#hydroxyl radicals
Explore tagged Tumblr posts
Text

The Science Research Diaries of S. Sunkavally, page 346.
#circular DNA#thymine dimers#nubigenous origin of life#Carl Woese#Drosophila melanogaster#estuaries#fecundity#nose length#odour detection#hydroxyl radicals#ferrous iron#RNA evolution#nitric oxide#cavitation#oceans#satyendra sunkavally#theoretical biology#manuscript#notes#notebook#cursive handwriting
4 notes
·
View notes
Link
1 note
·
View note
Text
In the same manner as hydroxyl, the nitrate radical is able to add to the double bond of olefins:
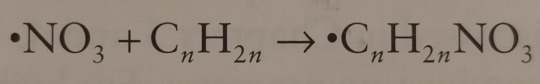
"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quote#environmental chemistry#nonfiction#textbook#hydroxyl#nitrate#radical#chemical bonding#chemical reactions#olefin
0 notes
Text
ROS generation occurs in several cellular compartments and as a result of the activities of specialized oxidases, such as NADPH oxidases, amine oxidases, and cell wall-bound peroxidases (Table 24.2).
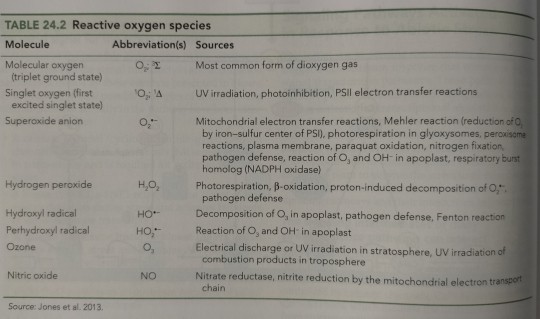
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#reactive oxygen species#ros#plant cells#oxidase#nadph#amine#cell wall#peroxidase#oxygen#molecular oxygen#singlet oxygen#superoxide#anion#hydrogen peroxide#hydroxyl#radical#perhydroxyl#ozone#nitric oxide
0 notes
Text
The invention is a powder that almost instantly kills thousands of waterborne bacteria when simply mixed with water and exposed to normal sunlight for a few seconds. It consists of nano-sized flakes of aluminum oxide, molybdenum sulfide, copper, and iron oxide, which combine with sunlight to form hydrogen peroxide and hydroxyl radicals, Phys.org reported.
These newly formed chemical byproducts work quickly to kill off any bacteria and then dissipate just as quickly.
The powder has several advantages over existing methods of cleaning drinking water. It does not use any chemicals that create lasting toxic byproducts, and it does not require ultraviolet light, which takes a long time and requires electricity, according to Phys.org.
In addition, the powder is recyclable. It can be removed from the now-clean water with a magnet, and researchers were able to reuse the same powder 30 times.
3 notes
·
View notes
Text
Unlocking the Power of Wellness: The Ultimate Hydrogen Water Machine by HydroGenie.
Introduction In an age where wellness technology is rapidly evolving, few innovations offer the scientific promise and practical benefits of hydrogen water. Leading the way in this space is the H2 Impact by HydroGenie, a revolutionary hydrogen water machine that combines advanced engineering with health-conscious design. From athletes to biohackers and wellness enthusiasts, people are turning to hydrogen water to enhance performance, reduce oxidative stress, and support cellular health. This article dives deep into what makes hydrogen water so beneficial, and how the HydroGenie H2 Impact is redefining at-home hydrogen therapy.
What Is Hydrogen Water? Hydrogen water is simply water (H2O) that has been infused with additional molecular hydrogen (H2). Unlike oxygen or vitamins, molecular hydrogen is a selective antioxidant. It targets only the most harmful free radicals, particularly hydroxyl radicals, which are associated with cell damage, aging, and inflammation.
Scientific studies show that drinking or inhaling molecular hydrogen can reduce oxidative stress, improve metabolism, enhance cognitive performance, and even support longevity. Unlike many antioxidants that can accumulate or interact poorly in the body, molecular hydrogen is safe, non-toxic, and naturally exhaled after it performs its job.
Introducing the HydroGenie H2 Impact: A Next-Gen Hydrogen Water Machine
The H2 Impact by HydroGenie isn't just another hydrogen water bottle or tablet-based infuser. It is a powerful and versatile hydrogen water machine designed to bring therapeutic hydrogen delivery to your home or wellness practice.
This 3-in-1 device offers:
Hydrogen water infusion for daily drinking
Hydrogen gas inhalation via nasal cannula
Topical hydrogen therapy through a gas cup
This modular approach allows users to tailor their hydrogen intake method based on need, whether it's rapid recovery, cognitive enhancement, skin hydration, or systemic antioxidant protection.
Key Features and Benefits of the H2 Impact Hydrogen Water Machine
Versatility in Hydrogen Delivery Most hydrogen devices focus on a single function: either water infusion or gas inhalation. The H2 Impact does all three:
Drink it: Infuse water or other beverages with H2 gas.
Inhale it: Use a nasal cannula to absorb hydrogen quickly.
Apply it: Use gas cup technology for joint relief or skin therapy.
Superior Hydrogen Concentration The H2 Impact delivers high concentrations of molecular hydrogen, ensuring that each session—whether inhaled or infused—delivers therapeutic benefits.
Portable and Durable With compact design and medical-grade materials, it’s suitable for home, office, gym, or travel use. Built to last and easy to operate.
Non-Flammable Safety Unlike devices that store hydrogen or use chemical reactions, the H2 Impact generates hydrogen on demand, reducing risk and increasing reliability.
Cost-Effective Long-Term Say goodbye to recurring purchases of tablets, hydrogen-rich bottled water, or single-function devices. The H2 Impact is an investment in daily wellness.
Why Hydrogen Therapy Matters
Hydrogen therapy is becoming a focal point in biohacking and preventative medicine. Why? Because hydrogen influences oxidative stress—the root cause of many chronic diseases. From fatigue and inflammation to neurodegeneration and cardiovascular disease, oxidative stress silently affects millions.
Hydrogen therapy addresses:
Energy loss and fatigue
Inflammatory joint pain
Exercise recovery needs
Mental fog and lack of focus
Premature skin aging
Immune system overactivation
By integrating a hydrogen water machine like H2 Impact into your lifestyle, you're not just drinking water—you’re fueling your body at the molecular level.
The Science Supporting Hydrogen Water
Numerous clinical and peer-reviewed studies highlight hydrogen's benefits. Key findings include:
Improved mitochondrial function
Reduction in blood lactate during exercise
Cognitive benefits in neurodegenerative disorders
Improved glucose and lipid metabolism in diabetics
Protection against radiation and chemotherapy damage
Molecular hydrogen also acts faster than traditional antioxidants, like Vitamin C or E, due to its small molecular size, which allows it to penetrate deeply into tissues and cells.
Everyday Use Cases: Who Benefits from the H2 Impact?
Athletes: Improve performance, hydration, and muscle recovery.
Busy Professionals: Boost mental clarity and fight fatigue.
Seniors: Support cognitive function and joint mobility.
Biohackers: Stack with other therapies like red light, cold plunges.
Families: Improve daily hydration and immunity.
Incorporating hydrogen therapy is as easy as sipping a glass of water or taking a 10-minute inhalation break. With HydroGenie, wellness meets simplicity.
Comparing Hydrogen Water Machines: What Sets HydroGenie Apart?FeatureH2 Impact by HydroGenieCompetitor ACompetitor BWater + Inhalation + TopicalYesNoNoPortable DesignYesPartialNoHigh H2 ConcentrationYesModerateModerateLow MaintenanceYesNoNoCost-EffectiveYesNoNo
HydroGenie’s multi-functionality and safety profile set it apart from overpriced, underpowered hydrogen machines.
How to Use the H2 Impact in Daily Life
Morning: Start your day with hydrogen-infused water to activate metabolism and mental clarity.
Pre-Workout: Inhale H2 gas for endurance and reduced lactic acid buildup.
Post-Workout: Infused drinks and topical hydrogen therapy aid muscle recovery.
Evening: Use hydrogen inhalation to wind down and support cellular repair during sleep.
Skin Routine: Apply hydrogen gas to your face or problem areas to reduce wrinkles, inflammation, or blemishes.
Customer Testimonials
"I've never had this much energy naturally. The H2 Impact replaced three supplements I used to take." — Jenna, CA
"As a triathlete, hydrogen therapy has helped my recovery tenfold. I use the machine daily." — Carlos, FL
"It’s like a spa, health hack, and hydration tool all in one. Worth every penny." — Michelle, NY
Maintenance & Longevity
The H2 Impact is designed for low-maintenance operation. Key points:
Regular water changes
Occasional filter replacement
No dangerous chemicals or combustible gases
Most users report seamless operation for years with minimal upkeep, making this device both powerful and practical.
Where to Buy the H2 Impact Hydrogen Water Machine
You can purchase the H2 Impact directly from HydroGenie’s official site: https://gethydrogenie.com/product/h2-impact/.
HydroGenie also offers:
Secure checkout
Warranty coverage
US-based support team
It’s more than a purchase—it’s an investment in your future health.
Final Thoughts: Is the H2 Impact Worth It?
Absolutely. The H2 Impact hydrogen water machine combines science-backed benefits with user-friendly features, offering a complete at-home wellness solution. Whether you’re aiming to optimize performance, extend longevity, or simply hydrate better, HydroGenie’s technology empowers you to do it efficiently and safely.
With a sleek design, multiple delivery methods, and powerful antioxidant impact, the H2 Impact is not just a wellness gadget—it’s a daily health ritual.
Call to Action
Ready to harness the healing power of molecular hydrogen? Visit HydroGenie’s official website and experience the difference a true hydrogen water machine can make.
0 notes
Text
Steps of DNA footprinting start by amplify region of interested DNA molecule by conventional PCR and label amplified DNA with radioactivity or fluorescence label probe and apply incubation with protein and immunoprecipitation After that apply DNA modification with DNA modifiers or cleavage agents such as (DNase-1 or Hydroxyl radicals) then run polyacrylamide gel electrophoresis with uniform ladder for control sample to allow prediction of exact location of binding site so footprinting is a gap pattern on polyacrylamide gel which mean protein bound DNA and protect DNA from cleavage agent (Watch Related Video in #geneticteacher) #geneticteacher
0 notes
Text
A Mini Review: The Activation of Biocarbon Materials and their Complexes to Persulfate

A Mini Review: The Activation of Biocarbon Materials and their Complexes to Persulfate in Biomedical Journal of Scientific & Technical Research
n most cases, the major oxidant species in persulfate systems are the sulfate radical(•SO4-) and the hydroxyl radical (•OH). Persulphates can be divided into two categories, namely, persulfate (PMS) and perdisulate (PDS). However, due to the unique structure, the O-O bond is difficult to break to form (•SO4-), so it needs to undergo a certain process to activate the persulfate. The current activation methods mainly include thermal activity method, alkali activation, transition metal ion activation, and ultraviolet activation [12]. However, the thermal activity method usually requires a high energy consumption, and the alkali activation and the transition metal ions activation often arise new problems in the cost and subsequent processing period. It is reported that carbon materials can effectively achieve activation of PS, but the cost is too expensive. Fortunately, BC have more advantages over other carbon materials [13], such as extensive source and lower cost. And recent works has reported that biomass carbon made from 300-500℃ calcination has good results on PS activation. The annual biomass waste production from various sources is very huge. As a more environmentally friendly carbon material, biomass carbon shows considerable potential for PS activation and much effort has been devoted to it. Other articles also summarize the application of biomass as a catalyst in some advanced oxidation processes of persulfate activation [14]. However, the effect of biomass catalyst in the advanced oxidation of sulfate radical remains to be systematically arranged. Hence, in this mini review, the recent mechanisms and application of biocarbon materials in activated persulfate are summarized and new insights into the future applications in activated PS are simultaneously provided. The present work also provides a direction for the better use of BC in the future.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01/ Follow on Blogger : https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#Journal on medical science#Open access clinical and medical journal#Journals on Medical Informatics#Biomedical Science and Research Journal#Biomedical Journal Impact Factor
0 notes
Text
Ozone Production and Destruction Using UV (Ultraviolet) Technology
Ozone Generation with UV Light
Ozone is one of the most powerful oxidizing agents available and is widely used in air purification and water treatment processes. While most commercial applications rely on ozone generators that use either air or oxygen as a feed gas via the corona discharge method, there are alternative technologies worth exploring.
One such alternative is the use of electrolytic ozone generators, which produce ozone directly from pure water, offering a chemical-free and efficient solution for specific applications.
Another method, though less common, involves the use of ultraviolet (UV) light. When UV light at a wavelength of 185 nm is passed through air or oxygen, it generates ozone. However, this method typically yields low ozone concentrations—around 0.5%—making it suitable primarily for air treatment. For water treatment, much higher ozone concentrations (typically above 6–8%) are required due to ozone's solubility limitations in water.
Ozone Destruction Using UV Technology
While ozone plays a crucial role in disinfection and oxidation processes, any residual ozone left in treated water can be harmful to both equipment and final products. This makes it essential to eliminate residual ozone before water enters downstream processes.
This is where UV destruction of ozone becomes valuable. UV light within the 240–280 nm range can effectively break down ozone molecules, offering a clean, chemical-free method of ozone removal. In particular, germicidal UV at 254 nm is capable of destroying ozone in water without leaving any byproducts.
To completely eliminate ozone concentrations up to 1000 ppb, a UV dosage of approximately 900 J/m² is typically required—considerably higher than the 300 J/m² dosage needed for standard disinfection.
Interestingly, UV-based ozone destruction also supports the generation of hydroxyl radicals (•OH), which are highly reactive but short-lived species. These radicals form through reactions such as:
O₃ + H₂O → O₂ + H₂O₂ (in the presence of UV light)
2 O₃ + H₂O₂ → 2 •OH + 3 O₂
This forms the basis of Advanced Oxidation Processes (AOP), which are used to enhance overall oxidation efficiency. AOPs are especially effective in reducing chemical oxygen demand (COD) by more than 75%, outperforming ozone alone in treating persistent organic contaminants.
Common Applications of UV-Ozone AOP Include:
COD reduction in industrial and municipal wastewater
TOC (Total Organic Carbon) reduction in process water
Removal of endocrine-disrupting compounds
BTX (Benzene, Toluene, Xylene) removal
Elimination of micropollutants from source water
0 notes
Text
Gallic Acid: A Natural Compound with Remarkable Benefits
Introduction
Gallic acid is a naturally occurring polyphenol found in various plants, fruits, and herbs. Known for its potent antioxidant, anti-inflammatory, and antimicrobial properties, this bioactive compound has been used in traditional medicine for centuries. In modern applications, gallic acid plays a crucial role in pharmaceuticals, food preservation, cosmetics, and scientific research. As demand for natural and sustainable solutions grows, gallic acid is increasingly recognized for its diverse applications and health benefits.

Chemical Composition and Natural Sources
Gallic acid, scientifically termed 3,4,5-trihydroxybenzoic acid, belongs to the hydroxybenzoic acid family, characterized by three hydroxyl (-OH) groups attached to a benzoic acid ring. This structure enhances its biological activity and therapeutic properties. Gallic acid is widely found in:
Fruits: Grapes, apples, bananas, blueberries, and strawberries
Plants and Herbs: Tea leaves, sumac, oak bark, and gallnuts
Beverages: Green tea, black tea, and red wine
Nuts: Walnuts, hazelnuts, and almonds
The presence of gallic acid in these natural sources contributes to their antioxidant and health-promoting effects. Extracted gallic acid is widely utilized in pharmaceuticals, food additives, and skincare formulations.
Health Benefits of Gallic Acid
Gallic acid has been extensively studied for its therapeutic potential. Some of its key benefits include:
1. Powerful Antioxidant Properties
Gallic acid effectively neutralizes free radicals, reducing oxidative stress and preventing cellular damage. This antioxidant function helps lower the risk of chronic diseases such as cancer, cardiovascular conditions, and neurodegenerative disorders like Alzheimer’s and Parkinson’s disease.
2. Potent Anti-Inflammatory Effects
Chronic inflammation is linked to conditions such as arthritis, diabetes, and heart disease. Gallic acid has been shown to suppress inflammatory responses, making it beneficial for managing inflammation-related health issues and supporting immune function.
3. Antimicrobial and Antiviral Activity
Gallic acid possesses strong antibacterial, antifungal, and antiviral properties. Research indicates that it inhibits harmful microorganisms such as E. coli and Staphylococcus aureus, making it valuable for food preservation, pharmaceuticals, and hygiene products.
4. Anti-Cancer Potential
Studies suggest that gallic acid may play a role in cancer prevention by inducing apoptosis (programmed cell death) in cancerous cells. It has shown promising effects in slowing tumor growth, particularly in breast, lung, and colon cancers, making it a focus of ongoing oncological research.
5. Neuroprotective Benefits
By reducing oxidative damage and inflammation in brain cells, gallic acid may help protect against cognitive decline and neurodegenerative diseases. Its neuroprotective properties make it a promising compound for brain health supplements and longevity research.
Industrial and Commercial Applications
Due to its multifunctional properties, gallic acid is widely used across various industries:
Pharmaceuticals: Incorporated into drug formulations for its antioxidant, anti-inflammatory, and anticancer benefits.
Food Industry: Acts as a natural preservative, improving food safety and extending shelf life.
Cosmetics and Skincare: Found in anti-aging creams, sunscreens, and serums for its protective effects against UV radiation and environmental pollutants.
Dyes and Inks: Historically used in the production of durable iron gall ink.
Chemical Industry: Used as a precursor for synthesizing bioactive compounds and specialty chemicals.
Future Prospects and Innovations
Ongoing research continues to explore the expanding applications of gallic acid in medicine, food technology, and cosmetics. Scientists are particularly interested in enhancing its bioavailability through advanced delivery systems, such as nanoparticles, to maximize its therapeutic effects. As interest in natural and plant-based compounds increases, gallic acid is expected to play an even greater role in health and industrial innovations.
Conclusion
Gallic acid is a naturally derived compound with vast potential in both health and industry. Its antioxidant, anti-inflammatory, antimicrobial, and anticancer properties make it invaluable in pharmaceuticals, food preservation, and cosmetics. With continuous scientific advancements and rising consumer demand for natural products, gallic acid is set to become a key component in sustainable and innovative developments across multiple sectors.
0 notes
Text
Piscine Naturelle: Where Technology Meets Nature in Advanced Pool Systems
Piscine Naturelle represents the forefront of innovation in pool systems, where cutting-edge technology seamlessly integrates with natural principles to create sustainable and aesthetically pleasing environments. By combining advanced filtration systems with natural elements, these pools not only enhance the beauty of outdoor spaces but also provide a healthier and more environmentally friendly alternative to traditional swimming pools. The absence of harsh chemicals ensures that the water remains safe for swimmers while supporting local biodiversity, making Piscine Naturelle's approach an attractive option for those seeking a more sustainable lifestyle.
The Evolution of Pool Filtration Technology
The evolution of pool filtration technology has led to the development of advanced systems that mimic natural processes to purify water. Systems like Origin Aqua's mineral+biome utilize beneficial microbes to outcompete pathogens, creating a clean and safe swimming environment without the need for chemical disinfectants. This approach not only improves water quality but also offers health benefits by incorporating thermal spa minerals like magnesium and selenium into the water. By leveraging such technologies, pool owners can enjoy superior water quality while minimizing their environmental footprint.

Integrating Advanced Oxidation Processes
Advanced Oxidation Processes (AOP) are revolutionizing the pool sanitation industry by providing a powerful and safe method for water disinfection. AOP systems create hydroxyl radicals, which are more potent oxidizers than chlorine or ozone, ensuring a healthier and safer swimming experience. These systems eliminate contaminants and toxic byproducts, making them ideal for sensitive swimmers and those with health limitations. By adopting AOP technology, pool owners can enjoy clear and clean water that feels better to the touch, while also reducing the risk of chemical exposure.
Sustainable Materials and Designs
Natural swimming pool commitment to sustainability extends beyond filtration systems to include the use of eco-friendly materials and designs. For instance, BioPoolTech's natural pools made from immersed wood offer a unique aesthetic while minimizing environmental impact. These pools reduce water and electricity consumption significantly, making them an attractive option for environmentally conscious homeowners. By combining sustainable materials with advanced filtration systems, Piscine Naturelle creates pools that not only enhance outdoor spaces but also contribute to a healthier environment.
Customization and Personalization
One of the key benefits of working with Piscine Naturelle is the ability to customize and personalize your pool system. Homeowners can collaborate with designers to create a unique space that reflects their personal style and complements their existing landscape. Whether it's incorporating specific types of plants, designing a unique shape, or adding custom stonework, the possibilities for personalization are vast. This flexibility allows homeowners to create a truly unique outdoor space that not only enhances their property but also provides a serene retreat from daily life.

The Future of Pool Design
As interest in sustainable living continues to grow, the demand for advanced pool systems that integrate technology with nature is likely to increase. Piscine Naturelle's innovative designs and commitment to environmental sustainability position them at the forefront of this trend. With advancements in technology and design, the possibilities for creating bespoke pools will continue to expand, offering homeowners the opportunity to craft truly unique and immersive outdoor experiences. Whether you're looking to create a serene oasis or simply want to elevate your property's beauty, Piscine Naturelle's advanced pool systems provide a compelling solution that combines aesthetics with ecological responsibility.
Conclusion: Embracing Sustainable Luxury
Piscine Naturelle's approach to pool design embodies the concept of sustainable luxury, where advanced technology and natural principles come together to create environments that are both beautiful and environmentally conscious. By embracing this approach, homeowners can enjoy the benefits of a superior swimming experience while contributing to a healthier planet. As the world moves towards more sustainable living practices, Piscine Naturelle's innovative pool systems offer a glimpse into the future of outdoor design, where technology and nature harmoniously coexist to create serene and sustainable oases.
0 notes
Text

TSRNOSS, p 489.
#fish#oxygen toxicity#turtle#leukemia#division rate of polymorphonuclear neutrophils#osteoporosis#leukocytes#amoeboid motion#NADase#superoxide#pyridine nucleotides#hydroxyl radicals#neutrophils
0 notes
Text
"Unlock the Power of Bitter Melon Peptides for Better Health"
What is bitter melon peptide? Tell you its effects and precautions According to statistics from the Ministry of Health and Welfare, diabetes is one of the top ten causes of death in Taiwan, and the number of patients is increasing year by year, with about 25,000 new patients each year. Currently, there are more than 2 million people in Taiwan suffering from diabetes. In recent years, bitter melon peptide has become a popular health food and is hailed as the savior of diabetes. Is it really so magical?
What is bitter melon peptide?
Bitter melon peptide (scientific name: bitter melon peptide) is a small molecule protein extracted from bitter melon. It has many health benefits such as promoting metabolism and regulating physiological functions. Because of its small molecules, it is easier to be absorbed by the human body. Some bitter melon peptides have been sequenced and patented. Choosing patented products is the best choice.
苦瓜苦瓜胜蛋白品牌推荐Nutritional ingredients of peptides
Known as "the pride of Taiwanese medical scientists", bitter melon peptides are composed of 68 amino acids, of which 50 to 68 amino acids can resist the damage of gastric acid and digestive enzymes, and can reduce blood sugar by 30%. Bitter melon peptide is called "plant insulin" because it can increase the sensitivity of insulin receptors, improve insulin resistance, help glucose enter cells, enhance the transport capacity of glucose transport protein "GLUT4", provide energy for cells, and maintain normal operation.
苦瓜苦瓜胜蛋白The efficacy of peptides
Lowering blood sugar Bitter melon peptides can regulate blood sugar like plant insulin. By binding to insulin receptors, they can effectively stabilize fasting blood sugar, promote insulin secretion, increase blood sugar utilization, reduce glucose absorption or enhance pancreatic β cell function. Experiments show that blood sugar can be reduced by 53% and glycosylated hemoglobin by 34% two hours after a meal. Lower body fat Studies have shown that bitter melon extract can reduce triglycerides, total cholesterol and low-density lipoprotein, promote fat metabolism, and reduce body fat accumulation. Body fat can be reduced by 25% in one month and 28% in two months苦瓜胜蛋白效果, total cholesterol can be reduced by 36%, and triglycerides can be reduced by 39%. Antioxidant Bitter melon peptide has significant Bitter melon peptide recommendation antioxidant effect. Its main components are galactose, glucose and arabinose. It has good antioxidant activity and hypoglycemic effect. Related studies show that the hydroxyl free radical scavenging rate of purified bitter melon polysaccharide at a concentration of 20 mg/mL can reach 82%.
The compound is more effective when combined with bitter melon peptides
Chromium yeastChromium yeast is an "accelerator" of insulin to control blood sugar. Studies have found that chromium yeast can improve insulin sensitivity and enhance insulin activity by 7 to 10 times. White Kidney Bean White Kidney Bean contains the highly effective ingredient "Phaselarmin" which can inhibit the decomposition of starch into glucose and reduce sugar absorption, reducing starch absorption by an average of 57%.
Eating suggestions and precautions Bitter melon peptide recommendation matters
It is recommended to eat 30 minutes before meals, which can stabilize sugar metabolism, regulate physiological functions, and reduce post-meal fatigue and appetite.
Avoid taking it at the same time as medication to prevent interactions between nutrients and medication.
Diabetic patients should consult a doctor before consumption, and should not replace medications with bitter melon peptides on their own. They should take medications on time as directed by the doctor, and avoid adjusting or stopping medications on their own to avoid causing greater harm to their health.
0 notes
Text
Considering the various hydrocarbon reactants, there are two principal mechanisms by which hydroxyl radicals initiate oxidation.
"Environmental Chemistry: A Global Perspective", 4e - Gary W. VanLoon & Stephen J. Duffy
#book quote#environmental chemistry#nonfiction#textbook#hydrocarbons#reactants#chemical reactions#hydroxyl#radicals#oxidation
0 notes
Text
The most common forms of ROS in plant cells are superoxide (O2•-), singlet oxygen (¹O2), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) (Figure 24.3).
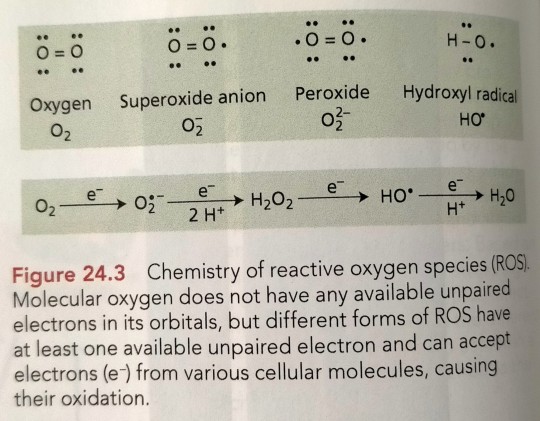
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#reactive oxygen species#ros#superoxide#singlet oxygen#hydrogen peroxide#hydroxyl#radicals#chemistry#oxidation
0 notes
Text
Chemical properties & pharmacological effects of Neferine
Abstract
Neferine is a natural bisbenzylisoquinoline alkaloid, mainly extracted from plants of the Nelumbo family (such as lotus seed embryo). In recent years, its wide range of biological activities (such as cardiovascular protection, anti-tumor, antioxidant, etc.) has attracted the attention of researchers. This paper reviews its chemical structure, pharmacological mechanism and potential applications, and looks forward to future research directions.
1. Introduction
Neferine has a long history in traditional medicine. Lotus seed heart (embryo) is recorded in the Compendium of Materia Medica as a medicinal material for clearing heat, calming the nerves and lowering blood pressure. As one of the main active ingredients of lotus seed heart, neferine exhibits diverse biological activities due to its unique bisbenzylisoquinoline structure. With the rise of natural product research, the pharmacological potential of neferine has gradually been revealed.
2. Chemical properties
Methyl neferine (C₃₈H₄₄N₂O₆, CAS number: 2292-16-2) belongs to the bis-benzylisoquinoline alkaloids, which are composed of two benzylisoquinoline units connected by ether bonds (Figure 1). It has a large molecular weight (624.77 g/mol) and high lipid solubility. It can be separated and purified from lotus seed germ by high performance liquid chromatography (HPLC) or mass spectrometry.
3. Pharmacological effects and mechanisms
3.1 Cardiovascular system protection
1. Anti-arrhythmic: By inhibiting the L-type calcium channel (LTCC) and delayed rectifier potassium channel (IK) of myocardial cells, it regulates the action potential duration and reduces the risk of arrhythmia.
2. Anti-hypertension and vasodilation: It activates the nitric oxide (NO) signaling pathway, promotes the release of NO by endothelial cells, and leads to relaxation of vascular smooth muscle.
3. Anti-thrombosis: Inhibits platelet aggregation induced by platelet activating factor (PAF) and reduces the risk of thrombosis.
3.2 Antioxidant and anti-inflammatory
- Scavenges free radicals: Neutralizes reactive oxygen species (ROS) through phenolic hydroxyl structure and reduces oxidative stress damage.
- Inhibits inflammatory factors: Downregulates NF-κB pathway, reduces the release of pro-inflammatory factors such as TNF-α and IL-6, and shows protective effects in acute lung injury models.
3.3 Anti-tumor activity
- Induce apoptosis: Activates mitochondrial apoptosis pathway (such as Bax/Bcl-2 imbalance, caspase-3 activation), inhibits breast cancer MCF-7 cell proliferation.
- Inhibits metastasis: Inhibits lung cancer A549 cell invasion and migration by blocking PI3K/Akt/mTOR pathway.
3.4 Neuroprotective effect
- Anti-Alzheimer's disease (AD): Reduce β-amyloid protein (Aβ) deposition, inhibit tau protein hyperphosphorylation, and improve cognitive dysfunction in mice.
4. Applications and challenges
4.1 Traditional and modern applications
- Traditional Chinese medicine uses: Lotus seeds are used to make tea to reduce internal heat and calm the nerves.
- Modern drug development: As a lead compound, explore the application of its derivatives in anti-tumor or cardiovascular diseases.
4.2 Safety and limitations
- Toxicity: Animal experiments show low acute toxicity (LD₅₀ > 2 g/kg), but long-term use may affect liver and kidney function.
- Bioavailability: High lipid solubility but poor water solubility, and the absorption rate needs to be improved through nanoformulation or structural modification.
5. Future research directions
1. Clinical transformation: Promote clinical trials to verify its efficacy on human diseases (such as hypertension and cancer).
2. Structural optimization: Enhance targeting and reduce side effects through chemical modification.
3. Multi-omics research: Combine metabolomics and proteomics to analyze its multi-target mechanism of action.
6. Conclusion
As a natural active molecule, neferine has multi-target and multi-pathway pharmacological properties and has significant potential in the treatment of cardiovascular diseases, tumors and neurodegenerative diseases. In the future, it is necessary to strengthen the connection between basic and clinical research and promote its application from laboratory to clinical application.
References :
1. Deng Y, et al. *Neferine inhibits colorectal cancer cell growth through ROS-mediated PI3K/Akt pathway*. Cancer Lett. 2019.
Liu CM, et al. *Neferine induces apoptosis by modulating Bcl-2 family proteins in human breast cancer cells*. Phytomedicine. 2020.
3. Chinese Pharmacopoeia Commission. *Pharmacopoeia of the People’s Republic of China*. 2020 Edition. article from:Chemical properties & pharmacological effects of Neferine - Kingherbs
0 notes