#education in chemistry
Explore tagged Tumblr posts
Text
That's just wrong!
Here's an excerpt from the GCSE Chemistry textbook we use in our school.

There are several crimes against chemistry in these statements. However, sometimes you have to simplify in order for students to understand the general idea, so there are always going to be lines in textbooks that make an experienced chemist mumble "I think you'll find it's a bit more complicated than that".
What makes me uneasy is when we teach stuff that's not just inaccurate but incorrect. Take the third bullet point, "The liquids become more viscous". The trouble is, they don't. To the unassisted human senses the viscosity of liquid hydrocarbons barely changes from hexane (6 carbon chain) to barbecue lighter fluid (11 to 14 carbons). You certainly wouldn't be able to measure any difference in a school lab. Motor oil (18 to 34 carbons) is a bit gloopy (to use a technical term) but still flows easily. If the carbon chains get slightly longer (20 to 40) you get waxes.
This evidence-free assertion has been taught in school chemistry forever. It may be plausible, but it's still wrong, and I can't believe I'm the first person to notice. It's far from the only example in school science. There are plenty in chemistry, but my favourite(!) is from biology - the way we teach the inheritance of eye colour is spectacularly bad.
This matters. We're teaching students "facts" that are easily disproved. We're making them do practicals that don't work because they haven't been tested or the instruction sheets are badly written (there's a whole book there, let alone a blog post). And if we aren't trustworthy, why shouldn't students be sceptical about science?
4 notes
·
View notes
Text











You guys wanna see a science Lego set? Well, here's Lego DNA!
With a scientifically accurate DNA model, and a historically accurate lab + 5 scientists!
Aims: to promote science to kids and honor Rosalind Franklin.
Less than 4,000 votes needed to get it considered as a real official Lego set to be sold worldwide!
If you like it, please support here and share with your friends: https://ideas.lego.com/projects/c92cd95b-49e7-46ec-b844-ac6482c51139
#chemistry#science#biology#design#diy#education#nature#environmental science#molecular biology#stem#women in stem#research#laboratory#women scientists#school#university#college#students#learning#lego art#lego photography#afol#lego sets#lego ideas#lego builds#lego moc#rosalind franklin#james watson#francis crick#university student
2K notes
·
View notes
Text
The Chemical Structure of Redstone
So I was curious about what the chemical structure of Redstone looks like, and Minecraft Education Edition, albeit unintentionally, gives us a canon look into what Redstone is made of:

In Minecraft Education Edition, putting a Redstone Block into a Material Reducer shows that it's composed of 31 Carbon, 31 Uranium, and 38 Unobtanium, which we can assume to be measured in grams
Dividing the Redstone Block into Redstone Dust, each Redstone Dust is then composed of approximately 3.4 Carbon, 3.4 Uranium, and 4.2 Unobtanium
Again assuming that's measured in grams, that's 0.17 cm³ of Uranium, 1.496 cm³ of Carbon, and ???³ of Unobtanium per Redstone Dust
So what does this tell us about the chemical structure of Redstone? Basing this on Redstone Dust's composition, we can estimate that each Redstone molecule is composed of 3 Carbon atoms, 3 Uranium atoms, 4 Unobtanium atoms, a little under half of the time it binds to an extra Uranium and/or Carbon, and 20% of the time it binds to an extra Unobtanium
This also has some horrifying implications for how Redstone works:
Redstone would be extremely volatile as the radioactive decay from Unobtanium and Uranium would occasionally release Helium ions through alpha radiation, sometimes breaking apart Carbon into two Beryllium atoms (as it absorbs the extra proton and neutron from the Uranium) or merging into Oxygen
So Redstone should, in theory, be extremely flammable and potentially explosive, which implies that cave static, or the player mining Redstone with an Iron Pickaxe, could lead to a spark that causes an explosive cave-in
As Unobtanium is just a placeholder for unobtainable elements (hence the name), I'm going to estimate Unobtanium in this case as Unbinilium, the placeholder name for element 120
Why?
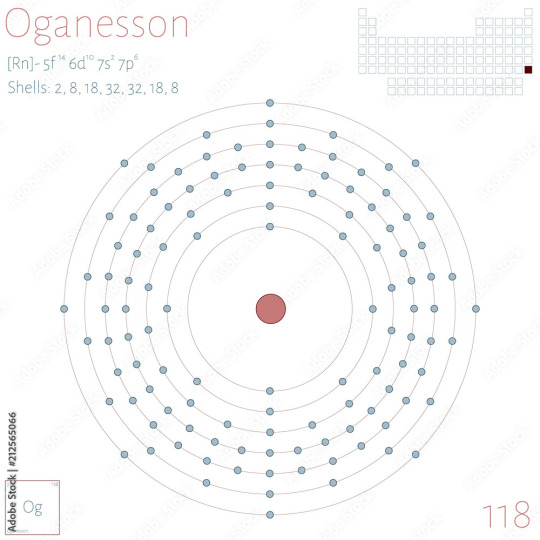
I'm estimating the Unobtanium as Redstone as being larger than the largest man-made element, Oganesson, which holds an impressive 118 protons
Each valence electron shell, from innermost to outermost, can bind with 2, 8, 18, 32, 32, 18, and 8 shells respectively, so I'd like Unobtanium to be an element we haven't discovered yet, and consequently I'd like to jump up to the next shell
While I could estimate with element 119's placeholder, Ununennium, it would have one electron in the next shell, so Unbinilium allows for easier chemical binding
So what does this molecule look like then? Well, horrifyingly...
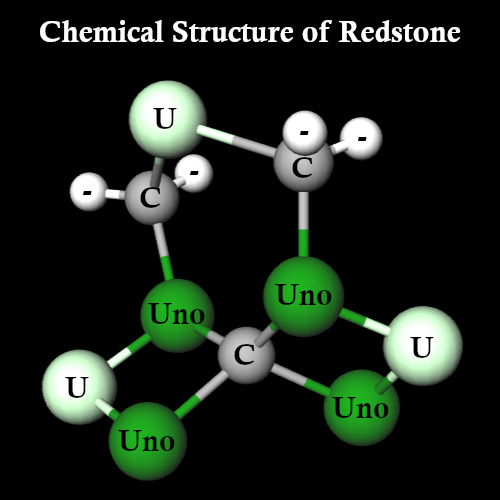
It looks like this. As Redstone forms in crystal lattices, and only two Carbon atoms are free to bind, I can absolutely see why it's so brittle that it breaks into powder.
This makes the structure of Redstone:
C3U3Uno4 (55% of molecules) C4U3Uno4 (13% of molecules) C3U4Uno4 (13% of molecules) C4U4Uno4 (7% of molecules) C3U3Uno5 (5% of molecules) C4U3Uno5 (3% of molecules) C3U4Uno5 (3% of molecules) C4U4Uno5 (1% of molecules)
An extremely radioactive, flammable, and explosive compound.
#redstone#minecraft#chemistry#physics#education#i would love if someone could submit what a 2D representation of these molecules would look like#i don't actually know how to make them
4K notes
·
View notes
Text










You're a maverick.
#sexeducationedit#aimee x isaac#aimee gibbs#isaac goodwin#sex education#mine#DUSTING OFF GOOD OL PHOTOSHOP FOR THEM IT'S THAT SERIOUS#their chemistry goes crazy
1K notes
·
View notes
Text

Magnesium creates a bright white flame when burned, so it's used in pyrotechnics, signal flares, and firestarters. It's also required for many enzymes to function, so eat your leafy greens (or cocoa)!
123 notes
·
View notes
Text

Part 3 of Louis getting precedingly more cursed somehow, S32 E3 concept art edition
#identity v#identity v fanart#matthias czernin#norton campbell#idv fool's gold#my art#Ofc this has to be the first thing I doodle when my wrist pain goes away hhahawaa#I have to question whether Matt was actually trying to create a pure life form cause this is more like a living kitchen appliance prototype#..or the superhero mascot for a 5th grade chemistry education program
163 notes
·
View notes
Text



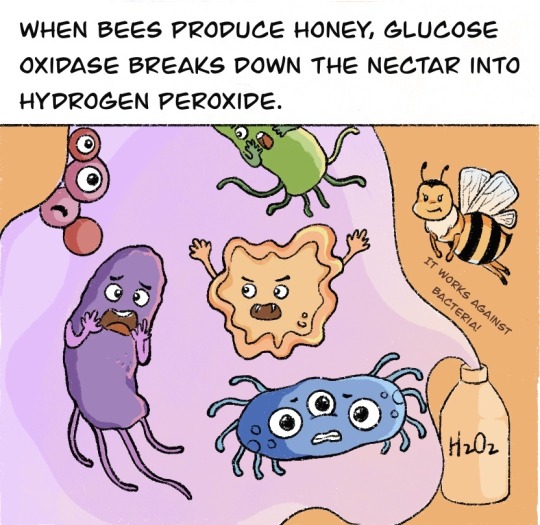



Do you know that a 3000-year-old honey was still edible? Does honey ever spoil?
#sciart#cute illustration#studyblr#sciencecommunication#biochemistry#stem education#chemistry#science#chemistry in real life#biology#food science#comics#cute comics
809 notes
·
View notes
Text

I'll Become a Chemist! (1964)
83 notes
·
View notes
Text

Hello all! I took a break from the Internet but I am back! Little update: I am now in year two of my degree, and I started organic chemistry! Very excited for this year's journey:) 🧪
#chemistry#college#science#stem academia#study aesthetic#studyblr#studyspo#biology#study blog#women in stem#education#rambles#tumblrpost#thank you#women in science#light academia#study notes#school#another day another slay#laboratory#study blr#study motivation
429 notes
·
View notes
Text

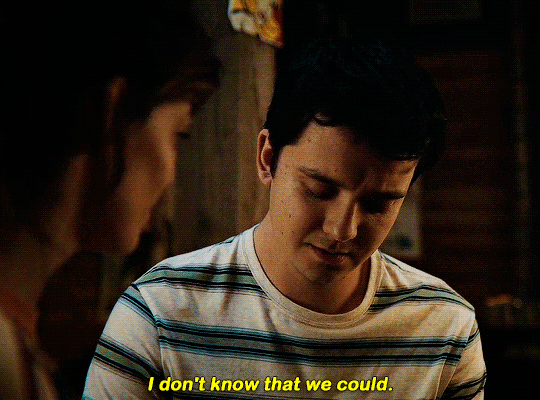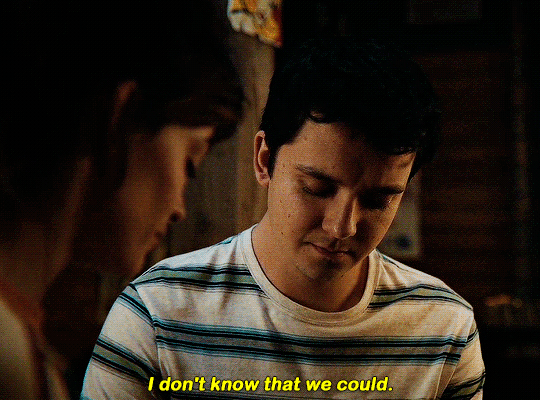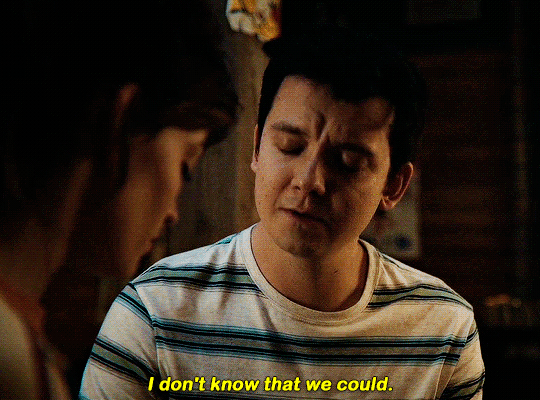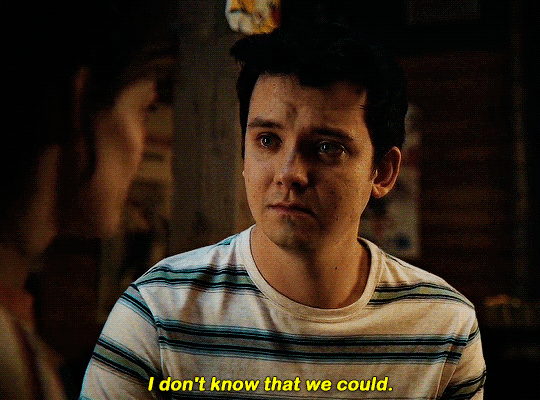

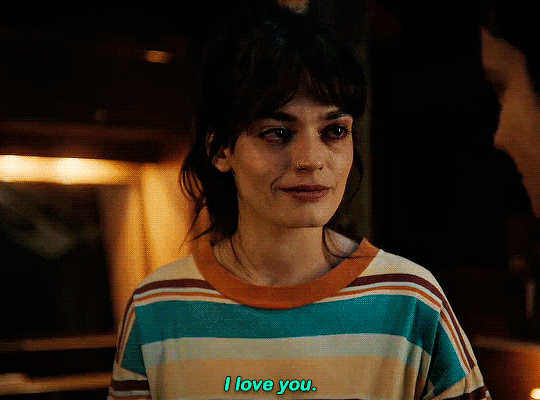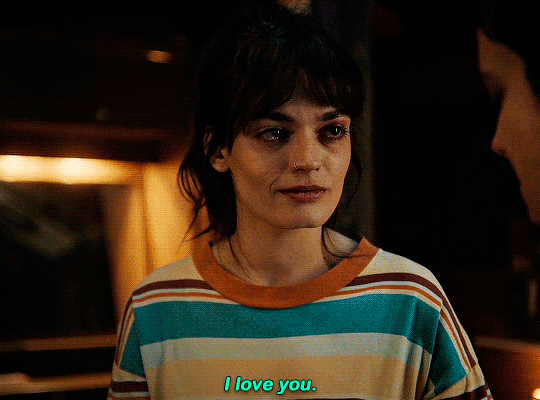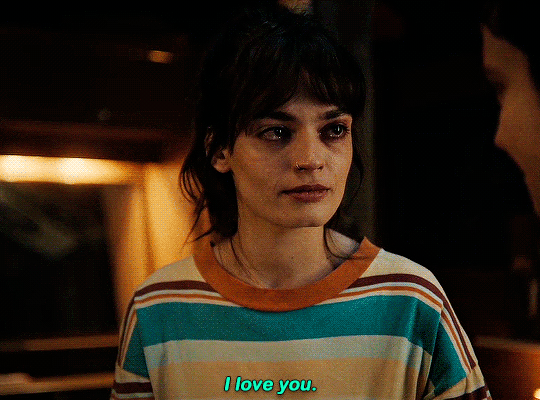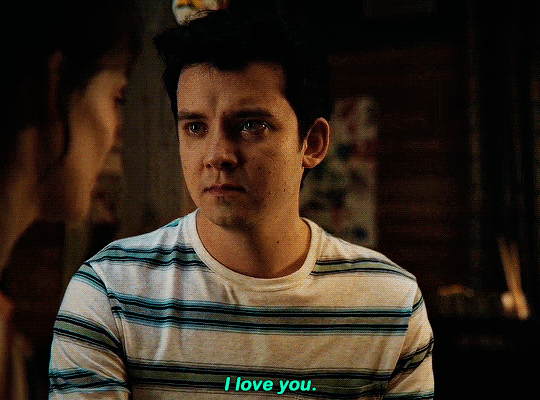
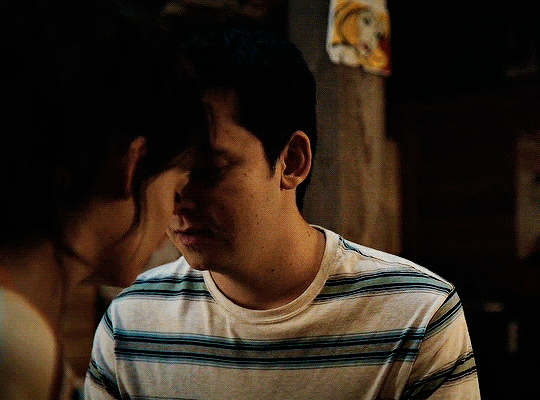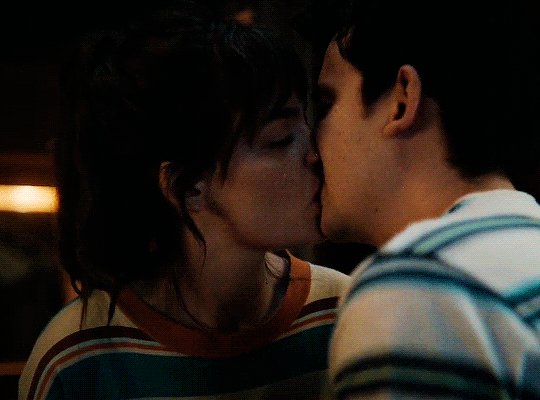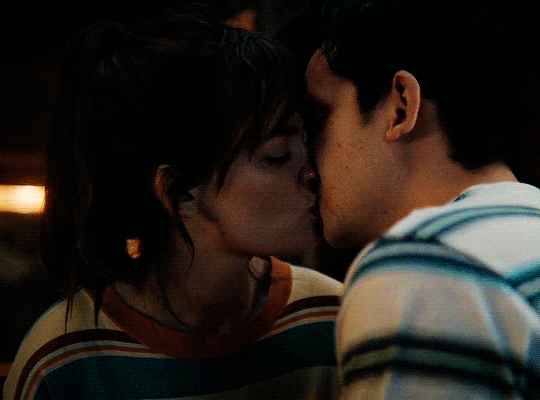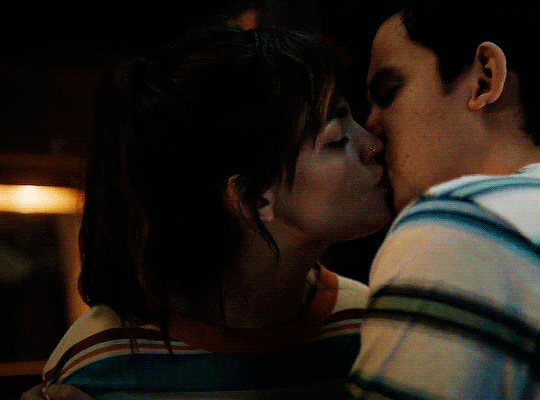
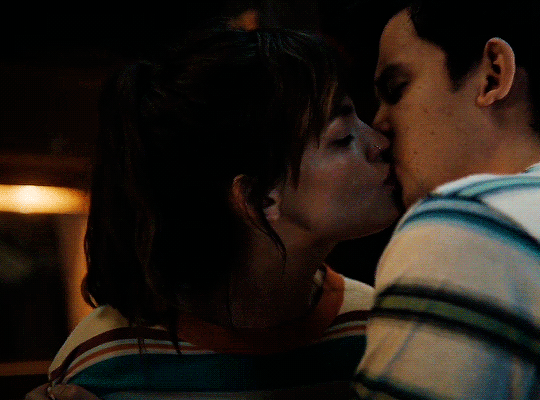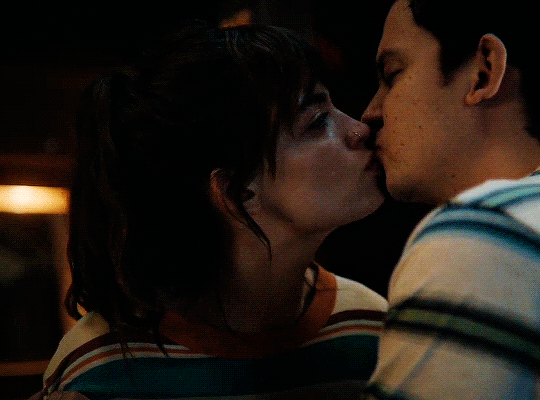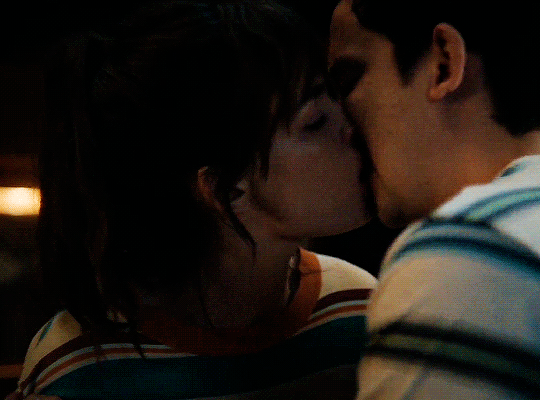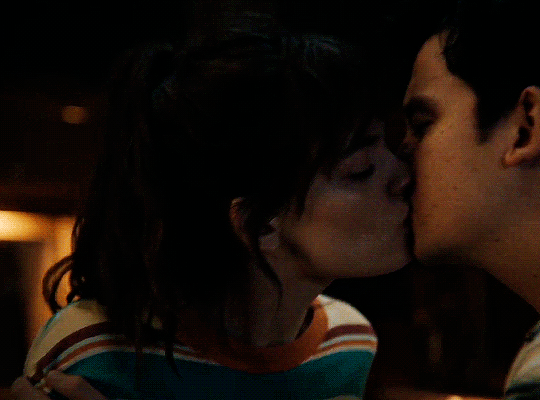












#could have been endgame#still soulmates idc#sex education#i love you#kiss#kisses#kissing#sexeducationedit#maeve x otis#otis x maeve#otis milburn#maeve wiley#emma mackey#asa butterfield#their chemistry though#gif#gifs#gifset#4x7#motis
407 notes
·
View notes
Text


monday/tuesday week six
energy levels can fluctuate and productivity along with it, and that is okay…
but it is annoying! especially when you’re trying to get used to the increased pace of uni courses, which is the boat i am currently in.
i tend to struggle with change and i am only just over two weeks in to my new course at a new campus with new lecturers and classmates, and with a new way of studying. thus, it makes sense that i am tired but is is slightly jarring to go from not ever really having struggled with understanding things for school to feeling a bit lost when the professor writes and talks fast and covers so much content that i don’t even have time to think about it, just try to get it written in my notes so that i can refer back to them later.
i do like to be challenged and this is definitely an experience that i will have great use of but right now, i am a bit too tired to appreciate that.
anyway, i hope you are not too stressed and remember to take care of yourself. rest is productive!
have a lovely day!
#study motivation#studyblr#uni#university#study aesthetic#study#study blog#studyspo#studying#study inspiration#stem academia#stem student#stemblr#stem#women in stem#stem aesthetic#stemeducation#stem studyblr#chemistry#science#school#education#self care
49 notes
·
View notes
Text
School chemistry vs. Real chemistry
Recently I’ve become interested in the concept of “microscale” chemistry and the philosophy of its approach to practical work in schools. It’s had a lot of publicity in the relevant bits of social media over the last years, and the reaction to it seems to have been largely positive. There are books dedicated to it, and training courses.
I don’t think it’s the right approach. I don’t think it’s enough for students simply to “do practicals” - there has to be a deeper philosophy to it, or we might as well stick to demonstrations and videos. The approach has led to great ideas (I wrote about one here), but overall I’m sceptical. The detailed critique will have to wait, however, because…
Working as a technician after years as a researcher in industry has convinced me that there is a difference between those who do chemistry and those who teach it, and that we need to bridge that gap. A line from the first article I linked to illustrates this:
“One pioneer of Microscale Chemistry in the UK, Stephen Breuer said, why make 5g of an organic chemical, use 0.1g for spectra and experiments and throw 4.9g away?”
Well. As an organic chemist I never tired of simply making compounds. The satisfaction of seeing that white powder in your flask never dimmed, and it was even more heart-warming if you could take the brown sludge from your first stage and purify it to a crystalline solid. Every synthetic chemist knows that feeling.
If we want some of our students to go on to be professional chemists then the joy of simply making stuff will draw them in. It doesn’t have to be organic - making copper sulfate is always popular, and suitable for students under 16. Growing crystals is a great activity for science clubs (I present the alum and chrome alum crystals I made for, ahem, research purposes). But you have to be able to see it, to have enough to scrape into a vial and weigh, and stick a label on it. Making a few mg, a thin layer on a filter paper, for analysis just won’t cut it. Doing chemistry on a larger scale also makes it easier for inexperienced chemists.

I can’t remember what drew me to chemistry in the first place. I ended up wanting to do it for a living because I found designing and making compounds interesting, challenging and fun. With properly designed practical work we can give today's school students a taste of that. We just have to make sure that teachers and those who write the syllabuses get it too.
P.S. This isn’t the only example of a disconnect between school chemistry and chemistry as she is practised in the real world. More examples to follow if I get the chance.
0 notes
Text
Do you wanna build a Lego science set? Here's Lego DNA!
With a scientifically accurate DNA model, and a historically accurate lab + 5 scientists!
Aims: to promote science to kids and young adults and honor Rosalind Franklin and her legacy!
3,800 votes needed (we already have 6,200!) to get it considered as a real official Lego set to be sold worldwide!
If you like it, please support via the link above or here: https://ideas.lego.com/projects/c92cd95b-49e7-46ec-b844-ac6482c51139
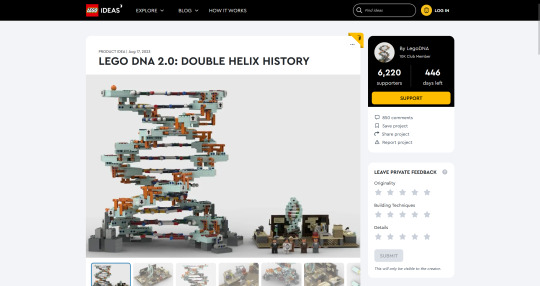

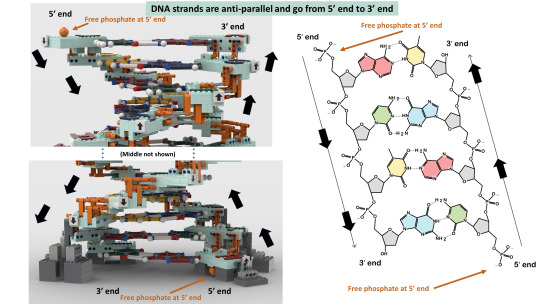



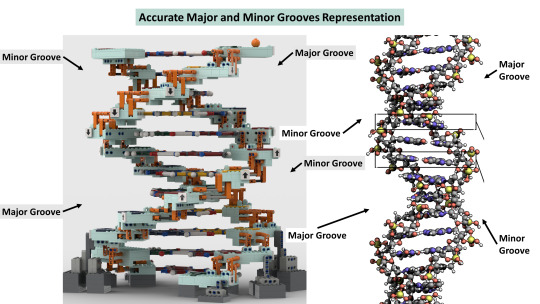



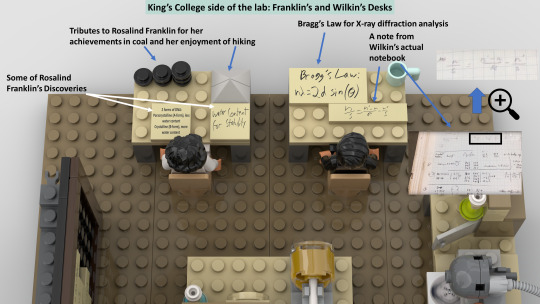



#chemistry#science#biology#design#diy#education#nature#environmental science#molecular biology#stem#women in stem#research#laboratory#women scientists#school#university#college#students#learning#lego art#lego photography#afol#lego sets#lego ideas#lego builds#lego moc#rosalind franklin#james watson#francis crick#university student
751 notes
·
View notes
Text

14 November 2024
Nuclear chem labs are usually really boring - to be safe, these samples can't be wildly radioactive, so measurements take ages - but this one was fun. Lab partner and I put a big piece of granite under the probe and the radiometer started clicking like crazy! We actually had to readjust the measuring range, it was so fun to see. Granite can be naturally radioactive! Then we measured spent nuclear fuel and the radiometer went almost dead silent (save for the background radiation of course) which was also cool. Spent fuel indeed!
#this is like#neither studyblr ish nor particularly educational but i still wanted to share :3#mine#op#studyblr#chemblr#chemistry#stemblr
60 notes
·
View notes
Text
New NileRed video “Turning paint thinner into cherry soda” highlights:




#if you haven’t watched his content before- no that is not clickbait#poison#chemistry#educational#sillies#videos#nilered
502 notes
·
View notes
Text

Xenon is rare and expensive to extract, but it's widely used in obnoxiously bright car headlights, camera flashes, arc lamps, and lasers. There are nine naturally-occurring isotopes of xenon, seven of which are stable and two of which have half-lives longer than any other element's isotope.
33 notes
·
View notes