#Antibody Drug Conjugates Market Growth
Explore tagged Tumblr posts
Text
Growing Antibody-Drug Conjugates Market Owing to Rising Demand for Targeted Cancer Therapy.

Antibody-drug conjugates (ADCs) are a type of bioconjugate consisting of monoclonal antibodies that are attached by chemical linkers to highly potent anti-cancer payloads. ADCs selectively target antigens that are highly expressed on tumor cells while sparing normal tissues through the use of antibodies. Linkers attached between the antibody and cytotoxic drug allow for the drug to be delivered unchanged until it reaches the intended tumor site, minimizing harm to healthy cells. ADCs have demonstrated clinical efficacy in treating various cancers including lymphoid malignancies, breast cancer, and solid tumors.
The Global Antibody-Drug Conjugates Market is estimated to be valued at US$ 5.38 Bn in 2024 and is expected to exhibit a CAGR of 14% over the forecast period 2023 to 2030. Key Takeaways: Key players operating in the Antibody-Drug Conjugates are AstraZeneca PLC, Daiichi Sankyo Company, Limited, Novasep, ADC Therapeutics SA, Alentis Therapeutics AG, F. Hoffmann-La Roche, Gilead Sciences, Inc., AbbVie Inc., Biosion USA, Inc., Astellas Pharma Inc., Duality Biologics (Suzhou) Co. Ltd., BioNTech SE, LaNova Medicines Ltd., Bliss Biopharmaceutical, Eisai Co., Ltd., ProfoundBio, Pfizer, Inc., ImmunoGen Inc., Mersana Therapeutics Inc., Sorrento Therapeutics Inc., Oxford BioTherapeutics Ltd, and Takeda Pharmaceutical Company Ltd. Growing demand for targeted cancer therapy with minimal side effects is expected to drive significant growth of the ADC market over the forecast period. Additionally, ongoing technological advancements in linker chemistry, increasing pipeline products and approvals are further fueling the market growth. Market Trends: The ADC market is witnessing increasing adoption of cleavable linkers that are stable in circulation but rapidly release the drug payload intracellularly upon internalization into target tumor cells. Additionally, the development of novel conjugation technologies such as DBCO-azide click chemistry is allowing for site-specific conjugation without effect on bioactivity and efficacy of ADCs. Market Opportunities: The significant opportunities in the ADC market are in developing ADCs for liquid and solid tumor indications with unmet medical needs. Additionally, optimization of physiochemical properties of molecules to improve pharmacokinetics is another key area that ADC developers are increasingly focusing on to enhance therapeutic index and efficacy of ADCs.
#Antibody Drug Conjugates Market Share#Antibody Drug Conjugates Market Growth#Antibody Drug Conjugates Market Analysis
0 notes
Text
Discover the latest advancements and market trends in the Antibody Drug Conjugates (ADCs) Market. Explore the growth drivers, key players, and innovative technologies shaping the future of ADCs in oncology and beyond.
#Antibody Drug Conjugates Market#Antibody Drug Conjugates Industry#Antibody Drug Conjugates Market size#Antibody Drug Conjugates Market share#Antibody Drug Conjugates Market demands#Antibody Drug Conjugates Market growth#Antibody Drug Conjugates Market analysis#Antibody Drug Conjugates Market report
0 notes
Text
#Antibody Drug Conjugates Market#Antibody Drug Conjugates Market Trends#Antibody Drug Conjugates Market Growth#Antibody Drug Conjugates Market Industry#Antibody Drug Conjugates Market Research#Antibody Drug Conjugates Market Report
0 notes
Text
Forecasting the Antibody Drug Conjugate Market: Trends and Outlook
Market Overview –
The antibody drug conjugate (ADC) market is a segment within the pharmaceutical industry that focuses on a class of targeted cancer therapies. ADCs combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs, offering a promising approach to cancer treatment. This market is driven by the increasing incidence of cancer worldwide, the need for more effective and targeted therapies, and advancements in biotechnology and drug delivery systems.
One of the key drivers of the ADC market is the demand for novel cancer treatments with improved efficacy and fewer side effects compared to traditional chemotherapy. ADCs offer a targeted approach, delivering cytotoxic drugs directly to cancer cells while sparing healthy tissues, thereby reducing systemic toxicity and enhancing patient outcomes.
Furthermore, the growing understanding of tumor biology and the identification of specific molecular targets have facilitated the development of ADCs tailored to different types of cancer. This personalized approach to treatment holds promise for patients with refractory or relapsed cancers who may not respond to conventional therapies.
The antibody drug conjugate market is experiencing rapid expansion, propelled by advancements in anti-drug conjugates. These innovative therapies combine the targeting precision of antibodies with potent anti-cancer drugs, offering promising treatment options for various cancers. With ongoing research and development efforts, the market for anti drug conjugates is poised for continued growth in the fight against cancer.
The COVID-19 pandemic has highlighted the importance of innovative therapies like ADCs in addressing unmet medical needs, especially in oncology. While the pandemic initially disrupted clinical trials and supply chains, the resilient nature of the biopharmaceutical industry has enabled continued research and development in this field.
However, challenges such as the complexity of ADC manufacturing, high development costs, and regulatory hurdles pose barriers to market growth. Nonetheless, with ongoing research and collaborations among pharmaceutical companies, academic institutions, and regulatory agencies, the ADC market is expected to witness significant expansion in the coming years, offering new hope to cancer patients worldwide.
With a predicted compound annual growth rate (CAGR) of 16.70% from 2022 to 2030, the antibody drug conjugate market, which was valued at USD 1.98 billion in 2021, is expected to rise from USD 2.31 billion in 2022 to USD 6.81 billion by 2030.
Segmentation –
As per MRFR report, the global antibody drug conjugate market is segmented on the basis of type, product, technology, application and end-user.
Based on type, it is segmented into drug/toxin, linker, monoclonal antibodies and others. Of these, the antibody drug conjugate linker is expected to have the maximum share in the antibody drug conjugate market.
Based on application, the antibody drug conjugate market is segmented into lymphoma, multiple myeloma, solid tumors, skin cancer, breast cancer, colon cancer, lung cancer, glioblastoma, ovary cancer, pancreas cancer, kidney cancer, prostate cancer and leukemia. Leukemia is further segmented into Chronic Lymphocytic Leukemia (CLL), Acute Lymphocytic Leukemia (ALL), Chronic Myeloid Leukemia (CML), and Acute Myeloid Leukemia (AML). Of these, breast cancer had the maximum share owing to its increasing prevalence.
Based on product, it is segmented into Kadcyla, Adcertis and others.
Based on technology, the antibody drug conjugate market is segmented into Immunomedics technology, Seattle Genetics technology, ImmunoGen technology and others.
Based on end-user, it is segmented into biopharmaceutical companies, biotechnology companies, academic research institutes, specialized cancer, and others.
Regional Analysis –
The antibody drug conjugate (ADC) market's regional dynamics depend on factors like research infrastructure, regulatory environment, and healthcare access. North America dominates, driven by robust research and development activities and favorable regulatory pathways. Europe follows, with a strong presence of biopharmaceutical companies and supportive policies for innovative therapies. Asia-Pacific is emerging as a significant market, fueled by investments in biotechnology and a growing patient population. Other regions, such as Latin America and Africa, are gradually gaining traction as awareness of ADC therapies increases. Market players must navigate regional differences in reimbursement policies and healthcare systems while capitalizing on opportunities for collaboration and expansion.
Key Players –
Antibody drug conjugate companies include ADC Therapeutics, Takeda Pharmaceutical Company Ltd., GlaxoSmithKline Plc, Hoffmann-La Roche Ltd., Daiichi Sankyo Company Ltd., Pfizer Inc., Seagen Inc., Gilead Sciences Inc., Astellas Pharma, among others.
Related Reports –
Steam Autoclave
Diagnostic Imaging
Neonatal Thermoregulation
Immunotherapy Drugs
For more information visit at MarketResearchFuture
#Antibody Drug Conjugate Market#Antibody Drug Conjugate Market Size#Antibody Drug Conjugate Market Share#Antibody Drug Conjugate Market Growth#Antibody Drug Conjugate Market Trends
0 notes
Text
Antibody Drug Conjugates Market Size, Share | Growth drivers 2023
According to Precision Business Insights, the global Antibody Drug Conjugates Market is poised to grow at a significant CAGR of 12.6% during forecast period 2023-29
The global antibody drug conjugates market size was valued at USD 6.84 billion in 2022 and is poised to grow at a significant CAGR of 12.6% during the forecast period 2023-29. It also includes market size and projection estimations for each of the five major regions from 2023 to 2029. The research report includes historical data, trending features, and market growth estimates for the future. Furthermore, the study includes a global and regional estimation and further split by nations and categories within each region. The research also includes factors and barriers to the antibody drug conjugates market growth, as well as their impact on the market's future growth. The report gives a comprehensive overview of both primary and secondary data.
View the detailed report description here - https://www.precisionbusinessinsights.com/market-reports/antibody-drug-conjugates-market
The global antibody drug conjugates market segmentation: 1) By Drugs : Kadcyla, Adcertis
2) By Mechanism Of Action : CD30 Antibodies, HER2 Antibodies
3) By Application : Breast Cancer, Lymphoma
The primary factors of the antibody drug conjugates market drivers are the increasing incidences of cancer. The antibody drug conjugates market report helps to provide the best results for business enhancement and business growth. It further helps to obtain the reactions of consumers to a novel product or service. It becomes possible for business players to take action for changing perceptions. It uncovers and identifies potential issues of the customers. It becomes easy to obtain the reactions of the customers to a novel product or service. It also enlightens further advancement, so it suits its intended market.
The antibody drug conjugates market researchreport gives a comprehensive outlook across the region with special emphasis on key regions such as North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America was the largest region in the antibody drug conjugates market report, accounting for the highest share in 2022. It was followed by Asia Pacific, and then the other regions. Request sample report at - https://www.precisionbusinessinsights.com/request-sample/?product_id=23717 The important profiles and strategies adopted by antibody drug conjugates market key players Roche Holding AG (Switzerland) Pfizer Inc. (U.S.) Bayer HealthCare (Germany) Progenics Pharmaceuticals (U.S.) Roche Holding AG (Switzerland) ImmunoGen, Inc. (U.S.) Celldex Therapeutics (U.S.) AbbVie Inc. (U.S.) Millennium Pharmaceuticals (U.S.) Agensys, Inc. (U.S.), covered here to help them in strengthening their place in the market.
About Precision Business Insights: We are a market research company that strives to provide the highest quality market research insights. Our diverse market research experts are enthusiastic about market research and therefore produce high-quality research reports. We have over 500 clients with whom we have a good business partnership and capacity to provide in-depth research analysis for more than 30 countries. In addition to deliver more than 150 custom solutions, we already have accounts with the top five medical device manufacturers.
Precision Business Insights offers a variety of cost-effective and customized research services to meet research requirements. We are a leading research service provider because of our extensive database built by our experts and the services we provide.
Contact:
Mr. Satya
Precision Business Insights | Toll Free: +1 866 598 1553
Email: [email protected] Kemp House, 152 – 160 City Road, London EC1V 2NX Web: https://precisionbusinessinsights.com/ | D U N S® Number: 852781747
#antibody drug conjugates market#antibody drug conjugates market size#antibody drug conjugates market industry share#antibody drug conjugates market growth drivers#antibody drug conjugates market trends analysis
0 notes
Text
Understanding Metastatic HER2-Positive Breast Cancer
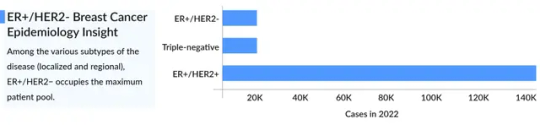
What is Metastatic HER2-Positive Breast Cancer?
HER2-positive breast cancer is a subtype identified by an overexpression of the HER2 protein, which accelerates cancer cell growth. When the cancer extends beyond the breast and nearby lymph nodes to distant organs, it is classified as metastatic HER2-positive breast cancer. Due to its aggressive nature, this condition typically requires specialized HER2-targeted therapies.
Symptoms of HER2-Positive Breast Cancer
The symptoms of HER2-positive breast cancer depend on the organs affected. Common signs include:
Persistent cough
Bone pain
Shortness of breath
Severe headaches
Jaundice
Unexplained weight loss Other indicators may include skin changes, swollen lymph nodes, and chronic fatigue. Early detection and symptom management are crucial for improving patient outcomes.
Prevalence of HER2-Positive Breast Cancer Cases
HER2-positive breast cancer accounts for approximately 15-20% of all breast cancer cases. A significant portion of these cases progress to stage 4 HER2-positive breast cancer, contributing to the overall HER2-positive metastatic breast cancer landscape. While advancements in treatment have led to better survival rates, this form of cancer remains a global challenge.
HER2-Targeted Therapies for Metastatic HER2-Positive Breast Cancer
Several leading pharmaceutical companies, such as Roche, Merck, and Immunomedics, have developed innovative HER2-targeted therapies. Notable treatments include:
Trastuzumab (Herceptin)
Pertuzumab (Perjeta)
Trastuzumab deruxtecan (Enhertu) These therapies have significantly improved survival rates and quality of life. Researchers are also exploring immunotherapies and combination treatments for enhanced effectiveness.
The HER2 Breast Cancer Pipeline: Future Prospects
The HER2 breast cancer pipeline continues to expand with promising new therapies undergoing clinical trials. Research efforts focus on:
Novel HER2 inhibitors
Antibody-drug conjugates
Advanced immunotherapies Companies like Roche and Merck are at the forefront of HER2-positive metastatic breast cancer research, aiming to develop more effective treatment options.
Conclusion
Advancements in the HER2-positive breast cancer market are revolutionizing treatment approaches, offering hope for improved survival rates. As HER2-positive metastatic breast cancer research progresses, early detection and targeted therapy remain crucial in managing the disease effectively. With ongoing clinical trials and new therapeutic innovations, the future holds promise for better treatment options and improved patient outcomes.
Another Reports Offered By Delveinsight
Liquid Biopsy for Cancer Diagnostics Market | Plasmodium Vivax Malaria Market | Polycystic Ovarian Syndrome Market | Short Bowel Syndrome Drugs Market | Somatotropin Deficiency Market | Temporomandibular Disorders Market | Testicular Neoplasm Market |Venous Ulcer Market | Adeno-Associated Viruses (AAV) Gene Therapy Market | Blastomycosis Market | Carcinoid Syndrome Market | Congenital Heart Defect Market | CXCR Inhibitors Market | Hip Replacement Devices Market | Myeloproliferative Neoplasms Market | Nocturia Market | Percutaneous Arterial Closure Device Market | Peripheral SpA Market | Psoriasis Vulgaris Market | Radial Artery Compression Device Market | Schistosomiasis Market | Type 1 Diabetes Market | Vital Sign Monitors Devices Market | Atherosclerosis Market | Avascular Necrosis Market | Gene Therapy in CNS Disorder Market | Pediatric Neuroblastoma Market | Spinal Trauma Devices Market | Surgical Lasers Market | Thyroid Cancer Market | Ventral Hernia Market
Contact Information
Kanishk
0 notes
Text
Bioconjugation Market Overview 2032
Bioconjugation Market Overview
The bioconjugation market is experiencing rapid expansion, driven by the increasing demand for targeted therapies, advancements in drug delivery technologies, and rising research investments in the pharmaceutical and biotechnology sectors. Bioconjugation refers to the chemical linkage of biomolecules, such as proteins, peptides, or nucleic acids, with other molecules, including drugs, polymers, or imaging agents, to enhance their therapeutic efficacy and diagnostic capabilities. This technique plays a critical role in modern medicine, particularly in developing antibody-drug conjugates (ADCs), nanoparticles, and enzyme-prodrug therapies.
The growing focus on precision medicine has significantly boosted the adoption of bioconjugation techniques, enabling the development of highly specific drug molecules with improved efficacy and reduced side effects. The increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases has further propelled the need for innovative therapeutic approaches. Additionally, advancements in bioconjugation chemistries, such as click chemistry and site-specific conjugation, have enhanced the efficiency, stability, and scalability of these processes, making them more attractive for pharmaceutical applications.
The global bioconjugation market is expected to grow substantially over the coming years, fueled by ongoing research and development activities, increasing collaborations between biotech companies and contract research organizations (CROs), and a rising number of clinical trials involving conjugated biomolecules. With the growing emphasis on personalized medicine and biologics, the market is set to witness a surge in demand for bioconjugation technologies across various therapeutic and diagnostic applications.
Regional Insights
North America
North America holds a dominant position in the bioconjugation market, primarily due to its strong pharmaceutical and biotechnology infrastructure. The presence of major players, extensive research activities, and high healthcare spending contribute to the region’s leadership. The United States, in particular, is at the forefront of bioconjugation research, with a significant number of clinical trials and approvals for ADCs and bioconjugated therapeutics. Government initiatives supporting biopharmaceutical innovation, along with investments in precision medicine, have further bolstered market growth in this region.
Europe
Europe is another significant market for bioconjugation, driven by strong research initiatives and well-established biotechnology firms. Countries such as Germany, the United Kingdom, and France are leading contributors, with a focus on drug discovery and development. Regulatory frameworks in the European Union have also encouraged the adoption of bioconjugated therapies, particularly in oncology and immunotherapy. Increased funding for research and collaborations between academia and industry have further supported market expansion in this region.
Asia-Pacific
The Asia-Pacific region is witnessing the fastest growth in the bioconjugation market, fueled by increasing investments in pharmaceutical R&D, growing biotechnology sectors, and rising healthcare expenditures. Countries such as China, Japan, and India are making significant strides in biopharmaceutical innovation, with an increasing number of bioconjugated drug approvals and research initiatives. The expansion of contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) in the region has also contributed to market growth by providing cost-effective solutions for drug development and manufacturing.
Rest of the World
The bioconjugation market is also expanding in regions such as Latin America and the Middle East, although at a relatively slower pace. Increasing healthcare awareness, improving regulatory frameworks, and a growing interest in biologics and precision medicine are driving market adoption in these areas. As global pharmaceutical companies expand their reach, these emerging markets are expected to see greater investments and technological advancements in the coming years.
Key Market Trends
Growing Demand for Antibody-Drug Conjugates (ADCs) ADCs have revolutionized cancer treatment by offering targeted drug delivery with minimal side effects. The increasing number of ADC approvals and ongoing clinical trials highlight the growing importance of bioconjugation in oncology. Companies are investing heavily in next-generation ADCs with improved linker chemistries and site-specific conjugation methods to enhance their efficacy and safety profiles.
Advancements in Bioconjugation Technologies Recent innovations in bioconjugation techniques, such as click chemistry, enzymatic conjugation, and chemo-selective ligation, have improved the efficiency and specificity of biomolecule conjugation. These advancements have expanded the applications of bioconjugation beyond therapeutics to include diagnostics, imaging, and biomarker detection.
Increasing Collaborations and Partnerships The growing complexity of bioconjugation processes has led to increased collaborations between pharmaceutical companies, biotech firms, and CROs. Strategic partnerships are helping companies accelerate drug development, optimize production processes, and expand their market reach. The outsourcing of bioconjugation services to specialized CROs and CDMOs has also contributed to cost efficiency and faster commercialization of novel therapeutics.
Expansion of Bioconjugation Applications Beyond Oncology While oncology remains the primary focus of bioconjugation, its applications are expanding to other therapeutic areas such as autoimmune diseases, infectious diseases, and neurological disorders. The development of novel bioconjugates for targeted drug delivery in conditions like rheumatoid arthritis and multiple sclerosis is gaining traction, paving the way for new treatment modalities.
Regulatory and Manufacturing Challenges Despite its promising growth, the bioconjugation market faces challenges related to regulatory approvals, manufacturing complexities, and scalability. Stringent regulatory requirements for ADCs and bioconjugated therapies require companies to adopt robust quality control measures and comply with Good Manufacturing Practices (GMP). Ensuring consistent product quality and large-scale production remains a key challenge for market players.
Frequently Asked Questions (FAQs)
1. What is bioconjugation, and why is it important? Bioconjugation is the process of chemically linking biomolecules to other compounds to enhance their therapeutic or diagnostic properties. It plays a critical role in targeted drug delivery, imaging, and biomarker detection, making it essential for modern medicine.
2. Which industries utilize bioconjugation technology? Bioconjugation is widely used in the pharmaceutical, biotechnology, diagnostics, and healthcare industries. It is particularly crucial for the development of antibody-drug conjugates, nanoparticle-based drug delivery systems, and imaging agents.
3. What are the key factors driving the growth of the bioconjugation market? The increasing demand for targeted therapies, advancements in conjugation technologies, rising R&D investments, and the growing number of bioconjugated drug approvals are major factors driving market growth.
4. What challenges does the bioconjugation market face? Regulatory hurdles, manufacturing complexities, and high costs associated with bioconjugation processes pose challenges for market expansion. Ensuring product stability, consistency, and scalability remains a key concern.
5. Which regions are leading the bioconjugation market? North America leads the market due to its strong biopharmaceutical sector, followed by Europe and the rapidly growing Asia-Pacific region. Emerging markets in Latin America and the Middle East are also showing potential growth opportunities.
For more insights on the Bioconjugation Market, visit the full report here: Bioconjugation Market Report
0 notes
Text
0 notes
Text
Antibodies Contract Manufacturing Market Size, Growth Outlook 2035
The global Antibodies Contract Manufacturing Market Size was estimated at 18.38 (USD Billion) in 2024. The Antibodies Contract Manufacturing Market Industry is expected to grow from 19.97 (USD Billion) in 2025 to 42.29 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 8.69% during the forecast period (2025 - 2034)
Market Overview The Antibodies Contract Manufacturing Market is experiencing robust growth, driven by the rising demand for monoclonal antibodies (mAbs), therapeutic antibodies, and biosimilars. Contract manufacturing organizations (CMOs) provide specialized services for antibody production, including cell line development, upstream and downstream processing, purification, and fill-finish services. The increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases, along with the growing adoption of biopharmaceutical outsourcing, is fueling market expansion.

Market Size and Share The global Antibodies Contract Manufacturing MarketSize was estimated at 18.38 (USD Billion) in 2024. The Antibodies Contract Manufacturing Market Industry is expected to grow from 19.97 (USD Billion) in 2025 to 42.29 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 8.69% during the forecast period (2025 - 2034). North America dominates the market due to the presence of leading biopharmaceutical companies, advanced bioprocessing technologies, and strong regulatory frameworks. The Asia-Pacific contract antibody production market is witnessing rapid growth due to increasing investments in biologics manufacturing facilities and the availability of cost-effective contract manufacturing services.
Market Drivers
Rising Demand for Monoclonal Antibodies: The growing application of mAbs in oncology, immunology, and infectious diseases is boosting demand for contract antibody production.
Growing Outsourcing Trends in Biopharmaceuticals: Companies are increasingly outsourcing antibody manufacturing services to CMOs to reduce production costs and focus on core R&D.
Advancements in Bioprocessing Technologies: Innovations in single-use bioreactors, cell culture optimization, and chromatography techniques are enhancing efficiency in antibody production.
Expanding Biosimilars Market: The increasing development of biosimilar antibodies is creating new opportunities for contract biologics manufacturing.
Challenges and Restraints
High Costs Associated with Large-Scale Antibody Production: The cost-intensive nature of biopharmaceutical manufacturing poses challenges for smaller companies.
Regulatory Compliance and Quality Control Issues: Strict guidelines from the FDA, EMA, and other regulatory agencies necessitate rigorous quality control in biologics contract manufacturing.
Limited Availability of Skilled Workforce: The shortage of bioprocessing experts can hinder market growth.
Market Trends
Adoption of Single-Use Bioprocessing Technologies: The shift towards disposable bioprocessing systems is improving flexibility and reducing contamination risks in contract antibody production.
Increasing Focus on Antibody-Drug Conjugates (ADCs): Contract manufacturers are expanding their capabilities to support the rising demand for ADCs in targeted cancer therapies.
Strategic Collaborations Between Biotech Firms and CMOs: Pharmaceutical companies are forming alliances with biologics CMOs to enhance antibody therapeutic production capabilities.
Regional Analysis
North America: The dominant region due to strong biopharmaceutical infrastructure, high investment in biologics manufacturing, and the presence of major CMOs.
Europe: Significant growth driven by increasing adoption of biosimilar contract manufacturing and stringent regulatory frameworks.
Asia-Pacific: Fastest-growing market, with countries like China, India, and South Korea emerging as key hubs for antibody contract manufacturing services.
Rest of the World: Moderate market expansion, with increasing interest in Latin America and the Middle East.
Segmental Analysis
By Product Type:
Monoclonal Antibodies (mAbs)
Polyclonal Antibodies
Antibody Fragments
Antibody-Drug Conjugates (ADCs)
By Service Type:
Cell Line Development & Optimization
Process Development & Scale-Up
Upstream & Downstream Processing
Analytical & Quality Control Services
Fill-Finish & Packaging
By End-User:
Biopharmaceutical Companies
Academic & Research Institutes
Contract Research Organizations (CROs)
Key Market Players
Charles River Laboratories
MyBioSource
AbCellera
AGC Biologics
Novavax
Lonza
MilliporeSigma
ProBioGen AG
Recent Developments
Expansion of Biologics Manufacturing Facilities: Leading CMOs are investing in new large-scale antibody production plants.
Launch of AI-Powered Bioprocessing Solutions: AI-driven process optimization platforms are enhancing efficiency in contract antibody production.
Strategic Mergers and Acquisitions: Companies are acquiring smaller biologics contract manufacturers to expand capabilities.
For more information, please visit us at marketresearchfuture
#Antibodies Contract Manufacturing Market Size#Antibodies Contract Manufacturing Market Share#Antibodies Contract Manufacturing Market Growth#Antibodies Contract Manufacturing Market Analysis#Antibodies Contract Manufacturing Market Trends#Antibodies Contract Manufacturing Market Forecast#Antibodies Contract Manufacturing Market Segments
0 notes
Text
The Difficult to Express Proteins Market is projected to grow from USD 4,591.9 million in 2024 to USD 9,149.64 million by 2032, reflecting a compound annual growth rate (CAGR) of 9% during the forecast period. The biotechnology and pharmaceutical industries are experiencing unprecedented growth, driven by advancements in drug discovery, therapeutic development, and innovative technologies. Among the many opportunities and challenges within these industries lies a specialized yet crucial segment: the difficult-to-express proteins market. This niche, though complex, has captured the attention of researchers, developers, and investors due to its immense potential in addressing unmet medical needs.Difficult-to-express (DTE) proteins are those that are challenging to produce in sufficient quantity, purity, or activity due to inherent structural or functional complexities. These proteins often include membrane proteins, multi-subunit complexes, glycosylated proteins, and those prone to aggregation or degradation. Their importance in drug development is immense, as they often serve as therapeutic targets or biologics in treating chronic diseases, rare disorders, and cancers.
Browse the full report at https://www.credenceresearch.com/report/difficult-to-express-proteins-market
Market Drivers
Several factors are driving the growth of the difficult-to-express proteins market:
Advancements in Expression Systems: The development of robust expression platforms, such as mammalian cell lines (CHO cells), insect cell systems, yeast, and engineered microbial hosts, has significantly improved the production of complex proteins. Technologies like CRISPR-Cas9 for genome editing have further enhanced these systems, enabling customized solutions for DTE proteins.
Growing Biopharmaceutical Demand: The increasing prevalence of chronic diseases, such as cancer, autoimmune disorders, and diabetes, has led to a surge in demand for biologics, including monoclonal antibodies, vaccines, and fusion proteins. Many of these are categorized as DTE proteins.
Emerging Therapies: The rise of novel therapeutic modalities, such as antibody-drug conjugates (ADCs), bispecific antibodies, and gene therapies, has expanded the demand for complex protein scaffolds. These innovations rely on the successful production of DTE proteins to ensure efficacy and safety.
Investment in R&D: Pharmaceutical companies and research organizations are investing heavily in developing innovative methods to overcome expression challenges. Continuous funding in this space has spurred the growth of novel solutions and technologies.
Challenges in the Market
Despite advancements, the DTE proteins market faces several hurdles:
High Costs: The production of DTE proteins often requires extensive optimization, specialized facilities, and expensive reagents, driving up costs.
Technical Complexity: Producing high-quality proteins with consistent results remains a challenge, especially for proteins requiring precise glycosylation or folding.
Regulatory Hurdles: The stringent regulatory landscape for biologics and biotherapeutics adds complexity to the commercialization process.
Key Players and Innovations
Prominent players in the difficult-to-express proteins market include Lonza, Thermo Fisher Scientific, Bio-Techne, and Merck KGaA. These companies are leading the charge by investing in cutting-edge technologies and offering tailored solutions.
Innovative approaches such as cell-free expression systems, AI-driven optimization, and synthetic biology are revolutionizing protein expression. For instance, cell-free systems enable rapid prototyping of proteins without the need for live cells, reducing the time and cost associated with traditional methods.
Future Outlook
The difficult-to-express proteins market is poised for substantial growth in the coming years. The integration of artificial intelligence and machine learning to optimize protein expression processes is expected to streamline production further. Additionally, the emergence of personalized medicine and precision therapies will likely create new opportunities for DTE proteins as biomarkers or therapeutic agents.
Collaboration between academic institutions, biotech firms, and contract research organizations (CROs) will also play a pivotal role in accelerating advancements. Governments and private investors are increasingly supporting initiatives aimed at overcoming the challenges associated with protein expression.
Key Player Analysis:
Sino Biological
Thermo Fisher Scientific
StressMarq Biosciences
Novasep Holding SAS
Rentschler Biopharma SE
Enzo Life Sciences
Research and Development Systems
LifeSensors Inc.
Lucigen
BioLegend Inc.
Segments:
Based on Protein Type
Proteases
Kinases
Membrane Proteins
Others
Based on Expression Technology
Cell-free Protein Synthesis
Prokaryotic Expression Systems
SUMO Fusion Systems
Gene Fusion System
Others
Based on Application
Drug Discovery
Protein Purification
Biopharmaceuticals
Protein Therapeutics
Disease Diagnostics & Monitoring
Based on the Geography:
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/difficult-to-express-proteins-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
0 notes
Text
CDMOs in the Spotlight: Revolutionizing the Production of Active Pharmaceutical Ingredients
The global active pharmaceutical ingredient CDMO market size was valued at USD 193.7 billion in 2030 and is projected to grow at a compound annual growth rate (CAGR) of 7.4% from 2024 to 2030. Increasing pharmaceutical R&D investments, patent expirations, and a rise in demand for generic drugs and biologic innovations propelling outsourcing are the factors driving the market.
The growth of small molecules, rising active pharmaceutical ingredient (API) complexity and the need to reduce costs are factors contributing to the rapid expansion of outsourcing services in the pharmaceutical sector. Only a few companies have achieved global reach and scale in the contract development and manufacturing organization (CDMO) sector, which is still fragmented. Besides, many companies are providing one-stop-shop solutions as an integrated source of APIs and formulations.
As the healthcare industry is undergoing a process of dynamic change, factors such as rapid technological advancements (e.g., automation & AI), the need for CROs/CMOs/CDMOs, and rising investments in research are influencing the healthcare market. The outsourcing of activities is benefitting many pharmaceutical companies in improving their operational efficiencies, expanding their geographical presence, decreasing resource costs, gaining therapeutic expertise, and enhancing on-demand services.
The COVID-19 pandemic placed unprecedented expectations on API makers, as evidenced by the substantial increase in demand for medications required to manage critically ill patients on mechanical ventilation. As a result, the sudden need to rapidly increase production has emphasized the need for adaptability for API CDMOs in maintaining drug supply, with some companies proving to be better prepared to withstand the pressures of quick scale-up than others. Growing demand for new therapies worldwide and an increase in the conduction of clinical research in the post-pandemic period are expected to support the market in the coming years.
Active Pharmaceutical Ingredient CDMO Market Report Highlights
The traditional active pharmaceutical ingredient segment dominated the market and accounted for the largest revenue share of 39.8% in 2023, due to the high adoption of traditional API in majority of pharmaceuticals
The innovative drugs segment held 73.7% of the revenue share in 2023. This is largely attributed to increasing FDA approvals for new molecular entities, and the increased focus on R&D by innovator API companies
The oncology segment led the market with the highest revenue share of 35.5% in 2023. This is due to the increasing demand for highly potent APIs for cancer therapy
The biotech segment is expected to grow at the fastest rate of 7.0% over the forecast period, owing to the high adoption of biopharmaceuticals in the treatment of chronic and infectious diseases
The clinical workflow segment is anticipated to witness the fastest CAGR of 7.3% over the forecast period. An increase in the number of clinical research studies supporting the demand for APIs is one of the key factors driving segment growth
In Asia Pacific, the market is expected to register the fastest CAGR of 9.1% over the forecast period. Due to the extreme growth in the number of pharmaceutical companies and contract manufacturing organizations in developing countries such as India and China, the region is likely to overtake Europe and North America in the near future
Active Pharmaceutical Ingredient CDMO Market Segmentation
Grand View Research has segmented the global Active Pharmaceutical Ingredient CDMO market report on the basis of product, synthesis, drug, application, workflow, and region:
API CDMO Product Outlook (Revenue, USD Million, 2018 - 2030)
Traditional Active Pharmaceutical Ingredient (Traditional API)
Highly Potent Active Pharmaceutical Ingredient (HP-API)
Antibody Drug Conjugate (ADC)
Others
API CDMO Synthesis Outlook (Revenue, USD Million, 2018 - 2030)
Synthetic
Biotech
API CDMO Drug Outlook (Revenue, USD Million, 2018 - 2030)
Innovative
Generics
API CDMO Workflow Outlook (Revenue, USD Million, 2018 - 2030)
Clinical
Commercial
API CDMO Application Outlook (Revenue, USD Million, 2018 - 2030)
Oncology
Hormonal
Glaucoma
Cardiovascular disease
Diabetes
Others
API CDMO Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
China
India
Japan
Australia
Thailand
South Korea
Latin America
Brazil
Mexico
Argentina
Colombia
Chile
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players
Cambrex Corporation
Recipharm AB
Thermo Fisher Scientific Inc. (Pantheon)
CordenPharma International
Samsung Biologics
Lonza
Catalent, Inc.
Siegfried Holding AG
Piramal Pharma Solutions
Boehringer Ingelheim International GmbH
Order a free sample PDF of the Active Pharmaceutical Ingredient CDMO Market Intelligence Study, published by Grand View Research.
0 notes
Text
Unlocking targeted therapies, the Antibody Drug Conjugates Market pioneers precision medicine in oncology. Advancements offer new hope and improved outcomes for patients battling cancer worldwide.
#Antibody Drug Conjugates Market#Antibody Drug Conjugates Industry#Antibody Drug Conjugates Market key players#Antibody Drug Conjugates Market growth rate#Antibody Drug Conjugates Market size#Antibody Drug Conjugates Market share
0 notes
Text
#Antibody Drug Conjugates Contract Manufacturing Market#Antibody Drug Conjugates Contract Manufacturing Market Trends#Antibody Drug Conjugates Contract Manufacturing Market Growth#Antibody Drug Conjugates Contract Manufacturing Market Research#Antibody Drug Conjugates Contract Manufacturing Market report
0 notes
Text
Antibody Drug Conjugates Market To Reach $24.01Bn By 2030
The global antibody drug conjugates market size is expected to reach USD 24.01 billion by 2030, registering a CAGR of 9.2% during the forecast period, according to a new report by Grand View Research, Inc. The presence of strong pipeline products and strategic initiatives undertaken by the key players are expected to drive the market growth during the forecast period. Key players such as ADC…
0 notes
Text
Antibody Drug Conjugates Market size was valued at USD 6.84 billion in 2022 and is expected to reach USD 11.87 billion by 2029, at a CAGR of 12.6% during the forecast period 2023-2029. ADCs are a type of biopharmaceutical used to treat cancer that consists of an antibody that selectively targets a tumour antigen and a cytotoxic agent linked together by a chemical linker. ADCs are designed to allow the cytotoxic substance to be targeted specifically to kill cancer cells while having a minimal effect on healthy tissue. Antibody drug conjugates are made up of three parts: an antibody specific for the target associated antigen, an antigen that has limited expression on normal cells, a cytotoxic agent that kills target cancer cells, and a chemical linker that connects the cytotoxic agent to the antibody.
#antibody drug conjugates market size#antibody drug conjugates market industry share#antibody drug conjugates market growth drivers#antibody drug conjugates market trends analysis
0 notes