coin doesn't have a 50-50 probability. It's not a new idea by any means, but the explanation is if you measured the mass of the coin, the force of the flip, the temperature of the coin & of the room, the force of any breeze, wind, or vibration in the air as it traveled, and so on, you could accurately determine within a small margin of error what side the coin will land on every time, and if you kept those constant it would flip on the same side every time. And that idea is also KIND OF the explanation for the conclusion in quantum physics that there is no free will.
A lot of people hear that and either clutch their pearls, roll their eyes, or aren't interested either way. (I mean, when you say some shit like that you're just going to immediately turn off any interest most people would have otherwise had but I'm digressing now). We all like to think we make decisions and choices, and then amateurs who want to talk about quantum mechanics alienate everyone by saying it's not true: you were always going to make these choices with no chance to make the other one.
But what I said in the first paragraph is something-like (but not exactly) what it means when you hear or read that according to quantum physics we have no free will. That if we had an unfathomable device that has been measuring all the variables of every single particle that was expelled during the Big Bang, with an also-sufficient/also-currently-unfathomable algorithm to plug those variables into, all within a computer that could do all of the calulations for BILLIONS of years, we could compute exactly where every particle was going and where it would end up, including those that make up the stars and planets, that make up the ground and oceans, that make up the animals and plants, that make up your brain and all of the proteins and neurotransmitters. That if it could all be measured and an algorithm sufficiently built then the decisions you make are already determined by the ongoing relationships and interactions the particles that make up your brain had in the past and are having right now.
However, humans cannot measure that, they likely never ever will.
Anyone that tells me they don't like quantum mechanics because something something affront to nature blah blah "they" don't believe in free will, etc. literally doesn't know it's just a rescale of the coin toss description. You still believe coin tosses are 50-50 because you aren't going to measure the variables used to receive an answer, you can still believe in free will because you can't measure the variables used to determine the ultimate path of all particles; I mean, I wouldn't become a theoretical physicist if that meant so much to you but I'm not your dad, do what you want.
Edit: I know I described the science mostly wrong, please check out the replies and reblogs for others' corrections and feel free to add corrections of your own for mine and others' learning, thank you.
#as has been pointed out to me you can't measure quantum functions in a meaningful way without collapse#I'm not a quantum physics person‚#(i mean‚ that is pretty clear)#but there are concepts that can be simplified to make them easier to process l#so that people interested can be pointed in the right direction and feel more familiar#before they find corrections and understand the bigger stuff‚ it's basically how middle school conventional science classes teach stuff#like i remember not really understanding dependant/independent variables#or not really understanding why endothermic/exothermic meant cold/hot#or how electrons don't really orbit atoms/have fixed places when attached to each other#we're taught simplified versions of things that are sometimes not the whole picture or sometimes wrong#to get you thinking in the right direction#anyway I'm not saying I crafted this post to be like that‚ just that I do understand some of the concepts aren't correct#it's just too complicated to get into specifics and that's the best way I can describe how I understand it#sorry if you do know better and bothered‚ I'll make any necessary edits if you message me and they don't over complicate the post#otherwise for everyone else go look this stuff up and get corrected#physics#quantum mechanics#quantum physics#op
104 notes
·
View notes
Text
Wait, are vampires ectothermic?
#bc on one hand theyre always described as having really cold skin kinda implying they cant regulate their own body temperature#and on the other hand (when they can turn into bats) bats are endothermic so would that cross over?#idk what traits cross over from bats really#bc its obviously not all traits#bc otherwise theyd literally just be bats#im so sorry this is pointless speculation im just combining my dumb interests#vampires
5 notes
·
View notes
Text
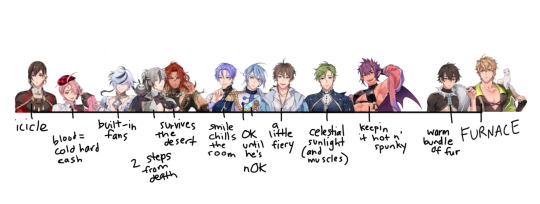
Resting body temperature headcanons based on pure speculation
#nu carnival#no i didn't put them at their proper heights they are all just existing randomly#yakumo is an icicle because i want him to be i need him to be#but does he technically go in the middle?? bc someone says “endothermic temperature regulation”#and yakumo will respond “who is that”#how can rei and Dante be similar resting temps but one is almost dead and the other has the energy to fight at the slightest provocation#i don't know#none of this is science#maybe Edmond got his baseline down after his desert training lol#something something muscles higher metabolism than fat something differing temperatures something#edmonds fat thighs are the only thing keeping him from heat stroke#kuya needs everything to be a crisp 17.6 degrees Celsius for optimal comfort#i don't even know why quincy is the king Master heat radiator 6000#i have no evidence#just a feeling#this entire scale was sparked by the lingering idea that yakumo would enjoy using quincy as a personal heater#they started this#as soon as i finished this i started changing my mind on everyone#gOOD THING temperature isn't a forever one time value----- huh---!!!!!#garu reminds me of those tiny dogs u hold and they're just somehow so warm and their lil hearts beat so fast and you're just wondering#how yall keeping cool under all that fur??? i need to get u some watermelons to lie on#the clan's all here!
47 notes
·
View notes
Text

#beakers#water#sodium hydroxide#copper sulfate#sodium chloride#temperature#chemistry#solutions#exothermic#endothermic#reactions
1 note
·
View note
Text
As you can see from figure 10.12, the first step is endothermic.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
1 note
·
View note
Text
The enthalpy change when a gas dissolves in a liquid has essentially two contributions, as shown in figure 10.3 on the next page.
Energy is required to open 'pockets' in the solvent that can hold gas molecules. The solvent must be expanded slightly to accommodate the molecules of the gas. This is an endothermic process since attractions between solvent molecules must be overcome. Water is a special case – it already contains open holes in its network of loose hydrogen bonds around room temperature. For water, very little energy is required to create pockets that can hold gas molecules.
Energy is released when gas molecules enter these pockets. Intermolecular attractions between the dissolved gas molecules and the surrounding solvent molecules lower the total energy, and energy is released as heat. The stronger the attractions are, the more heat is released. Water can form hydrogen bonds with some gases, such as NH3, whereas many organic solvents cannot. More heat is released when such a gas molecules is placed in a pocket in water than in organic solvents.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#gas#solubility#solute#solvent#solution#endothermic#exothermic#chemical reactions#enthalpy
1 note
·
View note
Text
I'm feeling very (post) biology major seeing everyone's walrus vs fairy takes. Just saw a post from a popular blogger say the discovery of fairies wouldn't be that big or interesting to biology, wtf are you talking about? Anyways, I'm dissecting the fairy
#im sorry if a walrus escaped the zoo and ended up outside my door it'd be in the news for like a day#derry girls literally did an episode like that but with a polar bear#how are yall not more curious about potential fairy biology and evolution?#shrews and hummingbirds are at the edge of size an endothermic animal can be so lets start wondering about fairy physio there
13 notes
·
View notes
Text
Endotherm (Thomas Wilkins) was introduced in The Invincible Iron Man #136, cover date July, 1980. He was created by Peter John Palmer, David Michelinie, and Alan Weiss. ("The Beginning of Endotherm", The Invincible Iron Man #136, Marvel Comic Event)

#nerds yearbook#real life event#first appearance#comic book#marvel#marvel comics#july#1980#iron man#invincible iron man#peter john palmer#david michelinie#alan weiss#tony stark#endotherm#thomas wilkins#sybil carmichal#colin campbell
6 notes
·
View notes