#Biotech Industry
Explore tagged Tumblr posts
Text
Pharmaceutical Water for Injection System Plant Manufacturers in India
The pharmaceutical industry relies on the highest standards of water purity, particularly when it comes to Water for Injection (WFI). WFI is an essential component in the production of injectables, parenteral drugs, and other pharmaceutical applications that demand the utmost sterility. In India, a growing number of manufacturers specialize in designing and installing Water for Injection system plants that comply with international regulatory standards such as the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and World Health Organization (WHO) guidelines.
Understanding Water for Injection (WFI)
Water for Injection (WFI) is highly purified water that is free from pyrogens, bacteria, and organic contaminants. It is primarily used for intravenous medications, diluents for injections, and cleaning pharmaceutical equipment. The production of WFI requires stringent treatment processes, including reverse osmosis (RO), ultrafiltration (UF), distillation, and sterilization.
A well-designed WFI system ensures consistent water quality that meets pharmacopeial standards while minimizing contamination risks. The leading pharmaceutical WFI plant manufacturers in India employ cutting-edge technologies to provide cost-effective, reliable, and compliant solutions.
Key Technologies Used in WFI Systems
Multiple Effect Distillation (MED): This method involves a series of distillation columns to remove impurities and pyrogens, ensuring the highest purity level.
Vapor Compression Distillation (VCD): Known for energy efficiency, VCD utilizes vapor recompression technology to produce WFI with minimal water wastage.
Reverse Osmosis (RO) and Ultrafiltration (UF) Combination: A non-distillation method where a combination of reverse osmosis and ultrafiltration is used to generate WFI.
Electrodeionization (EDI): A process that removes ionic contaminants without the need for chemical regeneration, ensuring continuous purified water production.
Key Features of a Pharmaceutical WFI System
Regulatory Compliance: Leading manufacturers design systems that adhere to global standards, including cGMP, USP, and EP guidelines.
High-Purity Output: The latest WFI systems ensure ultra-low conductivity, pyrogen-free water suitable for pharmaceutical applications.
Automated Control Systems: Advanced automation, including PLC and SCADA-based controls, ensures real-time monitoring and validation.
Sterile and Sanitary Design: Made from SS 316L stainless steel with electro-polished interiors, ensuring a contamination-free environment.
Energy Efficiency: Modern WFI systems are designed for low energy consumption and minimal environmental impact.
CIP/SIP Integration: Clean-in-place (CIP) and steam-in-place (SIP) processes maintain hygiene and prevent biofilm formation.
Top Pharmaceutical Water for Injection System Manufacturers in India
India has a robust network of manufacturers providing high-quality WFI systems that cater to the pharmaceutical, biotechnology, and healthcare industries. Here are some of the leading companies:
Swjal Process Pvt. Ltd.
Specializes in customized WFI solutions with a strong focus on automation and compliance.
Offers end-to-end solutions, including design, installation, and validation services.
Expertise in reverse osmosis, electrodeionization, and distillation-based WFI systems.
Hitech Water Solutions
Provides turnkey WFI plants with energy-efficient designs.
Ensures compliance with cGMP and international quality standards.
Offers preventive maintenance and service support.
Pure Water Technologies
Manufactures WFI systems with innovative energy-saving technologies.
Expertise in multiple effect distillation and vapor compression distillation.
Provides pharmaceutical-grade storage and distribution systems.
Pharmatech Process Equipments
Specializes in stainless steel WFI generation and storage solutions.
Offers integrated automation for seamless plant operation.
Compliant with WHO, US FDA, and European standards.
Aqua Purification Systems
Designs and installs complete WFI systems for pharmaceutical manufacturing.
Utilizes advanced purification techniques to meet stringent water quality requirements.
Provides on-site validation and documentation services.

Why Choose Indian WFI System Manufacturers?
India has emerged as a global hub for pharmaceutical equipment manufacturing, offering high-quality solutions at competitive prices. The key advantages of choosing Indian WFI system manufacturers include:
Cost-Effectiveness: Indian manufacturers provide world-class WFI systems at a fraction of the cost compared to Western suppliers.
Advanced Technology: The industry has embraced cutting-edge technologies such as AI-based monitoring and IoT-enabled predictive maintenance.
Regulatory Expertise: Indian manufacturers have extensive experience in meeting global pharmaceutical regulations.
Comprehensive Services: Many companies offer complete solutions, from consultation and design to installation, commissioning, and maintenance.
Scalability: Indian manufacturers cater to a wide range of production capacities, from small-scale biotech firms to large pharmaceutical plants.
Future Trends in WFI System Manufacturing
The pharmaceutical industry is witnessing continuous advancements in WFI system design and operation. Some of the upcoming trends include:
Sustainable Water Management: New systems focus on reducing water wastage and optimizing energy use.
Digital Integration: Smart monitoring solutions using AI and IoT are enhancing efficiency and reliability.
Modular Systems: Compact, pre-validated modular WFI units are gaining popularity for ease of installation.
Non-Distillation Methods: Membrane-based WFI generation is becoming a viable alternative to traditional distillation.
Conclusion
Pharmaceutical Water for Injection (WFI) systems play a crucial role in ensuring the safety and efficacy of injectable drugs. India’s leading manufacturers offer state-of-the-art WFI solutions that comply with global regulatory standards while maintaining cost efficiency and high performance. Companies like Swjal Process Pvt. Ltd. and other top manufacturers are setting new benchmarks in WFI system design, automation, and sustainability. As the pharmaceutical industry continues to grow, the demand for reliable and efficient WFI systems will only increase, making India a key player in global pharmaceutical water treatment solutions.
SWJAL PROCESS Pvt. Ltd. is a leading Pharmaceutical Water for Injection System Plant Manufacturer in Mumbai, India.
#wfi system#water for injection#pharmacutical WFI plant#water treatment process plant manufacturers#manufacturer#swjal process#biotech industry#pharmacutical industry
0 notes
Text
Navigating AI's Innovative Approaches In Biotechnology

Let’s take a step back in time.
Do you remember how things used to be? Back then, doctors didn’t have the advanced tools and knowledge we now enjoy. Many diseases we can treat today were once fatal, and people generally didn’t live as long because medical science was still in its infancy.
But look how far we’ve come! Today’s doctors can detect genetic issues, design treatments based on personal medical histories, and create vaccines that prevent the spread of diseases. What sparked such a massive shift?
Enter Artificial Intelligence in biotechnology. AI is making waves by assisting doctors in diagnosing patients, analyzing medical data, and even helping scientists manipulate DNA as easily as we write text in a document—well, almost that easily!
In this article, we’re diving into how AI is poised to revolutionize biotechnology. Are you ready?
Biotech companies and healthcare organizations today rely heavily on analyzing enormous amounts of data. To develop new biological processes, drugs, and understand DNA sequences, these technologies need faster and more precise data handling. This is where AI steps in.
Artificial Intelligence makes processes in biotech faster and more reliable. It reduces human error, which is crucial when it comes to handling life-changing data and research. AI is not just streamlining drug discovery but also advancing disease research. As the future unfolds, AI and biotechnology will continue to unlock groundbreaking innovations in healthcare, pushing the boundaries of what's possible.
Now, before we explore these exciting advancements, let’s clarify what AI in biotechnology really is.
What Is AI in Biotechnology?
Artificial Intelligence is transforming biotechnology by accelerating processes like drug discovery and research. Machine learning algorithms can sift through massive clinical trial data sets to identify drug targets and predict their effectiveness. AI also speeds up drug screening and automates data analysis, drastically shortening development timelines.
By leveraging AI’s analytical power, biotech companies gain valuable insights and reduce costs. Some major pharmaceutical companies are already investing heavily in AI. According to GlobalData, the pharma industry is expected to spend around USD 3 billion on AI for drug discovery by 2025—clear evidence of AI’s potential in biotech!
As traditional research methods hit their limits, AI is becoming indispensable in biotechnology, enabling revolutionary progress. With that in mind, let’s explore how AI is being used in various sectors of the biotechnology field.
How Is AI Used in Biotechnology?
AI is transforming the biotechnology industry in several ways, including:
3D Protein Structure Prediction Identifying the 3D structure of proteins is crucial but time-consuming. AI tools can quickly predict unknown protein structures from available data, making it easier to develop drugs that target those proteins. This breakthrough speeds up drug development and deepens our understanding of disease-related proteins.
Gene Editing and Genetic Coding AI enhances our ability to edit genes selectively, helping to eliminate harmful genes and enable personalized medicine. AI is significantly advancing our ability to treat genetic disorders and customize treatments based on individual genomes—an impressive leap in biotechnology.
AI-Powered Lab Assistants AI programs are acting as lab assistants, automating tedious tasks and performing complex data analyses. Robotic AI devices are already assisting in labs and hospitals, taking on routine duties so scientists can focus on innovation and discovery.
As AI is integrated into the biotech industry’s workflow, it accelerates the development of new treatments and ensures more precise results in clinical applications. Let’s explore how biotech companies are using AI to drive innovation!
How Can Biotech Companies Leverage AI for Innovation?
Biotech companies are using AI in several ways to accelerate research and innovation, including:
New Vaccines and Drugs AI is enabling faster identification of potential vaccines and drugs. Vaccine development, which traditionally took over a decade, has been shortened to just a few years with AI’s help. In the future, AI will continue to accelerate biopharmaceutical research.
Agricultural Biotechnology AI and robotics are improving crop yields by providing automated harvesting systems and data-driven insights into optimal growth conditions. Additionally, AI is helping biotech companies create hybrids and genetically modified organisms (GMOs), advancing agricultural research.
Personalized Medicine AI is revolutionizing medicine by analyzing patient genetics and symptoms to discover treatments tailored to individuals. This shift from "one-size-fits-all" treatments to personalized medicine is a game changer, especially for rare diseases.
As AI enhances the speed, accuracy, and efficiency of biotech workflows, it will continue to revolutionize the industry. On that note, let’s wrap up this article!
Conclusion
Artificial Intelligence in biotechnology is already offering immense benefits by accelerating drug discovery, enabling personalized medicine, and automating complex processes. AI’s ability to analyze big data and enhance human capabilities is transforming patient care and driving innovation in biotech. While challenges remain, the future is incredibly promising, as AI helps biotech make life-saving advances that will impact people worldwide.
Want to learn more about the exciting world of AI and biotech? Click here to explore the latest innovations!
0 notes
Text
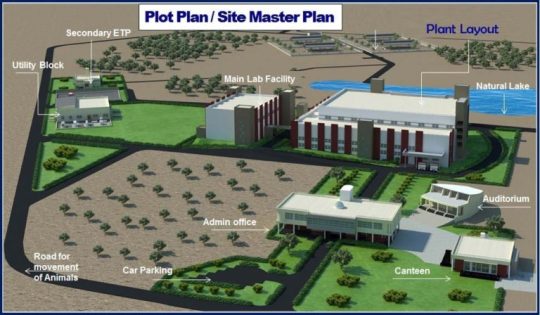
Navigate the dynamic world of biotech with confidence. Quantum Pharmatech's expert biotech management consulting empowers your business. We offer a comprehensive suite of services, from strategic planning and plant design to regulatory guidance.
0 notes
Text
I’ll be a doctor one day and all the pharmaceutical reps will be waiting in the lobby for hours begging for a chance to speak with me to push their samples to patients and I’ll have pharmaceutical companies buying free lunch for my employees every day just so they can sit w me at lunch and speak to me and I’ll also have a housewife/husband but instead it’ll be an office wife/husband and they’ll run the managerial aspects of my hospital for me . Among other things
#And that’s a VOW#Also depending on how involved I’ll be in the biotech industry maybe I’ll also be a medical director and spearhead sick research projects#I’ll def be research leaning I really do love it#But I don’t wanna do that shit on the sidelines like when I’m older I want to be directing that shit#I for sure wanna be involved in drug discovery I think it’s so fun#So many goals 😞😞#Also I’ll have a high turnover for patients and patients won’t wait forever but my staff will still be pleasant and not pushy#I also hate the red tape around healthcare services so they’ll fs be a lot more accessible#I have a lot of goals that are patient oriented I was just trying to sound conceited but it goes beyond that tbh#It’s rly funny watching pharma reps grovel but there’s more to life than being groveled for
74 notes
·
View notes
Text

BioTech Industries Borg Construct Aj^6
Source: The Essential Guide to Weapons and Technology (Del Rey, 1997)
#star wars#cybernetics#cyborgs#lobot#cloud city#biotech industries#borg construct aj^6#aj^6#computers#first appearance the empire strikes back#galactic civil war#essential guide to weapons and technology#essential guides
10 notes
·
View notes
Text

Normal Phenomena of Life
#Normal Phenomena of Life#Biotech-enabled Materials#Bio-centric Industry#Biophilic Culture#products#shop#green#typography#type#typeface#font#Univers Next#2024#Week 10#website#web design#inspire#inspiration#happywebdesign
6 notes
·
View notes
Text
I was having a hard time writing up something for work today (a piece about computational infrastructure for biomedical research) so I started a new doc and pretended I was writing a fanfic where Osgood was explaining the whole thing to Kate Stewart as part of a report.
BAM, unblocked.
Going to do that every time now. Too bad I can’t publish them as one-shots 😅
#writer life#unblocked#kate stewart#osgood#fanfic#two worlds collide#I guess UNIT has good reasons to be keeping tabs on the biotech industry#I wouldn’t be all that surprised if some of the tech we use was of alien origin#that would explain the garbage error messages
3 notes
·
View notes
Text
Professional Biotechnology Translation Services
Get accurate and reliable Professional Biotechnology Translation Services for your biotech needs. Our expert linguists specialize in translating clinical trials, research papers, patents, and more. We ensure precise, high-quality translations in over 100 languages, preserving the integrity of your content. Whether for global expansion or regulatory compliance, trust us for timely, confidential service. Contact us today to meet your biotech translation needs.
#Biotechnology Translations#Professional Biotechnology Translation Services#Biotechnology Translations Service#Biotechnology Industry Translations#Biotechnology Translation Services#Biotech Translation Services#Translation Services for Clinical Trials#Translation in Life Science#Life Science Translation
0 notes
Text

Elevate Research with DSS ImageTech’s Biotech Solutions
Find advanced solutions with DSS ImageTech – your trusted biotech partner. From top-notch lab equipment to innovative medical biotechnology tools, we drive progress in the life sciences industry. Visit DSS ImageTech now to explore our products and services. Elevate your research today! https://www.dssimage.com/
0 notes
Text
The Rising Importance Of Liquid Chromatography Mass Spectrometry In Pharma And Life Sciences Industries

What is LCMS and its Applications Liquid chromatography mass spectrometry (LCMS) is an analytical technique that combines the physical separation capabilities of liquid chromatography (LC) with the mass analysis capabilities of mass spectrometry (MS). LCMS works by separating and analyzing mixtures of chemicals that have been turned into charged particles by an applied electric current. This allows researchers to measure the molecular mass of molecules and identify unknown chemical structures. Some key applications of Liquid Chromatography Mass Spectrometry (LCMS) in the pharmaceutical and life sciences industries include drug discovery and development, quality control testing of drug products, metabolic studies, determination of drug impurities and degradation products, and clinical diagnostic applications like disease biomarker research. LCMS has become an indispensable tool for pharmaceutical R&D and manufacturing due to its high sensitivity, accuracy, and ability to detect trace quantities of analytes. Growth in Biosimilar and Generic Drug Markets The global biosimilars market is projected to grow from $7.5 billion in 2022 to over $55 billion by 2030, representing a CAGR of almost 28%. As patent expirations of big-brand biologics increase and regulatory pathways for biosimilar approvals evolve, more biosimilar drugs are launching and driving the market's expansion. The global generic drugs market size was estimated at $180 billion in 2021 and is predicted to surpass $300 billion by 2030. Over 85% of all prescriptions dispensed in the United States are for generic drugs. As more blockbuster drugs lose exclusivity and robust generic competition emerges, maintaining strict quality standards and performing robust product testing is crucial. Here again, LCMS serves as the gold standard method for generic drug testing across multiple core areas such as release testing, dissolution analysis, degradation product detection, and stability monitoring. Rising Importance of Precision Medicine Precision or personalized medicine aims to tailor treatments based on a patient's unique genetic, environmental, and lifestyle factors. It depends heavily on the rapidly advancing fields of genomics, proteomics, metabolomics, and diagnostics. LCMS has become an essential tool underpinning many precision medicine initiatives. Adoption of Combination Platforms Liquid chromatography mass spectrometry vendors are increasingly providing combination systems that integrate two or more techniques on a single platform. Examples include LC-Triple Quadrupole (TQ)-MS, LC-Quadrupole Time-of-Flight (Q-TOF)-MS, and Ultra Performance Liquid Chromatography (UPLC)-High Resolution MS (HRMS). These multi-technique platforms allow both targeted and non-targeted analysis of samples in a single run, adding versatility and efficiency gains.
Get more insights on, Liquid Chromatography Mass Spectrometry (LCMS)
For Deeper Insights, Find the Report in the Language that You want.
Japanese Korean
About Author:
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups.
(LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)
#Coherent Market Insights#Pharmaceutical Industry#Biotech Sector#Research and Development#Thermo Fisher Scientific
0 notes
Text
Optimizing Pharmaceutical and Biotech Processes with RO and EDI Systems
The pharmaceutical and biotech industries demand precision and reliability in every step of their processes. Among these, water purification stands as a cornerstone for manufacturing, research, and quality control. Reverse Osmosis (RO) and Electrodeionization (EDI) systems have become the gold standard for producing ultrapure water that meets stringent regulatory requirements. This article explores how these technologies enable efficiency, sustainability, and compliance in the industry.
The Need for High-Purity Water
Water plays a critical role in pharmaceutical and biotech operations, serving as a raw material, cleaning agent, and process facilitator. Regulatory frameworks such as the United States Pharmacopeia (USP) and European Pharmacopeia (EP) specify strict standards for water purity, including thresholds for microbial contamination, conductivity, and total organic carbon (TOC). Impure water can compromise drug safety, hinder research outcomes, and lead to costly recalls or regulatory penalties.
RO and EDI systems are tailored to address these challenges, ensuring consistent production of high-quality water suitable for applications ranging from drug formulation to laboratory analysis.
How RO and EDI Systems Work
Reverse Osmosis (RO): RO systems operate by forcing water through a semi-permeable membrane that filters out dissolved salts, organic compounds, and other contaminants. This technology serves as the primary purification step, achieving up to 99% rejection of impurities. Advanced RO systems now incorporate features like anti-scaling agents and energy recovery systems to enhance efficiency and durability.
Electrodeionization (EDI): EDI systems refine the water further, using ion-exchange resins and an electrical current to remove remaining ions. Unlike traditional deionization methods, EDI operates continuously without the need for chemical regenerants, making it both environmentally friendly and cost-effective.
Key Advantages of RO and EDI in Pharma and Biotech
Regulatory Compliance: RO and EDI systems consistently produce water that meets stringent standards for Purified Water, Highly Purified Water, and Water for Injection (WFI). This ensures seamless adherence to regulatory requirements worldwide.
Operational Efficiency: Continuous purification capabilities reduce downtime associated with maintenance and chemical handling, improving overall operational throughput.
Environmental Sustainability: By eliminating the need for harsh chemicals in regeneration, EDI reduces environmental impact. Energy-efficient RO systems further contribute to sustainability goals by optimizing water recovery and reducing power consumption.
Cost Savings: Automation and reduced reliance on consumables lower long-term operational costs, making these systems economically viable for facilities of all sizes.
Adaptability and Scalability: Modular RO and EDI units can be customized to meet the specific needs of small labs or large-scale manufacturing plants, ensuring flexibility in design and deployment.
Applications Across the Industry
RO and EDI systems have versatile applications in the pharmaceutical and biotech sectors:
Drug Manufacturing: High-purity water is essential for preparing solutions, diluents, and APIs. Any contaminants in the water can compromise the stability and efficacy of pharmaceutical products.
Cleaning and Sterilization: Water used for cleaning equipment and sterilizing containers must be free of microbial and ionic impurities to ensure compliance with Good Manufacturing Practices (GMP).
Cell Culture and Bioprocessing: The sensitive nature of cell cultures and bioprocesses demands ultrapure water to avoid adverse reactions that could compromise yields or product quality.
Laboratory and Analytical Testing: Techniques such as HPLC, GC-MS, and spectrophotometry rely on ultrapure water to avoid interference with test results, ensuring accuracy and reproducibility.
Technological Innovations Driving Excellence
The evolving needs of pharmaceutical and biotech industries have spurred significant advancements in RO and EDI systems:
Smart Monitoring Systems: Integration of IoT-enabled sensors allows real-time monitoring of water quality parameters, such as conductivity and TOC. These systems can trigger alerts and provide actionable insights for predictive maintenance.
Enhanced Membrane Performance: New-generation RO membranes offer improved fouling resistance, higher permeability, and extended lifespans, reducing both downtime and operating costs.
Energy Optimization: Advanced energy recovery devices in RO systems reduce power consumption, aligning with the industry's sustainability objectives.
Hybrid Configurations: Combining RO and EDI with complementary technologies like UV disinfection and ozone generation creates multi-barrier systems that deliver exceptional water quality and microbial control.
Case in Point: A Biotech Facility’s Transformation
A biotechnology firm specializing in vaccine production faced challenges with its aging water purification system, which required frequent maintenance and chemical handling. The company replaced its setup with an integrated RO-EDI system. This upgrade delivered remarkable results:
Improved Water Quality: Conductivity levels dropped below 0.1 µS/cm, ensuring compliance with USP standards.
Cost Reduction: Operational costs decreased by 25%, thanks to the elimination of chemical regenerants and reduced energy usage.
Increased Efficiency: Automated controls streamlined operations, allowing for uninterrupted water supply during peak production cycles.
Sustainability Gains: Water recovery rates improved by 15%, and the facility's carbon footprint was significantly reduced.
The Path Forward
As the pharmaceutical and biotech industries continue to evolve, the demand for reliable, efficient, and sustainable water purification systems will grow. RO and EDI technologies are well-positioned to meet these demands, offering a blend of performance, compliance, and environmental responsibility.
For organizations looking to optimize their water systems, adopting advanced RO-EDI solutions can provide a competitive edge. By ensuring water purity, these technologies not only enhance product quality and safety but also support operational excellence and sustainability goals. In a sector where precision is paramount, RO and EDI systems remain indispensable tools for success.
0 notes
Text
my mom mentioned bird flu and I chuckled and she was like "it's serious!!!"
i know! it's just the first ongoing comic I kept up with was Chew, a soft sci-fi soft supernatural cop mystery series finished a few years ago and I finally got up the emotional capacity to finish it soon. Except it's set in a world where bird flu (the '00s version) was a huge devastating pandemic and poultry is banned worldwide so ofc it would cause concerns again IRL when I'm finally ready to finish reading the series
I was laughing at the irony mom!
#also fingers crossed that investors come back to biotech#there's a lot of questions wrt lab grown meat vs standard animal ag BUT it is safer as long as regulations are being chopped up by trump#like. penicillin is a biological product and mushrooms farmed in a controlled monitored environment is going to be more safe and trustworth#than wild penicillium that you find out in your backyard ykwim? same for meat#a lot of contams in animal products come from animal poop. lab grown is the meat without the poop#if there was a viable product lab meat product put out while we're still in the era of cut regulations and funding to the fda and usda#investors could make it big!#(and the tech can really easily be sold off to the medical industry! we could end the need for organ donors in just a few decades!)#pull your money out of AI and put it back in biotech!!!!!!!!!!!
1 note
·
View note
Text
The pharmaceutical industry is a rapidly growing sector in India, and Andhra Pradesh has become a focal point for entrepreneurs seeking opportunities in this field. A Generic Pharma Franchise Company in Andhra Pradesh offers an ideal platform for individuals looking to establish a profitable business with minimal risk and maximum growth potential.
#Generic Pharma Franchise Company in Andhra Pradesh#Glenvox Biotech#The pharmaceutical industry#Andhra pradesh's leading generic pharma franchise company#best generic pharma franchise company in Andhra Pradesh#top 10 best generic pharma franchise industry in andhra pradesh#high quality generic pharma franchise cmpanies in andhra pradesh#generic pharma franchise business model in andhra pradesh#best generic pcd pharma franchise in andhra pradesh
0 notes
Text
applied to 100 jobs this month not even exaggerating i kept track🫠🫠🫠
#honestly devestating to my self esteem#biotech industry get better rn or i’ll kms#jk i’m just gonna become a barista i guess. bcs that’s what i wanted out of my 4 years of stupid hard work
0 notes
Text
Moshe Tanach, CEO and Co-Founder at NeuReality – Interview Series
New Post has been published on https://thedigitalinsider.com/moshe-tanach-ceo-and-co-founder-at-neureality-interview-series/
Moshe Tanach, CEO and Co-Founder at NeuReality – Interview Series
Moshe Tanach is the CEO & co-founder of NeuReality. Before founding NeuReality, Moshe served as Director of Engineering at Marvell and Intel, where he led the development of complex wireless and networking products to mass production. He also served as AVP of R&D at DesignArt Networks (later acquired by Qualcomm), where he contributed to the development of 4G base station products.
NeuReality’s mission is to simplify AI adoption. By taking a system-level approach to AI, NeuReality’s team of industry experts delivers AI inference holistically, identifying pain points and providing purpose-built, silicon-to-software AI inference solutions that make AI both affordable and accessible.
With your extensive experience leading engineering projects at Marvell, Intel, and DesignArt-Networks, what inspired you to co-found NeuReality, and how did your previous roles influence the vision and direction of the company?
NeuReality was built from inception to solve for the future cost, complexity and climate problems that would be inevitable AI inferencing – which is the deployment of trained AI models and software into production-level AI data centers. Where AI training is how AI is created; AI inference is how it is used and how it interacts with billions of people and devices around the world.
We are a team of systems engineers, so we look at all angles, all the multiple facets of end-to-end AI inferencing including GPUs and all classes of purpose-built AI accelerators. It became clear to us going back to 2015 that CPU-reliant AI chips and systems – which is every GPU, TPU, LPU, NRU, ASIC and FPGA out there – would hit a significant wall by 2020. Its system limitations where the AI accelerator has become better and faster in terms of raw performance, but the underlying infrastructure did not keep up.
As a result, we decided to break away from the big giants riddled with bureaucracy that protect successful businesses, like CPU and NIC manufacturers, and disrupt the industry with a better AI architecture that is open, agnostic, and purpose-built for AI inference. One of the conclusions of reimagining ideal AI inference is that in boosting GPU utilization and system-level efficiency, our new AI compute and network infrastructure – powered by our novel NR1 server-on-chip that replaces the host CPU and NICs. As an ingredient brand and companion to any GPU or AI accelerator, we can remove market barriers that deter 65% of organizations from innovating and adopting AI today – underutilized GPUs which leads to buying more than what’s really needed (because they run idle > 50% of the time) – all the while reducing energy consumption, AI data center real-estate challenge, and operational costs.
This is a once in a lifetime opportunity to really transform AI system architecture for the better based on everything I learned and practiced for 30 years, opening the doors for new AI innovators across industries and removing CPU bottlenecks, complexity, and carbon footprints.
NeuReality’s mission is to democratize AI. Can you elaborate on what “AI for All” means to you and how NeuReality plans to achieve this vision?
Our mission is to democratize AI by making it more accessible and affordable to all organizations big and small – by unleashing the maximum capacity of any GPU or any AI accelerator so you get more from your investment; in other words, get MORE from the GPUs you buy, rather than buying more GPUs that run idle >50% of the time. We can boost AI accelerators up to 100% full capability, while delivering up to 15X energy-efficiency and slashing system costs by up to 90%. These are order of magnitude improvements. We plan to achieve this vision with our NR1 AI Inference Solution, the world’s first data center system architecture tailored for the AI age. It runs high-volume, high-variety AI data pipelines affordably and efficiently with the added benefit of a reduced carbon footprint.
Achieving AI for all also means making it easy to use. At NeuReality, we simplify AI infrastructure deployment, management, and scalability, enhance business processes and profitability, and advance sectors such as public health, safety, law enforcement and customer service. Our impact spans sectors such as medical imaging, clinical trials, fraud detection, AI content creation and many more.
Currently, our first commercially available NR1-S AI Inference Appliances are available with Qualcomm Cloud AI 100 Ultra accelerators and through Cirrascale, a cloud service provider.
The NR1 AI Inference Solution is touted as the first data center system architecture tailored for the AI age, and purpose-built for AI inference. What were the key innovations and breakthroughs that led to the development of the NR1?
NR1™ is the name of the entire silicon-to-software system architecture we’ve designed and delivered to the AI industry – as an open, fully compatible AI compute and networking infrastructure that fully complements any AI accelerator and GPUs. If I had to break it down to the top-most unique and exciting innovations that led to this end-to-end NR1 Solution and differentiates us, I’d say:
Optimized AI Compute Graphs: The team designed a Programmable Graph Execution Accelerator to optimize the processing of Compute Graphs, which are crucial for AI and various other workloads like media processing, databases, and more. Compute Graphs represent a series of operations with dependencies, and this broader applicability positions NR1 as potentially disruptive beyond just super boosting GPUs and other AI accelerators. It simplifies AI model deployment by generating optimized Compute Graphs (CGs) based on pre-processed AI data and software APIs, leading to significant performance gains.
NR1 NAPU™ (Network Addressable Processing Unit): Our AI inference architecture is powered by the NR1 NAPU™ – a 7nm server-on-chip that enables direct network access for AI pre- and post-processing. We pack 6.5x more punch on a smaller NR1 chip than a typical general-purpose, host CPU. Traditionally, pre-processing tasks (like data cleaning, formatting, and feature extraction) and post-processing tasks (like result interpretation and formatting) are handled by the CPU. By offloading these tasks to the NR1 NAPU™, we displace both the CPUs and NIC. This reduces bottlenecks allowing for faster overall processing, lightning-fast response times and lower cost per AI query. This reduces bottlenecks and allows for faster overall processing.
NR1™ AI-Hypervisor™ technology: The NR1’s patented hardware-based AI-Hypervisor™ optimizes AI task orchestration and resource utilization, improving efficiency and reducing bottlenecks.
NR1™ AI-over-Fabric™ Network Engine: The NR1 incorporates a unique AI-over-Fabric™ network engine that ensures seamless network connectivity and efficient scaling of AI resources across multiple NR1 chips – which are coupled with any GPU or AI Accelerator – within the same inference server or NR1-S AI inference appliance.
NeuReality’s recent performance data highlights significant cost and energy savings. Could you provide more details on how the NR1 achieves up to 90% cost savings and 15x better energy efficiency compared to traditional systems?
NeuReality’s NR1 slashes the cost and energy consumption of AI inference by up to 90% and 15x, respectively. This is achieved through:
Specialized Silicon: Our purpose-built AI inference infrastructure is powered by the NR1 NAPU™ server-on-chip, which absorbs the functionality of the CPU and NIC into one – and eliminates the need for CPUs in inference. Ultimately the NR1 maximizes the output of any AI accelerator or GPU in the most efficient way possible.
Optimized Architecture: By streamlining AI data flow and incorporating AI pre- and post-processing directly within the NR1 NAPU™, we offload and replace the CPU. This results in reduced latency, linear scalability, and lower cost per AI query.
Flexible Deployment: You can buy the NR1 in two primary ways: 1) inside the NR1-M™ Module which is a PCIe card that houses multiple NR1 NAPUs (typically 10) designed to pair with your existing AI accelerator cards. 2) inside the NR1-S™ Appliance, which pairs NR1 NAPUs with an equal number of AI accelerators (GPU, ASIC, FPGA, etc.) as a ready-to-go AI Inference system.
At Supercomputing 2024 in November, you will see us demonstrate an NR1-S Appliance with 4x NR1 chips per 16x Qualcomm Cloud AI 100 Ultra accelerators. We’ve tested the same with Nvidia AI inference chips. NeuReality is revolutionizing AI inference with its open, purpose-built architecture.
How does the NR1-S AI Inference Appliance match up with Qualcomm® Cloud AI 100 accelerators compare against traditional CPU-centric inference servers with Nvidia® H100 or L40S GPUs in real-world applications?
NR1, combined with Qualcomm Cloud AI 100 or NVIDIA H100 or L40S GPUs, delivers a substantial performance boost over traditional CPU-centric inference servers in real-world AI applications across large language models like Llama 3, computer vision, natural language processing and speech recognition. In other words, running your AI inference system with NR1 optimizes the performance, system cost, energy efficiency and response times across images, sound, language, and text – both separately (single modality) or together (multi-modality).
The end-result? When paired with NR1, a customer gets MORE from the expensive GPU investments they make, rather than BUYING more GPUs to achieve desired performance.
Beyond maximizing GPU utilization, the NR1 delivers exceptional efficiency, resulting in 50-90% better price/performance and up to 13-15x greater energy efficiency. This translates to significant cost savings and a reduced environmental footprint for your AI infrastructure.
The NR1-S demonstrates linear scalability with no performance drop-offs. Can you explain the technical aspects that allow such seamless scalability?
The NR1-S Appliance, coupling our NR1 chips with AI accelerators of any type or quantity, redefines AI infrastructure. We’ve moved beyond CPU-centric limitations to achieve a new level of performance and efficiency.
Instead of the traditional NIC-to-CPU-to-accelerator bottleneck, the NR1-S integrates direct network access, AI pre-processing, and post-processing within our Network Addressable Processing Units (NAPUs). With typically 10 NAPUs per system, each handling tasks like vision, audio, and DSP processing, and our AI-Hypervisor™ orchestrating workloads, streamlined AI data flow is achieved. This translates to linear scalability: add more accelerators, get proportionally more performance.
The result? 100% utilization of AI accelerators is consistently observed. While overall cost and energy efficiency vary depending on the specific AI chips used, maximized hardware investment, and improved performance are consistently delivered. As AI inference needs scale, the NR1-S provides a compelling alternative to traditional architectures.
NeuReality aims to address the barriers to widespread AI adoption. What are the most significant challenges businesses face when adopting AI, and how does your technology help overcome these?
When poorly implemented, AI software and solutions can become troublesome. Many businesses cannot adopt AI due to the cost and complexity of building and scaling AI systems. Today’s AI solutions are not optimized for inference, with training pods typically having poor efficiency and inference servers having high bottlenecks. To take on this challenge and make AI more accessible, we have developed the first complete AI inference solution – a compute and networking infrastructure powered by our NAPU – which makes the most of its companion AI accelerator and reduces market barriers around excessive cost and energy consumption.
Our system-level approach to AI inference – versus trying to develop a better GPU or AI accelerator where there is already a lot of innovation and competition – means we are filling a significant industry gap for dozens of AI inference chip and system innovators. Our team attacked the shortcomings in AI Inference systemically and holistically, by determining pain points, architecture gaps and AI workload projections — to deliver the first purpose-built, silicon-to-software, CPU-free AI inference architecture. And by developing a top-to-bottom AI software stack with open standards from Python and Kubernetes combined with NeuReality Toolchain, Provisioning, and Inference APIs, our integrated set of software tools combines all components into a single high-quality UI/UX.
In a competitive AI market, what sets NeuReality apart from other AI inference solution providers?
To put it simply, we’re open and accelerator-agnostic. Our NR1 inference infrastructure supercharges any AI accelerator – GPU, TPU, LPU, ASIC, you name it – creating a truly optimized end-to-end system. AI accelerators were initially brought in to help CPUs handle the demands of neural networks and machine learning at large, but now the AI accelerators have become so powerful, they’re now held back by the very CPUs they were meant to assist.
Our solution? The NR1. It’s a complete, reimagined AI inference architecture. Our secret weapon? The NR1 NAPU™ was designed as a co-ingredient to maximize AI accelerator performance without guzzling extra power or breaking the bank. We’ve built an open ecosystem, seamlessly integrating with any AI inference chip and popular software frameworks like Kubernetes, Python, TensorFlow, and more.
NeuReality’s open approach means we’re not competing with the AI landscape; we’re here to complement it through strategic partnerships and technology collaboration. We provide the missing piece of the puzzle: a purpose-built, CPU-free inference architecture that not only unlocks AI accelerators to benchmark performance, but also makes it easier for businesses and governments to adopt AI. Imagine unleashing the full power of NVIDIA H100s, Google TPUs, or AMD MI300s – giving them the infrastructure they deserve.
NeuReality’s open, efficient architecture levels the playing field, making AI more accessible and affordable for everyone. I’m passionate about seeing different industries – fintech, biotech, healthtech – experience the NR1 advantage firsthand. Compare your AI solutions on traditional CPU-bound systems versus the modern NR1 infrastructure and witness the difference. Today, only 35% of businesses and governments have adopted AI and that is based on incredibly low qualifying criteria. Let’s make it possible for over 50% of enterprise customers to adopt AI by this time next year without harming the planet or breaking the bank.
Looking ahead, what is NeuReality’s long-term vision for the role of AI in society, and how do you see your company contributing to this future?
I envision a future where AI benefits everyone, fostering innovation and improving lives. We’re not just building technology; we’re building the foundation for a better future.
Our NR1 is key to that vision. It’s a complete AI inference solution that starts to shatter the cost and complexity barriers hindering mass AI business adoption. We’ve reimagined both the infrastructure and the architecture, delivering a revolutionary system that maximizes the output of any GPU, any AI accelerator, without increasing operational costs or energy consumption.
The business model really matters to scale and give end-customers real choices over concentrated AI autocracy as I’ve written on before. So instead, we’re building an open ecosystem where our silicon works with other silicon, not against it. That’s why we designed NR1 to integrate seamlessly with all AI accelerators and with open models and software, making it as easy as possible to install, manage and scale.
But we’re not stopping there. We’re collaborating with partners to validate our technology across various AI workloads and deliver “inference-as-a-service” and “LLM-as-a-service” through cloud service providers, hyper scalers, and directly with companion chip makers. We want to make advanced AI accessible and affordable to all.
Imagine the possibilities if we could boost AI inference performance, energy efficiency, and affordability by double-digit percentages. Imagine a robust, AI-enabled society with more voices and choices becoming a reality. So, we must all do the demanding work of proving business impact and ROI when AI is implemented in daily data center operations. Let’s focus on revolutionary AI implementation, not just AI model capability.
This is how we contribute to a future where AI benefits everyone – a win for profit margins, people, and the planet.
Thank you for the great interview, readers who wish to learn more should visit NeuReality.
#2024#4g#accelerators#ADD#adoption#ai#AI adoption#AI chips#AI industry#ai inference#AI Infrastructure#ai model#AI models#AI systems#ai training#amd#amp#APIs#applications#approach#architecture#audio#bank#benchmark#biotech#Building#Business#business model#carbon#carbon footprint
0 notes
Text
High-Quality Fermenters & Bioreactors | India’s Biotech Supplier | Inses Instruments & Services
For top-notch fermenters and bioreactors in India's biotech industry, trust Inses Instruments & Services. Offering reliable, high-tech equipment for all biotech applications. Contact today.
#Fermenters for biotech industry#Bioreactor suppliers India#Biotech fermenters#Bioreactor solutions India#Biotechnology equipment suppliers India#Biotech equipment suppliers#Fermenters & bioreactors India
0 notes