#tumor antigen release
Explore tagged Tumblr posts
Text
youtube
#Sonodynamic therapy#ferroptosis#FSP1 inhibition#cancer immunotherapy#reactive oxygen species#lipid peroxidation#cell membrane targeting#ultrasound therapy#tumor microenvironment#anti-tumor immunity#programmed cell death#combination therapy#oncology innovation#tumor antigen release#immune cell infiltration#precision oncology#therapeutic synergy#cancer treatment#immune activation#cancer breakthroughs.#Youtube
0 notes
Text
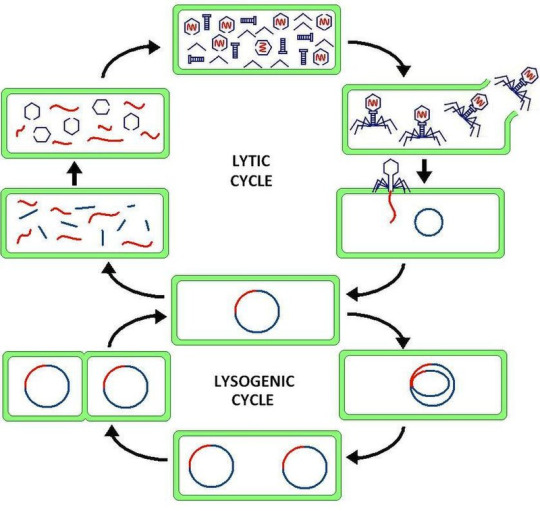
Virus latency (or viral latency) is the ability of a pathogenic virus to lie dormant (latent) within a cell, denoted as the lysogenic part of the viral life cycle.[1] A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny (the lytic part of the viral life cycle) without the host becoming reinfected by new outside virus, and stays within the host indefinitely.[2]
Episomal latency refers to the use of genetic episomes during latency. In this latency type, viral genes are stabilized, floating in the cytoplasm or nucleus as distinct objects, either as linear or lariat structures. Episomal latency is more vulnerable to ribozymes or host foreign gene degradation than proviral latency (see below).
Advantages of episomal latency include the fact that the virus may not need to enter the cell nucleus, and hence may avoid nuclear domain 10 (ND10) from activating interferon via that pathway. Disadvantages include more exposure to cellular defenses, leading to possible degradation of viral gene via cellular enzymes.[12]
Proviral latency: A provirus is a virus genome that is integrated into the DNA of a host cell
All interferons share several common effects: they are antiviral agents and they modulate functions of the immune system. Administration of Type I IFN has been shown experimentally to inhibit tumor growth in animals, but the beneficial action in human tumors has not been widely documented. A virus-infected cell releases viral particles that can infect nearby cells. However, the infected cell can protect neighboring cells against a potential infection of the virus by releasing interferons. In response to interferon, cells produce large amounts of an enzyme known as protein kinase R (PKR). This enzyme phosphorylates a protein known as eIF-2 in response to new viral infections; the phosphorylated eIF-2 forms an inactive complex with another protein, called eIF2B, to reduce protein synthesis within the cell. Another cellular enzyme, RNAse L—also induced by interferon action—destroys RNA within the cells to further reduce protein synthesis of both viral and host genes. Inhibited protein synthesis impairs both virus replication and infected host cells. In addition, interferons induce production of hundreds of other proteins—known collectively as interferon-stimulated genes (ISGs)—that have roles in combating viruses and other actions produced by interferon.[13][14] They also limit viral spread by increasing p53 activity, which kills virus-infected cells by promoting apoptosis.[15][16] The effect of IFN on p53 is also linked to its protective role against certain cancers.[15]
Another function of interferons is to up-regulate major histocompatibility complex molecules, MHC I and MHC II, and increase immunoproteasome activity. All interferons significantly enhance the presentation of MHC I dependent antigens. Interferon gamma (IFN-gamma) also significantly stimulates the MHC II-dependent presentation of antigens. Higher MHC I expression increases presentation of viral and abnormal peptides from cancer cells to cytotoxic T cells, while the immunoproteasome processes these peptides for loading onto the MHC I molecule, thereby increasing the recognition and killing of infected or malignant cells. Higher MHC II expression increases presentation of these peptides to helper T cells; these cells release cytokines (such as more interferons and interleukins, among others) that signal to and co-ordinate the activity of other immune cells.[17][18][19]
Epstein–Barr virus lytic reactivation (which can be due to chemotherapy or radiation) can result in genome instability and cancer.[5]
HSV reactivates upon even minor chromatin loosening with stress,[7] although the chromatin compacts (becomes latent) upon oxygen and nutrient deprivation.[8]
Cytomegalovirus (CMV) establishes latency in myeloid progenitor cells, and is reactivated by inflammation.[9] Immunosuppression and critical illness (sepsis in particular) often results in CMV reactivation.[10] CMV reactivation is commonly seen in patients with severe colitis.[11]
viral latency is so fucked up
11 notes
·
View notes
Text
A Dive into CAR-T Cell Therapy
Imagine training your own soldiers to fight cancer. Not just any soldiers, but elite warriors genetically modified to recognize and demolish the enemy with laser-like precision. That's the essence of CAR-T cell therapy, a revolutionary approach turning heads in the medical world. In the fight against cancer, CAR-T cell therapy embodies this very concept, harnessing the body's own immune system to wage war against malignant cells. T cells are the special forces of your immune system, constantly scanning for and eliminating threats. But sometimes, cancer cells outsmart them, hiding in plain sight. CAR-T cell therapy steps in, equipping these T-cells with a unique weapon: a Chimeric Antigen Receptor (CAR). Think of it as a GPS that locks onto a specific protein on cancer cells, guiding the T-cells straight to their target.
How does it work? Here's the simplified version:
Recruitment: First, T cells are extracted from your blood. Modification Station: In the lab, scientists use a virus or other tools to insert the CAR gene into the T cells' DNA. This equips them with the cancer-targeting GPS. Bootcamp Boost: The modified T cells are grown in a special environment, multiplying into a powerful army. Redeployment: The CAR-T cell troops are infused back into your bloodstream, ready to seek and destroy.
Sounds amazing, right? But like any powerful technology, CAR-T comes with its own set of challenges. The treatment process is complex and expensive, and there can be serious side effects like cytokine release syndrome, where the immune system goes into overdrive. So, is CAR-T a miracle cure? Not yet. But for some patients with aggressive blood cancers like leukemia and lymphoma, it has shown remarkable results, offering hope where other treatments have failed. Researchers are constantly working to improve the safety and efficacy of CAR-T, making it a potential game-changer for even more cancers in the future. CAR-T cell therapy has demonstrated remarkable efficacy in the treatment of certain types of hematologic malignancies, including acute lymphoblastic leukemia (ALL) and certain subtypes of non-Hodgkin lymphoma. Clinical trials have shown unprecedented response rates and durable remissions in patients who have exhausted all other treatment options. Furthermore, ongoing research is exploring the potential of CAR-T cell therapy in treating solid tumors, extending its therapeutic reach to a broader spectrum of cancers. The future is bright for CAR-T. It's a testament to the power of human ingenuity and our ongoing quest to conquer one of humanity's greatest foes. While there's still a way to go, this groundbreaking therapy is a beacon of hope, reminding us that even the seemingly impossible can become reality.
3 notes
·
View notes
Text
Bonne nouvelle... les heures du cancer qui rapportent beaucoup, car le cancer fait vivre (très très bien) plus de gens qu'il n'en fait mourir, a sonné.
L'Histotripsy comme démontré dans cette étude, fonctionne très bien. Il s'agit de bombarder d'ultra-sons à partir d'échographies les tumeurs, l'enveloppe se liquéfie et alors le système immunitaire adaptatif refonctionne et détruit les cellules cancéreuses !
0 notes
Text
Medical Oncologist in Kolkata - Dr. Pallabika Mandal

Side effects of colorectal cancer:
Any aggravation or irregularity in the colon, rectum, and rear end might bring about any of the accompanying side effects:
Rectal dying with torment or without torment
Butt-centric torment and consuming sensation
Butt-centric irregularities and swellings
Butt-centric mucous release
Butt-centric discharge release
Tingling
Obstruction
Looseness of the bowels
Mucus or discharge release
Stomach pain
Procedure and treatments of colorectal cancer:
Some standard diagnostic tests and procedures that doctors may recommend for diagnosis are:
Antigen test.
Liver function test.
CEA test.
Endoscopic ultrasound.
Colonoscopy and biopsy.
Several kinds of procedures are available to treat colorectal cancer. Treatments are based on the infected area, size, and cancer phases. Common treatments like surgery, chemotherapy and radiation therapy are also recommended to cure. Other treatments are:
colonoscopy (Removing polyps)
Endoscopic mucosal resection.
Minimally invasive surgery (laparoscopic surgery).
Colorectal surgery is useful for earlier-stage cancer that hasn't spread. Therapy for colon cancer usually involves surgery to remove cancer. The three main types of surgery for colorectal cancer are open, laparoscopic, and robotic. Open surgery for colorectal cancer has been the crucial method for removing tumors. Recently modified technologies are laparoscopic and robotic to treat colorectal cancer.
It can also be treated using medications that can be given by mouth or directly into the bloodstream. All the methods of treatment depend on the patient's general health and medical conditions.
The most notable name for the best general surgeon in Kolkata is Dr. Gaurav Kumar, who has been constantly working in Narayana Superspeciality for the last 5 years, in the department of GI surgery, Minimal Invasive Surgery & General surgery. He mentions the importance of letting your patients know about their diseases and making them aware of what their diagnosis means, explaining the risk factors, and so on to make sure that there is full awareness of decisions and clarity during the whole procedure, leaving no place for misunderstanding.
VISIT NOW: https://drpallabikamandal.com/
0 notes
Text
Medical Oncologist in Kolkata - Dr. Sanjoy Roy

Side effects of colorectal cancer:
Any aggravation or irregularity in the colon, rectum, and rear end might bring about any of the accompanying side effects:
Rectal dying with torment or without torment
Butt-centric torment and consuming sensation
Butt-centric irregularities and swellings
Butt-centric mucous release
Butt-centric discharge release
Tingling
Obstruction
Looseness of the bowels
Mucus or discharge release
Stomach pain
Procedure and treatments of colorectal cancer:
Some standard diagnostic tests and procedures that doctors may recommend for diagnosis are:
Antigen test.
Liver function test.
CEA test.
Endoscopic ultrasound.
Colonoscopy and biopsy.
Several kinds of procedures are available to treat colorectal cancer. Treatments are based on the infected area, size, and cancer phases. Common treatments like surgery, chemotherapy and radiation therapy are also recommended to cure. Other treatments are:
colonoscopy (Removing polyps)
Endoscopic mucosal resection.
Minimally invasive surgery (laparoscopic surgery).
Colorectal surgery is useful for earlier-stage cancer that hasn't spread. Therapy for colon cancer usually involves surgery to remove cancer. The three main types of surgery for colorectal cancer are open, laparoscopic, and robotic. Open surgery for colorectal cancer has been the crucial method for removing tumors. Recently modified technologies are laparoscopic and robotic to treat colorectal cancer.
It can also be treated using medications that can be given by mouth or directly into the bloodstream. All the methods of treatment depend on the patient's general health and medical conditions.
The most notable name for the best general surgeon in Kolkata is Dr. Gaurav Kumar, who has been constantly working in Narayana Superspeciality for the last 5 years, in the department of GI surgery, Minimal Invasive Surgery & General surgery. He mentions the importance of letting your patients know about their diseases and making them aware of what their diagnosis means, explaining the risk factors, and so on to make sure that there is full awareness of decisions and clarity during the whole procedure, leaving no place for misunderstanding.
VISIT NOW: https://drsanjoyroy.com/
0 notes
Text
Medical Oncologist in Kolkata - Dr. Sayan Paul

Side effects of colorectal cancer: Any aggravation or irregularity in the colon, rectum, and rear end might bring about any of the accompanying side effects: Rectal dying with torment or without torment Butt-centric torment and consuming sensation Butt-centric irregularities and swellings Butt-centric mucous release Butt-centric discharge release Tingling Obstruction Looseness of the bowels Mucus or discharge release Stomach pain
Procedure and treatments of colorectal cancer: Some standard diagnostic tests and procedures that doctors may recommend for diagnosis are: Antigen test. Liver function test. CEA test. Endoscopic ultrasound. Colonoscopy and biopsy. Several kinds of procedures are available to treat colorectal cancer. Treatments are based on the infected area, size, and cancer phases. Common treatments like surgery, chemotherapy and radiation therapy are also recommended to cure. Other treatments are: colonoscopy (Removing polyps) Endoscopic mucosal resection. Minimally invasive surgery (laparoscopic surgery). Colorectal surgery is useful for earlier-stage cancer that hasn't spread. Therapy for colon cancer usually involves surgery to remove cancer. The three main types of surgery for colorectal cancer are open, laparoscopic, and robotic. Open surgery for colorectal cancer has been the crucial method for removing tumors. Recently modified technologies are laparoscopic and robotic to treat colorectal cancer. It can also be treated using medications that can be given by mouth or directly into the bloodstream. All the methods of treatment depend on the patient's general health and medical conditions. The most notable name for the best general surgeon in Kolkata is Dr. Gaurav Kumar, who has been constantly working in Narayana Superspeciality for the last 5 years, in the department of GI surgery, Minimal Invasive Surgery & General surgery. He mentions the importance of letting your patients know about their diseases and making them aware of what their diagnosis means, explaining the risk factors, and so on to make sure that there is full awareness of decisions and clarity during the whole procedure, leaving no place for misunderstanding.
VISIT NOW: https://www.drsayanpaul.com/
0 notes
Text
T Cell Therapy Market Analysis Report - Size & Share 2030
T-cell therapy is revolutionizing the field of cancer therapy by giving hope to patients suffering from previously untreatable diseases. This innovative therapy establishes the power of immune cells that target and destroy cancerous cells. The industry in this field is expected to undergo significant growth due to the increase in technological advancements, more regulatory approvals, and a growing pipeline of promising therapies.
Market Overview
The global market for T-cell therapy is growing at a very high rate, mainly due to the rapid growth in the prevalence of cancer and the limited availability of conventional treatment modalities. This market is primarily driven by the development of chimeric antigen receptor T cell therapy, wherein T cells are engineered to recognize antigens particular to cancer.
The T cell therapy market size was US$ 2,754.0 million in 2022 and will grow at a rate of US$ 9,035.01 million by 2030; the market is expected to register a CAGR of 16.0% during 2022–2030.
Key Market Drivers
Rising Cancer Incidence: Increasing rates of cancers across the world have created a dire need for effective and targeted therapies. T-cell therapy is promising in such a scenario, especially in advanced or refractory cancers.
Technological Advancements: Continuous advancement in cell engineering and genetic modification methodology makes it possible to develop more potent and specific T-cell therapy.
The potential improvements in therapeutic outcomes and widened therapeutic windows are within innovations.
Regulatory Approvals: The potential of T cell therapy is increasingly being acknowledged by all regulatory bodies, with approved treatments gaining foot. Regulatory support has accelerated the adoption and created new opportunities in the market.
Prosperous Pipeline of Therapies: With a strong pipeline of T cell therapies in development, targeting a huge variety of cancer types, is designed to overcome the shortcomings of existing therapies and help provide a much better and longer-lasting response.
Market Segmentation
By Modality
Research and Commercialized
By Therapy Type
CAR T-cell Therapy
T-cell Receptor-based
By Indication
Hematologic Malignancies and Solid Tumors
By Region
North America
Europe
Asia-Pacific
South and Central America
Middle East and Africa
Market leaders and key company profiles
Immunocore Holdings Plc
Legend Biotech Corp
Janssen Global Services LLC
Gilead Sciences Inc
Bristol-Myers Squibb Co
Bluebird Bio Inc
Novartis AG
JW (Cayman) Therapeutics Co Ltd
Cartesian Therapeutics Inc
Challenges and Opportunities
Although the T-cell therapy market appears to be extremely promising, some serious challenges are still limiting its wide use at this moment:
High Cost of Treatment: T-cell therapies are expensive and therefore not accessible to many patients.
Side Effects These therapeutic approaches can cause severe side effects, including cytokine release syndrome and neurotoxicity, and hence have to be observed and managed very closely.
Manufacturing Complexity - The process for T cell therapies is highly complex and requires highly skilled labor with proper facilities.
However, the T cell therapy market does offer a lot of opportunities:
Combination Therapies—Combining T-cell therapy with other treatment modalities, such as chemotherapy or immunotherapy, can improve the effectiveness of the treatment and overcome some forms of resistance.
Allogeneic T Cell Therapies: Allogeneic T-cell therapies, derived from donor cells, are more accessible and scalable compared to autologous therapies.
Targeted Therapies: T-cell therapies targeted toward particular tumor antigens may lead to higher precision in the treatment and less off-target effects.
Conclusion
The T cell therapy market is at the edge of its revolutionizing change, where this therapy might change the fate of the treatment of cancer. R and D continued to progress and enhance, and more innovative and effective therapies can expectably roll out. With such challenges and opportunities, the T-cell therapy market can realize its goal to make improvements in patient outcomes and save lives.
FAQs-
Which is the largest regional market for T Cell Therapy?
Ans: - Asia Pacific is the largest regional market for T Cell Therapy.
Which are the top companies to hold the market share in the T Cell Therapy market?
Ans: These are the top companies that will hold the market share including Holdings Plc, Legend Biotech Corp, Janssen Global Services LLC, Gilead Sciences Inc, Bristol-Myers Squibb Co, Bluebird Bio Inc, Novartis AG, JW (Cayman) Therapeutics Co Ltd, Cartesian Therapeutics Inc, and Innovent Biologics Inc.
What growth rate will the market be projected to grow during the forecast period of 2022 to 2030?
Ans: - The T Cell Therapy market is likely to register a growth rate of 16.0% during the forecasting period of 2030.
How big is the T Cell Therapy market?
Ans: - The size of the global T Cell Therapy market was valued at US$ 2,754.0 million in 2022 and is expected to reach US$ 9,035.01 million by 2030.
What are the key segments of the T Cell Therapy market?
Ans: - The T Cell Therapy market is segmented into Modality, Therapy Type, Indication, and region.
About Us-
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
0 notes
Text
Creative Biolabs: Creative Thoughts to Shake Up mRNA R&D
Creative Biolabs offers top-notch mRNA technology support to fuel emerging therapeutic discoveries.
Recent headlines have been dominated by the transformative potential of mRNA-based therapies, extending beyond infectious diseases to encompass cancer, genetic disorders, and autoimmune conditions. This surge in interest underscores the importance of reliable and innovative partners in mRNA research and development. Creative Biolabs stands out as a pivotal player, providing comprehensive solutions that streamline the drug development process and enhance the efficacy of mRNA therapeutics.
One of the key offerings of Creative Biolabs is the mRNA technology platform, which provides a kaleidoscopic toolbox for mRNA research and development. The platform integrates the lipoplyplex (LPR) technique to produce mRNA modified by specific histidylated liposomes and polymers and encoding a wide variety of tumor antigens, ensuring a streamlined development workflow, improved mRNA delivery, and reduced overall cost.
A decisive component of successful mRNA therapeutics is the delivery system. Recognizing that one size does not fit all, Creative Biolabs offers customized mRNA delivery vehicles tailored to the unique requirements of each project. Their ability to customize delivery vehicles enables researchers to optimize the therapeutic index and overcome the specific challenges associated with the candidate mRNA drugs, whether they be targeted delivery, controlled release, or enhanced cellular uptake.
"We help scheme LNP formulations to make sure that the desired therapeutic mRNA reaches its intended destination within the body, maximizing therapeutic potential while minimizing side effects. Researchers can rely on our lipid nanoparticle technology to advance their mRNA-based treatments from the laboratory to next stage trials with confidence." According to a scientist at Creative Biolabs.
Furthermore, Creative Biolabs excels in developing lipid nanoparticles, one of the prevalent options of a successful delivery system, as an essential carrier to transport mRNA therapeutics into cells with their LNP technology designed to optimize the delivery efficiency and stability of mRNA molecules, allowing for one-stop, customized LNP preparation and scalable proprietary manufacturing.
Overall, Creative Biolabs is dedicated to pushing the boundaries of mRNA technology, empowering researchers to advance their mRNA-related research projects, and playing a crucial role in driving innovation in the field of mRNA therapeutics.
To learn more about Creative Biolabs' mRNA technology services, please visit: https://mrna.creative-biolabs.com.
About In an era where precision medicine and rapid drug development are paramount, Creative Biolabs' contributions are not just relevant—they are revolutionary. Their comprehensive mRNA technology platform, coupled with pioneering lipid nanoparticle and delivery system solutions, positions them as a leader in the mRNA therapeutics arena. By partnering with Creative Biolabs, scientists and pharmaceutical companies can accelerate their research, bringing life-changing therapies to patients faster and more efficiently than ever before.
0 notes
Text
CAR T-Cell Therapy Market to Reach $20.3B by 2033, CAGR 14.7%
CAR T-Cell therapy Market: CAR T-cell therapy is revolutionizing oncology by offering a cutting-edge approach to treating certain cancers. This personalized immunotherapy involves modifying a patient’s T-cells to express chimeric antigen receptors (CARs) that specifically target and destroy cancer cells. It has shown remarkable success in treating blood cancers such as leukemia and lymphoma, providing hope for patients with otherwise limited options. As clinical trials expand, researchers are exploring its potential to combat solid tumors, pushing the boundaries of cancer treatment and transforming outcomes for countless individuals.
To Request Sample Report : https://www.globalinsightservices.com/request-sample/?id=GIS31609 &utm_source=SnehaPatil&utm_medium=Article
The rapid advancements in CAR T-cell therapy highlight its promise and challenges. Efforts to enhance efficacy, reduce side effects like cytokine release syndrome, and make the therapy more accessible are shaping the next generation of treatments. With increasing approvals and innovations in manufacturing processes, this therapy is poised to become a cornerstone of personalized medicine. Its success exemplifies the power of biotechnology to turn groundbreaking research into life-saving solutions, heralding a new era in the fight against cancer.
#CARTCellTherapy #CancerTreatment #Immunotherapy #PersonalizedMedicine #BiotechInnovation #BloodCancerCare #RevolutionInOncology #CancerBreakthroughs #FutureOfMedicine #CancerResearch #TCellEngineering #AdvancedTherapies #HopeForCancerPatients #BiotechRevolution #OncologyAdvancements
0 notes
Text
What is a Urology Test?
A urology test refers to any diagnostic procedure or medical examination performed to evaluate the function and health of the urinary tract system and male reproductive organs. These tests are used to diagnose conditions related to the kidneys, bladder, urethra, ureters, and in men, the prostate and testicles.
يشير اختبار المسالك البولية إلى أي إجراء تشخيصي أو فحص طبي يتم إجراؤه لتقييم وظيفة وصحة الجهاز البولي والأعضاء التناسلية الذكرية. تُستخدم هذه الاختبارات لتشخيص الحالات المتعلقة بالكلى والمثانة والإحليل والحالب، وفي الرجال البروستاتا والخصيتين.
Common urology tests include:
Urinalysis: A basic test that examines the urine for abnormalities, such as infection, blood, or protein.
Ultrasound: Imaging test used to visualize the kidneys, bladder, and reproductive organs.
Cystoscopy: A procedure where a thin tube with a camera is inserted through the urethra to inspect the bladder and urethra.
Urodynamic Testing: Evaluates how well the bladder and urethra are storing and releasing urine.
Prostate-Specific Antigen (PSA) Test: Blood test used to screen for prostate cancer.
CT Scan or MRI: Advanced imaging to look for kidney stones, tumors, or other structural abnormalities.

What is URS in Urology?
URS (Ureteroscopy) is a medical procedure used in urology to examine the inside of the ureters (the tubes that connect the kidneys to the bladder) and, in some cases, the kidneys. URS is typically used to diagnose and treat conditions like kidney stones, tumors, or strictures within the ureters.
URS (تنظير الحالب) هو إجراء طبي يستخدم في طب المسالك البولية لفحص داخل الحالب (الأنابيب التي تربط الكلى بالمثانة)، وفي بعض الحالات، الكلى. يُستخدم URS عادةً لتشخيص وعلاج حالات مثل حصوات الكلى أو الأورام أو التضيقات داخل الحالب.
During URS:
A thin, flexible tube called a ureteroscope is inserted into the bladder and then guided up into the ureter and kidney.
The ureteroscope has a light and a camera, allowing the urologist to view the inside of the ureter and kidney.
If stones are found, they can be removed or broken up using specialized tools.
The procedure is usually done under general anesthesia, and patients can often go home the same day.

URS is minimally invasive and is often preferred over more invasive surgical options for treating kidney stones and other urological issues.
The Urology and Neurourology Department at Fakeeh University Hospital treats patients with problems with the bladder, prostate, kidneys, and reproductive organs in men. The latter may include problems with erections, ejaculation, and infertility.
يُعالج قسم المسالك البولية والأعصاب في مستشفى فقيه الجامعي المرضى الذين يعانون من مشكلات في المثانة والبروستاتا والكلى والأعضاء التناسلية للذكور. وقد تتضمن مشكلات الأعضاء التناسلية هذه مشكلات الانتصاب، والقذف، والعقم.
يتم إيلاء اهتمام خاص بالجوانب الوظيفية لطب المسالك البولية، مثل الاختلال الوظيفي في الجهاز البولي (تسرب البول، صعوبة التبول) وآلام الحوض المزمنة (ألم في منطقة الأعضاء التناسلية والشرج). كما تُوفَّر أعلى مستويات الخدمات لتشخيص مشكلات المسالك البولية وعلاجها
Particular attention is given to the functional aspects of urology, like urinary dysfunctions (leak of urine, difficulty to pass urine) and chronic pelvic pain (pain in the genital and anal area). A high standard of diagnosis and treatment of urological problems is provided.
One of the standout departments at Fakeeh University Hospital Dubai مستشفى فقيه الجامعي دبي is the Urology and Andrology Department. Whether you’re dealing with kidney stones, urinary tract infections, prostate issues, or other urological conditions, the expert team at Fakeeh University Hospital is equipped with the latest technology and expertise to provide effective treatment. They offer a range of diagnostic and therapeutic services, including advanced procedures like URS (Ureteroscopy).
For more information on their urology services, you can visit their dedicated page here.
Trust your health with the specialists at Fakeeh University Hospital Dubai مستشفى فقيه الجامعي دبي, where patient care and medical excellence come first.

Why Choose Fakeeh University Hospital?
State-of-the-Art Facility: The hospital is equipped with cutting-edge technology and modern infrastructure, ensuring that patients receive top-tier medical care in a comfortable and safe environment.
Expert Medical Team: The hospital boasts a highly qualified team of doctors, surgeons, nurses, and support staff, all dedicated to delivering personalized care. The medical professionals here are not only experts in their fields but also committed to staying updated with the latest medical advancements.
Comprehensive Care: Fakeeh University Hospital offers a wide range of specialties under one roof, making it a convenient choice for all your healthcare needs. From routine check-ups to complex surgeries, the hospital is designed to cater to patients with varying medical conditions.
🔗Click Here To Learn More
Spotlight on the Urology and Andrology Department
The Urology and Andrology Department at Fakeeh University Hospital Dubai مستشفى فقيه الجامعي دبي is one of the most advanced in the region. Here’s why it stands out:
Comprehensive Urological Services: The department provides a full spectrum of urological care, addressing conditions such as kidney stones, urinary tract infections, prostate disorders, bladder issues, and male infertility.
Advanced Diagnostic Tools: The hospital utilizes the latest diagnostic technologies, such as high-definition imaging and minimally invasive techniques, to ensure accurate diagnosis and effective treatment plans.
Minimally Invasive Procedures: The Urology Department is skilled in performing minimally invasive procedures like URS (Ureteroscopy). This procedure allows for the treatment of kidney stones and other urological conditions with minimal discomfort and quicker recovery times compared to traditional surgery.
Patient-Centered Approach: The team at Fakeeh University Hospital understands that urological conditions can be sensitive and sometimes challenging to discuss. They prioritize patient comfort, privacy, and clear communication throughout the treatment process.

Why Trust Your Urological Care to Fakeeh University Hospital?
Holistic Approach: The hospital doesn’t just treat the symptoms but looks at the overall well-being of the patient, ensuring a holistic approach to healthcare.
Collaborative Care: The Urology Department works closely with other specialties within the hospital, such as nephrology and oncology, to provide coordinated care for patients with complex conditions.
Continuous Support: From diagnosis through treatment and follow-up, the team at Fakeeh University Hospital is dedicated to providing continuous support to their patients, ensuring the best possible outcomes.

If you’re facing urological issues or need expert advice, don’t hesitate to reach out to the Urology and Andrology Department at Fakeeh University Hospital. For more detailed information, including how to book an appointment, visit their Urology and Andrology page.
Choosing Fakeeh University Hospital Dubai مستشفى فقيه الجامعي دبي means entrusting your health to one of Dubai’s most respected medical institutions, where your well-being is the top priority.
🏥 Click Here To Book An Appointment
0 notes
Text
A new way to reprogram immune cells and direct them toward anti-tumor immunity
New Post has been published on https://thedigitalinsider.com/a-new-way-to-reprogram-immune-cells-and-direct-them-toward-anti-tumor-immunity/
A new way to reprogram immune cells and direct them toward anti-tumor immunity
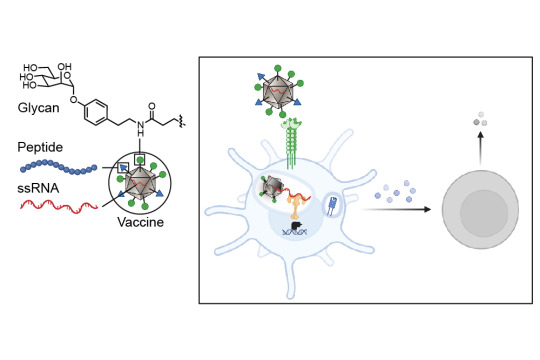

A collaboration between four MIT groups, led by principal investigators Laura L. Kiessling, Jeremiah A. Johnson, Alex K. Shalek, and Darrell J. Irvine, in conjunction with a group at Georgia Tech led by M.G. Finn, has revealed a new strategy for enabling immune system mobilization against cancer cells. The work, which appears today in ACS Nano, produces exactly the type of anti-tumor immunity needed to function as a tumor vaccine — both prophylactically and therapeutically.
Cancer cells can look very similar to the human cells from which they are derived. In contrast, viruses, bacteria, and fungi carry carbohydrates on their surfaces that are markedly different from those of human carbohydrates. Dendritic cells — the immune system’s best antigen-presenting cells — carry proteins on their surfaces that help them recognize these atypical carbohydrates and bring those antigens inside of them. The antigens are then processed into smaller peptides and presented to the immune system for a response. Intriguingly, some of these carbohydrate proteins can also collaborate to direct immune responses. This work presents a strategy for targeting those antigens to the dendritic cells that results in a more activated, stronger immune response.
Tackling tumors’ tenacity
The researchers’ new strategy shrouds the tumor antigens with foreign carbohydrates and co-delivers them with single-stranded RNA so that the dendritic cells can be programmed to recognize the tumor antigens as a potential threat. The researchers targeted the lectin (carbohydrate-binding protein) DC-SIGN because of its ability to serve as an activator of dendritic cell immunity. They decorated a virus-like particle (a particle composed of virus proteins assembled onto a piece of RNA that is noninfectious because its internal RNA is not from the virus) with DC-binding carbohydrate derivatives. The resulting glycan-costumed virus-like particles display unique sugars; therefore, the dendritic cells recognize them as something they need to attack.
“On the surface of the dendritic cells are carbohydrate binding proteins called lectins that combine to the sugars on the surface of bacteria or viruses, and when they do that they penetrate the membrane,” explains Kiessling, the paper’s senior author. “On the cell, the DC-SIGN gets clustered upon binding the virus or bacteria and that promotes internalization. When a virus-like particle gets internalized, it starts to fall apart and releases its RNA.” The toll-like receptor (bound to RNA) and DC-SIGN (bound to the sugar decoration) can both signal to activate the immune response.
Once the dendritic cells have sounded the alarm of a foreign invasion, a robust immune response is triggered that is significantly stronger than the immune response that would be expected with a typical untargeted vaccine. When an antigen is encountered by the dendritic cells, they send signals to T cells, the next cell in the immune system, to give different responses depending on what pathways have been activated in the dendritic cells.
Advancing cancer vaccine development
The activity of a potential vaccine developed in line with this new research is twofold. First, the vaccine glycan coat binds to lectins, providing a primary signal. Then, binding to toll-like receptors elicits potent immune activation.
The Kiessling, Finn, and Johnson groups had previously identified a synthetic DC-SIGN binding group that directed cellular immune responses when used to decorate virus-like particles. But it was unclear whether this method could be utilized as an anticancer vaccine. Collaboration between researchers in the labs at MIT and Georgia Tech demonstrated that in fact, it could.
Valerie Lensch, a chemistry PhD student from MIT’s Program in Polymers and Soft Matter and a joint member of the Kiessling and Johnson labs, took the preexisting strategy and tested it as an anticancer vaccine, learning a great deal about immunology in order to do so.
“We have developed a modular vaccine platform designed to drive antigen-specific cellular immune responses,” says Lensch. “This platform is not only pivotal in the fight against cancer, but also offers significant potential for combating challenging intracellular pathogens, including malaria parasites, HIV, and Mycobacterium tuberculosis. This technology holds promise for tackling a range of diseases where vaccine development has been particularly challenging.”
Lensch and her fellow researchers conducted in vitro experiments with extensive iterations of these glycan-costumed virus-like particles before identifying a design that demonstrated potential for success. Once that was achieved, the researchers were able to move on to an in vivo model, an exciting milestone for their research.
Adele Gabba, a postdoc in the Kiessling Lab, conducted the in vivo experiments with Lensch, and Robert Hincapie, who conducted his PhD studies with Professor M.G. Finn at Georgia Tech, built and decorated the virus-like particles with a series of glycans that were sent to him from the researchers at MIT.
“We are discovering that carbohydrates act like a language that cells use to communicate and direct the immune system,” says Gabba. “It’s thrilling that we have begun to decode this language and can now harness it to reshape immune responses.”
“The design principles behind this vaccine are rooted in extensive fundamental research conducted by previous graduate student and postdoctoral researchers over many years, focusing on optimizing lectin engagement and understanding the roles of lectins in immunity,” says Lensch. “It has been exciting to witness the translation of these concepts into therapeutic platforms across various applications.”
#antigen#applications#author#Bacteria#Biological engineering#Cancer#cancer cells#cell#Cells#chemistry#collaborate#Collaboration#deal#Design#design principles#development#Diseases#display#Fight#Fundamental#fungi#hiv#human#human cells#immune cells#immune response#immune system#immunology#Institute for Medical Engineering and Science (IMES)#it
1 note
·
View note
Text
Growing Antibody-Drug Conjugates Market Owing to Rising Demand for Targeted Cancer Therapy.

Antibody-drug conjugates (ADCs) are a type of bioconjugate consisting of monoclonal antibodies that are attached by chemical linkers to highly potent anti-cancer payloads. ADCs selectively target antigens that are highly expressed on tumor cells while sparing normal tissues through the use of antibodies. Linkers attached between the antibody and cytotoxic drug allow for the drug to be delivered unchanged until it reaches the intended tumor site, minimizing harm to healthy cells. ADCs have demonstrated clinical efficacy in treating various cancers including lymphoid malignancies, breast cancer, and solid tumors.
The Global Antibody-Drug Conjugates Market is estimated to be valued at US$ 5.38 Bn in 2024 and is expected to exhibit a CAGR of 14% over the forecast period 2023 to 2030. Key Takeaways: Key players operating in the Antibody-Drug Conjugates are AstraZeneca PLC, Daiichi Sankyo Company, Limited, Novasep, ADC Therapeutics SA, Alentis Therapeutics AG, F. Hoffmann-La Roche, Gilead Sciences, Inc., AbbVie Inc., Biosion USA, Inc., Astellas Pharma Inc., Duality Biologics (Suzhou) Co. Ltd., BioNTech SE, LaNova Medicines Ltd., Bliss Biopharmaceutical, Eisai Co., Ltd., ProfoundBio, Pfizer, Inc., ImmunoGen Inc., Mersana Therapeutics Inc., Sorrento Therapeutics Inc., Oxford BioTherapeutics Ltd, and Takeda Pharmaceutical Company Ltd. Growing demand for targeted cancer therapy with minimal side effects is expected to drive significant growth of the ADC market over the forecast period. Additionally, ongoing technological advancements in linker chemistry, increasing pipeline products and approvals are further fueling the market growth. Market Trends: The ADC market is witnessing increasing adoption of cleavable linkers that are stable in circulation but rapidly release the drug payload intracellularly upon internalization into target tumor cells. Additionally, the development of novel conjugation technologies such as DBCO-azide click chemistry is allowing for site-specific conjugation without effect on bioactivity and efficacy of ADCs. Market Opportunities: The significant opportunities in the ADC market are in developing ADCs for liquid and solid tumor indications with unmet medical needs. Additionally, optimization of physiochemical properties of molecules to improve pharmacokinetics is another key area that ADC developers are increasingly focusing on to enhance therapeutic index and efficacy of ADCs.
#Antibody Drug Conjugates Market Share#Antibody Drug Conjugates Market Growth#Antibody Drug Conjugates Market Analysis
0 notes
Text
The demand for antibody drug conjugate was valued at USD 9984.20 million in 2023 and is expected to reach USD 26594.21 million in 2032, growing at a CAGR of 11.50% between 2024 and 2032.The Antibody-Drug Conjugate (ADC) market represents a groundbreaking intersection of biotechnology and pharmacology, offering a novel approach to cancer treatment. ADCs are sophisticated biopharmaceuticals designed to deliver cytotoxic drugs directly to cancer cells, minimizing damage to healthy tissues. This targeted therapy approach has gained substantial attention in recent years due to its potential to enhance the efficacy and safety profiles of cancer treatments. As the ADC market continues to expand, it is poised to revolutionize oncology and drive significant advancements in personalized medicine.
Browse the full report at https://www.credenceresearch.com/report/antibody-drug-conjugate-market
Market Overview
The global ADC market has witnessed rapid growth, driven by increasing cancer prevalence, technological advancements in drug development, and rising demand for targeted therapies. According to recent market research, the ADC market was valued at approximately USD 4.8 billion in 2022 and is expected to reach over USD 12 billion by 2028, growing at a compound annual growth rate (CAGR) of 16.7% during the forecast period.
This growth is fueled by the rising incidence of cancer worldwide, coupled with the limitations of conventional cancer therapies, such as chemotherapy and radiation, which often result in severe side effects. ADCs, by contrast, offer a more precise mechanism of action, delivering cytotoxic agents directly to cancer cells while sparing healthy tissues. This specificity reduces adverse effects and improves patient outcomes, making ADCs a promising option in the oncology landscape.
Technological Advancements
Advancements in ADC technology have been pivotal in driving market growth. Early-generation ADCs faced challenges such as low therapeutic indices, off-target toxicities, and limited efficacy. However, recent innovations have addressed these issues, leading to the development of more stable linkers, improved antibody engineering, and the use of highly potent cytotoxic agents.
Modern ADCs utilize cleavable linkers that release the drug payload only in the presence of specific enzymes or conditions within the cancer cell, ensuring targeted drug delivery. Additionally, advancements in monoclonal antibody engineering have enhanced the ability of ADCs to bind selectively to tumor-specific antigens, further improving their therapeutic potential.
Key Market Players
Several pharmaceutical companies are at the forefront of the ADC market, investing heavily in research and development to bring new ADCs to market. Notable players include:
1. Seagen Inc.: Known for its pioneering work in ADCs, Seagen’s ADC technology has led to the successful development of drugs like Adcetris, used in the treatment of Hodgkin lymphoma and other CD30-expressing lymphomas.
2. Roche: A global leader in oncology, Roche has developed Kadcyla, an ADC used in the treatment of HER2-positive breast cancer. Kadcyla combines the HER2-targeting properties of trastuzumab with a cytotoxic agent, offering a targeted approach to treating this aggressive form of breast cancer.
3. AstraZeneca: With the development of Enhertu, an ADC for HER2-positive breast cancer, AstraZeneca has further solidified its position in the ADC market. Enhertu’s unique design allows it to deliver a higher drug-to-antibody ratio, enhancing its therapeutic efficacy.
Challenges and Opportunities
Despite the promising outlook, the ADC market faces several challenges. The complexity of ADC development, high production costs, and stringent regulatory requirements can hinder market growth. Additionally, issues related to drug resistance and the need for personalized approaches to treatment pose ongoing challenges.
However, these challenges also present opportunities for innovation. Companies are exploring new ADC technologies, such as bispecific ADCs that target multiple antigens, and the use of alternative payloads to overcome drug resistance. Furthermore, ongoing research into biomarker-driven patient selection is expected to enhance the precision of ADC therapies, aligning them with the principles of personalized medicine.
Future Outlook
The future of the ADC market looks promising, with continued advancements in technology and a growing pipeline of ADC candidates in clinical trials. As more ADCs receive regulatory approval and enter the market, the adoption of this targeted therapy is expected to increase, offering new hope to cancer patients worldwide.
Moreover, the expanding application of ADCs beyond oncology, such as in the treatment of autoimmune diseases, presents additional growth opportunities. As research in this area progresses, ADCs may become a cornerstone of targeted therapy across various therapeutic areas.
Key Players
Seagen Inc.
Takeda Pharmaceutical Company Ltd.
AstraZeneca Plc.
F. Hoffmann-La Roche Ltd.
Pfizer Inc.
ImmunoGen Inc.
Gilead Sciences Inc.
Daiichi Sankyo Company Ltd.
Segmentation
By Product
Kadcyla
Enhertu
Adcetris
Padcev
Trodelvy
Polivy
Others
By Disease Type
Breast Cancer
Blood Cancer
Others
By Linker Type
Non-Cleavable
Cleavable
By Target
HER2
CD22
CD30
Others
By Payload Type
MMAE/Auristatin
Calicheamicin
Maytansinoids
Others
By Region
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/antibody-drug-conjugate-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
CAR T-Cell Therapy: Challenges and Future Directions

CAR T-cell therapy, short for Chimeric Antigen Receptor T-cell therapy, represents a groundbreaking advancement in cancer treatment. This innovative approach harnesses the power of a patient's own immune system to target and destroy cancer cells, offering new hope for patients with certain types of cancer, particularly those who have not responded to traditional therapies.
The process begins with the extraction of T-cells, a type of white blood cell crucial to the immune system, from the patient’s blood. These T-cells are then genetically modified in a laboratory to express a chimeric antigen receptor (CAR) on their surface. This receptor is designed to specifically recognize and bind to antigens on the surface of cancer cells. Once the T-cells are engineered and multiplied, they are infused back into the patient’s bloodstream, where they seek out and attack the cancer cells.
CAR T-cell therapy has shown remarkable success, particularly in treating hematologic malignancies such as certain types of leukemia and lymphoma. For instance, it has been approved for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) in children and young adults, as well as certain types of non-Hodgkin lymphoma in adults. Clinical trials and real-world studies have demonstrated impressive remission rates, offering a lifeline to patients with otherwise limited treatment options.
However, CAR T-cell therapy is not without challenges. One of the most significant is the potential for severe side effects. Cytokine release syndrome (CRS) is a common and potentially life-threatening reaction, resulting from the rapid activation and proliferation of CAR T-cells and the subsequent release of large amounts of cytokines. Symptoms can range from high fever and flu-like symptoms to more severe complications affecting multiple organs. Another concern is neurotoxicity, which can cause confusion, seizures, and other neurological issues.
Additionally, the therapy’s high cost and complex manufacturing process pose barriers to widespread accessibility. The personalized nature of CAR T-cell therapy means that it must be tailored to each individual patient, which is both time-consuming and expensive. Efforts are ongoing to streamline production and reduce costs, as well as to expand the therapy’s applicability to solid tumors and other cancer types.
Despite these challenges, CAR T-cell therapy remains a beacon of hope in the oncology field. Continued research and clinical advancements are likely to enhance its efficacy, safety, and accessibility, potentially transforming the landscape of cancer treatment and providing new avenues for patient care.
0 notes