#coronavirus vaccine research post-SARS
Explore tagged Tumblr posts
Text
Quotes from Coronavirus Vaccine Designers and Researchers since SARS-COV1
Coronavirus Vaccine History Back in 2004, SARS vaccine trial spotlights continued peril by Helen Pearson was published in the science press. But public-health experts remain concerned that a second wave of infections could erupt, either from human contact with infected animals or by the virus escaping from laboratory samples.Pearson, Helen SARS vaccine trial spotlights continued peril. Nature…
View On WordPress
#animal testing#caution#coronavirus vaccine discovery#coronavirus vaccine exploration#coronavirus vaccine investigations#coronavirus vaccine progress#coronavirus vaccine research#coronavirus vaccine research post-SARS#findings from research#following SARS-COV1#human trials#immunity#Reasons for starting vaccine research#SARS aftermath#SARS epidemic#SARS experience#SARS legacy#transparency#vaccine development#vaccine development trends#vaccine studies#warnings
0 notes
Text
COVID-19's long-term effects on the body: an incomplete list
COVID’s effect on the immune system, specifically on lymphocytes:
NYT article from 2020 (Studies cited: https://www.biorxiv.org/content/10.1101/2020.05.18.101717v1, https://www.biorxiv.org/content/10.1101/2020.05.20.106401v1, https://www.unboundmedicine.com/medline/citation/32405080/Decreased_T_cell_populations_contribute_to_the_increased_severity_of_COVID_19_, https://www.medrxiv.org/content/10.1101/2020.06.08.20125112v1)
https://www.biorxiv.org/content/10.1101/2022.01.10.475725v1
https://www.science.org/doi/10.1126/science.abc8511 (Published in Science)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9057012/
https://www.forbes.com/sites/williamhaseltine/2022/04/14/sars-cov-2-actively-infects-and-kills-lymphoid-cells/
https://www.cleveland.com/news/2022/10/in-cleveland-and-beyond-researchers-begin-to-unravel-the-mystery-of-long-covid-19.html
SARS-CoV-2 infection weakens immune-cell response to vaccination: NIH-funded study suggests need to boost CD8+ T cell response after infection
https://www.merckmanuals.com/professional/hematology-and-oncology/leukopenias/lymphocytopenia
https://thetyee.ca/Analysis/2022/11/07/COVID-Reinfections-And-Immunity/
Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection
https://www.frontiersin.org/articles/10.3389/fimmu.2022.1034159/full
https://www.n-tv.de/politik/Lauterbach-warnt-vor-unheilbarer-Immunschwaeche-durch-Corona-article23860527.html (German Minister of Health)
Anecdotal evidence of COVID’s effects on white blood cells:
https://twitter.com/DrJohnHhess/status/1661837956875956224
https://x.com/TristanVeness/status/1661565201345564673
https://twitter.com/TristanVeness/status/1689996298408312832
Much more if you speak to Long Covid patients directly!
Related information of interest:
China approves Genuine Biotech's HIV drug for COVID patients
COVID as a “mass disabling event” and impact on the economy:
https://www.ctvnews.ca/health/report-says-long-covid-could-impact-economy-and-be-mass-disabling-event-in-canada-1.6306608
https://x.com/inkblue01/status/1742183209809453456?s=20
COVID’s impact on the heart:
https://www.dailystar.co.uk/news/world-news/deadly-virus-could-lead-heart-31751263 (Research from: Japan's Riken research institute)
https://www.brisbanetimes.com.au/national/queensland/unlike-flu-covid-19-attacks-dna-in-the-heart-new-research-20220929-p5bm10.html
https://www.mdpi.com/2077-0383/12/1/186
https://medicalxpress.com/news/2023-04-mild-covid-effects-cardiovascular-health.html
https://publichealth.jhu.edu/2022/covid-and-the-heart-it-spares-no-one
https://www.bhf.org.uk/informationsupport/heart-matters-magazine/news/coronavirus-and-your-health/is-coronavirus-a-disease-of-the-blood-vessels (British Heart Foundation)
COVID’s effect on the brain and cognitive function:
https://www.openaccessgovernment.org/article/brain-infection-by-sars-cov-2-lifelong-consequences/171391/
https://www.cidrap.umn.edu/covid-19/study-shows-covid-leaves-brain-injury-markers-blood
https://www.theguardian.com/world/2020/jul/08/warning-of-serious-brain-disorders-in-people-with-mild-covid-symptoms
Cognitive post-acute sequelae of SARS-CoV-2 (PASC) can occur after mild COVID-19
Neurologic Effects of SARS-CoV-2 Transmitted among Dogs
https://journals.lww.com/nsan/fulltext/2022/39030/neurological_manifestations_and_mortality_in.4.aspx
https://www.salon.com/2023/06/17/new-evidence-suggests-alters-the-brain--but-the-extent-of-changes-is-unclear/
https://www.scientificamerican.com/article/covid-virus-may-tunnel-through-nanotubes-from-nose-to-brain/
https://neurosciencenews.com/post-covid-brain-21904/
https://www.thelancet.com/journals/lanpsy/article/PIIS2215-0366(22)00260-7/fulltext
https://medicalxpress.com/news/2022-08-covid-infection-crucial-brain-regions.html
https://news.ecu.edu/2022/08/04/covid-parkinsons-link/
Covid as a vascular/blood vessel disease:
https://www.salon.com/2020/06/01/coronavirus-is-a-blood-vessel-disease-study-says-and-its-mysteries-finally-make-sense/
https://www.salon.com/2023/12/27/brain-damage-caused-by-19-may-not-show-up-on-routine-tests-study-finds/
https://www.nih.gov/news-events/news-releases/sars-cov-2-infects-coronary-arteries-increases-plaque-inflammation
https://www.mdpi.com/2077-0383/12/6/2123
https://www.sciencedaily.com/releases/2021/10/211004104134.htm (microclots)
Long Covid:
Post-COVID-19 Condition in Canada: What we know, what we don’t know, and a framework for action
https://www.ctvnews.ca/health/coronavirus/more-than-two-years-of-long-covid-research-hasn-t-yielded-many-answers-scientific-review-1.6235227
https://www.cbc.ca/news/canada/london/cause-of-long-covid-symptoms-revealed-by-lung-imaging-research-at-western-university-1.6504318
https://www.cbc.ca/news/canada/montreal/long-covid-study-montreal-1.6521131
https://news.yale.edu/2023/12/19/study-helps-explain-post-covid-exercise-intolerance
Other:
- Viruses and mutation: https://typingmonkeys.substack.com/p/monkeys-on-typewriters
Measures taken by the rich and world leaders
Heightened risk of diabetes
https://jamanetwork.com/journals/jama/fullarticle/2805461
https://www.nature.com/articles/d41586-022-00912-y
Liver damage:
https://timesofindia.indiatimes.com/city/mumbai/46-of-covid-patients-have-liver-damage-study/articleshow/97809200.cms?from=mdr
tl;dr: covid is a vascular disease, not a respiratory illness. it can affect your blood and every organ in your body. every time you're reinfected, your chances of getting long covid increase.
avoid being infected. reduce the amount of viral load you're exposed to.
the gap between what the scientific community knows and ordinary people know is massive. collective action is needed.
#putting this somewhere at least as reference for... somebody hopefully#covid#disability#y'all. it is bleak out there but some very good people are doing their best to help#we need as many people aware and helping as possible
464 notes
·
View notes
Text
Deaths Are Up Post-Covid, and So Are Funeral Stocks: Prognosis - Published Aug 19, 2024
The Business of Death Aussies, Americans, and Brits — and no doubt people in many other nations — are dying faster than before the pandemic.
Even though Covid waves are becoming less deadly, thanks mostly to increased immune protection from vaccinations and prior infections, the coronavirus remains a significant killer. And stubbornly high all-cause mortality rates indicate that its direct and indirect effects are helping drive a sustained increase in death and disease around the globe.
It’s depressing news, I know.
With death comes bereavement, and there’s been a lot of that since SARS-CoV-2 began spreading widely in late 2019. The number of officially reported Covid fatalities (7.1 million worldwide) doesn’t fully explain the trend in excess deaths. (Neither do Covid vaccines, since body bags were piling up months before the shots were released, and multiple studies show the immunizations protect against severe illness and death).
There’s no silver lining to the tragic loss of life. But if one group sees an upside, it’s those providing funerals, cremations, and burials. Publicly traded companies handling funerals and related services have handed investors an average 79% return since Jan. 1, 2020 — outpacing the 60% gain in the MSCI All Country World Index, one of the broadest measures of the global equity market.
The US highlights the morbid picture. In the two decades before the pandemic, the number of deaths had been climbing at an average clip of almost 1% a year — reflecting population growth and aging, and the devastating opioid epidemic — for a crude rate in 2019 of 869.7 deaths for every 100,000 Americans.
Covid catapulted the rate well beyond 1,000 in 2020 and 2021 before the rate dropped back to just over 984 in 2022. Last year, there were 927.4 deaths per 100,000 people in the US — almost 12% above the 20-year average — for nearly 3.1 million deaths all up.
The coronavirus directly and indirectly contributed to many of them. For instance, a jump in drug overdoses and alcohol use–related diseases during the pandemic likely added to fatalities from unintentional injuries and chronic liver disease in 2023, according to a study this month. Covid also led to more cardiometabolic disease, and age-adjusted mortality rates for diabetes, heart disease, and stroke were above pre-pandemic levels.
Last month, researchers reported similar findings in Australia, where emergency departments have taken longer to hospitalize patients arriving in ambulances — a sign of health-system stress associated with a greater risk of patients dying up to 30 days after their initial medical encounter.
Mortality rates in England have also stayed persistently high since Covid hit, likely reflecting the direct effects of the illness, pressures on the National Health Service, and disruptions to chronic disease detection and management, researchers said in a study in January.
“The greatest numbers of excess deaths in the acute phase of the pandemic were in older adults,” Jonny Pearson-Stuttard and colleagues wrote. “The pattern now is one of persisting excess deaths, which are most prominent in relative terms in middle-aged and younger adults.”
Almost five years into the pandemic, dodging SARS-CoV-2 still remains one of the best ways to avoid adding to the toll — and the frequency of funerals. —Jason Gale
#covid#mask up#pandemic#covid 19#wear a mask#coronavirus#sars cov 2#still coviding#public health#wear a respirator
33 notes
·
View notes
Text
long post containing a lockdown infodump so LOCK IN. there will be stim gifs!
tl;drs will be included

so i’ve been doing a lot of research on covid, especially on why we went into lockdown in the first place?
covid was so unknown at the time, having ONE viral relative: SARS (the epidemic in 2003/04, no cases since). so i researched SARS. (scroll

checkpoint one: SARS
tl;dr, SARS didnt burn itself out, it was still contained due to human intervention, but it had very little asymptomatic cases and was not known to spread until symptom onset.
tl;dr two, covid was most transmissible before its symptom onset, and had LOTS of transmittable asymptomatic cases.
SARS-CoV-1 is an abbreviation for severe acute respiratory syndrome, coronavirus one. it caused a global epidemic in 2003-04, and a case hasnt been reported since then. covid is SARS-CoV-2. the two were about 80% similar, but those differences are key in covids boom.
im not gonna go in-detail about the specific mutations that cause these things (but i do know them, i think u guys might get bored) but covid had a much higher asymptomatic case rate (as high as 40%, estimated by wastewater) and still remained contagious. SARS on the otherhand had very little asymptomatic cases, and it did not remain contagious.

this is really important to consider, as 50-70% of SARS victims needed oxygen supplementation, and 20-30% were in the ICU. 13% of cases died. this is a lot compared to the 15-20% of hospitalizations due to covid, and 3-5% needing critical care.
quarantining and isolation was a lot easier when the virus wasnt spread until symptom onset, and most of the time caused severe enough illness to warrant hospitalization.
there was no cure or vaccine for SARS, you just had to wait it out and treat its symptoms.

checkpoint two: COVID
tl;dr, covid was a more severe illness with an extremely contagious nature. nobody knew what to do, and the american leadership added more strain due to the fact that trump tried to downplay the virus.
now that we know that covid was very unknown at the time, its parent virus had no cure and no vaccine, and covid bumped the transmission into gear, we can actually understand why lockdowns happened.
covid wasn’t mild like the flu or the common cold, but was still extremely contagious. shelter-in-place orders were placed so that the virus didn’t spread as quickly and mutate to become either more contagious, more deadly, or both, as more cases means more mutations.

i live in the united states, so im going to focus a little bit on that. right-wing ideology had gotten much more severe since 2003, and former president donald trump is, well, an idiot. he made false claims about the virus and his administration was focused on downplaying the situation rather than ramping up on medical supplies and telling the people what to do.
the election year had a lot to do with the pandemic, especially with america as a large world leader, and most right-wingers would die for their beloved trump. they refused to listen to anyone on the left-leaning.
we went into lockdown due to global unpreparedness, world leader unpreparedness, and general lack of knowledge.

checkpoint three: what would another lockdown need?
another lockdown would still need relevant political interference, which, hooray! is still happening in the united states. if you research the social aspects of any new diseases, right-wing folk tend to say they’re not falling for a “ploy to get biden back in office”. this includes not wearing masks, not quarantining, not getting vaccines, etc.
for a known virus to cause a pandemic, it would need to mutate so fast that it becomes extremely different from its parent. it would need to transmit human-to-human, have many asymptomatic cases, and still manage to cause severe infection in previously healthy people.
i’m not really counting on monkeypox 1b to cause a pandemic, but idk! things always happen :3 i am however counting on bird flu, as it clearly has less of a watchful eye over it, has never transmitted h2h before, and causes severe illness.
like and subscribe
#pro rq 🌈🍓#radqueer#rq community#rq please interact#rq safe#rq 🌈🍓#rqc🌈🍓#permalockdown#translockdown#trans2020#perma2020#⌒🍰 IMPORTANT
5 notes
·
View notes
Text
By Lambert Strether of Corrente.
On May 25 of this year, JAMA published Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection (“Definition”), an “original investigation” whose authors were drawn from the RECOVER Consortium, an initiative of the National Institutes of Health (NIH)[1]. This was an initially welcome development for Long Covid sufferers and activists, since questions had arisen about what exactly patients were getting for the billion dollars RECOVER was appropriated. From STAT:
The federal government has burned through more than $1 billion to study long Covid, an effort to help the millions of Americans who experience brain fog, fatigue, and other symptoms after recovering from a coronavirus infection.
There’s basically nothing to show for it.
The National Institutes of Health hasn’t signed up a single patient to test any potential treatments — despite a clear mandate from Congress to study them.
Instead, the NIH spent the majority of its money on broader, observational research that won’t directly bring relief to patients. But it still hasn’t published any findings from the patients who joined that study, almost two years after it started.
(The STAT article, NC hot take here on April 20, is worth reading in full.) Perhaps unfairly to NIH — one is tempted to say that the mountain has labored, and brought forth a coprolite — a CERN-level headcount may explain both RECOVER’s glacial pace, and its high cost:

That’s a lot of violin lessons for a lot of little Madisons!
“Definition” falls resoundingly into the research (and not treatment) bucket. In this post, I will first look at the public relations debacle (if debacle it was) that immediately followed its release; then I will look at its problematic methodology, and briefly conclude. (Please note that I feel qualified to speak on public relations and institutional issues; very much less so on research methodology, which actually involves (dread word) statistics. So I hope readers will bear with me and correct where necessary.)
The Public Relations Debacle
Our famously free press instantly framed “Definition” as a checklist of Long Covid (LC) symptoms. Here are the headlines. For the common reader:
12 key symptoms define long Covid, new study shows, bringing treatments closer CNN Long COVID is defined by these 12 symptoms, new study finds CBS Scientists Identify 12 Major Symptoms of Long Covid Smithsonian These 12 symptoms may define long COVID, new study finds PBS News Hour These Are the 12 Major Symptoms of Long COVID Daily Beast
(We will get to the actual so-called “12[2] Symptoms” when we look at methodology.) And for readers in the health industry:
For the first time, researchers identify 12 symptoms of long covid Chief Healthcare Executive 12 symptoms of long COVID, FDA Paxlovid approval & mpox vaccines with Andrea Garcia, JD, MPH AMA Update Finally! These 12 symptoms define long COVID, say researchers ALM Benefits Pro
With these last three, we can easily see the CEO handing a copy of their “12 symptoms” article to a doctor, the doctor double-checking that headline against the AMA Update’s headline, and incorporating the NIH-branded 12-point checklist into their case notes going forward, and the medical coders at the insurance company (I love that word, “benefits”) nodding approvingly. At last, the clinicians have a checklist! They know what to do!
We’ll see why the whole notion of a checklist with twelve items is wrong and off-point for what “Definition” was actually, or at least putatively, trying to do, but for now it’s easy to see why the press went down this path (or over this cliff). Here is the press release from NIH that accompanied “Definition”‘s publication in JAMA:
Researchers examined data from 9,764 adults, including 8,646 who had COVID-19 and 1,118 who did not have COVID-19. They assessed more than 30 symptoms across multiple body areas and organs and applied statistical analyses that identified 12 symptoms that most set apart those with and without long COVID: post-exertional malaise, fatigue, brain fog, dizziness, gastrointestinal symptoms, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic cough, chest pain, and abnormal movements.
They then established a scoring system based on patient-reported symptoms. By assigning points to each of the 12 symptoms, the team gave each patient a score based on symptom combinations. With these scores in hand, researchers identified a meaningful threshold for identifying participants with long COVID. They also found that certain symptoms occurred together and defined four subgroups or “clusters” with a range of impacts on health
So there are 12 symptoms, right? Just like the headline says? Certainly, that’s what a normal reader would take away. And if a temporally pressed reporter goes to the JAMA original and searches on “12”, they find this:
Using the full cohort, LASSO identified 12 symptoms with corresponding scores ranging from 1 to 8 (Table 2). The optimal PASC score threshold used was 12 or greater
And if the reporter goes further and finds Table 2 (we’ll get there when we look at methodology), they will see, yes, 12 symptoms (in rank order identified by something called LASSO).
So it’s easy to see how the headlines were written as they were written, and how the newsroom wrote the stories as they did. The wee problem: The twelve symptoms are not meant to be used clinically, for diagnosis.[3], Lisa McCorkell was the patient representative[4] for the paper, and has this to say:


Nevertheless, the “12 symptoms” are out of the barn and in the next county, and as a result, you get search results like this:
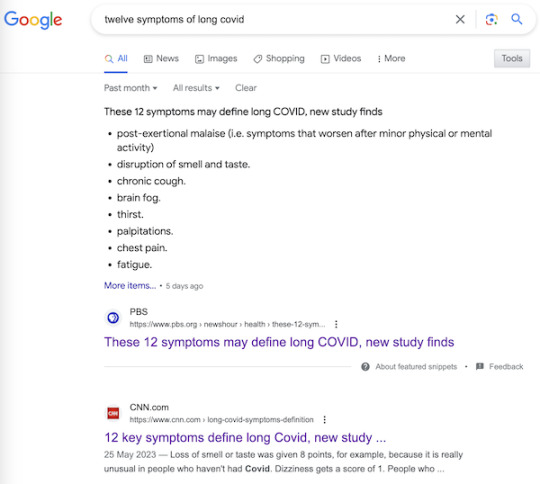
It’s very easy to imagine a harried ER room nurse hearing “12 Symptoms” on the TV news[5], doublechecking with a Google search, and then making clinical decisions based on a checklist not fit for purpose. Or, for that matter, a doctor.
Now, to be fair to the authors, once one grasps the idea that symptoms, even clusters of symptoms, can exist, and still not be suitable for diagnosis by a clinician, the careful language of “Definition” is clear, starting with the title: “Development of a Definition.” And in the Meaning section of the Abstract:
A framework for identifying PASC cases based on symptoms is a first step to defining PASC as a new condition. These findings require iterative refinement that further incorporates clinical features to arrive at actionable definitions of PASC.
Well and good, but do you see “framework” in the headlines? “Iterative”? “First step”? No? Now, I’d like to exonerate the authors of “Definitions” — “They’re just scientists!” — for that debacle, but I cannot, completely. The authors are well-compensated, sophisticated, and aware professionals; PMC, in fact. I cannot believe that the Cochrane “fools gold” antimask study debacle went unobserved at NIH, especially in the press office. How was it possible that “Definitions” was simply… printed as it was, and no strategic consideration given to shaping the likely coverage?[6] One obvious precautionary measure would have been a preprint, but for reasons unknown to me, NIH did not do that. A second obvious precautionary measure would have been to have the patient representative approve the press release. Ditto. Now let us turn to methodology.
The Problematic Methodology
First, I will look at issues with Table 2, which presents the key twelve-point checklist, and names the algorithm (although without explaining it). After that, I will branch out to a few larger issues. Again I issue a caveat that I’m not a Long Covid maven or a statistics maven, and I hope readers will correct and clarify where needed.
Here is Table 2:
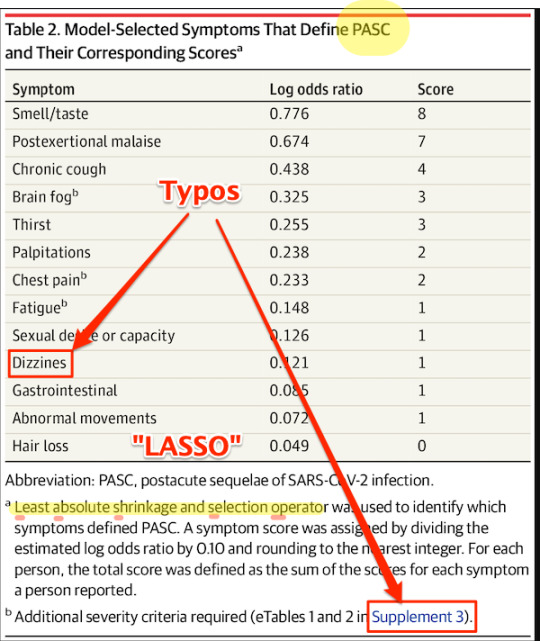
First, some copy editing trifles (highlighted). On “PASC”: As WebMD says: “You might know this as ‘long COVID.’ Experts have coined a new term for it: post-acute sequelae SARS-CoV-2 infection (PASC).” Those lovable scamps, always inventing impenetrable jargon! (Bourdieu would chuckle at this.) On “Dizzines”: Come on. A serious journal doesn’t let a typo like that slip through (maybe they’re accustomed to fixing the preprints?). On “Supplement 3”: The text is highlighted as a link, but clicking it brings up the image, and doesn’t take you to the Supplement. These small errors are important[7], because they indicate that no editor took more than a cursory look at the most important table in the paper. On “LASSO,” hold that thought.
Second, the Covid Action Network points out that some obvious, and serious, symptoms are missing from the list:
[T]he next attempts at diagnostic criteria should take into account existing literature that shows more specifically defined symptoms for Long Covid, from objective findings. (E.g. PoTS, Vestibular issues, migraine, vs more vague symptoms like “headache” or “dizziness.) [The Long Covid Action Project (LCAP)] noticed that while [Post-Extertional Malaise (PEM)] was used as a specific symptom with a high score to produce PASC-positive results, other suites of symptoms, like those in the neurologic category, could have produced an equal or higher score than PEM if questionnaires had not separated neuro-symptoms into multiple subtypes and reduced their total scores. This alone could have created a more scientifically accurate picture of the Long Covid population.
Third, these symptoms — missing, from the patient perspective; to be iterated from the researcher’s perspective, at least one would hope — are the result of “Definition”‘s methodology:


Fourth, I would argue focus on the “most clearly provable effects” — as opposed to organ damage — is a result of the “LASSO” algorithm named in Table 2. I did a good deal of searching on LASSO, and discovered that most of the examples I could find, even the “real world” ones, were examples of how to run LASSO programs, as opposed to selecting the LASSO algorithm as opposed to others. So that was discouraging. I believe — reinforcing the caveats, plural, given above — that I literally searched on “LASSO” “child of five” (“Explain it to me like I’m five”) to finally come up with this:
Lasso Regression is an essential variable selection technique for eliminating unnecessary variables from your model.
This method can be highly advantageous when some variables do not contribute any variance (predictability) to the model. Lasso Regression will automatically set their coefficients to zero in situations like this, excluding them from the analysis. For example, let’s say you have a skiing dataset and are building a model to see how fast someone goes down the mountain. This dataset has a variable referencing the user’s ability to make basketball shots. This obviously does not contribute any variance to the model – Lasso Regression will quickly identify this and eliminate these variables.
Since variables are being eliminated with Lasso Regression, the model becomes more interpretable and less complex.
Even more important than the model’s complexity is the shrinking of the subspace of your dataset. Since we eliminate these variables, our dataset shrinks in size (dimensionality). This is insanely advantageous for most machine learning models and has been shown to increase model accuracy in things like linear regression and least squares.
Since LC is said to have over 200 candidates for symptoms, you can see why a scientist trying to get their arms around the problem would be very happy to shrink those candidates to 12. But is that true to the disease?
Because LASSO (caveats, caveats) has one problem. From the same source:
One crucial aspect to consider is that Lasso Regression does not handle multicollinearity well. Multicollinearity occurs when two or more highly correlated predictor variables make it difficult to determine their individual contributions to the model.
Amplifying:
Lasso can be sensitive to multicollinearity, which is when two or more predictors are highly correlated. In this case, Lasso may select one of the correlated predictors and exclude the other [“set their coefficients to zero”], even if both are important for predicting the target variable.
As Ted Nelson wrote, “Everything is deeply intertwingled” (i.e., multicollinear), and if there’s one thing we know about LC, it’s that it’s a disease of the whole body taken as a system, and not of a single organ:
There are some who seek to downplay Long Covid by saying the list of 200 possible symptoms makes it impossible to accurately diagnose and that it could be encompassing illnesses people might have gone on to develop anyway, but there are sound biological reasons for this condition to affect the body in so many different ways.
Angiotensin-converting enzyme receptor 2 (ACE2) is the socket SARS-CoV-2 plugs into to infect human cells. The virus can use other mechanisms to enter cells=, but ACE2 is the most common method. ACE2 is widely expressed in the human body, with highest levels of expression in small intestine, testis, kidneys, heart, thyroid, and adipose (fat) tissue, but it is found almost everywhere, including the blood, spleen, bone marrow, brain, blood vessels, muscle, lungs, colon, liver, bladder, and adrenal gland
Given how common the ACE2 receptor is, it is unsurprising SARS-CoV-2 can cause a very wide range of symptoms.
In other words, multicollinearity everywhere. Not basketball players vs. skiiers at all.
So is LASSO even the right algorithm to handle entwinglement, like ACE2 receptors in every organ? Are there statistics mavens in the readership who can clarify? With that, I will leave the shaky ground of statistics and Table II, and raise two other issues.
First, it’s clear that the population selected for “Definitions” is unrepresentative of the LC population as a whole:
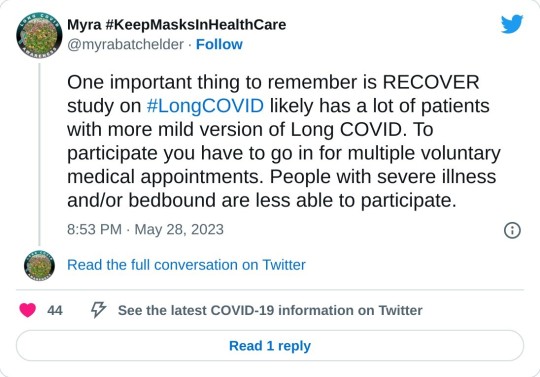
If the patients in “Definition” are not so ill, that might also account for Table 2’s missing symptoms.
Second, “Definition”‘s questionnaires should include measures of severity, and don’t:

Conclusion
The Long Covid Action Project (materials here) is running a letter writing campaign: “Request for NIH to Retract RECOVER Study Regarding 12 Symptom PASC Score For Long Covid.” As of this writing, “only 3,082 more until our goal of 25,600.” You might consider dropping them a line.
Back to the checklist for one moment. One way to look at the checklist is — we’re talking [drumroll] the PMC here — as a set of complex eligibility requirements, whose function is, as usual, gatekeeping and denial:
what they did is create basically a means test to figure out a dx but for smthg that is still not fully understood. it's premature and rly limited, & this will only further aid ppl already dismissive of lc — Wendi Muse (@MuseWendi) June 3, 2023
If you score 12, HappyVille! If you score 11, Pain City! And no consideration given to the actual organ damage in your body. And after the last three years following CDC, I find it really, really difficult to give NIH the benefit of the doubt. If one believed that NIH was acting in bad faith, one would see “Definition” as a way to keep the funding gravy train rolling, and the “12 Symptoms” headlines as having the immediate and happy outcome of denying care to the unfit. Stay safe out there, and let’s save some lives!
NOTES
[1] Oddly, the JAMA paper is not yet listed on RECOVER’s publications page.
[2] “12” is such a clickbait-worthy brainworm. “12 Days of Christmas,” “12 apostles,” “12 steps,” “12 months,” “12 signs of the zodiac,” etc. One might wonder where if the number had been “9” or “14” the uptake would have been so instant.
[3] To be fair to the sources, most of them mention this: Not CBS, Chief Health Care Executive, or the Daily Beast, but CNN in paragraph 51, Smithsonian (9), PBS (20), AMA Update (10), and Benefits Pro (17).
[4] There was only one patient representative for the paper:
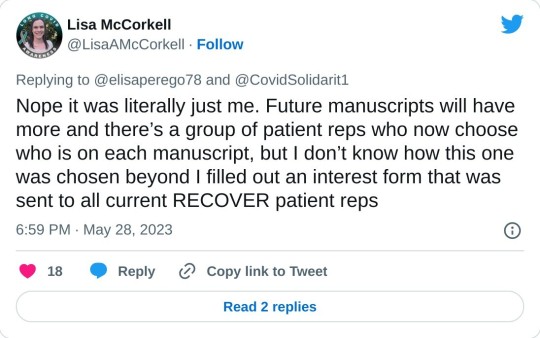
One seems low, especially given the headcount for the project.
[5] I was not able to find a nursing journal that covered the story.
[6] Unless it was, of course.
[7] Samuel Johnson: “When I take up the end of a web, and find it packthread, I do not expect, by looking further, to find embroidery.”
#long covid#naked capitalism#lambert strether#national institutes of health#covid pandemic#covid 19#long covid action project#long covid awareness day
5 notes
·
View notes
Text
Known COVID-19 Health Complications
Last Updated September 8, 2023
Repeat Infections
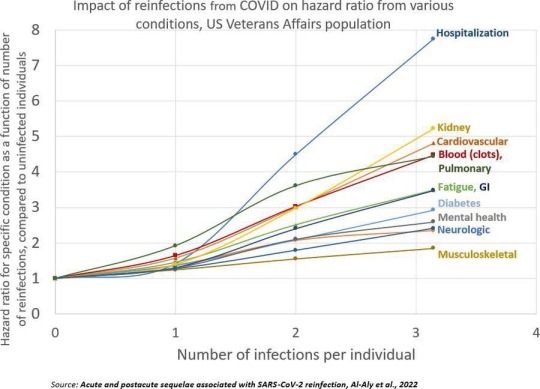
Summary: Repeat infections, even if mild during the acute phase, cause cumulative damage to the body and increase your risk of developing health complications or Long COVID. You should aim to limit the number of times you are infected as much as possible, even if you are not currently high risk (Note: Health complications post-COVID-19 infection can make you high risk) and have been vaccinated.
Published Research
Acute and postacute sequelae associated with SARS-CoV-2 reinfection | Nature Medicine Bowe, B., Xie, Y, & Al-Aly, Z. (2022).
Articles & Reports
Repeat COVID-19 infections increase risk of organ failure, death – Washington University School of Medicine in St. Louis (wustl.edu) Sauerwein, K. (2022).
Why Getting COVID-19 Multiple Times Is Risky For Your Health | Time Park, A. (2022).
Heart & Cardiovascular Damage
Summary: COVID-19 increases your risk of heart failure, heart attacks, strokes, pulmonary embolism, palpitations, arrhythmia, myocarditis, blood clots (thrombosis), etc. post-infection. Inflammation during the acute phase of a COVID-19 infection can damage the heart and blood vessels.
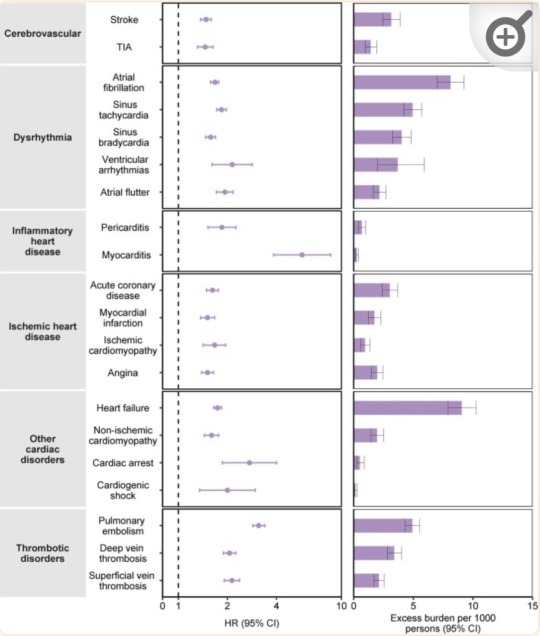
“Risks and 12-month burdens of incident post-acute COVID-19 cardiovascular outcomes in participants without any history of cardiovascular outcomes prior to COVID-19 exposure compared to the contemporary control cohort.” (Xie et al., 2022)
Published Research
Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts | Science Translational Medicine Guarnieri, J. W., Dybas, J. M., ... Wallace, D. C. (2023).
Long-term cardiovascular outcomes of COVID-19 - PMC (nih.gov) Xie, Y., Xu, E., Bowe, B., & Al-Aly, Z. (2022).
Articles & Reports
Blood Clotting Proteins Might Help Predict Long COVID Brain Fog - Scientific American Reardon, S. (2023, September 1).
SARS-CoV-2 can damage mitochondrion in heart, other organs, study finds | CIDRAP (umn.edu) Van Beusekom, M. (2023, August 9).
Your vascular system and COVID | Heart and Stroke Foundation Heart and Stroke Foundation. (2023).
COVID, heart disease and stroke | Heart and Stroke Foundation Heart and Stroke Foundation. (2023, April 17).
How does coronavirus affect your heart? - BHF British Heart Foundation. (2023, March 21).
COVID-19 and Heart Damage: What You Should Know (clevelandclinic.org) Cleveland Clinic. (2022, May 10).
Heart Problems after COVID-19 | Johns Hopkins Medicine Post, W. S., & Gilotra, N. A. (2022).
COVID and the Heart: It Spares No One | Johns Hopkins | Bloomberg School of Public Health (jhu.edu) Desmon, S., & Al-Aly, Z. (2022, March 14).
COVID-19 takes serious toll on heart health—a full year after recovery | Science | AAAS Wadman, M. (2022, February 9).
Brain & Neurological Damage
Summary: COVID-19 infection increases your risk of developing cognitive impairments, mental health issues, poor memory, early onset dementia, and permanent loss of smell due to brain damage and the atrophy of brain matter. "Brain fog" and problems concentrating are common complaints post-infection that have also been linked to brain damage. Damage to blood vessels due to inflammation during the infection may be responsible for this by restricting oxygen flow to the brain. COVID-19 may also directly infect the brain.
Published Research
Biology | Free Full-Text | Vascular Dysfunctions Contribute to the Long-Term Cognitive Deficits Following COVID-19 (mdpi.com) Shabani, Z., Liu, J., & Su, H. (2023).
Frontiers | COVCOG 2: Cognitive and Memory Deficits in Long COVID: A Second Publication From the COVID and Cognition Study (frontiersin.org) Guo, P., Ballesteros, B. A., Yeung, S. P., Liu, R., Saha, A., Curtis, L., Kaser, M., Haggard, M. P., & Cheke, L. G. (2022).
COVID-19 and cognitive impairment: neuroinvasive and blood‒brain barrier dysfunction - PMC (nih.gov) Chen, Y., Yang, W., Chen, F., & Cui, L. (2022).
Comparison of post-COVID depression and major depressive disorder | medRxiv Perlis, R. H., Santillana, M., Ognyanova, K., Green, J., Druckman, J., Lazer, D., & Baum, M. A. (2021).
Articles & Reports
Long COVID May Impair Memory, Cognition for Months (healthline.com) Rossiaky, D. (2022).
COVID Variants Can Affect the Brain in Different Ways - Neuroscience News (2023).
The hidden long-term cognitive effects of COVID-19 - Harvard Health Budson, A. E. (2021). Harvard Medical School.
Long Covid: Even mild Covid is linked to damage to the brain months after infection (nbcnews.com) Ryan, B. (2022). NBC News.
COVID-19 Can Affect the Brain Even Long After an Infection | Time Ducharme, J. (2023). Time.
Lung Damage
Summary: COVID-19 infections can cause lung damage or scarring, and can trigger pneumonia, bronchitis, ARDS, and sepsis. Additionally, some people experience shortness of breath (dyspnea) and difficulty exercising as a post-acute sequela after infection, or multiple infections.
Published Research
At a crossroads: COVID-19 recovery and the risk of pulmonary vascular disease - PMC (nih.gov) Cascino, T. M., Desai, A. A., & Kanthi, Y. (2021).
[Pulmonary manifestations in long COVID] - PubMed (nih.gov) Sommer, N., & Schmeck, B. (2022).
Residual Lung Abnormalities after COVID-19 Hospitalization: Interim Analysis of the UKILD Post-COVID-19 Study - PubMed (nih.gov) Stewart, I., Jacob, J., George, P. M., Molyneaux, P. L., Porter, J. C., Allen, R. J., Aslani, S., Baillie, J. K., Barratt, S. L., Beirne, P., Bianchi, S. M., Blaikley, J. F., ...Jenkins, G. R. (2023).
Articles & Reports
Even mild cases of COVID-19 may cause long-term lung damage - UPI.com HealthDay News. (2022). United Press International.
COVID-19 Lung Damage | Johns Hopkins Medicine Galiatsatos, P. (2022).
Immune System & Autoimmune Diseases
Summary: COVID-19 infection can impair the functioning of your immune system. This means that those who have previously been infected are potentially immunocompromised (higher risk). For some people, the way COVID-19 impairs their immune system results in the onset of autoimmune diseases.
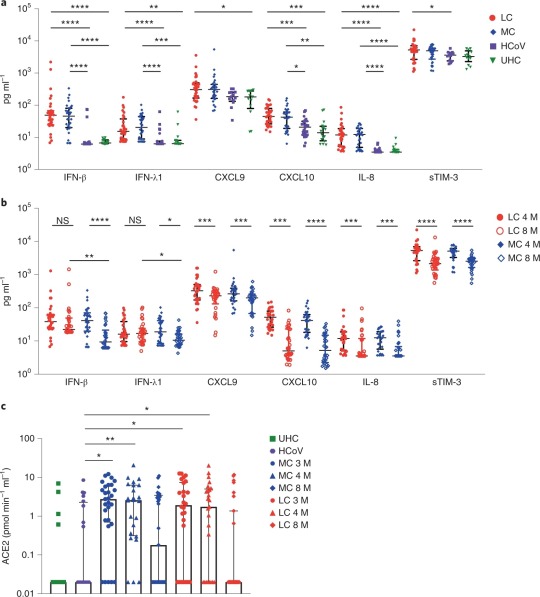
“Elevated levels of proinflammatory cytokines that persist more than 8 months following convalescence.” (Phetsouphanh et al., 2022)
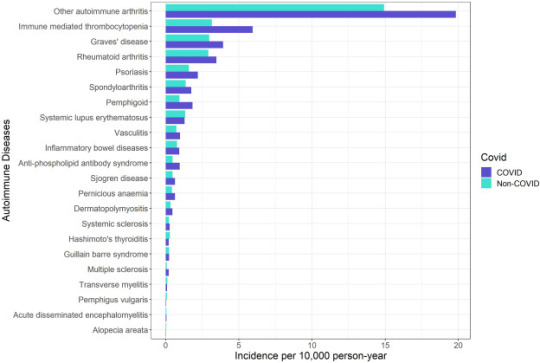
“Crude incidence of each autoimmune disease by COVID-19 and non-COVID groups.” (Peng et al., 2023)
Published Research
Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection | Nature Immunology Phetsouphanh, C., Darley, D. R., Wilson, D. B., Howe, A., Munier, M. L., Patel, S. K., Juno, J. A., Burrell, L. M., Kent, S. J., Dore, G. J., ... & Matthews, G. V. (2022).
Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection | BMC Medicine | Full Text (biomedcentral.com) Ryan, F. J., Hope, C. M., Masavuli, M. G., Lynn, M. A., Mekonnen, Z. A., Yeow, A. E. L., Garcia-Valtanen, P., Al-Delfi, Z., Gummow, J., Furguson, C., ... Lynn, D. J. (2022).
Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study - eClinicalMedicine (thelancet.com) Peng, K., Li, X., Yang, D., Chan, S. C. W., Zhou, J., & Wan, E. Y. F. (2023).
Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection | BMC Medicine | Full Text (biomedcentral.com) Winheim, E., Rinke, L., Lutz, K., Reischer, A., Leutbecher, A., Wolfram, L., Rausch, L., Kranich, J., Wratil, P. R., Huber, J. E., Baumjohann, D., ... Krug, A. B. (2021).
Articles & Reports
How COVID-19 Changes the Immune System | Time Park, A. (2023, August 18).
How COVID-19 alters the immune system -- ScienceDaily ScienceDaily. (2021, October 28).
Impacts of COVID on the immune system (medicalxpress.com) Herrero, L. (2022, September 19).
COVID-19's impact on the immune system, and how this may affect subsequent infections - ABC News Smith, B. (2022, December 1).
COVID-19 can derange immune system; survivors have autoimmune diseases (usatoday.com) Szabo, L. (2021, March 2).
Long COVID & PASC
Summary: Long COVID is an umbrella term that refers to the onset of disabling symptoms/conditions resulting from any of the previously mentioned organ, immune system, and vascular damage sustained during infection. These conditions are also referred to as "post-acute sequelae of COVID-19" (PASC). Vaccination can reduce the damage experienced by decreasing inflammation during an infection, but Long COVID/PASC can affect anyone. This is especially true in the case of multiple infections. Your risk of developing Long COVID, or worse/new symptoms, increases with each additional infection.
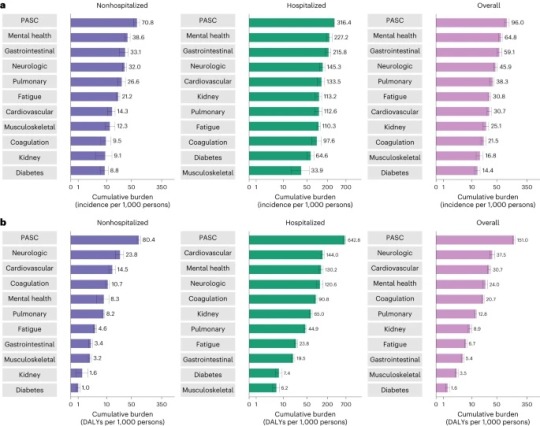
“Cumulative incidence and DALYs of postacute sequelae overall and by organ system at 2 years after infection.” (Bowe et al., 2023)

Published Research
T cell apoptosis characterizes severe Covid-19 disease - PubMed (nih.gov) André, S., Picard, M., Cezar, R., Roux-Dalvai, F., Alleaume-Butaux, A., Soundaramourty, C., Cruz, A. S., Mendes-Frias, A., Gotti, C., … Estaquier, J. (2022).
SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC) | Nature Immunology Proal, A. D., VanElzakker, M. B., Aleman, S., Bach, K., Boribong, B. P., Buggert, M., Cherry, S., Chertow, D. S., Davies, H. E., Dupont, C. L., ... Wherry, E. J. (2023).
The immunology of long COVID | Nature Reviews Immunology Altmann, D. M., Whettlock, E. M., Liu, S., Arachchillage, D. J., & Boyton, R. J. (2023).
Long COVID: major findings, mechanisms and recommendations | Nature Reviews Microbiology Davis, H. E., McCorkell, L., Vogel, J. M., & Topol, E. J. (2023).
Long COVID prevalence and impact on quality of life 2 years after acute COVID-19 | Scientific Reports (nature.com) Kim, Y., Bae, S., Chang, H., & Kim, S. (2023).
Postacute sequelae of COVID-19 at 2 years | Nature Medicine Bowe, B., Xie, Y., & Al-Aly, Z. (2023).
Articles & Reports
Long COVID | NIH COVID-19 Research National Institutes of Health. (2023, June 8).
Long COVID or Post-COVID Conditions | CDC Centers for Disease Control and Prevention. (2023, July 20).
The Most Important Question About Long COVID | Harvard Medical School Pesheva, K. (2023, August 9).
Nearly One in Five American Adults Who Have Had COVID-19 Still Have "Long COVID" (cdc.gov) Centers for Disease Control and Prevention. (2022, June 22).
2 notes
·
View notes
Text
Digital Virology: AI’s Expert Eye Identifies Infections - Technology Org
New Post has been published on https://thedigitalinsider.com/digital-virology-ais-expert-eye-identifies-infections-technology-org/
Digital Virology: AI’s Expert Eye Identifies Infections - Technology Org
Microscopy plus machine learning and AI equals a quick, cheap and automated way to detect not only coronavirus, but other emerging pathogens or cellular responses to drugs.
Digital virology, AI in medical research – illustrative rendering. Image credit: Minttu Polso/Aalto University
Before there were vaccines or good ways to diagnose SARS-CoV-2, scientists around the world were scrambling to develop methods for understanding the biology of the virus. While the pandemic opened plenty of scientific opportunities and questions, doors, and borders were also shut almost everywhere.
One of the millions of people who were stuck during early 2020 was computer scientist Christian Guckelsberger. Due to starting a postdoc on AI and creativity in Finland, he instead found himself part of an international biomedical research project to harness machine learning to identify the coronavirus from patient blood samples.
“It was like being thrown into cold water. I’m not a medical person and hadn’t thought about these kinds of things since high school biology,” says Guckelsberger, now an assistant professor of creative technologies at Aalto University and FCAI.
“But this project gave me the feeling of doing something relevant and putting my mind to best use at a critical time.”
Amid pandemic travel restrictions, Guckelsberger’s involvement in the timely, multidisciplinary project allowed him to come to Finland and embark on what he thought would be a two-month project. It ended up lasting an entire year and sparked a renewed fascination for biomedical research and the people doing it.
What Guckelsberger and colleagues developed during that year is a way to automatically classify digital microscopy images of the interactions between antibodies and virus antigens in lab-grown cells. A computer essentially learns to detect if the patient has anti-coronavirus antibodies.
Beyond a diagnosis, the method also gives researchers insight into what features in cells indicate a positive result, what kind of antibody responses are present and allows them to make predictions about the likelihood of a COVID-19 antibody-positive sample from the image alone.
The same sample images classified by a computer were also shown to expert virologists, who rated them as positive or negative for coronavirus antibodies. “Our approach can match the classification level of human experts,” says Guckelsberger, “and it’s much faster. Plus, it can tell us when there are ambiguous results that should be given a closer look by an expert eye.”
The results of the project, recently published in Cell Reports Methods, also show that the method is comparable, and in some ways superior, to widely-used assays like ELISA.
“We used cells, rather than purified virus proteins, as the basis for our assay, which is closer to real physiology,” says lead author Vilja Pietiäinen of the Institute for Molecular Medicine Finland (FIMM) at the University of Helsinki.
“Because everything is completely automated, we have high throughput, but we also get the digital images that can be shown to a virologist or a pathologist, without them having to go to a microscope. The results can even be checked on a mobile device. And we can count the number of infected cells, so we have the quantitative data as well as the visuals.”
During the early days of the pandemic, the research team was able to form quickly thanks to earlier international and local collaborations on virology, imaging and drug response studies, explains Pietiäinen.
“At that point, we needed a high-throughput assay for antibody testing that would indicate if a person had a SARS-CoV-2 infection. Since then, there has been a lot of improvement on SARS-CoV-2 diagnosis, detection, and antibody response,” such as the widely familiar polymerase chain reaction (PCR) test or the antigen test (such as the nose swab) that directly measures the presence of the virus in the body.
The test developed by Pietiäinen, Guckelsberger and colleagues, by contrast, measures antibodies, which tells us how the immune system recognizes the virus and produces different types of antibodies against it.
Machine-learning-based analysis, automated workflow. Image credit: Minttu Polso/Aalto University
“When you only have a few samples, know very little about a disease or might not have access to a high-level biosafety lab, our pipeline can be really valuable,” says Guckelsberger, adding that it can be used anywhere regardless of location, sample preparation equipment or type of microscope. In fact, the pipeline is versatile to test on any germ.
“We designed the test to be used for any emerging pathogen, increasing our readiness for future pandemics,” says Pietiäinen. “Certain components should be optimized for each new virus, but the beauty of the assay is that it can be used for different purposes. It’s already being used to study zoonotic viruses like the Puumala virus.”
Other automated cell-based assays, followed by AI-guided image analysis methods, are being used in the research group to study the drug responses to SARS-CoV-2 as well as to identify drugs that can kill patient-derived cancer cells ex vivo.
Beyond publishing their work and contributing to a better understanding of the pandemic, Guckelsberger and Pietiäinen share a common insight that this project taught them.
“When big questions come up in the world, we as scientists can’t work alone in silos. Experts from different fields, different universities and countries need to come together with a shared aim—in our case, data scientists, clinicians, computer scientists, biochemists,” says Pietiäinen.
“Working in a big team, which is not something we do often in computer science, was fascinating,” echoes Guckelsberger.
“One big challenge was communicating from different perspectives of expertise, for example making sense of what is happening at both ends of the pipeline from wet lab procedures to parameters to data and images. At the same time, this was a fantastic learning experience, and one that I wish to have more of in the future.”
While they employed well-established machine learning for each component of the pipeline, Guckelsberger says making the connection between biologists and computer scientists was one of the real advances. Using technology to resolve biological questions was a big takeaway for Pietiäinen, too.
“Combining microscopy with machine learning, not just for SARS-CoV-2, but to see personalized responses to drugs or to see the cellular phenotypes of rare genetic diseases, is powerful. A picture is worth a thousand words, that is also the case here.”
Source: Aalto University
You can offer your link to a page which is relevant to the topic of this post.
#A.I. & Neural Networks news#Aalto University#ai#Analysis#antibodies#antigen#approach#artificial intelligence (AI)#Biology#Biotechnology news#blood#Cancer#cancer cells#cell#Cells#challenge#computer#Computer Science#coronavirus#covid#creativity#data#detection#Disease#Diseases#drug#drugs#employed#equipment#eye
1 note
·
View note
Text
📆 22 Sep 2023 📰 Post-Vax Covid Variants Thrive via Shared Mechanisms 🗞 Mirage
In the paper titled, SARS-CoV-2 Variants Evolve Convergent Strategies to Remodel the Host Response, scientists carried out an unprecedented, systematic comparative study using the most infectious COVID-19 variants, namely Alpha, Beta, Gamma, Delta and Omicron to identify specific viral mutations responsible for hijacking a common host pathway, thereby leading to increased transmissibility, infectivity and survival. Specifically, they discovered a convergence in potent suppression of interferon-stimulated genes through several viral proteins, including Orf6 and Orf9b, which serve as innate immune antagonist proteins capable of blocking innate host immune response.
The study, led by the laboratories of Nevan Krogan, Ph.D., Director of the Quantitative Biosciences Institute (QBI) at the School of Pharmacy at UC San Francisco, Senior Investigator at Gladstone Institutes, was a collaborative effort that involved 16 institutions in six countries, including University College London (UCL) in London, England (Greg Towers and Clare Jolly) and Icahn Mount Sinai (Adolfo Garcia-Sastre and Lisa Miorin) among others.

"Unfortunately, we continue to see new mutations and strains of SARS-CoV-2 despite innovations in new vaccines," said Krogan, who founded the QBI Coronavirus Research Group (QCRG). "We evaluated each of the viral variants in isolation and discovered that there was a common mechanism involving several viral proteins, including Orf6 and Orf9b, that potently suppresses innate immunity. This finding is consistent with our investigation of early SARS-CoV-2 variants where certain viral proteins were highly expressed in infected cells which helped the virus infect our cells. With our additional research across SARS-CoV-2 variants, we now see this as a crucial finding that, if targeted effectively, could be turned into a significant vulnerability for this virus, which also has important implications for management of future pandemics."
Mehdi Bouhaddou, PhD, assistant professor Department of Microbiology, Immunology, and Molecular Genetics at the University of California, Los Angeles (UCLA) who contributed to the research during his prior post-doctoral fellowship at UCSF commented, "We believe the future approach to managing pandemics will require a drug combination cocktail, similar to treatment regimens for HIV, that will include vaccines and antiviral innovations to target the virus. Specifically, combination therapy approaches to target the adaptive immune response (e.g., vaccines, antibody treatments) and another inhibiting viral innate immune antagonist proteins (e.g., Orf6 and Orf9b) or activating the innate immune response, could be the most effective. Perhaps with this approach, we may be able to get ahead of viruses before they reach pandemic levels."
0 notes
Text
Second Takes - Andrea Thorn - University of Hamburg
Sharing some of our #SBGrid developer tales from the last year. This one from February 2023.
Andrea Thorn had been a junior group leader for a less than a year when, in early January 2020, researchers in China identified the cause of a mysterious contagious illness with pneumonia-like symptoms. Less than a week later, they released its genetic code, which revealed the novel virus to be a close cousin to the SARS coronavirus (SARS-CoV-1) that caused an outbreak in 2002-2003.

The rapid scientific response gave Thorn an idea for a talk she was due to give over coffee and cake to colleagues in her building at the Julius Maximilian University of Würzburg in Germany. She ran the only lab there focused on new computational methods for experimental structural biology. Most scientists in the building were infectious disease specialists.
"I needed to explain to them how my work was relevant," she says. Thorn works on ways to better model the atomic structures of RNA and proteins to make them useful for structure-based drug discovery, something that quickly would become more important as researchers rushed to respond to COVID-19.
Molecular models are a foundation of modern drug and vaccine development. They enable scientists to identify how to break the cycle of infection by targeting the right spot in the right protein. But even the most carefully determined structures reported in the most prestigious journals are imperfect interpretations of experimental data, Thorn says. Small errors can have large consequences in the search for a drug with the right fit.
When Thorn was preparing for her talk, no structures from the SARS-CoV-2 virus had been published yet. But more than 100 structures from SARS-CoV-1 were available in the worldwide Protein Data Bank (wwPDB). The structures ranged from a key protease in viral replication to the spike protein needed to attach and enter host cells.
From a random sample of five, Thorn found three SARS structures to illustrate the point of her research: Most protein structures can be improved with additional analysis.
Her talk was well received, germinating the seed of another idea that first seemed outlandishly bold and then became increasingly urgent as COVID-19 spread and the death toll soared. Thorn floated the idea over dinner with her mentor, Arwen Pearson in Hamburg: Analyze and correct all SARS structures. Pearson encouraged her, as did another senior colleague, Elspeth Garman of Oxford University.
In March 2020, as the first SARS-CoV-2 structures were being reported, Thorn first got her own small group on board and within a week pulled together a team of like-minded and mostly junior structural biology developers from all over the globe as the “Coronavirus Structural Task Force”. Many of them were international specialists in their respective fields, she notes.
They met online every weekday and posted daily updates to GitHub for anyone to access. Every Wednesday, when new wwPDB structures were released, their automated pipeline identified new coronavirus structures and assessed the quality of a representative sample of models and experimental data. When they improved a structure, they sent it back to the original authors to update the wwPDB entry, no strings attached.
For their web site (https://insidecorona.net), the group wrote blog posts to share with colleagues the larger story emerging from aggregated data of multiple structures. They wrote explanatory pieces for the public. They posted a 3D printable model of SARS-CoV-2.

Coronavirus Structural Task Force website: https://insidecorona.net. Image credit: Thomas Splettstößer
A year into the pandemic, structural biologists had released a total of 1,146 SARS structures covering 18 proteins from both SARS-CoV-1 and SARS-CoV-2. Most were derived by X-ray crystallography (73%) and single particle cryo-electron microscopy (cryo-EM) (24%) (Nature Structural & Molecular Biology, January 2021).
The task force's analytical pipeline deployed software programs from contributors inside and outside the Task Force. Among them were two tools previously developed by Thorn—AUSPEX, used for experimental X-ray data to detect sample measurement and processing problems (Acta Crystallographica, 2017) and, for experimental cryo-EM data, HARUSPEX, a neural network tool to automatically distinguish between nucleic acids and protein and to assign secondary protein structure elements (Angewandte Chemie, International Edition, 2020).
"We didn't do any peer reviewed publication until 2021," Thorn says about the task force. "We just wanted to fight the pandemic. The whole thing was fast. We pushed and pushed and pushed against corona so that drug developers and vaccine developers would have the right data. You know, it's been fantastic. It was born out of spontaneous willingness. I wish science would always be like this."
The task force published a description of its work (Nature Structural & Molecular Biology, May 2021). In 2023, Thorn says a dozen articles are in progress for peer-reviewed journals to summarize the combined information and results of their work, as well as to note questions yet to be answered about SARS-CoV-2. The task force will wind down in summer 2023. Thorn is strategizing on the next steps for a tenured academic position.
Thorn grew up in Germany in an intellectual family. Her father is a chemist, her mother an artist and entrepreneur. She is the third-born child with three brothers.
She calls her path to structural biology straightforward. One of her early science-related memories goes back to age 3, when her father explained surface tension to her with the gravity-defying demonstration of floating a needle on water. "My dad showed that to me with his always huge confidence that I would understand everything," Thorn says.
The family moved four times when Thorn was in elementary school. She supplemented the patchwork early education with natural curiosity. She read voraciously from the family library, made observations with a home microscope, and conducted chemistry experiments on the sly. In her mother's atelier, she also painted on large blank canvases.
In her teens, she and her friends were early adopters of live action role-playing games that have subsequently become popular in Germany. Participants assume the roles of characters and act out scenarios in a collaborative storytelling experience. To prepare for her roles, she researched scenarios ranging from futuristic military campaigns in Libya to glial cell retrieval from patients. "I read up to 600 pages a day when I was a teenager," she says.
In school, Thorn's studies became concentrated in chemistry and art. She continued first to a bachelor's degree and a master's degree, a prerequisite for her PhD.
She excelled in the lab and in computational chemistry, but her breakthrough lessons came after she failed quantum chemistry. She sought a tutor in the solid-state physics department, where her academic track record was unknown. A professor she consulted for a referral, Helmuth Zimmermann, volunteered to oversee weekly tutoring for Thorn and other classmates who needed help.
"Arguably those tutoring sessions did more for my education than everything else that semester," she says. "I'd never used maths before to describe things in nature in that way and fell in love with that."
One day, in response to Thorn's curiosity, the professor explained his research, which was measuring crystals to determine the structure of molecules. "I was hooked, because that meant the maths that I just learned could be applied far beyond class," she says. "It meant you could use them to find out the structure of crystal lattices and, with the structure of crystal lattices, the structure of molecules."
She skipped her prescribed medicinal chemistry classes in favor of crystallography lectures, which were not in her curriculum plan. One day, her crystallography lecturer refused to answer her question about SHELX, a crystal structure refinement program, saying she wasn't smart enough to understand.
"I was so angry, I printed out the SHELX manual, which is 100 pages, and I read the whole fricking thing." She came to the next class armed with new and more detailed questions. "It turned out he didn't know so much about crystallography," she says.
When it came time to choose a PhD group, Thorn had a list. The list included George Sheldrick at University of Göttingen, who developed SHELX. On the way to a holiday live role-play destination, she stopped by his lab to scout it out informally. Unexpectedly, she had a long conversation with him that ended with an immediate offer of doing her PhD thesis in his group.
She thrived in the high-performing lab and found fellow role-play enthusiasts. When she was teaching her first classes as a PhD student, a friend came to collect her for lunch. He noted her stance in front of the students (who were much older than her) looked like the last weekend's live role play when she had played a commanding leader with feet planted and shoulders squared as she led her warriors into battle.
In 2011, Thorn graduated with her doctorate and four new first-author publications from her thesis. The morning after her defense, she married her childhood sweetheart, a chemist. Within a year, she had secured a prestigious three-year Marie Curie fellowship for independent research at the MRC Laboratory of Molecular Biology in Cambridge, followed by a brief but productive stint at University of Oxford.
Homesick for Germany and their parents, she returned with high expectations of becoming an assistant professor, something that remains a goal. She secured outside funding and became a group leader at University of Würzburg in 2019 and moved to Universität Hamburg in 2020.
The task force may be winding down, but its value lingers in Thorn's mind as an unmet need in science. She has been thinking about the volume of data being generated by the structural biology community and how to combine the data for more meaningful interpretation and usefulness. She also takes it as an example of what is possible when experts across the globe start to collaborate on an important problem.
"We are generating data like crazy," she says, “beyond the capacity of individuals to assimilate and make sense of details from different techniques across labs. "In Germany, we have this beautiful word, Datennachnutzung. It means you are using experimental data for a second time to find new insights. And that's something we absolutely need to do."
-Carol Cruzan Morton
1 note
·
View note
Text
📆 July 2023 📰 Rare link between coronavirus vaccines and Long Covid–like illness starts to gain acceptance 🗞️ Science
But in recent months, what some call Long Vax has gained wider acceptance among doctors and scientists, and some are now working to better understand and treat its symptoms.
“You see one or two patients and you wonder if it’s a coincidence,” says Anne Louise Oaklander, a neurologist and researcher at Harvard Medical School. “But by the time you’ve seen 10, 20,” she continues, trailing off. “Where there’s smoke, there’s fire.”
Symptoms can include persistent headaches, severe fatigue, and abnormal heart rate and blood pressure. They appear hours, days, or weeks after vaccination and are difficult to study. But researchers and clinicians are increasingly finding some alignment with known medical conditions. One is small fiber neuropathy, a condition Oaklander studies, in which nerve damage can cause tingling or electric shock–like sensations, burning pain, and blood circulation problems. The second is a more nebulous syndrome, with symptoms sometimes triggered by small fiber neuropathy, called postural orthostatic tachycardia syndrome (POTS). It can involve muscle weakness, swings in heart rate and blood pressure, fatigue, and brain fog.
German Minister of Health Karl Lauterbach acknowledged in March that though rare, Long Covid–like symptoms after vaccination are a real phenomenon. He said his ministry was working to organize funding for studies, although none has been announced so far.

Science first wrote about these health concerns in January 2022, describing efforts by scientists at the National Institutes of Health to study and treat affected individuals. A study including 23 people was posted as a preprint in May 2022 but never published. Following Science’s story, almost 200 people contacted the journal sharing their postvaccination symptoms.
Research has since proceeded slowly. This is “a challenging outcome to monitor,” Tom Shimabukuro of the U.S. Centers for Disease Control and Prevention told a government advisory committee in January. Still, more than two dozen case studies have accumulated describing POTS or small fiber neuropathy following a COVID-19 shot, regardless of the vaccine manufacturer.
An immune overreaction to SARS-CoV-2 spike protein, which COVID-19 vaccines use to induce protective antibodies, is one possible cause of these symptoms. One theory is that after vaccination some people generate another round of antibodies targeting the first. Those antibodies could function somewhat like spike itself: Spike targets a cell surface protein called the angiotensin-converting enzyme 2 (ACE2) receptor, enabling the virus to enter cells. The rogue antibodies might also bind to ACE2, which helps regulate blood pressure and heart rate, says Bernhard Schieffer, a cardiologist at the University of Marburg. If those antibodies disrupt ACE2 signaling, that could cause the racing heart rates and blood pressure swings seen in POTS.
Small fiber neurons also have the ACE2 receptor on their surface, so in theory rogue antibodies could contribute to neuropathy. But Matthew Schelke, a neurologist at Columbia University who has treated small fiber neuropathy in both Long Covid and postvaccine patients, says pinning down a connection won’t be easy. Even when unusual antibodies turn up in someone’s blood, “it’s extremely difficult to know if any of these are pathogenic or if they are just bystanders,” he says. Other immune system components that fuel inflammation may also harm nerves, he notes.
1 note
·
View note
Text
SARS-CoV-2 infection and Ad26.COV2.S vaccination may increase risk of stroke post-vaccination
In a recent study posted to medRxiv* preprint server, researchers examined the factors associated with stroke following coronavirus disease 2019 (COVID-19) vaccination. Study: Factors Associated with Stroke after COVID-19 Vaccination: A Statewide Analysis. Image Credit: BaLL LunLa/Shutterstock Background Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are effective…

View On WordPress
0 notes
Text
#COVID-19#coronavirus#immunity#vaccines#medicine#biology#health#sciences#research and development#sars cov 2#article#link#inforgraphics#my posts
1 note
·
View note
Text
COVID killed by sunlight
No direct evidence of airborne spread
Surface spread very rare
Asymptomatic spread low
Masks ineffective
Lockdowns don’t work
Herd immunity real
Miniscule risk to children
Survival rate 99.97%+
1. “When researchers at the National Biodefense Analysis and Countermeasures Center exposed SARS-CoV-2 in simulated saliva to artificial sunlight (equivalent to a sunny day), 90% of viruses were inactivated within seven minutes.” https://www.forbes.com/sites/jvchamary/2020/06/29/light-coronavirus/
1. “The present study is the first to demonstrate that UVB levels representative of natural sunlight rapidly inactivate SARS-CoV-2 on surfaces”… https://academic.oup.com/jid/article/222/2/214/5841129
2. “[The CDC] has reversed an update made to its coronavirus guidance that stated COVID-19 is an airborne virus. The health agency said that update, posted to its website… was made in error.” (It later said it was “possible.”) https://www.huffingtonpost.co.uk/entry/coronavirus-airborne-in-aerosols-cdc-says_n_5f689b89c5b6480e8972428d?
2. “There is absolutely no evidence that this disease is airborne”… https://bc.ctvnews.ca/absolutely-no-evidence-that-covid-19-is-airborne-b-c-health-official-says-1.4964156
3. “[The CDC] just released a new scientific brief that says your risk of contracting COVID-19 from a surface is about 1 in 10,000. That means, on average, you have a 0.01% chance of actually picking up the virus from, say, touching a counter.” https://news.yahoo.com/risk-getting-covid-19-surface-194600830.html
4. “[A] meta-analysis of 54 studies with 77,758 total participants… found that secondary attack rates were higher for symptomatic people than asymptomatic people — an 18% rate for symptomatic people and a 0.7% rate for asymptomatic people.” https://tallahasseereports.com/2020/12/31/asymptomatic-covid-spread-unlikely-but-possible-according-to-study/
5. “We did not find evidence that surgical-type face masks are effective in reducing laboratory-confirmed influenza transmission, either when worn by infected persons (source control) or by persons in the general community.” Analysis of COVID mask data. https://wwwnc.cdc.gov/eid/article/26/5/19-0994_article
5. “Researchers in Denmark reported… that surgical masks did not protect the wearers against infection with the coronavirus in a large randomized clinical trial.” https://fee.org/articles/new-danish-study-finds-masks-don-t-protect-wearers-from-covid-infection/
5. “Recommendation to wear a surgical mask when outside the home among others did not reduce, at conventional levels of statistical significance, incident SARS-CoV-2 infection compared with no mask recommendation.” https://www.msn.com/en-us/health/medical/first-randomized-control-trial-shows-face-masks-did-not-reduce-coronavirus-infections-with-statistical-significance/ar-BB1b8zo2
5. The top 10 states in case rates over the past 7 days all still have mask mandates

5. “In the first 20 days after implementing mask mandates, new cases slowed by 0.5 percentage points… COVID-19 death rates dropped by 0.7 percentage points…” Thus, not even a 1% difference in one CDC study. https://eu.usatoday.com/story/news/factcheck/2021/03/12/fact-check-cdc-study-links-mask-mandates-slowed-covid-infections/6938262002/
6. “A study evaluating COVID-19 responses around the world found that mandatory lockdown orders early in the pandemic may not provide significantly more benefits to slowing the spread of the disease than other voluntary measures”… https://www.newsweek.com/covid-lockdowns-have-no-clear-benefit-vs-other-voluntary-measures-international-study-shows-1561656
6. “We do not question the role of all public health interventions, or of coordinated communications about the epidemic, but we fail to find an additional benefit of stay-at-home orders and business closures.” https://onlinelibrary.wiley.com/doi/10.1111/eci.13484
6. “188 countries that have declared at least one case, only those counting a minimum of 10 deaths due to Covid-19 were included… Stringency of the measures settled to fight pandemia, including lockdown, did not appear to be linked with death rate.” https://www.frontiersin.org/articles/10.3389/fpubh.2020.604339/full#SM6
6. “[Tel Aviv researchers] found no statistical correlation between the severity of a lockdown and the number of Covid-19 fatalities in the country.” https://www.israel21c.org/distancing-not-lockdowns-prevents-covid-19-deaths-says-study/
6. “We in the [WHO] do not advocate lockdowns… The only time we believe a lockdown is justified is to buy you time to reorganize, regroup, rebalance your resources, protect your health workers who are exhausted, but by and large, we’d rather not do it.” https://www.msn.com/en-us/health/medical/who-official-urges-world-leaders-to-stop-using-lockdowns-as-primary-virus-control-method/ar-BB19TBUo
6. “During the study period, states allowed restaurants to reopen for on-premises dining in 3,076 (97.9%) U.S. counties. Changes in daily COVID-19 case & death growth rates were not statistically significant”… https://www.cdc.gov/mmwr/volumes/70/wr/mm7010e3.htm?s_cid=mm7010e3_w
7. “Fauci has been saying that the country needs to vaccinate 70% to 85% of the population to reach herd immunity from Covid-19. But he inexplicably ignores natural immunity. If you account for previous infections, herd immunity is likely close at hand.” https://www.wsj.com/articles/herd-immunity-is-near-despite-faucis-denial-11616624554
7. “Experts believe that if no other measures are taken, herd immunity could kick in when between 50% and 70% of a population gains immunity via vaccination… the number most people are looking at (for herd immunity) is about 60-65%.” https://graphics.reuters.com/HEALTH-CORONAVIRUS/HERD%20IMMUNITY%20(EXPLAINER)/gjnvwayydvw/
8. The CDC reports that 251 patients under 17 years old have died with COVID since the pandemic began.
There are 74.2 million Americans under 18. That is a survival rate for that age demographic of about 99.9997%. The survival rate for 18-29 is about 99.997%.

9. The average age of COVID death is 78.6 years old. The average age of life expectancy for those patients was 78.6 years old. Overall survival rate 99.97%.
“CDC data also show that Americans, regardless of age group, are far more likely to die of something other than COVID-19.”

1K notes
·
View notes
Text
Also preserved in our archive
By Vijay Kumar Malesu
In a recent pre-print study posted to bioRxiv*, a team of researchers investigated the predictive role of gut microbiome composition during acute Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in the development of Long Coronavirus Disease (Long COVID) (LC) and its association with clinical variables and symptom clusters.
Background LC affects 10–30% of non-hospitalized individuals infected with SARS-CoV-2, leading to significant morbidity, workforce loss, and an economic impact of $3.7 trillion in the United States (U.S.).
Symptoms span cardiovascular, gastrointestinal, cognitive, and neurological issues, resembling myalgic encephalomyelitis and other post-infectious syndromes. Proposed mechanisms include immune dysregulation, neuroinflammation, viral persistence, and coagulation abnormalities, with emerging evidence implicating the gut microbiome in LC pathogenesis.
Current studies focus on hospitalized patients, limiting generalizability to milder cases. Further research is needed to explore microbiome-driven predictors in outpatient populations, enabling targeted diagnostics and therapies for LC’s heterogeneous and complex presentation.
About the study The study was approved by the Mayo Clinic Institutional Review Board and recruited adults aged 18 years or older who underwent SARS-CoV-2 testing at Mayo Clinic locations in Minnesota, Florida, and Arizona from October 2020 to September 2021. Participants were identified through electronic health record (EHR) reviews filtered by SARS-CoV-2 testing schedules.
Eligible individuals were contacted via email, and informed consent was obtained. Of the 1,061 participants initially recruited, 242 were excluded due to incomplete data, failed sequencing, or other issues. The final cohort included 799 participants (380 SARS-CoV-2-positive and 419 SARS-CoV-2-negative), providing 947 stool samples.
Stool samples were collected at two-time points: weeks 0–2 and weeks 3–5 after testing. Samples were shipped in frozen gel packs via overnight courier and stored at −80°C for downstream analyses. Microbial deoxyribonucleic acid (DNA) was extracted using Qiagen kits, and metagenomic sequencing was performed targeting 8 million reads per sample.
Taxonomic profiling was conducted using Kraken2, and functional profiling was performed using the Human Microbiome Project Unified Metabolic Analysis Network (HUMAnN3).
Stool calprotectin levels were measured using enzyme-linked immunosorbent assay (ELISA), and SARS-CoV-2 ribonucleic acid (RNA) was detected using reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Clinical data, including demographics, comorbidities, medications, and symptom persistence, were extracted from EHRs.
Machine learning models incorporating microbiome and clinical data were utilized to predict LC and to identify symptom clusters, providing valuable insights into the heterogeneity of the condition.
Study results The study analyzed 947 stool samples collected from 799 participants, including 380 SARS-CoV-2-positive individuals and 419 negative controls. Of the SARS-CoV-2-positive group, 80 patients developed LC during a one-year follow-up period.
Participants were categorized into three groups for analysis: LC, non-LC (SARS-CoV-2-positive without LC), and SARS-CoV-2-negative. Baseline characteristics revealed significant differences between these groups. LC participants were predominantly female and had more baseline comorbidities compared to non-LC participants.
The SARS-CoV-2-negative group was older, with higher antibiotic use and vaccination rates. These variables were adjusted for in subsequent analyses.
During acute infection, gut microbiome diversity differed significantly between groups. Alpha diversity was lower in SARS-CoV-2-positive participants (LC and non-LC) than in SARS-CoV-2-negative participants.
Beta diversity analyses revealed distinct microbial compositions among the groups, with LC patients exhibiting unique microbiome profiles during acute infection.
Specific bacterial taxa, including Faecalimonas and Blautia, were enriched in LC patients, while other taxa were predominant in non-LC and negative participants. These findings indicate that gut microbiome composition during acute infection is a potential predictor for LC.
Temporal analysis of gut microbiome changes between the acute and post-acute phases revealed significant individual variability but no cohort-level differences, suggesting that temporal changes do not contribute to LC development.
However, machine learning models demonstrated that microbiome data during acute infection, when combined with clinical variables, predicted LC with high accuracy. Microbial predictors, including species from the Lachnospiraceae family, significantly influenced model performance.
Symptom analysis revealed that LC encompasses heterogeneous clinical presentations. Fatigue was the most prevalent symptom, followed by dyspnea and cough.
Cluster analysis identified four LC subphenotypes based on symptom co-occurrence: gastrointestinal and sensory, musculoskeletal and neuropsychiatric, cardiopulmonary, and fatigue-only.
Each cluster exhibited unique microbial associations, with the gastrointestinal and sensory clusters showing the most pronounced microbial alterations. Notably, taxa such as those from Lachnospiraceae and Erysipelotrichaceae families were significantly enriched in this cluster.
Conclusions To summarize, this study demonstrated that SARS-CoV-2-positive individuals who later developed LC exhibited distinct gut microbiome profiles during acute infection. While prior research has linked the gut microbiome to COVID-19 outcomes, few studies have explored its predictive potential for LC, particularly in outpatient cohorts.
Using machine learning models, including artificial neural networks and logistic regression, this study found that microbiome data alone predicted LC more accurately than clinical variables, such as disease severity, sex, and vaccination status.
Key microbial contributors included species from the Lachnospiraceae family, such as Eubacterium and Agathobacter, and Prevotella spp. These findings highlight the gut microbiome’s potential as a diagnostic tool for identifying LC risk, enabling personalized interventions.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference: Preliminary scientific report. Isin Y. Comba, Ruben A. T. Mars, Lu Yang, et al. (2024) Gut Microbiome Signatures During Acute Infection Predict Long COVID, bioRxiv. doi:https://doi.org/10.1101/2024.12.10.626852. www.biorxiv.org/content/10.1101/2024.12.10.626852v1.full
#mask up#public health#wear a mask#pandemic#wear a respirator#covid#still coviding#covid 19#coronavirus#sars cov 2#long covid#AI
9 notes
·
View notes
Photo

The Pandemic After the Pandemic
Long COVID isn’t going away, and we still do not have a way to fully prevent it, cure it, or really to quantify it.
The world was slow to recognize long COVID as one of the most serious consequences of the coronavirus. Six months into the pathogen’s tear across the globe, SARS-CoV-2 was still considered an acute airway infection that would spark a weeks-long illness at most; anyone who experienced symptoms for longer could be expected to be dismissed by droves of doctors. Now long COVID is written into CDC and WHO documents; it makes a cameo in the newest version of President Joe Biden’s National COVID-19 Preparedness Plan.
But for all we know now about long COVID, it is still not enough. Researchers still don’t know who’s most at risk, or how long the condition might last; whether certain variants might cause it more frequently, or the extent to which vaccines might sweep it away. We do not have a way to fully prevent it. We do not have a way to cure it. We don’t even have a way to really quantify it: There still isn’t consensus on how common long COVID actually is. Its danger feels both amorphous and unavoidable. People already struggle to deal with well-known risks, let alone fuzzy, slippery ones. “You can be too afraid of what you don’t understand or just say, ‘It’s not well defined; I’m not going to think about it,’” says Erin Sanders, a nurse practitioner and clinical scientist at MIT. Concern, when we let it, can act like a gas. It expands to fill the space we give it.
But even if long COVID’s prevalence turns out to be a single-digit percentage of SARS-CoV-2 infections—proportionally much smaller than most experts estimate—in absolute terms “that is not small,” says Ziyad Al-Aly, the director of the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System. Millions of people have already developed long COVID; many of them, an untold fraction, have not recovered. This is the challenge of chronic illness: When people join its ranks, they do not always exit. With each new case of long COVID, the virus’s burden balloons.
“I worry, now that everyone is moving to the post-pandemic world, we’re going to sweep all these patients under the rug,” Al-Aly said. Long COVID struggled to gain a toehold in the national consciousness; now it threatens to be one of the first major COVID impacts to slip back into the margins.
Researchers have known for many months that long COVID is more a category than a monolith. Al-Aly very roughly likens it to the way we talk about cancer—an umbrella term for diseases that are related but that require distinct diagnoses and treatments. Long COVID has hundreds of possible symptoms. It can batter the brain, the heart, the lungs, the gut, all of the above, or none of the above. The condition can start from a silent infection, an ICU-caliber case, or anything in between. It can begin days, weeks, or months after the virus first infects someone, and its severity can fluctuate over time. “We lump all of that into one broad thing,” Al-Aly said. “It is not.”
The condition’s root causes, accordingly, are also diverse. In some cases, long COVID may be collateral damage from the war waged between virus and immune system; in others, it might sprout out of a chronic SARS-CoV-2 infection or, conversely, a quick viral encounter that sets bodily systems on the fritz. These hypotheses aren’t comprehensive or mutually exclusive: There are only so many ways for bodies to run smoothly, and infinite ways to throw those processes out of whack.
All of this means that even diagnosing long COVID—an essential step toward understanding it—is still a battle. We don’t have a clear-cut, consensus clinical definition, a single name for the condition, or a standardized set of tests to catch it. Even the CDC and the WHO can’t agree on how long a person must be sick before they meet the condition’s criteria. Some researchers and health-care providers favor one agency’s definition; others, dissatisfied with both, come up with their own. And “there are still doctors out there that do not think long COVID exists,” says Alexandra Yonts, a pediatric-infectious-disease specialist at Children’s National Hospital, in Washington, D.C.
In an ideal experimental world, to understand long COVID’s risks, researchers would systematically survey large swaths of the population over long periods of time, watching to see who gets infected, who goes on to develop the condition, what form it takes, and how it impacts people’s health, says Shruti Mehta, an infectious-disease epidemiologist at the Johns Hopkins Bloomberg School of Public Health who is studying long COVID. But few institutions have the resources for such an undertaking, which could span many months or years. So many researchers have to make do with the limited data sets that are already available to them. As a result, some studies end up biased toward patients who were hospitalized, while others wind up favoring people who have the time, means, and trust in the health-care system to sign up for long-term studies. Neither group fully captures long COVID’s wide-ranging toll. The situation’s especially tough for pediatric patients, who might be too young to articulate the severity of their symptoms and are often excluded from long-COVID studies. Long COVID certainly exists in kids, but it may not perfectly mirror what goes on in adults: Children’s susceptibility to the virus is different, and their bodies are so rapidly changing, says Yonts, who runs a pediatric-long-COVID clinic in D.C.
All told, the study of long COVID has become, as Sanders of MIT puts it, “a data disaster.” Some researchers estimate that a single-digit percentage of SARS-CoV-2 infections bloom into long COVID; Al-Aly is one of them. Others, meanwhile, favor larger numbers, with a few even insisting that the rates are actually more than half. Most of the experts said they feel comfortable working in the 10 to 30 percent range, which is where many studies seem to be starting to converge. Finding one answer is tricky, without knowing how many forms long COVID can take—some could be more common than others. Formally splitting the disease into subdivisions could help address some of these ambiguities. But we don’t know nearly enough to start slicing and dicing, says Bryan Lau, an infectious-disease epidemiologist working with Mehta and Priya Duggal.
If researchers aren’t comprehensively capturing who currently has long COVID, they can’t say for certain who’s most likely to get it either. Many researchers have found that women contract long COVID more frequently than men. Others have uncovered evidence that people who end up infected with gobs of the coronavirus, or who produce antibodies that attack the body’s own tissues, also seem to tilt toward long COVID. Chronic health issues, including diabetes, could up a person’s chances of getting sick and staying sick as well. So might a lingering Epstein-Barr virus infection. But some of these trends are still being confirmed, experts said, and the extent to which they toggle risk up or down isn’t known. And it’s definitely too early to pinpoint any of these factors as long-COVID causes. “For acute COVID, we know what the risk factors are,” Akiko Iwasaki, an immunologist studying long COVID at Yale, said. “For long COVID, it’s much less clear.”
Still, a couple of other variables feel a bit more nailed down. “The risk is high in people who need hospitalization or ICU care,” Al-Aly said. Deepti Gurdasani, an epidemiologist at Queen Mary University of London, says she’s fairly confident that the nature of a person’s exposure to SARS-CoV-2 plays a role as well: Heavier and more frequent viral encounters seem to tip the scales toward symptoms that last and last. That’s a concern for people in essential occupations, who “aren’t able to shield themselves,” she said.
If these last few factors directly affect how and whether long COVID unspools, vaccination—which reliably staves off hospitalization and, to a lesser degree, infection—could be a partial preventive. Several studies have shown that shots do seem to muzzle long-COVID rates. They don’t, however, eliminate long COVID’s odds. To date, experts have yet to find any demographic that has been spared from the condition, despite persistent myths that certain groups, particularly kids, are somehow immune. “We’ve seen it in children of all ages,” says Laura Malone, a pediatric neurologist at the Kennedy Krieger Institute, in Baltimore. Some of her patients are toddlers. The virus isn’t pulling any punches either. Every iteration we’ve encountered so far, Omicron included, seems capable of causing long COVID. “No one is not at risk,” Al-Aly said.
To this day, most countries do not keep a running tally of long-COVID cases. But ballparks of the burden are staggering. Some 2 percent of all U.K. residents—not just those with documented infections—might currently have long COVID, according to the Office for National Statistics. Another analysis estimates that up to 23 million Americans have developed the condition since the pandemic’s start. More will join them. But Davis worries that those numbers will continue to be left off of national dashboards, and thus out of the public eye. Now that the federal government has tightened the boundaries of its concern to hospitalizations and deaths, the public does not even really have to look away from the national perspective on long COVID: There is next to nothing to see.
As people rack up different combinations of shots and infections with different variants, what worsens or soothes long COVID is also getting harder to understand. Many of the experts think long COVID is essential to study, it’s too complex for them to want to tackle themselves. Meanwhile, long COVID remains the pandemic’s looming specter. We are told there is risk, but not exactly how much; we are told that avoiding long COVID would be ideal, but lack the practical guidance to do so—the virus is so widespread that eventual infection, for many people, feels almost inevitable.
At the same time, as researchers look deeper and deeper into the bodies of infected people, they’re only seeing more damage. With each passing month, more studies emerge documenting how the coronavirus alters the function of vital organs such as the heart and the brain. The public has been cultured to think that most SARS-CoV-2 infections are trivial, and the repercussions brief, especially for the young, healthy, and privileged. But long COVID breaks the binary of severe and mild. “It’s going to continue to affect people, even people who are protected from severe illness during the acute phase of infection,” Michael Peluso, an infectious-disease physician and long-COVID researcher at UC San Francisco, said.
No matter where the true numbers on long-COVID risk sit, they are too large to ignore. “Whether it’s 10 percent or 50 percent, at both levels you have to do something about it,” Gurdasani said. Statistics will help sharpen and clarify the condition’s boundaries, and are still worth seeking out. They will not, however, change long COVID’s threat, at its core.
Davis, who is nearing her second anniversary of developing long COVID, feels this deeply. She is still experiencing cognitive dysfunction and memory loss. Her heart still races when she stands. “You cannot live your life like you used to,” she said. “Your life just becomes this shell.” For individuals, for societies, “this is not going away.” Even after much of the world puts the pandemic in its rearview, long COVID will keep filling hospitals and clinics. It will dot the pages of scientific texts, and linginfer in the bodies of millions of people worldwide. Hospitalizations and ICU admissions are not the only COVID outcomes that can buckle a health-care system.
That strain is already being felt by the health-care workers on long COVID’s front lines. Yonts, the Children’s National pediatrician, said that she’s currently booking patients “out to Memorial Day.” COVID’s global crisis can, in some ways, end when we decide to treat it as done. But that is not an option for a growing fraction of the planet, who cannot put COVID fully behind them. “This is going to be the pandemic after the pandemic,” Gurdasani said.
Source: Katherine J. Wu (The Atlantic). Image credit: Leo Correa/Redux.
119 notes
·
View notes
Link
Fears that our immune system could swiftly forget its encounter with the SARS-CoV-2 virus are increasingly unfounded with an Australian study revealing our blood is still capable of mounting a strong response eight months post-infection.
This is good news for those concerned that COVID-19 vaccines won't deliver the period of protection needed to manage the virus's spread throughout the population.
"This has been a black cloud hanging over the potential protection that could be provided by any COVID-19 vaccine and gives real hope that, once a vaccine or vaccines are developed, they will provide long-term protection," says Monash University immunologist Menno van Zelm.
While it's still too early to tell just how long immunity to this specific coronavirus might last, we can be confident time will probably be on our side.
Continue Reading.
239 notes
·
View notes