#oxidations
Explore tagged Tumblr posts
Text
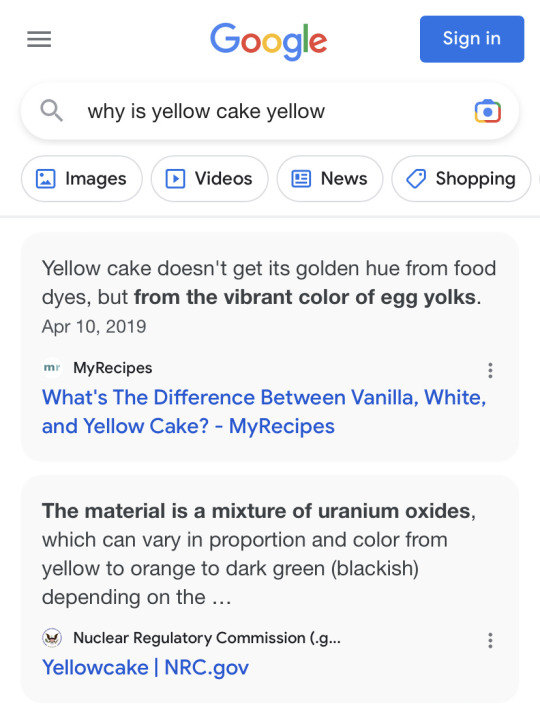
Getting conflicting information here
#cake just isn’t the same without uranium oxide#it’s the worst ingredient to get when you’re baking from scratch
28K notes
·
View notes
Text
When sodium hypochlorite (bleach) solution is added to luminol, a chemical reaction occurs that releases energy in the form of light. This is called chemiluminescence. The bleach solution acts as an oxidizing agent, which means it takes electrons away from the luminol molecule. This causes the luminol molecule to become excited, and it releases the energy as light.
🎥 Courtesy: Kendra Frederick
The luminol molecule is made up of two amino groups, a carbonyl group, and an azo group. The amino groups are electron-rich, while the carbonyl group is electron-poor. The azo group is a conjugated system, which means that the electrons in the double bonds can move freely from one atom to another.
When sodium hypochlorite (bleach) solution is added to luminol, the bleach molecules react with the amino groups of the luminol molecule. This reaction takes electrons away from the luminol molecule, which causes the luminol molecule to become oxidized. The oxidized luminol molecule is in an excited state, which means that it has more energy than it normally does.
The excited luminol molecule then releases the extra energy as light. This light is called chemiluminescence. The light emitted by the chemiluminescence reaction is blue because the luminol molecule has a blue fluorescence.
The chemiluminescence reaction between luminol and sodium hypochlorite is catalyzed by the presence of a metal ion, such as iron or copper. The metal ion helps to stabilize the excited state of the luminol molecule, which makes it more likely to release the extra energy as light.
The chemiluminescence reaction is very sensitive to impurities, so it is important to use pure chemicals. The reaction can also be affected by the pH of the solution. The optimal pH for the reaction is around 9.
The chemiluminescence reaction between luminol and sodium hypochlorite can be used to detect blood, as the iron in hemoglobin can catalyze the reaction. The reaction is also used in some commercial products, such as glow sticks and emergency lights.
I hope you enjoyed learning about this. ❤️🙏
#chemiluminescence#luminol#bleach#science#chemistry#scienceexperiment#glowing#light#excitedstate#oxidation#chemicalreact
5K notes
·
View notes
Text

I like to think that I'm funny
#inanimate insanity#pickle ii#pickle inanimate insanity#hotel oj#carbon oxides#taco ii indirect mention
617 notes
·
View notes
Text

Jazz’s turn, same style as Prowl here.
You know who’s next 🌵
#myart#transformers animated#transformers#tfa Jazz#maccadam#traditional art#alcohol markers#distress oxide
514 notes
·
View notes
Text

I’ve been creating some T-Shirt designs for pride month and am considering printing this to wear for my first London Pride 🏳️🌈🤣🌈
#good omens#aziraphale#go#michael sheen#gayer than a treeful of monkeys on nitrous oxide#tshirt#designing#good omens fanart#fanart#angel#beautiful gay little angel
484 notes
·
View notes
Text
diy mild cleaning solution
650 notes
·
View notes
Text
Of course I love how Benoit Blanc is so shamelessly, fabulously, himself. How he’s gayer than a tree full of monkeys on nitrous oxide [GNU Terry Pratchett]. How he doesn’t hide that his brain is peculiar, for better or for worse. How he picks the cases he likes.
But I also love that in Glass Onion Rian Johnson shows us that Blanc can choose to be himself because he’s protected by his privilege of rich, upper-class, white man.
Which in turn leads me to notice how in both movies Blanc uses his privilege to protect a working-class woman and a Black woman, but he always refuses to be their saviour. He just creates the conditions in which they can choose to be themselves and save themselves—and punish their oppressors.
[Edit: A couple of people pointed out that Marta is actually white, even if the Thrombeys would probably disagree. I’d add that Helen isn’t exactly working-class, she’s simply less rich than the Disruptors shitheads. Anyway: I apologise—I’ve edited the post.]
#knives out#glass onion#glass onion spoilers#benoit blanc#benoit blanc is the opposite of batman#white savior complex#and lack thereof#rian johnson#checking your privilege#the monkeys on nitrous oxide pun is in good omens (the book)#gnu terry pratchett
7K notes
·
View notes
Text

Daredevil is in the trunk, he didn't fit.
#rottmnt#rottmnt raph#rottmnt raphael#red hood#jason todd#hollow knight hornet#hk hornet#dc red hood#my art#listen i know they're all red but THAT'S NOT ON PURPOSE#i guess red is just the color of 'angry'#i wonder what that says about me#haha#is copper red or orange?#actually its GREEN#(when oxidized)#draw your comfort characters#hollow knight#dc comics
519 notes
·
View notes
Text
It's the scent of autumn, oxidation: you can smell it on your skin, that sunburn perfume.
Margaret Atwood, Aflame
#Margaret Atwood#Aflame#Dearly#autumn#autumn quotes#fall#fall quotes#oxidation#smell#perfume#sunburn#Canadian literature#poetry#poetry quotes#poem#quotes#quotes blog#literary quotes#literature quotes#literature#book quotes#books#words#text
347 notes
·
View notes
Note
I'm curious about your Lucifer design, I'd love to read notes about it 👀
Thanks for asking! I actually would love to talk about it, I can include my concept art as well:

My thought process is I see all angels using birds as their basis for their comprehendible forms! So his is meant to play off a cockatiel since his cheeks and hair already remind me of one! I pushed that further with the tailcoat looking like feathers, giving him more feathery hair, and his mouth having curvature similar to a beak
But also I see him being visually stuck as a snake as a punishment for what he did before becoming fallen, a reminder of what he's done and why he's where he is now...so the two features mix into his look! His face and body have scales, his eyes are more narrow, and his coat's hood is meant to look similar to the one of a snake as well
As for the colors I wanted to go little further with it! I felt it'd make sense if he was more cream and brownish red to symbolize being the one to cause the 'bite of the forbidden fruit' aka 'the apple'. When apples are bitten and left alone they oxidize, so I took inspiration from that for the changes. Meanwhile his staff is a literal depiction of this act, his colors a more subtle one, it's all tied together ^^
#veearts#answered#hazbin hotel#lucifer morningstar#hazbin hotel lucifer#hazbin hotel redesign#redesign#hazbin hotel fanart#fan art#lilith morningstar#hazbin hotel lilith#I drew him aside lilith since I was using them both for my charlie brainstorming#i will just lastly add the oxidization color palette is also a nod to his state after being casted out and left alone#you leave a bitten apple by itself and it'll start to rot#i think it's all a very fun narrative#i have more in mind but I want to save that for future explorative pieces through upcoming art 👀
454 notes
·
View notes
Text

Inhaling laughing gas. Wonders of electricity and the elements. Late 19th century.
Science History Institute
157 notes
·
View notes
Text
in the good omens fandom we don't say "they are gay". we say:

#im dyinggg what is that😭😭#gayer than a tree full of monkeys on nitrous oxide#okay🤨#good omens book#good omens#good omens book quotes#aziraphale#crowley#aziracrow#crowly x aziraphale#good omens season 2#neil gaiman#terry pratchett#quotes#book quotes
445 notes
·
View notes
Text

Yeah.
487 notes
·
View notes
Text

@joemomrgneissguy SPACE MINING. HO BOY.
So when mining comes into a conversation, there are several 'laws' of mining and processing that I like to consider that people tend to forget:
Location and rarity of commodity
Location and rarity of extraction techniques/reagents
What is necessary for this operation to work?
Where does the finished product go?
Some of these are extraneous. Theoretically, we don't have to care that iron is common on earth and might be present on the moon, so it changes the conversation from "why?" to "how would we?". Same with extraction and reagents. If you don't care how expensive it is to ship- for example: water and carbon dioxide to the moon because you want to process He-3, nothing can stop you.
However, what will stop planning, is processing. Blowing up a rock is easy. Collecting the rock and breaking it into a usable form is not. If there isn't a plan for exactly what commodity is being mined and how to separate it and all the equipment that needs to be made to get it into a usable form, and a plan to get that equipment into space. God help the poor bastard.
And fundamentally, no matter HOW you turn it, people use the finished product. If there are no people where you are mining the Thing, you need to have a way for the Thing to get back to the people who need it. WHY are you mining the Thing? What is economic about the Thing being made? and Is it worth the money?
[angry geologist rant under the cut]
So the thing about space and asteroids is metals come in native form a lot of the time because there's nothing to oxidize them; it makes processing simpler and the density increases profit. This is usually what people talk about when they go off about space mining: Ohh, if we just reach this asteroid 400 years away there's so much Gold and Platinum! Ohh, if we just crashed a FUCKING ASTEROID INTO EARTH OR MARS we could be so rich!
However this is a LIE for two reasons: It's actually harder to process straight sulfides or straight metal because they aren't brittle. Instead of breaking into smaller pieces you can separate and process, they jam the crusher. Universities with mining departments often have huge chunks of impressive high-grade sitting around that were donated by companies when they jammed their fucking system. If you can't break it down, it's a useless fucking clump of rock.
Secondly, even if you have native metals clumped together like an iron-nickel asteroid, unless you want an iron-nickel product, you have to separate them. Since it's not brittle, you would have to pour a bunch of hydrochloric on it and wait for the reaction to dissolve the outer surface.
And all this is assuming the metals are on Earth. If not, you have to figure out how to do this in space. How much HCl will you need? How are you going to fly it up there? How are you going to break it down? How are you going to replace parts when they inevitably break?
The big "commodity" on the moon is Helium-3, which is extremely rare on Earth. (So yes, we have a need, and yes, there's substantial reason to mine it in a place where it's more accessible.) The logic starts breaking down around "getting it back" and "how does the operation work": In moon quantities (up to 15 parts per billion (ppb)), you have to mine about 150 tons to extract 1g of He-3. That's not unreasonable, to be honest, since economic gold hovers around 7-12 ppb. And technically you'd only have to heat the rock to 600-700 C. However, things do melt at those temperatures. Then you have to get it back to earth. Either a SpaceX-style return and come back, or a drop shipments- It's just insane to me though that we would use SO MANY RESOURCES to rip up the fucking moon, even with an automated system, when if you look at He-3 we already produce what equals 11 pounds of He-3 yearly from Oil and Gas deposits, it's just not collected.
I have more beef with planets that are theoretically resource-rich, but people just- don't care about getting them back to Earth? Venus has significant metal-Sulfides and Tellurides in its atmosphere, which is why people joke about the "floating oxygen colonies" on Venus. But congratulations! You've colonized a planet that is inaccessible to human technology because anything we've ever designed will dissolve. Same with Europa. To design something that works on Venus - not to mention extracts things in the proper form to be used in human conditions - and/or get them back to Earth means redesigning how we think of the properties of the periodic table.
With extraction, we play a lot with oxidation states, and one of the rules is to stay within Earth's aqueous conditions. If you oxidize anything too much, your solution will want to vaporize to oxygen. Reduce anything too much, and your solution will want to vaporize to hydrogen gas.
So, if you design anything on Earth designed for conditions on Venus, it will be unstable. If you design anything on Venus meant for Earth, it will be unstable.
Which is kind of the end of my rant, I guess. Don't crash something into Earth unless you can process it. If you can process it in space, can you get it back? Who's responsible when the thing breaks? Why the fuck is money being spent when 9 times out of 10 we have it here on earth with the conditions we're familiar with?
If we've somehow depleted Earth enough that we need resources from other planets, which would insinuate we have not figured out how to recycle our own metals, which is untrue, and likewise we have no business in space anyway- Where did all our resources go? Are we leaving for those other planets? Do we have faster-than-light travel to collect the new resources in a timely manner?
There isn't even water in space half the time and if you do have a colony on Mars and tech bros are going to process all the hematite to build their shitty underground Martian city, are they shipping water from the north and south poles to do this? Have they figured out how to renew the carbon filters that are going to be needed to get all the waste and organics out of it once it's used?
In my opinion, it's all just fucking stupid. Space mining tries to answer a question that doesn't need to be asked with people who don't know how mineral processing works who haven't thought what the logistics require and don't care that entropy demands even minerals in stasis don't last forever. But it's ~new~ and the dollar signs on metallic asteroids gleam in their eyes and I want to take out Elon Musk's kneecaps.
#Apparently a team in Europe proposed a way to separate Fe2O3 while producing oxygen. Which is definitely a step forward.#But I still say the actual water and reagents used to process rock to element are non-circular enough that it's a huge hindrance.#Anyway! Space mining! Quickest way to expose a techbro dipshit is ask where they'll get the water for iron oxide separation.#Fix our own planet and close the circuits in hydrometallurgy and then we can talk about space mining.#mining#geology#mineral processing#I hope this was actually legible and coherent lol. I didn't spend as much time on it as I did on the Gold one.#I hate space mining and gold mining for the greed and colonialist mindset.
127 notes
·
View notes
Text

"for every atom belonging to me as good as belongs to you"
from the fic iron oxide (i am once again telling you to read it)
#yippee!! i spent so long on this i'm rlly happy with it :]#life changing fic fr#sth#sonic#sonic the hedgehog#metal sonic#metonic#sonic art#sonic fanart#sonic the hedgehog fanart#iron oxide#art#fanart#digital art#artists on tumblr#vixenart
759 notes
·
View notes
Text



Sweet baby Rottweiler skeleton. Natural death, cleaned using oxidation.
301 notes
·
View notes