#solubility in the plasma membrane
Explore tagged Tumblr posts
Text

TSRNOSS, p381.
#nitrogen#solubility in the plasma membrane#cerebral activity#glucocorticoids#uptake of glucose#myocytes of light muscle#humming birds#New England#ethyl alcohol#old people#bacterial cell colonies#freezing damage#enzyme kinetics#plant pathology#nematodes#cytoplasmic streaming#onchiostyle#fovea#march hemoglobinuria#satyendra sunkavally#theoretical biology#manuscript#notebooks#cursive handwriting
0 notes
Text
The Physiology Of The Liver
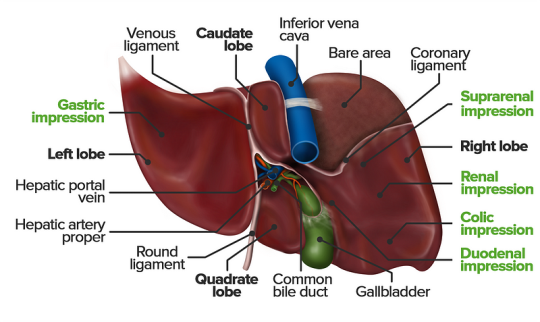
The liver is a vital organ responsible for numerous functions including metabolism, immunity, digestion, detoxification, and vitamin storage. It weighs around 2% of an adult’s body weight and is unique due to its dual blood supply from the portal vein (75%) and the hepatic artery (25%).
Cellular Structure
The liver’s functional unit is the lobule, which is hexagonal in shape. Each corner of the hexagon has a portal triad consisting of the portal vein, hepatic artery, and bile duct. The lobule is composed mainly of hepatocytes, which have distinct apical and basolateral membranes. Hepatocytes are categorized into three zones based on their function and blood supply:
Zone I (periportal region): Closest to the blood supply, involved in oxidative metabolism (e.g., gluconeogenesis, bile formation).
Zone II (pericentral region): Sits between Zones I and III.
Zone III: Farthest from the blood supply, primarily involved in detoxification and biotransformation.
Blood and bile flow in opposite directions within the liver. The space of Disse, between the hepatocytes and the sinusoidal lumen, contains Kupffer cells (macrophages) and Ito cells (fat-storing stellate cells).
Development
The liver develops from endodermal cells of the foregut as the hepatic diverticulum around the fourth week of embryonic development. It undergoes complex differentiation influenced by various pathways (e.g., Wnt/β-catenin, FGF). By the sixth week, the liver participates in hematopoiesis, and hepatocytes begin bile production by the 12th week.
Organ Systems and Functions
The liver interacts with multiple body systems:
Digestive and Metabolic Roles: Aids in digestion, stores fat-soluble vitamins, and handles cholesterol.
Hematological Functions: Produces clotting factors and proteins.
Detoxification: Metabolizes drugs and other xenobiotics through phase I (oxidation, reduction, hydrolysis) and phase II (conjugation) reactions.
Bilirubin Metabolism: Converts heme to unconjugated bilirubin, then conjugates it for excretion.
Hormonal and Protein Synthesis: Involved in thyroid hormone activation and synthesis of nearly all plasma proteins.
Related Testing
Liver function tests (LFTs), including ALT, AST, bilirubin, alkaline phosphatase, and gamma-glutamyl transpeptidase (GGT), help assess liver health. Imaging techniques like ultrasound, CT, and MRI are also employed to identify liver abnormalities.
Pathophysiology
Cirrhosis results from chronic liver injury (e.g., due to alcoholism, hepatitis B and C), leading to fibrosis and necrosis. It causes symptoms like portal hypertension, coagulopathy, and jaundice. Hepatitis viruses (A, B, C, D, E), autoimmune diseases (e.g., primary biliary cholangitis), and metabolic conditions (e.g., non-alcoholic fatty liver disease) also contribute to liver pathology.
Clinical Significance
Understanding liver physiology helps manage conditions like viral hepatitis, alcoholic liver disease, benign liver lesions, and liver cancers. Early detection through appropriate testing and management strategies is essential for preventing end-stage liver disease and improving patient outcomes
As academic students and researchers navigate the challenges of their assignments and research endeavors, Expert Academic Assignment Help stands ready to provide professional guidance and assistance. Whether you require support with assignment writing, research paper assistance, or essay help, our team of experts is dedicated to helping you achieve academic excellence. Reach out to us today at [email protected] and let us support you on your academic journey. We wish you success and professional excellence.
#medical students#healthcare#nursing school#nursing student#medicine#medical help#academic assignments#university student#medical university#university life#university#studying#study motivation#study blog#studyblr community#study inspiration#studyspo#studyblr#student#study aesthetic#medical student#aesthetic#medical school#case study
2 notes
·
View notes
Text
Changes in pressure affects how the mixture of gases in your blood interact (changes in partial pressures). Depending on the atmospheric pressure, a gas can change how much force and space it takes! And will do until finding electrochemical equilibrium!
“Dalton’s law of partial pressures states that in a mixture of gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases [20]. Therefore, air (20.9% O2, 79.1% N2) at 1 ata total pressure is made up of oxygen at a partial pressure (p) of 0.209 ata and nitrogen at 0.791 ata. At depth, when the ambient pressures increase so do the partial pressures of the constituent gases (e.g. at 20 msw, the partial pressure of nitrogen in air is 3 × 0.791 = 2.373 ata). Originally devised in 1803 by William Henry, Henry’s law states that at a constant temperature, the amount of gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid [20].
The consequence of these physical properties to the diver is that, when breathing gas under pressure, the constituents will dissolve in the body fluids (plasma, cytoplasm and lipids) proportional to the depth underwater since the alveolar/blood interface facilitates gaseous diffusion.” [Source]
“The effects of nitrogen narcosis are highly variable among divers with all divers being significantly impaired while breathing air at 60 to 70 meters, whereas some divers are affected at 30 meters. The effects are not progressive with time while depth is maintained, but symptoms progress and new symptoms develop as a diver descends deeper to greater pressures. The narcotic symptoms observed are quickly reversible upon ascent.
The symptoms seen in nitrogen narcosis begin first with effects of the higher function such as judgment, reasoning, short-term memory, and concentration. The diver may also experience a euphoric or stimulating feeling initially similar to mild alcohol intoxication. Further increases in the partial pressure of nitrogen in the blood from descending deeper lend to impairments in manual dexterity and further mental decline including idea fixation, hallucinations, and finally stupor and coma. Death can result from unconsciousness associated with severe narcosis or from severely impaired judgment leading to an accident of some form during the dive. Other factors have been linked to increased risk of nitrogen narcosis during dives while breathing compressed air and they include alcohol, fatigue, anxiety, and hypothermia. The concentration of carbon dioxide in the blood is thought to have an additive, rather than synergistic effect to nitrogen narcosis.” [Source]
“Gaseous anaesthetics when solubilised in the lipid-rich membranes of neurons cause physical swelling on the membranes (up to 5%) leading to dysregulation of cell surface proteins and affect ion channel function which can be reversed, in part, by compression [56,65].”
“Increased Pn2 leads to nitrogen narcosis, which causes impaired cognition and predisposes to accidents. Nitrogen is poorly soluble in water and blood, but is much more soluble in lipids and hence cell membranes, and importantly, neurological tissues. The uptake half-life of nitrogen is shortest in well-perfused tissues. Therefore the brain, which is well perfused and lipid-rich, takes up large amounts of nitrogen very rapidly as a diver breathing a gas with a fixed percentage of nitrogen, such as air, descends and the pressure increases.” [Source]
“Anaesthetic agents such as hyperbaric nitrogen may bind competitively to cellular proteins, directly to ion channels or other hydrophobic sites within the cell [67,68]. Anaesthetic protein interactions occur that utilise hydrophobic pockets on protein surfaces through which the narcotic agent could interact. […] Protein kinase C (PKC), guanine nucleotide-binding proteins, GABAA and ligand-gated ion channels on sensory and motor neurons have all been cited as target proteins for narcotic agents including nitrogen”.
Basically the current consensus is that Nitrogen just gets inside the cells and swells them under higher pressure, simply put! But the rate at what happens vary from person to person, and some minority of people have a susceptibility for it to happen much much sooner than most!
If someone gets a scientific paper explaining if there’s a genetic susceptibility it would be really cool <3
I know people on tumblr looove stories of underwater cave diving, but I haven't seen anyone talk about nitrogen narcosis aka "raptures of the deep"
basically when you want to get your advanced scuba certification (allowing you to go more than 60 feet deep) you have to undergo a very specific test: your instructor takes you down past the 60+ foot threshold, and she brings a little underwater white board with her.
she writes a very basic math problem on that board. 6 + 15. she shows it to you, and you have to solve it.
if you can solve it, you're good. that is the hardest part of the test.
because here's what happens: there is a subset of people, and we have no real idea why this happens only to them, who lose their minds at depth. they're not dying, they're not running out of oxygen, they just completely lose their sense of identity when deep in the sea.
a woman on a dive my instructor led once vanished during the course of the excursion. they were diving near this dropoff point, beyond which the depth exceeded 60 feet and he'd told them not to go down that way. the instructor made his way over to look for her and found a guy sitting at the edge of the dropoff (an underwater cliff situation) just staring down into the dark. the guy is okay, but he's at the threshold, spacing out, and mentally difficult to reach. they try to communicate, and finally the guy just points down into the dark, knowing he can't go down there, but he saw the woman go.
instructor is deep water certified and he goes down. he shines his light into the dark, down onto the seafloor which is at 90 feet below the surface. he sees the woman, her arms locked to her sides, moving like a fish, swimming furiously in circles in the pitch black.
she is hard to catch but he stops her and checks her remaining oxygen: she is almost out, on account of swimming a marathon for absolutely no reason. he is able to drag her back up, get her to a stable depth to decompress, and bring her to the surface safely.
when their masks are off and he finally asks her what happened, and why was she swimming like that, she says she fully, 100% believed she was a mermaid, had always been a mermaid, and something was hunting her in the dark 👍
#I love chemistry#and physiology is such a beautiful subject#getting into the physiology of diving and the adaptation the body does was really fun#thanks for the information OP
105K notes
·
View notes
Text
Coenzyme Q10 Detection Technology
In 1957, Prof. Grane of the Institute of Enzyme Research of the University of Wisconsin isolated a new quinone compound from the lipid extract of bovine heart mitochondria [1]. The compound is an orange-yellow crystal with a melting point of 48-49 ℃, capable of reversible oxygenation and reduction, and mainly involved in mitochondrial electron transfer. The compound is coded as Q-275 (Q is the initials of quinone, and 275 is the maximum absorption at 275 nm).
In 1958, American scholar Folkers and his team synthesized a series of coenzyme Q compounds, confirmed the structure of Q-275 and named it coenzyme Q10 [2]. In 1961, Mitchell, a British chemist, proposed the theory of "chemotaxis" in the study of energy conversion in living organisms and revealed the role of coenzyme Q10 in the energy conversion system of mitochondria [3], and was awarded the Nobel Prize in Chemistry in 1978. Since then, people have gradually recognized coenzyme Q10, and its applications have been widely and deeply studied.

Coenzyme Q10 (CoQ10), also known as ubiquinone, is chemically known as 2,3-dimethoxy-5-methyl 6-deca-isopentadienylbenzoquinone and consists of a benzoquinone ring and polyisoprene side chains. The number of isoprene units in the coenzyme Q series varies by species, with humans having 10 units. Coenzyme Q10 is available in both oxidized (ubiquinone, CoQ10, Ubiquinone) and reduced (ubiquinol, CoQ10H2, Ubiquinol) forms, and its chemical structure is shown in Figure 1.
Fig. 1 Chemical structures of oxidized (a) and reduced (b) forms of coenzyme Q10
Coenzyme Q10 is an important component of the mitochondrial respiratory chain, where it acts as an electron carrier and participates in electron transfer and ATP production. Furthermore, the cellular functions of coenzyme Q10 are multifaceted: it is present in all cell membranes, it limits the toxic effects of free radicals, it is a component of low-density lipoprotein (LDL), and it is involved in the aging process. Its deficiency is associated with a variety of diseases, such as mitochondrial disease, cardiovascular disease, age-related diseases, tumors, liver disease, kidney disease, etc. Panthenol is also a powerful antioxidant. Panthenol is also a powerful antioxidant, preventing lipid peroxidation in biological membranes [4].
With the deepening of the research on coenzyme Q10, the application of coenzyme Q10 is becoming more and more extensive. In addition to its use as a drug, it also has many applications in nutraceuticals, cosmetics and dietary supplements. Coenzyme Q10 is an endogenous substance, but its concentration in living organisms is very low. The analysis and determination of CoQ10 is important for the clinical diagnosis of diseases and the quality control of drugs and health products. In recent years, many analytical methods for the determination of coenzyme Q10 have been developed, which are summarized and discussed in this paper.
1 Coenzyme Q10 extraction and sample preparation
Coenzyme Q10 is insoluble in water and methanol at room temperature, slightly soluble in ethanol, soluble in acetone, 1-propanol, and soluble in organic solvents such as hexane and chloroform. Pharmaceuticals and dietary supplements such as tablets, capsules and softgels can be dissolved in ethanol, 1-propanol and other solvents, and analyzed by ultrasonication or filtration.
The isolation and enrichment of coenzyme Q10 from complex biological matrices is a laborious process.
Conventional liquid-liquid extraction is the most commonly used extraction method. This method is simple and has a large processing capacity, but has the disadvantage of high solvent consumption and some solvents can interfere with subsequent detection. Often the solvent is evaporated under N2 protection after extraction and redissolved in a mobile phase or other solvent. Whole blood samples were immediately dosed with the anticoagulants heparin or EDTA, and the plasma was centrifuged at low temperature and stored at -80°C. The plasma was then analyzed for the presence of coenzyme Q10 in the plasma. Coenzyme Q10 was extracted from plasma as follows [5]: Methanol was added to the plasma to precipitate the proteins, and the plasma was extracted with hexane. The mixture was rotated and shaken for 15 min, then centrifuged for 5 min, and the supernatant was extracted and the solvent was evaporated. The supernatant was dissolved in acetonitrile before analysis.
Coenzyme Q10 was extracted from animal heart tissue [6]. The extraction of coenzyme Q10 from animal heart tissue [6] was performed by precise weighing, transferring to homogenization tubes containing lysis medium A (containing garnet and zirconia beads), adding 1-propanol and the antioxidant 2,6-di-tert-butyl-4-methylphenol (BHT), shaking, centrifugation, and collection of the supernatant, which was analyzed immediately. The extraction of coenzyme Q10 from muscle tissue is most often done directly using muscle homogenate, or sometimes mitochondria are extracted from the tissue under ice-cold conditions, and then the mitochondrial suspension is diluted with 1-propanol, centrifuged, and the organic layer is extracted with ethanol and hexane [7]. The one-step extraction method is to use a suitable organic solvent to extract coenzyme Q10 while precipitating proteins. Yang et al. [8] studied the one-step precipitation of plasma proteins with different organic solvents (methanol, ethanol, acetonitrile, and acetone), and found that acetone was the best precipitant, and the extraction yield ranged from 71.00% to 93.07%, and was simpler than the operation of liquid-liquid extraction.
The solid phase extraction (SPE) technique can also be used for the extraction of coenzyme Q10. On-line SPE techniques are less time-consuming, less expensive, and reduce sample loss and contamination problems. The technique is usually automated using a programmable on/off valve [9]. However, protein precipitation is required prior to extraction.
Molecularly imprinted polymers (MIPs) are specialized molecular recognition techniques that have been developed in recent years. Molecularly imprinted polymers (MIPs) are formed by mixing template molecules with functional monomers, cross-linkers and initiators. After polymerization, the template molecules are removed and binding sites and cavities complementary to the templates in size, shape and function are formed [10], allowing selective recognition and adsorption of molecules structurally similar to the templates.
Contin et al. [10] synthesized MIP using coenzyme Q0 as the template, methacrylic acid as the functional monomer, acetonitrile as the pore-forming agent, ethylene glycol dimethylacrylate as the cross-linking agent, and benzoyl peroxide as the initiator. MIP was used as an adsorbent for solid-phase extraction of coenzyme Q10 from liver samples using dispersive solid-phase extraction. In addition, MIP synthesized in the same way could be used as the filling adsorbent for solid-phase extraction of coenzyme Q10 in urine. In addition, the MIP synthesized by the same method can also be used as the filling adsorbent of polypropylene columns for solid-phase extraction of coenzyme Q10 in urine, and the columns can be reused four times [11]. Compared with the traditional solid-phase extraction, MIP as a polymer adsorbent for solid-phase extraction has the advantages of simple synthesis, low cost, good stability, porous, and high selectivity for target molecules [11].
Sometimes it is necessary to maintain the original oxygenated and reduced state of coenzyme Q10 in the samples during the extraction process, which causes great difficulties due to the oxidizability of CoQ10H2. In this case, the temperature can be controlled at a low temperature of 4 ℃ during the extraction process [6,12], shortening the extraction time and using anhydrous extract will increase the stability of CoQ10H2 [13], and the use of HCl-acidified ethanol as a diluent can also prolong the stability of CoQ10H2 and prevent the auto-oxidation of CoQ10H2 [12]. BHT is an antioxidant often added in the extraction of plasma and tissue samples, which can prevent the oxidation of CoQ10H2 [6,12,14]. However, the addition of BHT to CoQ10H2 extracts from dietary supplements and pharmaceuticals was found to increase the oxidation of CoQ10H2 [13,15]. The difference in matrix composition between plasma samples and dietary supplements may be the main reason for the loss of antioxidant capacity of BHT [13].
Biological samples for coenzyme Q10 extraction include plasma, leukocytes or platelets, muscle, fibroblasts and urine [16]. Muscle biopsy is the best choice for studying coenzyme Q10 status in mitochondrial diseases, but it is very invasive; the correlation between the levels of coenzyme Q10 and tissues in plasma, blood cells and urine has been controversial, but the determination of coenzyme Q10 in these samples has an important role in therapeutic monitoring [16]. However, the determination of coenzyme Q10 in these samples is important for therapeutic monitoring [16].
The methods used to extract coenzyme Q10 from biological samples are summarized in Table 1.
Table 1 Extraction methods of Coenzyme Q10
Simple operation, large processing capacity, high solvent consumption, some solvents may interfere with the subsequent detection.
Plasma, animal heart, muscle homogenate, mitochondria
Online Solid Phase Extraction
Less time-consuming and costly, reducing sample loss and contamination.
plasma (medicine)
Molecular Blotting Techniques
Low cost, good stability, high selectivity for target molecules, and can be combined with solid phase extraction.
Animal liver, urine
2 The main assay for Coenzyme Q10
2.1 High Performance Liquid Chromatography (HPLC)
HPLC is currently the main analytical method for analyzing coenzyme Q10 in various matrices. The main detectors coupled with HPLC are ultraviolet (UV), tandem mass spectrometry (MS/MS), electrochemistry (ECD), fluorescence (FL), chemiluminescence (CL), etc. The separation effect of HPLC is good, and each detector has its own characteristics.
2.1.1 HPLC-UV
HPLC-UV is the most commonly used method for the determination of coenzyme Q10, and has become the national standard for drugs and health foods [17, 18]. It has been widely used for the determination of coenzyme Q10 in pharmaceuticals [15, 19-22], health foods or dietary supplements [15, 20], plasma [14, 23] and tissues [10]. Conventional C18 or C8 reversed-phase chromatographic columns can separate either one form of coenzyme Q10 (usually oxidized) or both oxidized and reduced forms.
Liposomes are a new type of pharmaceutical dosage form formed by the self-assembly of lipids (mainly phospholipids and cholesterol) with a bilayer structure similar to that of a cell membrane, which can encapsulate hydrophilic or hydrophobic drugs. Ruiz-Garcia et al. [21] prepared a small monolayer of liposomes encapsulating coenzyme Q10, phosphatidylserine, and fat-soluble vitamin C (6-o-palmitoyl-L-ascorbic acid) by thin-film hydration. The prepared samples were freeze-dried, solubilized in chloroform and determined by HPLC-DAD at two analytical wavelengths.
Clementino et al. [22] prepared lecithin/chitosan nanoparticles encapsulating simvastatin and coenzyme Q10. The chitosan-modified liposomes showed higher stability and narrower particle size distribution. The content of simvastatin, simvastatin hydroxylate and coenzyme Q10 was quantified by reversed-phase HPLC-UV method to account for possible degradation products. The encapsulation rate was determined and the in vitro release of the drugs was studied. According to the study, the serious side effects of statins, such as rhabdomyolysis, were associated with the decrease of coenzyme Q10, so the co-encapsulation of these two drugs is of great significance.
Coenzyme Q10, as a fat-soluble vitamin coenzyme, is often measured in conjunction with other fat-soluble vitamins. Franke et al. [14] analyzed 25 substances including 25-OH-vitamin D3, 25-OH-vitamin D2, retinol, tocopherols, carotenoids (including their stirrup isomers), and oxidized and reduced coenzyme Q10 in plasma on a fusion-nucleated 2.6 μm particle size C18 column in tandem with a C30 column, which is good at separating carotenoid isomers, and in conjunction with a six-pass valve. D2, retinol, tocopherols, carotenoids (including their stirrup isomers), and oxidized and reduced coenzyme Q10 in plasma. The switching of the six-way valve allows coenzyme Q10 to flow from the C18 column to the detector while the carotenoid isomers are eluted on the C30 column, avoiding the difficulty of separating these two substances on the same column. In addition, if a pressure-resistant UV-Vis detector is added between the C18 and C30 columns, it is possible to separate all substances without switching the six-way valve, but special software is required to control the two detectors and to acquire and process the data. It has also been reported that retinol, six carotenoids, two tocopherols, and coenzyme Q10 (10 fat-soluble vitamins) can be measured in human plasma using a MYC30 column, and the total amount of the oxidized form of coenzyme Q10 was measured by oxidizing coenzyme Q10H2 first with FeCl3 [23].
The HPLC-UV method is highly accurate and reproducible, with LOD generally on the order of μg-mL-1 and sometimes on the order of ng-mL-1 with highly sensitive detectors [14].
2.1.2 HPLC-MS/MS: HPLC-MS/MS has been developed rapidly and applied more and more widely. This method utilizes the high separation efficiency of HPLC for complex samples combined with the high sensitivity and high selectivity of mass spectrometry, which can detect low content samples under the background of complex matrix, and is widely used in the analysis and determination of target compounds in biological samples.
The main types of tandem mass spectrometry are triple quadrupole mass spectrometry [5,7,11,25,26], quadruple linear ion trap mass spectrometry [13,24], and hybrid quadruple orbit trap mass spectrometry [12], etc. Most of them use electrospray ionization, multiple reaction monitoring (MRM), and positive ionization mode. Due to the low sensitivity of [M + H]+ analysis of coenzyme Q10, ammonium adducts, i.e., [M + NH4]+, are often used to improve the sensitivity of the mass spectrometric response. By adding a certain amount of ammonium acetate to the mobile phase, [NH4]+ forms a stable five-membered chelated ammonium cation with coenzyme Q10 [8]. The formation of Li adducts has also been reported to greatly increase the sensitivity [24].
The electrostatic field orbitrap mass spectrometry (Otbitrap) is a new type of high-resolution mass spectrometry, which has the advantages of high resolution, high mass accuracy, and wide dynamic range, etc. Pandey et al. [12] applied HPLC-hybrid quadruple orbitrap mass spectrometry (Q-Orbitrap) to rapidly determine the redox state of coenzyme Q9 and coenzyme Q10. Two scanning modes, full MS/AIF and tSIM/data-dependent secondary scanning (tSIM/ddMS/MS), were compared, and it was found that full MS/AIF had higher signal sensitivity and good peak shape. During sample preparation, coenzyme Q9 and coenzyme Q10 were extracted with BHT-containing hexane to limit the oxidation of the reduced form, and the Kinetex C18 column, with fused-core SiO2 packing and smaller particle size (2.6 μm), was found to have higher column efficiency, better resolution, and good peak shape. Oxidized and reduced forms of coenzyme Q9 and coenzyme Q10 were analyzed in brain, heart, liver, adipose tissue, and muscle of healthy mice with a small amount of sample (<5 mg) and a very short analysis time (4 min). the LOD ranged from 0.01 to 0.49 ng mL-1 .
Due to the complexity of the biological sample matrix and the low concentration of coenzyme Q10, sample pretreatment is very important. Becerra et al. [11] analyzed coenzyme Q10 in human urine by molecularly imprinted polymer solid-phase extraction (MIP-SPE) coupled with HPLC-MS/MS. The pretreatment process concentrates the coenzyme Q10 by at least 5-fold. The high degree of sample purification reduces the ion suppression caused by the matrix effect of mass spectrometry. The analytical system does not interfere with protein or white blood cell elevations, which is important in cases of coenzyme Q10 deficiency with renal impairment.
The HPLC-MS/MS method uses a lot of internal standards, and the selection of suitable internal standards is also an effective way to eliminate matrix effects. Commonly used internal standards include coenzyme Q9 [5, 11, 25], coenzyme Q4 [12], and the isotopes of coenzyme Q10, coenzyme Q10-2 H6 [7] and coenzyme Q10-2 H9 [26], which are structurally similar to coenzyme Q10. Structural analogs of coenzyme Q10, such as coenzyme Q4 and coenzyme Q9, have many advantages. They are also endogenous ubiquinones and are present in human plasma at very low concentrations, or at least at levels that do not interfere with their use in analytes at the concentrations required for analysis, and therefore do not interfere with the quantification of analytes. In addition, it separates well from coenzyme Q10 [5]. A potential source of error in mass spectrometry is ion suppression, especially in electrospray ionization mass spectrometry, where the response signal of the analyte is altered and often suppressed if an interfering substance interferes with the ionization of the analyte on the surface of the droplet, or if there is competition. The use of an isotope internal standard is a good solution to the problem of ion suppression. By co-eluting the isotope internal standard with the analyte, the effects of various effects can cancel each other out, and the matrix effect can be minimized and the sample recovery can be better [7].
2.1.3 HPLC-ECD
Electrochemical detectors (ECDs) are widely used because of their high sensitivity, good selectivity and low price. Coenzyme Q10 can undergo a reversible redox reaction and can be detected by an ECD.
The commonly used detection methods are coulometric or voltammetric analysis. Different voltages are set according to the redox potentials of the substances to be measured. For oxidized coenzyme Q10, it is usually reduced to its reduced form first, and then oxidized as the original reduced coenzyme Q10 in the sample. This method can measure both oxidized and reduced coenzyme Q10 simultaneously.
Yubero et al. [27] used HPLC-ECD to determine coenzyme Q10 in urine and gave reference values for the pediatric population. An ESA Coulochem II electrochemical detector was used, and the cell voltages were -600 mV and +600 mV. The amount of coenzyme Q10 in urine fluctuated greatly at different times of the day, and the morning urine with the smallest fluctuation was chosen as the sample. The results were expressed as the amount of coenzyme Q10 per gram of particulate protein. The reference standards for children are: 2-10 years old: 24-109 nmol; 11-17 years old: 43-139 nmol. This assay provides a noninvasive method for assessing renal coenzyme Q10 status in patients with renal disease, but it is not currently available and requires up to 30 mL of urine per sample.
Schou-Pedersen et al. [6] determined reduced and oxidized coenzyme Q10 in canine plasma and cardiac tissue by HPLC-ECD and compared it with HPLC-MS/MS. The ECD was performed by fluid dynamic voltammetry using an RS6011 ultra-analytical cell at a voltage setting of 500 mV. A guard cell at -600 mV was used prior to the analytical cell to reduce oxidized coenzyme Q10 eluting from the column. Mass spectrometry was performed using a Waters Micromass Quattro micro API triple quadrupole mass spectrometer with multiple reaction monitoring (MRM) and the internal standard CoQ10-2 H9. Both methods used the same column with slightly different mobile phase ratios and additives. The results showed that CoQ10H2 was approximately 30% lower in the HPLC-MS/MS method than in the HPLC-ECD method, which may be due to differences in the calibration stock solutions or to accelerated oxidation during storage or analysis in the LC-MS/MS system. Therefore, the two methods are not interchangeable. In terms of sensitivity, the sensitivity of the two methods was comparable for coenzyme Q10H2, whereas the sensitivity of the HPLC-ECD method was higher for coenzyme Q10.
2.1.4 HPLC-FL and HPLC-CL
HPLC with a fluorescence (FL) detector is widely used for the determination of various substances in biological samples due to its high selectivity and sensitivity. Coenzyme Q10 is not a fluorescent substance and needs to be derivatized before determination. Nohara et al. [28] measured CoQ10 and CoQ10H2 in blood by post-column derivatization using HPLC using 2-cyanoacetamide and CoQ10 and CoQ10H2 heated under alkaline conditions to produce fluorescent products. The fluorescence emission and excitation wavelengths were 442 nm and 549 nm, respectively.
HPLC coupled with a chemiluminescence (CL) detector has also been reported for the determination of coenzyme Q10.Kishikawa et al. [29] used dithiothreitol (DTT) as a reductant to reduce quinone to semiquinone radicals, and semiquinone radicals converted dissolved oxygen to superoxide anion, which reacted with luminal to form CL.Accordingly, coenzyme Q10 was determined in plasma by HPLC-CL, and other components in plasma were not interfered with. Coenzyme Q10 in plasma was determined by HPLC-CL, and other components of plasma were not interfered.
Both methods require a reaction coil between the column and the detector, and require two or three pumps to mix the various reaction reagents with the coenzyme Q10-containing eluent after the column and then into the reaction coil, which is a cumbersome operation. In recent years, the literature in this area is relatively scarce.
2.2 Spectrophotometric and fluorescent methods
The Enzyme Labeler, also known as Microplate Reader, is an instrument for reading and analyzing the results of Enzyme Linked Immunosorbent Assay (ELISA) experiments. The basic principle of ELISA is similar to that of spectrophotometer or photoelectric colorimeter, using plastic microplates instead of cuvettes, usually 48-well, 96-well, or larger, with low reagent consumption, high speed, and good repeatability. Multifunctional enzyme labeling instrument often has a variety of detection functions such as absorbance, fluorescence, chemiluminescence, etc., in the medical and health inspection has been widely used.
Fukuda et al. [30] developed a rapid microtiter plate method for the determination of coenzyme Q10 using the redox cycle of quinone. Coenzyme Q10 was reduced to ubiquinone radical by NaBH4, and then the ubiquinone radical was oxidized to ubiquinone and superoxide anion radical by dissolved oxygen. The superoxide anion radical converts 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride (INT) into a pink methanol dye. It has a strong absorbance at 510 nm, which increases with increasing concentrations of coenzyme Q10. The absorbance of Mazanine dye was measured quickly and easily by a microplate reader. As an application of this method, the content of coenzyme Q10 in cosmetics was successfully determined with an LOD of 14.8 nmol-L-1 . The proposed method can be used for the rapid high-throughput analysis of ubiquinone-containing products.
Fei et al. [31] developed a new method for the determination of coenzyme Q10 in serum and urine of Alzheimer's disease patients by fluorescence spectrophotometry, also using a microplate reader. The method is based on the fact that the chemical derivative between ethyl cyanoacetate (ECA) and coenzyme Q10 is fluorescent and can be detected by fluorescence spectrophotometer (FS-ECA) at λex/em = 450/515 nm. The results showed that serum and urine levels of coenzyme Q10 were significantly lower in Alzheimer's patients than in age-matched controls. This method has similar LOD and LOQ as the HPLC-UV method.
The FS-ECA method has some advantages over the HPLC-UV method, such as easier sample preparation, faster detection speed, and similar accuracy and specificity [31].
The important role of liposomes as a new drug dosage form for co-administration and targeted delivery was described in the literature [21,22], and liposomes can also be used as micro-containers to protect and concentrate reagents.Román-Pizarro et al. [32] prepared a new type of magnetic liposomes (MLs) containing hydrophobic magnetic gold nanoparticles (Fe3 O4 @ AuNPs) and the long-wavelength fluorophore cresyl violet for the determination of coenzyme Q10 in foodstuffs. AuNPs) and a long-wavelength fluorophore, cresyl violet, were used for the determination of coenzyme Q10 in food. First, the MLs were introduced into the flow-through system using a flow injection device and retained in front of the detector for 300 s by means of a solenoid device to achieve preconcentration. Then, a coenzyme Q10 solution containing the surfactant Triton X-100 was injected into the flow-through system. The surfactant caused the solubilization of the MLs and the release of cresyl violet, which was oxidized by coenzyme Q10, resulting in a decrease in the fluorescence signal. The concentration of coenzyme Q10 is directly proportional to the decrease in fluorescence signal. The LOD of this method is lower than that of the LC-UV method, but the equipment required is simpler and less expensive.
2.3 Electrochemical analysis
The redox properties of CoQ10/CoQ10H2 allow the determination of coenzyme Q10 by electrochemical analysis. The methods are generally voltammetric, such as cyclic voltammetry (CV) [33], differential pulse voltammetry (DPV) [34], square wave voltammetry (SWV) [35], etc. The samples can be pharmaceuticals, dietary supplements, animal and plant tissues, etc. The samples can also be used for the determination of CoQ10/CoQ10H2. Samples can be pharmaceuticals, dietary supplements, plant and animal tissues, etc.
Li et al. [34] investigated the electrochemical reduction mechanism of coenzyme Q10 at a silver electrode and developed a DPV method for the direct determination of coenzyme Q10 in plant and animal tissues. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) revealed that the reduction of coenzyme Q10 under anoxic conditions is a reversible one-electron, one-proton reduction and forms a stable semiquinone radical (coenzyme Q10H-), which is quenched by oxygen in an oxygen-filled environment. This is the reason why coenzyme Q10H2 is able to scavenge oxygen radicals due to its antioxidant function. Under the optimized experimental conditions, the DPV method can be used to determine coenzyme Q10 in complex samples, and it is rapid, sensitive, and highly selective, with an LOD of 3.33 × 10 -8 mol-L-1 .
Graphene, a single atom thick planar sheet composed of carbon atoms heterogeneously linked by sp2 in a honeycomb lattice, is a new type of sensor material [35]. Screen printing is a practical technique for manufacturing low-cost disposable sensors [35].
The new graphene sensor developed by this technology can be used for the determination of coenzyme Q10 and α-lipoic acid. The MnO2-modified screen-printed graphene electrode (MnO2/SPGE) has a larger capacitance and electrically active surface area, which facilitates electron transfer and significantly improves the oxidation performance of coenzyme Q10 and α-lipoic acid. The MnO2-modified screen-printed graphene electrode coupled with square wave dissolution voltammetry (SWV) can be used for the simultaneous determination of coenzyme Q10 and α-lipoic acid in dietary supplements with high sensitivity and practicality.
The electrochemical mechanism of coenzyme Q10 is complicated by the different electrodes and media. In a 1.1:1 methanol-ethanol solution, the electrochemical reaction of coenzyme Q10 at the glassy carbon electrode was controlled by adsorption, and the sensitivity of the determination could be improved by pre-enrichment [33]. In anaerobic ethanol solution, the cathodic process of coenzyme Q10 at the silver electrode was one-electron-single proton reduction [34], while the oxidation on MnO2/SPGE showed two-electron-single proton transfer [35]. Michalkiewicz [36] investigated the anodic oxidation of oxidized coenzyme Q10 in acetic acid solution using a glassy-carbon electrode and a carbon-fiber microelectrode coupled with voltammetry, respectively. The oxidized coenzyme Q10 in acetic acid solution was studied by
The results show clear oxidation peaks or waves in the potential range above 1.5 V. The presence of these signals cannot be linked to the well-known redox pair CoQ10/CoQ10H2, but may be attributed to the irreversible and diffusion-controlled two-electron oxidation of methoxy in coenzyme Q10 (formation of two additional quinone groups at the 2 and 3 positions of the ring). the total number of electrons involved in the CoQ10 anodic oxidation is much greater than two, suggesting that the oxidation also takes place in the unsaturated isopentadienyl side chain. The total number of electrons involved in CoQ10 anodic oxidation is much higher than two, suggesting that oxidation also occurs in the unsaturated isoprene side chain. The oxidation of the oxidized coenzyme CoQ10 has been rarely reported, is much less readily accessible than that of coenzyme Q10H2, and the mechanism of oxidation has yet to be demonstrated.
2.4 Other analytical methods
Supercritical fluids are substances whose temperature and pressure exceed the critical point, in a state of gas-liquid indistinguishability, with a density close to that of liquids, and a viscosity close to that of gases, and a high diffusion coefficient. Supercritical fluids with high diffusivity and low viscosity, very suitable for mobile phase. The supercritical fluid with more research and application is supercritical CO2. Supercritical fluid chromatography-tandem mass spectrometry (SFC-Ms/Ms) with supercritical CO2 as mobile phase can be used for the determination of coenzyme CoQ10 in rat plasma [8]. The method uses one-step acetone method to precipitate the protein and extract CoQ10 from the sample. Due to the low sensitivity of [M + H]+ of CoQ10 in mass spectrometry, methanol containing ammonium acetate was used as a post-column compensating solvent to provide [M + NH4]+ and improve the sensitivity. Supercritical CO2 is non-toxic, non-flammable, and relatively inexpensive, so it is widely used. Due to its non-polar nature, it is well suited for the analysis of fat-soluble compounds and can greatly reduce the use of organic solvents.
Other analytical methods include: high performance thin layer chromatography (HPTLC) [37], Fourier transform near infrared spectroscopy (FT-NIR) [38], nuclear magnetic resonance hydrogen spectroscopy (1 H- NMR) [39], etc. HPTLC is simple and rapid, but the sensitivity is not very high, and can be used for the analysis of coenzyme Q10 in raw materials and pharmaceutical preparations. FT-NIR does not require complex sample pretreatment, but requires a certain number of samples to establish a calibration model and obtain results through complex statistical analysis, and is generally used for the initial screening of the target analyte. 1 H- NMR also does not require complex sample pretreatment, can be both calibrated models, and obtained through complex statistical analysis, and is generally used for the initial screening of target analytes. FT-NIR does not require complex sample pretreatment, but requires a certain number of samples to establish a calibration model and obtain the results through complex statistical analysis, and is generally used for the initial screening of target analytes.1 H-NMR also does not require complex sample pretreatment, and can be used both qualitatively and quantitatively, with low sensitivity, and can be used for routine analysis of preparations. These methods can be used as a useful supplement to the quantitative analysis of coenzyme Q10.
3 Simultaneous determination of oxidized and reduced coenzyme Q10
In many methods, the total amount of coenzyme Q10 is determined by adding oxidizing agents such as FeCl3 to oxidize coenzyme Q10H2 to the oxidized form, and then the total amount of the oxidized form is determined. However, coenzyme Q10 coexists in both oxidized and reduced forms in biological matrices, and sometimes it is necessary to determine the two forms of coenzyme Q10 in biological samples, drugs and supplements separately. Commonly used methods include HPLC-UV [14, 15], HPLC-MS/MS [12, 13, 24, 25, 40], HPLC-ECD [6, 41], HPLC-FL [28], and so on.
Coenzyme Q10H2 standards are sometimes not readily available and can be obtained by reducing oxidized coenzyme Q10 with reducing agents such as NaBH4 [12-14,24,25,28,40,41]. The reduction of coenzyme Q10 at low concentrations may be incomplete, even if the amount of NaBH4 is 8800-fold excess [13], so the reduction process needs to be controlled at a certain concentration range. The reaction is usually carried out at low temperature and in the dark, and sometimes ED-TA is added to the solution after the reaction [12,13,15,25,41], which mainly binds to the metal ions that catalyze the oxidation process and acts as an antioxidant. Even if a standard of coenzyme Q10H2 is available, it may be partially oxidized and needs to be reduced before use [15], or the absorbance of the stock solution (ε = 4010) can be measured spectrophotometrically at 290 nm to determine the exact concentration [6].
Whether the quantitative results were expressed as the total amount of coenzyme Q10 or as the oxidized and reduced amounts, respectively, could affect the sample preparation. In order to maintain the reduced state of coenzyme Q10H2, in addition to the rapid operation at low temperature and the addition of antioxidants such as BHT during the preparation process, researchers have different opinions on whether the extracted solution should be re-dissolved by evaporating the solvent in the presence of N2. Some of them evaporate the solvent and re-dissolve it during sample pretreatment [12,14,15,24], while some scholars believe that there will be significant oxygenation of Coenzyme Q10H2 during solvent evaporation, so the extracted solution should be immediately dissolved in the presence of N2 [12,14,15,24].
Determination [6 ,13 ,25 ,28 ,40 ,41].
In fact, coenzyme Q10H2 is highly unstable during extraction and determination.Yamashita et al.[41] found that coenzyme Q10H2 was stable only at -78 °C, and the rate of oxidation of coenzyme Q10H2 to coenzyme Q10 increased with increasing storage time and temperature.Claessens et al.[25] found that significant oxidation of coenzyme Q10H2 to coenzyme Q10 occurred after 2 h in 1-propanol extracts of human plasma, so routine analysis was limited to 12 samples per batch in order to keep the total run time within 2 h. In addition, coenzyme Q10H2 is not stable at the same temperature as coenzyme Q1010. Claessens et al. [25] found that in 1-propanol extracts of human plasma, significant oxidation of coenzyme Q10H2 to coenzyme Q10 occurred after 2 h. Therefore, routine analyses were limited to 12 samples per batch in order to keep the total run time within 2 h. The results of this study are summarized below.
Due to the uncontrolled nature of oxidation, it has been suggested that almost all mitochondrial coenzyme Q10H2 is oxidized during sample pretreatment, and therefore quantification of total coenzyme Q10 in isolated mitochondria does not require ubiquinol oxidation [7]. Nevertheless, attempts have been made to control the rate of oxidation of coenzyme Q10H2 during analysis or to know the extent of oxidation. The choice of internal standards has helped to realize this desire. Structural analogs of coenzyme Q10 and coenzyme Q10H2, such as diethyl- or dibutyl-coenzyme Q10 [14] and dipropoxy-coenzyme Q10 [40], are sometimes used as internal standards in the analysis of coenzyme Q10 and coenzyme Q10H2. They are structurally very similar to CoQ10 and CoQ10H2 and exhibit the same chemical behavior as the analytes, especially with respect to artificial oxidation, which makes it possible to accurately back-calculate the original CoQ10H2/CoQ10 ratio [14].
The CoQ10H2/CoQ10 ratios in biological tissues varied, and Claessens et al. [25] showed that the plasma CoQ10H2/CoQ10 ratios in healthy volunteers without nutrient supplementation ranged from 22.3 to 64.4, with an average of 41.7, whereas Yamashita et al. [41] showed that the plasma CoQ10H2/CoQ10 ratio was about 95/5, suggesting that plasma coenzyme Q10 is mainly in the reduced form. These results indicate that plasma coenzyme Q10 exists mainly in the reduced form.
Changes in the CoQ10H2/CoQ10 ratio have important physiological significance and are associated with many functional disorders and diseases. Measurement of the CoQ10H2/CoQ10 ratio is useful in exploring the mechanisms of many diseases. Oxidative stress has been defined as a disturbance of the pro-oxidant-antioxidant balance in favor of the former, and is considered to be a causative factor in aging and degenerative diseases such as cardiac diseases, diabetes mellitus and cancer [41]. There is a consensus that the CoQ10H2/CoQ10 ratio is an important parameter in the assessment of oxygenation stress [24, 25, 41].
Another study showed that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TC-DD) damaged mouse liver in a dose-dependent manner. Tang et al. [40] investigated the mechanism, and found that exposure of mouse liver samples to TCDD resulted in a decrease in the total amount of coenzyme Q10, a decrease in the level of coenzyme Q10H2, and an increase in the CoQ10H2/total CoQ10 ratio. This may be due to the inhibition of succinate dehydrogenase in the electron transport chain. In addition, the decrease in the total amount of CoQ10 implies that CoQ10 is degraded by external environmental influences, which was confirmed by Temova Rakua et al [42]. They found that oxidized coenzyme Q10 was degraded during storage of dietary supplements and drugs containing coenzyme Q10, and that oxidized coenzyme Q10 was converted to reduced coenzyme Q10H2, especially in the presence of antioxidants such as vitamin C.
4 Summary
Coenzyme Q10 is an important electron carrier and antioxidant component of the mitochondrial respiratory chain and is widely found in human cells. Coenzyme Q10 deficiency may be associated with a variety of diseases. Although it is an endogenous substance, it can be used as a drug or dietary supplement to treat or ameliorate certain related diseases. Therefore, the selection of efficient isolation and analytical methods is of physiological and clinical importance. Liquid-liquid extraction is the most common method for the extraction of coenzyme Q10, while the extraction of reduced coenzyme Q10H2 requires temperature control and the addition of antioxidants. Solid-phase extraction and molecular blotting techniques have also been applied in the extraction of coenzyme Q10 from biological samples, which have greatly improved the extraction efficiency and detection sensitivity.
Coenzyme Q10 can be detected by a variety of methods, and currently the most commonly used method is HPLC. In clinical and pharmaceutical analysis, miniaturization of instrumentation by reducing column diameter and length and particle size is one of the major trends in improving separations [43]. HPLC-UV is easy to use as a standard method, has good stability, is not very sensitive, but is generally sensitive enough to meet the requirements for the simultaneous analysis of a variety of components.
HPLC-MS/MS has high sensitivity and good selectivity, and has unique advantages for the analysis of coenzyme Q10 in complex matrices, such as biological samples, but the operation of the instrument is complicated and the price is expensive. The electrochemical analysis method is simple, fast and sensitive, and has certain applications in the analysis of coenzyme Q10. The HPLC-ECD method is convenient for the simultaneous determination of oxidized and reduced Coenzyme Q10. Coenzyme Q10 co-exists in both oxidized and reduced forms in almost any sample. Some analytical methods are capable of determining both the total amount of coenzyme Q10 and the oxidized and reduced forms, while others can only determine the total amount, depending on the HPLC separation. The characteristics of the various methods, their determination formats and their applications in samples are shown in Table 2.
Table 2 Comparison of different analytical methods for Coenzyme Q10
Easy and fast to use, sometimes requires color development or derivatization, matrix may be interfering
Coenzyme Q10 is mainly present in reduced form in organisms, and the ratio of CoQ10H2/CoQ10 is clinically important, with greater bioavailability of CoQ10H2 in drugs and dietary supplements. Therefore, the simultaneous determination of oxidized and reduced coenzyme Q10 is a future development. The distribution of the two forms, their interconversion and their biological significance will be a focus of future research, which also brings opportunities and challenges to the study of extraction and analytical methods for both forms of coenzyme Q10.
In order to meet the clinical needs, coenzyme Q10 can be prepared by microbial fermentation [44] or chemical synthesis in addition to extraction from biological samples. Chemical synthesis is divided into total synthesis [45] and semi-synthesis [46], and the intermediate of semi-synthesis is ganiol. Microbial fermentation can be used for large-scale industrial production.
References:
[1] Crane FL, Hatefi Y, Lester RI, et al. Isolation of a quinone from beef heart mitochondria [J].Biochim.Biophys.Acta, 1957 , 25 ( 1 ): 220-221.
[2] Wolf DE , Hoffman CH, Trenner NR, et al. Coenzyme Q. I. Structural studies on the coenzyme Q group [J]. J. Am. Chem. Soc, 1958 , 80 (17) :4752.
[3] Mitchell P. Coupling of phosphorylation to electron and hydrogen trans- fer by a chemi-osmotic type of mechanism [J].Nature,1961 ,191 :144- 148.
[4] Pallotti F,Bergamini C ,Lamperti C ,et al. The roles of coenzyme Q in disease:direct and indirect involvement in cellular functions [J]. Int.J.Mol.Sci,2022 ,23(1) :128.
[5] Visconti GL, Mazzoleni L, Rusconi C ,et al. Determination by UPLC/ MS-MS of coenzyme Q10 (CoQ10) in plasma of healthy volunteers be- fore and after oral Determination by UPLC/ MS-MS of coenzyme Q10 (CoQ10) in plasma of healthy volunteers be- fore and after oral intake of food supplements containing CoQ10[J].
J. Anal. Bioanal. Tech, 2015 ,S13 :011.
[6] Schou-Pedersen AMV,Schemeth D,Lykkesfeldt J. Determination of re- duced and oxidized coenzyme Q10 in canine plasma and heart tissue by HPLC-ECD : a comparison with LC-MS/MS quantification [J].Antioxi- dants,2019 ,8(8) :253.
[7] Itkonen O,Suomalainen A,Turpeinen U. Mitochondrial coenzyme Q10 determination by isotope-dilution liquid chromatography - tandem mass spectrometry〔J〕.Clin.Chem,2013 ,59(8) :1260-1267.
[8] Yang R,Li Y,Liu C ,et al. An improvement of separation and response applying post-column compensation and one-step acetone protein pre cipitation for the determination of coenzyme Q10 in rat plasma by SFC- MS/MS [J].J. Chromatogr.B ,2016 ,1031 :221-226.
[9] Bompadrea S,Tulipanib S,Romandini S,et al. Improved HPLC col- umn-switching determination of coenzyme Q and Vitamin E in plasma [J]. 2008 ,32(1-4) :257-262.
[10] Contin M,Flor S,Martinefski M,et al. The use of coenzyme Q0 as a template in the development of a molecularly imprinted polymer for the selective recognition of coenzyme Q10[ J].Anal.Chim.Acta,2014 ,807 :67-74.
[11] Becerra CG,Baez F,Lucangioli S,et al. Miniaturized imprinted solid phase extraction to the selective analysis of Coenzyme Q10 in urine [J]. Chromatogr.B ,2019 ,1116 :24-29.
[12] Pandey R,Riley CL,Mills EM,et al. Highly sensitive and selective determination of redox states of coenzymes Q9 and Q10 in mice tis- sues. Application of orbitrap mass spectrometry [J].Anal.Chim.Acta, 2018 ,1011 :68-76.
[13] Vass A,Deák E ,Dernovics M. Quantification of the reduced form of coenzyme Q10 , ubiquinol, in dietary supplements with HPLC-ESI- MS/MS [J]. food Anal.Methods,2015 ,8(2) :452-458.
[14] Franke AA, Morrison CM, Custer LJ, et al. Simultaneous analysis of circulating 25-hydroxy-vitamin D3 ,25-hydroxy-vitamin D2 ,retinol, to- copherols,carotenoids,and oxidized and reduced coenzyme Q10 by high performance liquid chromatography with photo diode-array detec- tion using C18 and C30 columns alone or incombination [J].J. Chromatogr A,2013 ,1301 :1-9.
[15] Temova Rakua,Kristl A,Rokar R. Quantification of reduced and oxi- dized coenzyme Q10 in supplements and medicines by HPLC-UV [J].Anal.Methods,. 2020 ,12(20) :2580-2589.
[16] Yubero D,Allen G,Artuch R,et al. The value of coenzyme Q10 de- termination in mitochondrial patients [J].J.Clin.Med,2017 ,6(4) : 1-10.
[17] National Pharmacopoeia Commission, ed. Chinese Pharmacopoeia (Part II) [S]. Beijing: China Pharmaceutical Science and Technology Publishing House ,2020 :1457.
[18] GB/T 22252-2008. Determination of Coenzyme Q10 in Health Food [S].
[19] Grace AC,Prabha T,Jagadeeswaran M,et al. Analytical method develop- ment for simultaneous determination of ubidecarenone and vitamin E ace- tate in capsule dosage form by HPLC [J].Int.J.Pharm.Pharm.Sci, 2019 ,11(1) :79-84.
[20] Rakusa ZT,Srecnik E ,Roskar R. Novel HPLC-UV method for simul- taneous determination of fat-soluble vitamins and coenzyme Q10 in medicines and supplements [J].Acta.Chim.Slov,2017 ,64(3) :523 - 529.
[21] Ruiz-Garcia M, Perez-Lozano P, Mercade-Frutos D, et al. Development and validation of a new high-performance liquid chromatography method for the simultaneous quantification of coenzyme Q10 ,phos- phatidylserine, and vitamin C from a cutting-edge liposomal vehiculi- zation [J].ACS Omega,2019 , 4(22) :19710-19715.
[22] Clementino A,Sonvico F. Development and validation of a RP-HPLC method for the simultaneous detection and quantification of simvasta- tin ′s isoforms and coenzyme Q10 in lecithin/chitosan nanoparticles [J].J.Pharm.Biomed.Anal,2018 ,155 :33-41.
[23] Boulet L,Alex B ,Clavey N,et al. Simultaneous analysis of retinol,six carotenoids,two tocopherols,and coenzyme Q10 from human plasma by HPLC [J]. J. Chromatogr.B ,2020 ,1151 :122158.
[24] Kotnik D,Jazbec-Krizman P,Krizman M,et al. Rapid and sensitive HPLC-MS/MS method for quantitative determination of CoQ10 [J]. Research on Precision Instrument and Machinery ( RPIM) ,2013 ,2 (1) :6-13.
[25] Claessens AJ,Yeung CK,Risler LJ,et al. Rapid and sensitive analysis of reduced and oxidized coenzyme Q10 in human plasma by ultra per- formance liquid chromatography-tandem mass spectrometry and appli- cation to studies in healthy human subjects [J].Ann.Clin.Biochem, 2016 ,53(2) :265-273.
[26] Mathieu RE ,Riley CP. Quantitation of ubiquinone (coenzyme Q10) in serum/plasma using liquid chromatography electrospray tandem mass spectrometry (ESI-LC-MS/MS) [J].Methods.Mol.Biol,2016 , 1378 :61-69.
[27] Yubero D,Montero R,Ramos M,et al. Determination of urinary coen- zyme Q10 by HPLC with electrochemical detection:Reference values for a paediatric population [J].Biofactors,2015 ,41(6) :424-430.
[28] Nohara Y, Suzuki J, Kubo H. Determination of ubiquinone in blood by high-performance liquid chromatography with post-column fluorescence- cence derivatization using 2-cyanoacetamide[J].J. Fluoresc,2011 ,21 (6) :2093-2100.
[29] Kishikawa N,Ohkubo N,Ohyama K et al. Selective determination of ubiquinone in human plasma by HPLC with chemiluminescence reac- tion based on the redox cycle of quinine[ J].Anal.Bioanal.Chem,2011 ,400(2) :381-385.
[30] Fukuda M, Liu Q, Kishikawa N, et al. Development of ultrafast colori- metric microplate assay method for ubiquinone utilizing the redox cy- cle of the quinine [J].Microchem.J,2019 ,150(C) :104104.
[31] Fei X, Yu Y, Di Y, et al. A rapid and non-invasive fluorescence meth- od for quantifying coenzyme Q10 in blood and urine in clinical analy- sis [J]. Clin. Lab. Anal,2020 ,34(4) :e23130.
[32] Román-Pizarro V,Fernández-Romero JM,Gómez-Hens A. Automatic determination of coenzyme Q10 in food using cresyl violet encapsula- ted into magnetoliposomes [J].Food Chemistry,2017 ,221 :864-870.
LIU Yuhong,GUO Bin,TU Yifeng [33]. Determination of coenzyme Q10 by adsorption voltammetry[J]. Journal of Analytical Testing ,2021 ,40(8) :1224-1229.
[34] Li D,Deng W,Xu H,et al. Electrochemical investigation of coenzyme Q10 on silver electrode in ethanol aqueous solution and its determina- tion using differential pulse voltammetry〔J〕.J.Lab.Autom,2016 ,21 (4) :579-589.
[35] Charoenkitamorn K, Chaiyo S, Chailapakul O, et al. Low-cost and dis- posable sensors for the simultaneous determination of coenzyme Q10 and α- Lipoic acid using manganese (IV) oxide-modified screen-prin- ted graphene [J].Anal.Chim.Acta,2018 ,1004 :22-31.
[36] Michalkiewicz S. Anodic oxidation of oxidized forms of coenzymes Q10 and Q0 on carbon electrodes in acetic acid solutions [J].Bioel- ectrochemistry,2011 ,82(2) :103-111.
[37] Kulkarni MB ,Joshi AM,Patil RV.A novel HPTLC method for simul- taneous determination of co-enzyme Q10 and α-tocopherol in bulk and pharmaceutical formulation [J].Int.J.Pharm.Pharm.Sci,2018 , 10(10) :134-141.
[38] Rácz A,Vass A,Héberger K,et al. Quantitative determination of co- enzyme Q10 from dietary supplements by FT-NIR spectroscopy and statistical analysis [J].Anal.Bioanal.Chem, 2015 , 407 ( 10 ): 2887-2898.
[39] Monakhova YB ,Ruge I,Kuballa T,et,al. Rapid determination of co- enzyme Q10 in food supplements using 1H NMR spectroscopy.Int.J.Vitam.Nutr.Res,. 2013 ,83(1) ,67-72.
[40] Tang Z,Li S,Guan X,et al.Rapid assessment of the coenzyme Q10 redox state using ultrahigh performance liquid chromatography tandem mass spectrometry[J].Analyst,2014 ,139(21) :5600-5604.
[41] Yamashita S, Yamamoto Y. Simultaneous Detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress [J]. Anal. Biochem,1997 ,250(1) :66-73.
[42] Temova Rakua, Kristl A, Rokar R. Stability of reduced and oxidized coenzyme Q10 in finished products [J]. Antioxidants, 2021 , 10 (3) :360.
[43] Lucangioli S,Martinefski M,Tripodi V. Coenzyme Q10 analytical de- termination in biological matrices and pharmaceuticals [J]. Front.Biosci.Scholar,2016 ,8(2) :321-330.
[44] Fan JB ,Xu W,Xu Xi,et al. Production of Coenzyme Q10 by mi- crobes:an update [J].World J.Microbiol.Biotechnol,2022 ,38(11) :194.
[45] Nguyen T,Mac H,Pham P. Preparation of Key Intermediates for the Syntheses of Coenzyme Q10 and Derivatives by Cross-Metathesis Re- actions [J]. Molecules,2020 ,25 :488.
[46] Atla SR,Raja1 B ,Dontamsetti BR.A new method of synthesis of co- enzyme Q10 from isolated solanesol from tobacco waste [J]. Int.J.Pharm.Pharm.Sci,2014 ,6(8) :499-502.
#coenzymecoq10 #Coenzyme Q10 #Q10 #coq10
0 notes
Text
Dried Fig
Dried figs are one of the valuable agricultural products that are cultivated in many parts of the world. Iran is known as one of the most important producers in the world with a suitable climate and a long history in the cultivation and production of figs. Estahban is a city in the Fars province of Iran, which has gained international fame in the production of dried figs due to its unique weather conditions and fertile soil.
First part: product introduction
Estahban dried figs for export are obtained from high-quality figs produced in this region. Fig is a tree with sweet and delicious fruits, and it continues using its natural and nutritious properties by drying. Estahban, as one of Iran’s main fig production centers, produces high-quality figs that are very suitable for export due to the favorable weather conditions and fertile soil.
Second part: Benefits of dried figs
Enhance Digestive Health Fiber is great for digestion, and figs are loaded with dietary fiber, which aids healthy bowel movement and relieves constipation the fiber in figs also treats diarrhea and soothes the entire digestive system.
Improve Heart Health Figs reduce the triglyceride levels in the blood and contribute to improving heart health Triglycerides are fat particles in the blood that are a leading cause of heart diseases. Figs also contain phenols and omega-3 and omega-6 fatty acids that decrease the risk of heart disease.
Lower Cholesterol Figs contain pectin, a soluble fiber that is known to reduce cholesterol levels. The fiber in figs clears the excess cholesterol in your digestive system and carries it to the bowels to eliminate it
Prevent Colon Cancer Regular consumption of figs can lower the risk of colon cancer. The fiber in figs helps to eliminate the waste in the body quickly, which works well for the prevention of colon cancer. The numerous seeds in figs contain high levels of mucin that collects wastes and mucus in the colon and flushes them out.
Cure Anemia A lack of iron in the body can cause iron-deficiency anemia. Dried figs contain iron, which is a key component of hemoglobin. Consuming dried figs was found to improve the hemoglobin levels in the blood. Lower Sugar Levels in Diabetic Patients Dried Figs have amazing properties that help regulate blood glucose levels.
Prevent Breast Cancer Figs are amongst those fruits that contain the highest amount of fiber. And it was found that women who consumed more dietary fiber during adolescence and early adulthood were at a lesser risk of falling prey to breast cancer.
Strengthen Bones Figs contain calcium, potassium, and magnesium, all of which aid bone health. Calcium is crucial to maintaining healthy bones and figs are one of the best sources of it. Figs contain potassium that counteracts the increased urinary calcium loss caused by high-salt diets. This prevents your bones from thinning out.
Rich in Antioxidants Figs are a powerhouse of antioxidants, and they neutralize the free radicals in your body and fight diseases. The riper a fig is, the more antioxidants contains. Dried Figs are a rich source of phenolic antioxidants. The antioxidants in figs enrich the lipoproteins in plasma and shield them from further oxidation.
Prevent Hypertension When you consume less potassium and more sodium, it disturbs the sodium-potassium balance in your body, paving the way for hypertension. Figs help restore this balance as they are rich in potassium.
Treat Asthma Figs moisturize the mucous membrane and drain the phlegm, thereby relieving asthma symptoms. They also contain phytochemical compounds that fight the free radicals, which otherwise trigger asthma.
Prevent Venereal Disease The consumption or application of fig extracts is known to provide relief from sexually transmitted diseases in many cultures. Figs are known to have been used as a calming balm for venereal diseases.
Reduce Throat Pain Figs contain high mucilage that heals and protects against sore throat. These fruits are soothing to the throat, and their natural juices relieve pain and stress in the vocal cords. Also, figs are a natural cure for tonsillitis. They help in reducing the swelling and irritation caused due to the condition. Make a paste of the figs with warm water and apply it to your throat. It will reduce pain and soothe your throat.
Prevent Coronary Heart Disease The antioxidants in figs, as well as their blood pressure-lowering properties, eliminate the free radicals in the body, which otherwise block the coronary arteries, leading to coronary heart disease. Also, the presence of potassium, omega-3s, and omega-6s in figs helps in preventing heart attacks.
Dried figs are a good Source of Energy Adding dried figs to your diet is a sure shot way to increase your energy levels. The carbohydrates and sugar present in figs increase the percentage of energy in your body
SOURCE: BENMARYFOODS
0 notes
Text
Revolutionizing Disease Detection | Unveiling the Potential of Diagnostic Exosome Biomarkers in Healthcare
The global diagnostic exosome biomarkers market is expected to secure US$ 172.1 Million in 2022. From 2022-2032, the market is anticipated to display a CAGR of 16.3% while garnering a market value worth US$ 904.1 Million.
The fusion of plasma membrane with the internal vesicle fusion leads to the secretion of nanovescicles called exosomes into the extracellular environment. The exosomes are released in easily accessible body fluids such as urine and blood and hence acts as a precious source of biomedical tool. As cancer is a booming research area, exosomes may act as a very useful biomarkers for the diagnosis and prognosis of malignant tumor.
The application of exosome as a potential biomarker for the various neurodegenerative disorders is also under investigation. All this is expected to create a new market where the industry payers can focus on new product developments.
Diagnostic Exosome Biomarkers Market: Drivers and Restraints
Exosomes are the biomimetic nanovectors for a variety of nucleic acid, chemicals and proteins. Exosome-encapsulated curcumin, delivered by the intranasal route is efficient in preventing brain inflammation, and specific gene silencing miRNAs enclosed in targeted exosomes and delivered systemically have shown promising therapeutic effects.
Exosomes biomarkers fix in the ideal theranostic approach as they can act as biomarkers or vectors of therapeutic molecules. The theranostic approach is very prominent in personalized medicine where the individual is monitored and diagnosed for a particular mode of treatment. The exosome biomarkers also help in providing targeted drug delivery system thereby providing a very potential market.
Diagnostic Exosome Biomarkers Market: Overview
The extracellular vesicles called exosomes are sized around 100 nm in diameter, which are released from many different cell types. Exosomes are produced by different mechanism and hence differ from other class of extracellular vesicles and microvesicles that are different in size too.
Exosomes contains a range of biomolecules including membrane-bound and soluble proteins, microRNA, lipids and noncoding RNA. Therefore exosomes are a good source of disease biomarkers for early diagnosis and/or prediction of disease progression.
Diagnostic Exosome Biomarkers Market: Region-wise Outlook
In terms of geography, diagnostic exosome biomarkers market has been divided into seven regions including North- America, Eastern Europe, Western Europe, Asia- Pacific excluding Japan, Japan Middle-East & Africa and Latin America. North America is expected to remain the dominating region while Asia Pacific is expected to emerge as a fastest growing region.
In 2016, the National Institutes of Health is seeking grant applications for projects investigating the potential of exosomes and extracellular vesicles (EVs), as well as their cargo, as biomarkers for cancer risk assessment, detection, diagnosis, and prognosis. Such research funding and government support is expected to accelerate the growth of the diagnostic exosome biomarkers market.
For More Information: https://www.futuremarketinsights.com/reports/diagnostic-exosome-biomarkers-market
Diagnostic Exosome Biomarkers Market: Key Market Participants
The diagnostic exosome biomarker market players are expanding their laboratory capacities to fit in to the increasing demand. Exosome Diagnostics moved into its new ISO 13485certified facility, built for the company’s patented liquid-biopsy sample processing and analysis technologies.
Some of the diagnostic exosome biomarkers market participants are 101Bio, AMS Biotechnology Limited, BioRegenerative Sciences, Inc., Cell Guidance Systems LLC, Codiak BioSciences, Evomic Science LLC, ExoCyte Therapeutics Pte Ltd, Exosome Diagnostics, Inc, Exovita biosciences, Immune Therapy Holdings AB, Lonza Group, Norgen Biotek Corp., ReNeuron Group plc and Therapeutic Solutions International, Inc.
The research report presents a comprehensive assessment of the market and contains thoughtful insights, facts, historical data, and statistically supported and industry-validated market data. It also contains projections using a suitable set of assumptions and methodologies. The research report provides analysis and information according to market segments such as geographies, application, and industry.
0 notes
Text
THE EXCELLENT RANGE OF LECTIN CONJUGATES AND THEIR APPLICATION
WHAT ARE LECTIN CONJUGATES?
Proteins (building blocks) that attach to cells and specific carbohydrate groups on proteins or cell membrane proteins are known as lectin conjugates. They are further classified according to their amino acid sequences and biological characteristics. Lectins contain 120 amino acids that are involved in carbohydrate binding.
Because of its carbohydrate binding, lectin is employed in glycobiology to analyse cell surface glycoproteins. Lectins are synthesised in laboratories after being extracted from plant or animal components.
The capacity of Lectins to form precipitates with glycoconjugates is due to the fact that most lectin proteins are composed of non-covalently linked subunits. Agglutination of cells by Lectins is uncommon in nature and thus extremely difficult to detect.
Lectins enable scientists to investigate a wide range of biological structures and functions. Some Lectins bind to mannose or glucose residues, while others bind only to galactose residues due to their complex binding requirements. Some Lectins also require sugar-binding at specific oligosaccharide sites.
LECTINS APPLICATION
Lectins are being used in clinical laboratories to type blood cells. There is the extensive usage of Lectin in specialist applications such as-
• As chemotherapeutic agents
• In fractionation of animal cells as mitogens.
• While investigating cellular surfaces
• Lectins isolate specific cells or viruses with a mixture and study determined processes amongst several.
LECTIN IN ANIMALS
Regulate cell adhesion
• Glycoprotein synthesis is regulated by Lectins
• They can also regulate blood protein levels.
• Recognition of galactose residues on the surface of mammalian liver cells responds better to Lectins.
LECTIN IN PLANTS
Plants are naturally rich in lectins. Dietary lectins are found in protein sources such as beans and legumes, peanuts, lentils, wheat, uncooked kidney beans, fruits, and vegetables. Conversely, lectin-free diet items include pasture-raised meats, cooked sweet potatoes, cruciferous vegetables, asparagus, garlic, and onion.
Lectin activity and function in plants are both unknown. The content of Lectin in plant seeds decreases as they mature. Plant Lectin has the ability to recognise hydrophobic noncarbohydrate ligands.
Adenine, auxins, cytokinin, indole-3-acetic acid, and water-soluble porphyrins are examples. Because these compounds behave as phytohormones, their interactions may be psychologically significant.
Furthermore, the plasma membranes of human EL4 tumour cells are labelled with horseradish peroxidase-conjugated wheat-germ agglutinin. After the labelled intact cells are disrupted, plasma-membrane refinement is observed by ultrastructural examination of the various fractions for positive effect product on the membrane vesicles.
LECTINS AND OTHER CARBOHYDRATE-BINDING PROTEINS
Cellular proteoglycans, glycoproteins, and glycolipids include a wide range of oligosaccharides. Fluorescent carbohydrate-binding derivativesMicroscopy PROTEINS and flow cytometry both use proteins to identify intracellular glycoconjugates. This is done to isolate glycoproteins on protein blots and cause agglutination of specific cell types. Lectins can also be used to detect cancer since they have changed surface glycoproteins.
LECTINS INTERCONNECTING

Biotechnology has narrowed down biorecognition molecules with diagnostic potentials in light of the different diseases that impact the human species. Particular Lectin content binds with mono- or oligosaccharides with high affinity via no covalent connection via hydrogen bonds.
Lectins from viruses, bacteria, fungi, algae, mammals, and plants recognise carbohydrates in cells, tissue sections, and biological fluids. These are useful tools for diagnostic purposes. To find medicines and inhibitors, sialic acid-specific lectins such as Influenza Virus Hemagglutinin are being studied. These can remove or inhibit sialic acid in host cells, preventing it from binding.
Similarly, strong anti-HIV activity in vitro has been associated with bacterial Lectins. Large levels of algal Lectins are attracting interest for biomedical uses such as anti-HIV, anti-inflammatory, antibacterial, and antinociceptive properties. Animal Lectins are important in psychological processes such metastatic cancer, apoptotic pathways, and immunomodulation.
LECTIN INDUCED MECHANISMS OF INFLAMMATION RESPONSES
Immune systems act in two specific ways called; innate and adaptive responses. These responses are activated by a group of cells and molecules that promote the destruction of aggressive agents. Neutrophils, eosinophils, basophils, and mono/macrophages can generate and release molecules called cytokines.
These molecules modulate the activation of immune cells, inflammation, and humoral responses. Biomolecules like these are the answer for adjustment of immune conditions and therapeutic applications in regards to immune response-related diseases.
Lectins are thought to contribute to the development of diseases such as celiac disease, autoimmune diseases, rheumatoid arthritis, obesity, cardiovascular disease, and type 2 diabetes. This happens through translocation across the intestinal barrier and activation of the adaptive immune system. Common high-lectin foods include grains, legumes, and nightshades.
Lectins aren’t digestible. They bind to cell membranes lining the digestive tract, where they may disrupt metabolism and cause damage. Lectin sensitivity is the body’s delayed immune response that can occur some hours to even days after these foods are consumed.
Symptoms associated with lectin sensitivities include:
•Bloating and abdominal cramps
•Painful or swollen joints
•Tiredness
•Skin problems
•Hormonal fluctuations
•Nausea
•Allergies or allergy-like symptoms
•Neurological symptoms
The highest concentrations of lectins are found in healthy foods like legumes, grains, and nightshade vegetables. Fortunately, there are ways to reduce the lectin content of these healthy foods to make them safe to eat.
Research studies have shown that by cooking, sprouting, or fermenting foods high in lectins, their lectin content can easily be reduced to negligible amounts.
Foods That Are High in Lectins
1. Red kidney beans
Raw kidney beans contain high levels of a lectin called phytohaemagglutinin. Eating them raw or undercooked can cause severe nausea, vomiting, and diarrhea. As few as five beans can cause a response.
A hemagglutinating unit (hau) is a measure of lectin content. When in raw form, red kidney beans contain 20,000–70,000 hau. Once cooked, however, they contain only 200–400 hau, which is considered a safe level.
In cooked form, they are valuable and nutritious food.
2. Soybeans
Soybeans have several health benefits but are another food that also contains high levels of lectins.
As with red kidney beans, cooking soybeans almost eliminates their lectin content, provided they are cooked at high temperatures. Studies show that soybean lectins are almost completely deactivated when at 100°C for at least 10 minutes.
3. Wheat
Raw wheat, including wheat germ, is high in lectins, with around 300 mcg of wheat lectins per gram. (Whole-wheat flour has a much lower lectin content at about 30 mcg per gram). Lectins are almost completely eliminated by cooking and processing, and as most whole-wheat products consumed are cooked, it’s not likely that lectins pose a major risk to health.
4. Tomatoes
Tomatoes are part of the nightshade family. They are high in fiber, a good source of potassium and vitamin K1, and high in vitamin C. (One tomato provides about 20% of the daily recommended value.
Tomatoes also contain lectins, though there is little evidence that they have any adverse effects on humans. Some people have linked tomatoes and other nightshade vegetables to inflammation, such as arthritis. No formal research has supported this link.
5. Potatoes
Potatoes are also members of the nightshade family and a good source of vitamins and minerals. Potato skins are particularly high in antioxidants, such as chlorogenic acid, which has been linked to a reduced risk of heart disease and type 2 diabetes. As with tomatoes, adverse effects have been experienced by some when eating potatoes. Studies have shown that this could be linked to lectins.
6. Peanuts
Peanuts are an excellent source of protein, unsaturated fats, and many vitamins and minerals.
Peanuts do contain Lectin, and one study found that peanut lectins increased growth in cancer cells. With evidence that peanut lectins can enter the bloodstream, this has led some people to believe that lectins could increase the risk of cancer spreading in the body. However, the above study was carried out using very high doses of pure lectins placed directly onto cancer cells.
No studies have investigated their exact effects on humans. Evidence of their health benefits appears to be stronger than that of any risks.
DRUG DELIVERY USING LECTIN SOURCE OF PROTEIN
Chemical agent therapies often come across as barriers when the need for increasing dosages and action of metabolism reduces the effectiveness of treatments. Delivery of drugs to specific targets requires a new and an effective strategy to combat side effects and chemical reactions.
Lectin medicated bio adhesion constitutes specified interactions with receptor-like structures in the cell membrane, binding directly to targeted cells. Therefore Lectins can interact differently with distinct cells and act as drug carriers to desired tissue and cells. For it to be a tool in drug delivery Lectins, need to be avid binding, low toxicity, and site-specific molecules.
To conclude, Lectins from diversified sources with distinct carbohydrate recognition events play a vital role in many biotechnological applications/disease therapies. The uses in vitro and in vivo display Lectins with protective effects against viruses and microorganisms. Lectins are a highly potent modulator of an immune response, mitosis, proli9, healing, drug delivery therapies, and cancer regression.
Histochemistry, biosensors, detect diseases, and infections against glycans alterations on cells or tissue surfaces, and serum samples can be isolated using Lectin-based technology and techniques. There is potential to unravel new interpretations in the biological effects, pathways, and biotechnological potential of Lectins. They are focusing on their achievements in therapeutic applications and health effects.
Want to learn more about Lectin Conjugate, its usage, and health benefits? Contact our experts at Helvetica Health Care today!
1 note
·
View note
Text
The Natural Way - How to Reduce High Cholesterol
Cholesterol is a wax-like substance present in the cell membranes of body tissues and is carried in the blood plasma. It is a sterol (alcohol and steroid combination), also called atherosclerotic plaque. The body requires cholesterol to form and sustain the plasma membrane, help with bile production, and aid the metabolism of fat-soluble vitamins. However, having high blood cholesterol levels is not always good and can even cause an increased risk of cardiovascular health problems such as a heart attack or stroke.
Over time cholesterol builds up on the artery walls, a condition called atherosclerosis. Atherosclerosis was thought to be an affliction of the elderly until the 1950s. When American pathologists were sent to Korea by the Pentagon to study the bodies of soldiers who died during the conflict, they autopsied around 2000 soldiers and found that approximately 75% had waxy, yellow deposits on the walls of their arteries; a shocking statistic considering the average age of the soldiers was 21. Their findings astonished the scientific community by highlighting the onset of heart disease in the very young. That’s why our focus here is to help you know a natural way to lower high cholesterol. https://unbelievablehealth.com/the-natural-way-how-to-reduce-high-cholesterol
13 notes
·
View notes
Text

Hormones are chemical substances that act like messengers in the body. They are produced by glands, ovaries and testes, and released into the bloodstream. From the blood they can reach any of their target destinations, where they serve different purposes, including regulating long-term behavioural patterns. They can affect such things as growth, development, reproduction, sexual function, metabolism, mood, etc.
Here is a diagram of how hormones move through the body. From the secreting cell hormone enters the bloodstream and moves along it to further enter the target cell. There can be multiple target cells for just one hormone.

When reaching the target cell, hormones bind to receptors. Receptors are proteins embedded into the plasma membrane that are shaped to fit specific molecules (substrate-specific receptors). Later some other reactions take place producing second messengers. In the end, those messages activate various cellular responses. This diagram demonstrates the work of water-soluble hormones that are unable to pass through the membrane that has hydrophobic layers. Fat-soluble hormones pass through the cell membrane directly and produce their effect by binding to receptors inside the cell.

Source: https://oakcrestapbio.wordpress.com/endocrine-system/how-hormones-work/
Hormones are very powerful - very little amount is needed to perform its function. That is why any discrepancies from the normal levels are dangerous. For example, high levels of oestrogen (e.g. from taking birth control pills) can put you at higher risk of blood clots, stroke and even thyroid dysfunction, while low levels of it interfere with sexual development, can cause more frequent UTIs, irregular menstrual cycles and many more.
Some signs of hormonal imbalance include low libido, insomnia or poor-quality sleep, unexplained weight gain, headaches, fertility problems, skin problems, weak bones, mood swings, heavy and painful periods, etc. It is always best to consult a specialist if you experience one or more of those to get an accurate diagnosis, because many of those can be caused by other diseases as well.

#neuroscience#biology#science#brain#diseases#hormones#hormonal imbalance#estrogen#birth control#low libido#insomnia#headaches#acne#mood swings#weight gain#hormonal signaling#cells#plasma membrane#binding#receptor
3 notes
·
View notes
Text

The Research Notebooks of S. Sunkavally. Page 118.
#axon conduction#low temperature#Kodiak bears#island effect#non-ionic surfactants#cholesterol#hydrophobic regions#phospholipid#sheet formation#plasma membrane#lateral diffusion#oxygen solubility#frog skin#range of molecualr speeds#Boltzmann distribution#theoretical biology#cursive#handwriting#manuscript#notebooks
1 note
·
View note
Text
Reduce Cholesterol Level Without Drug
Cholesterol is a wax-like substance that is present in the cell membranes of body tissues and is carried in the blood stream plasma. It is a sterol; a combination of alcohol and steroid and is also called atherosclerotic plaque. The body requires cholesterol in order to form and sustain cell membranes, help with the production of bile and aid the metabolism of fat soluble vitamins. Over time, cholesterol builds up on the artery walls and this condition is known as atherosclerosis. Atherosclerosis was thought to be an affliction of the elderly until the 1950’s when American pathologists were sent to Korea by the Pentagon to study the bodies of servicemen who died during the conflict. They autopsied around 2000 soldiers and found that approximately 75% had waxy, yellow deposits on the walls of their arteries; a shocking statistic considering the average age of the soldiers was 21. Their findings astonished the scientific community as it highlighted the onset of heart disease in the very young. LDL and HDL Cholesterol There are two different types of cholesterol; low-density lipoproteins (LDL) and high-density lipoproteins (HDL). HDL is commonly known as ‘good’ cholesterol and LDL is recognised as ‘bad’ cholesterol. LDL has been markedly linked with heart disease, whereas HDL is thought to reduce the chance of a heart attack. It has been estimated that approximately 25% of Americans are at risk from heart disease due to atherosclerosis, and around 10% have such high levels that doctors are left no choice than to prescribe cholesterol reducing drugs. Foods that Lower LDL Cholesterol There are a number of foods which are believed to significantly lower LDL cholesterol. Fiber-rich foods are said to be particularly beneficial and will not only lower ‘bad’ cholesterol, but also help the bowel to function properly, lowering the risk of colon and bowel cancer. There are many other foods that can help the body fight back against these dangerous deposits. Fiber-rich foods, particularly oat bran, barley and wheat bran. They can be eaten as a cereal for breakfast and sprinkled onto other foods. Pearl barley can be added to soups. Apples and pears also have considerable amounts of soluble fiber and should be enjoyed on a daily basis Beans and pulses are high in fiber and low-fat. They also contain lecithin, a nutrient that lowers cholesterol. Try to incorporate kidney, fava, borlotti and other dried whole-foods into your diet; there are many different types of lentils and pulses that are delicious in soups and stews Avocado contains prolific amounts of monounsaturated fat, which helps to reduce LDL cholesterol and has many other health benefits including anti-cancer properties Raw carrots are rich in a fiber called pectin that is renowned for minimizing cholesterol. There are a number of fruits that also contain pectin, including; apples, citrus fruits, strawberries, raspberries and other red or black berries Shitake mushrooms are widely used by the Japanese and include a compound called lentinan, which not only lowers cholesterol, but is also thought to be anti-cancerous and may help to boost the immune system Garlic is a super food and is well known for its blood-thinning properties. It also contains a substance allicin which is thought to prevent the body retaining LDL cholesterol and research has shown that the equivalent of one clove per day can lessen ‘bad’ cholesterol by 10-15% in the majority of people Root ginger has been used in Chinese medicine for centuries and has numerous health benefits. It can be added to stir-fry’s (using healthy sesame oil) and other vegetable dishes Nuts are naturally high in omega-3 fatty acids and are known to significantly reduce blood cholesterol; walnuts, pecans, hazelnuts and almonds are especially beneficial Sesame seeds are rich in phytosterols. These compounds are said to substantially lessen LDL cholesterol. Other foods containing phytosterols include; celery, lettuce, asparagus, spinach, tomatoes, ginger, squash and strawberries Safflower, canola, soybean, and olive oil are
monounsaturated and are an excellent source of omega-3 fatty acids. Research indicates that they can decrease atherosclerotic plaque by up to 15% when eaten regularly Salmon, tuna, sardines and mackerel contain considerable amounts of omega-3 fatty acids, which have many health benefits and are essential for brain and eye function Prunes are a wonderful source of antioxidants and fiber, which is known to reduce LDL cholesterol Alfalfa sprouts contain a substance called saponin, which is thought to obstruct and inhibit the formation of atherosclerotic plaque in the arteries If you have high cholesterol or a family tendency towards atherosclerosis it is best to avoid processed and prepared foods, fried food, food containing animal fats or animal products, high-fat dairy products and food containing either saturated fat or trans fats. Research has revealed the key to lowering LDL cholesterol is a healthy, balanced diet that incorporates plenty of soluble fiber and at least five portions of fruit or vegetables a day. This combined with regular cardiovascular exercise should help keep the heart healthy and provide other health benefits as well numerous
https://www.getresponse.com/?a=7DveqCnP3H

#Health
1 note
·
View note
Text
Going through questions:
Chédiak–Higashi syndrome[1] (CHS) is a rare autosomal recessive disorder that arises from a mutation of a lysosomal trafficking regulator protein,[2] which leads to a decrease in phagocytosis. The decrease in phagocytosis results in recurrent pyogenic infections, albinism, and peripheral neuropathy.
Defect in neutrophil phagosome-lysosome fusion. Pyogenic bacteria = staph, strep, pneumococcus.
Cytotoxic (CD8+) T cells have a PD-1 receptor. Tumor cells can express programmed death ligand-1 (PD-L1), which binds to the PD-1 receptors on cytotoxic T cells. PD-L1 binding to the PD-1 receptor prevents cytotoxic T cells from doing their apoptotic function. This is how cancer cells evade destruction by cytotoxic T cells. The binding of PD-L1 to PD-1 receptor causes T cell exhaustion (inability of the T cell to induce apoptosis). Monoclonal antibodies against PD-1 or PD-L1 prevent PD-L1 interaction with PD-1 receptor, so cancer cells can't evade cytotoxic T cell destruction. Pembrolizumab, nivolizumab = PD-1 antibodies. Atezolizumab = PD-L1 antibody.
T lymphocytes release interferon gamma, which activates macrophages to express MHC-> Th1 differentiation. Viral and bacterial infection result in interferon gamma release from T lymphocytes and NK cells. Interferon Gamma Release Assay (IGRA) tests for latent TB; measures amount of interferon gamma released by T lymphs in response to M. tuberculosis antigens; measures cell-mediated immunity.
Mast cells release tryptase. Tryptase is released by mast cells in anaphylactic reactions. Serum tryptase level is used to diagnose anaphylaxis after the pt is stabilized.
The FceRI IgE on mast cells and basophils bind to the Fc portion of circulating IgE antibodies. Antigen binds to multiple IgEs, which causes aggregation of the FceRI receptors (the IgEs on basophils and mast cells)-> degranulation of histamine and tryptase from basophils and mast cells. Ok so this is more complex than I remember learning. I thought it was the IgEs that aggregate. It's specifically the FceRIs (high affinity IgEs) that aggregate. FceRI receptor on mast cells bind to the circulating IgEs. The the FceRIs aggregate via the antigen and that causes degranulation. Aggregation of the FceRIs activates a tyrosine kinase, which then causes degranulation.
Question 14 of my first immunology quiz has an explanation I want to draw a table from
Etanercept is a TNF-alpha inhibitor it links the Fc portion of human IgG1 to soluble TNF alpha receptors, functioning as a fusion protein. It functions as a decoy receptor, which binds all TNF alpha to keep it from binding to TNF alpha receptors. It treats rheumatoid arthritis when MTX fails to work.
Question 13 also has a figure I want to copy.
Azathioprine and mycophenolate inhibit nucleotide synthesis. This prevents B cells and T cells from proliferating. These are immunosuppresants. Azathioprine-> 6 mercaptopurine-> 6 Thioguanine active metabolites; the Thioguauanine metabolites inhibit purine synthesis. If you can't replicate your DNA/RNA, you can't reproduce, so azathioprine prevents B and T cells from proliferating. Activated T lymphs make IL-2, so less IL-2 will be made as well. Immunoglobulins come from plasma cells, which form from B cells. So azathioprine also reduces immunoglobulins (because azathioprine prevents the T cells from proliferating and you need T cells to activate B cells with the costimulatory response).
I got a question right because I remembered from watching an OnlineMedEd video yesterday that MHC Class I receptor is edited in the endoplasmic reticulum. Part of MHC Class I receptor is beta 2 microglobulin. Cells that are infected break up the pathogen in their cytoplasm and then send pieces of the pathogen (antigens) into the endoplasmic reticulum via the TAP receptor. Inside the ER, antigen is added to MHC Class I, then the MHC Class I-Antigen complex is sent to the cell membrane. TAP = Transporter Associated with Antigen Processing. Mutations in the TAP1 gene, which codes for TAP, lead to inability to bring antigen into the ER. So the pt gets granulomatous skin ulcers and respiratory infections even though lymphocyte and immunoglobulin levels are normal.
Pneumococcal conjugate vaccine (PCV13) induces a more robust immune response than the pneumococcal polysaccharide vaccine (PPSV23) because the conjugate vaccine causes B and T cell recruitment-> memory. The polysaccharide vaccine (PPSV23) doesn't create memory cells. PCV13 has a conjugated protein whereas PPSV23 doesn't. Pneumovax = PPSV23; Prevnar = PCV13.
In the blood vessels, bradykinin relaxes smooth muscle, causing vasodilation. But in smooth muscle that is not in the vasculature, bradykinin actually causes contraction of that non-vascular smooth muscle. So, bradykinin dilates blood vessels but constricts smooth muscle that is not in the blood vessels. That’s why it causes cough–it causes constriction of smooth muscle in the bronchi. Since bradykinin is degraded by ACE, when you inhibit ACE with ACEIs such as lisinopril, you stop breakdown of bradykinin–> increased vasodilation and bronchoconstriction. That’s why ACEIs can cause angioedema and cough.
Bradykinin vasodilates-> angioedema (fluid leaves blood vessels).
Hereditary angioedema is AD; can also be due to ACEIs. In hereditary angioedema, pts lack C1 esterase inhibitor, which is normally involved in break down of bradykinin. So no C1 esterase inhibitor-> increased bradykinin-> angioedema. Dx hereditary angioedema with low serum C1 esterase inhibitor and C4 levels. C1 esterase inhibitor suppresses C1-> no classic complement pathway. Kininogen is converted to bradykinin by kallikrein. C1 esterase inhibitor inhibits kallikrein-> decreased bradykinin. So of course without C1 esterase inhibitor, you have increased kallikrein-> more bradykinin.
In germinal centers in lymph nodes, B cells undergo somatic hypermutation, which is what allows the B cells that can respond to a particular antigen to proliferate. Somatic hypermutation = immunoglobulin mutation.
Serum sickness = type III hypersensitivity reaction (antigen-antibody complexes in tissues); occurs 1 to 2 weeks after being exposed to non-human proteins. Pts present with vasculitis, arthralgia, fever, rash, low C3 and C4. Histologically, you see fibrinoid necrosis and infiltrating neutrophils. Low complement occurs because complement-fixing IgM and IgG are in the immune complexes.
Hyper IgM syndrome = X-linked recessive deficiency of CD40L (CD40 Ligand located in T cells and necessary to interact with CD40R on B cells); causes recurrent pulmonary and sinus infections, GI infection, infection with opportunistic bugs (e.g., cryptosporidium), failure to thrive. Pts have lots of IgM, but little IgG, IgA, and IgE. B cells have trouble class switching (because the CD40L-CD40R interaction is necessary to create the costimulatory signal that causes B cell class switching and proliferation). Class switching occurs when heavy chain constant region genes are spliced out so the B cell can create the isotype of immunoglobulin it needs to make. That doesn't happen in Hyper-IgM syndrome because the CD40R-CD40L initeraction necessary to cause that can't happen when there's deficient DC40L. CD40 deficiency is autosomal recessive form of this disease. If B cells don't class switch, they stay as IgM-secreting cells.
Severe Combined Immunodeficiency (SCID) = autosomal recessive adenosine deaminase (ADA) deficiency. Adenosine deaminase deficiency leads to accumulation of adenosine in T cells and B cells (lymphocytes)-> cellular and humoral immunodeficiency. Tx: gene therapy; hematopoietic stem cell transplant. X-linked SCID is more commone than ADA deficiency. No lymphocytes-> bacterial, viral, and fungal infections.
Henoch-Schonlein Purpura (HSP) = small vessel leukocytoclastic angiitis + deposition of IgA-C3 complexes in skin and GI tract. It's a hypersensitivity reaction related to recent infection. Pts have a purpuric rash, arthralgia, abdominal pain. Can lead to acute glomerulonephritis. Occurs in kids.
#immunology#anaphylaxis#IGRA#interferon gamma#chediak higashi#chediak higashi syndrome#allergic reaction#TNF alpha#etanercept#PPSV23#PCV13#pneumococcal vaccine#serum sickness#hyper IgM syndrome#SCID#Henoch Schonlein pupura#HSP#Henoch Schonlein
2 notes
·
View notes
Text
“ Many clinically important drugs affect the plasma membrane. For some anesthetics, such as chloroform, ether, halothane, and nitrous oxide, potency is directly correlated with their lipid solubility. High lipid solubility speeds the drug’s entry into cells and enhances its ability to block ion channels or change other properties of plasma membranes. These changes reduce the sensitivity of neurons and muscle cells. However, some common anesthetics have relatively low lipid solubility. For example, the local anesthetics procaine and lidocaine affect nerve cells by blocking sodium ion channels in their plasma membranes. This blockage reduces or eliminates the responsiveness of these cells to painful (or any other) stimuli. “
- Fundamentals of Anatomy and Physiology, 11th e; Frederic H. Martini
7 notes
·
View notes
Photo

EXOCYTOSIS
Proteins synthesized in the endoplasmic reticulum and trafficked to the Golgi apparatus
From the Golgi, the proteins are sorted and can follow different paths:
3a) Constitutive secretory pathway
Also called default pathway as it does not require a signal for cargo to enter this pathway
Can transport soluble proteins or plasma membrane proteins
Transport vesicles fuse with the plasma membrane and release their contents (plasma membrane proteins become accessible to the extracellular space)
3b) Regulated secretory pathway
Found mainly in specialized cells that release molecules like hormones, neurotransmitters, etc.
Secretory vesicles are blocked from fusing with the plasma membrane without a proper signal
An extracellular ligand binds a cellular receptor which causes an intracellular signal that releases the block on the secretory vesicles
Secretory vesicles then fuse with the plasma membrane and release their cargo
2 notes
·
View notes
Text
Marta mielczarek

These conjugates have a longer plasma half-life and a higher bioavailability (Engelbrecht 2011). 2009).Ĭonjugation of drug with fatty acids increases its lipid solubility what facilitates permeation into the cell membrane. It is known that they inhibit the formation of the tumor growth promotor 13-hydroxy-octadecadienoic acid (13-HODE) and they have a cardioprotective effect, which can reduce the cardiotoxicity of DOX (Sauer et al. They can play an important role in delay of the cancer progression by modulating hormone receptors, Akt kinase, and nuclear factors к B as well as being the target for ROS (Das 2004 Narayanan et al. ω-3 fatty acids (e.g., DHA) can bind to cognate receptors on cancer cells and then exert a targeting effect (Sauer et al. The most important ω-3 PUFAs are: α-linolenic ( cis-9,12,15-octadecatrienoic, LNA) and cis-7,7,10,13,16,19-docosahexaenoic (DHA). In addition, compared with normal cells, PUFAs are more avidly taken up by tumor cells (Sauer et al. One of the most important compounds that affect cell metabolism are polyunsaturated fatty acids (PUFAs). They display uncontrolled growth and usually require a large amount of various nutrients (Jaracz et al. It is well known that cancer cells differ from normal cells. To design a tumor-targeting drug, it is crucial to understand the tumor cell microenvironment. The improvement in the effectiveness of anticancer properties of DOX though conjugation or derivatization could be an alternative option to reduce time and costs required to develop a new anticancer agent (Hidayat et al. The use of DOX is associated with very high risks, such as cardiomyopathy and congestive heart failure (Lenaz and 6 Weiss 1992 Johnson et al. 2003) and binding to lipids in cell membrane resulting in the changes of its permeability (Pessah et al. The anticancer action of DOX is also mediated by chelating of iron, zinc and copper ions, formation of reactive oxygen species (ROS) (Minotti et al. 1975), or topoisomerase II (Binaschi et al. 1981), inhibition of RNA and DNA polymerases (Zunina et al. These compounds can act independently or in combination with other medicines (combined therapy) and can be used in treatment of cancer diseases (Xu and Mao 2016 Narang and Desai 2009).ĭoxorubicin (DOX) is a multidirectional chemotherapy agent (Gewirtz 1999), which mechanism of action includes intercalation and alkylation of DNA (Young et al. Availability of new technologies related to research on tumor pathogenesis designated new strategies of searching active compounds, which can be used as medications. Nowadays many different active substances are used to inhibit the proliferation of cancer cells, but still there is a need to find substances, which act specifically as anticancer factors. They show high cytotoxicity toward the tumor cell lines and moderate cytotoxicity towards the normal cell line.Īpplication of chemotherapy seems to be crucial in the fight against cancer diseases. Among the all derivatives, the conjugates formed by the amide and ester linkages ( 4, 5) were found to be more promising compared with conjugates ( 2, 3) formed only by the amide linkage. We found that all studied conjugates exhibit lower cytotoxicity but higher selectivity than DOX. To explain the basic mechanism of cell death induction the Annexin V-FITC/IP flow cytometry analysis was investigated. Lactate dehydrogenase (LDH) assay was performed for all compounds to assess the level of cell damage. In addition, a cytotoxic capacity against tumor cells for tested compounds was expressed as a selectivity factor (selectivity index, SI). The cytotoxic activity was established by calculation of the inhibitory concentration IC 50. For all compounds 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the cytotoxic effect on human cancer cell lines (SW480, SW620, and PC3) and Chinese hamster lung fibroblasts (V79) that were used as a control. The structures of the compounds were confirmed by Proton Nuclear Magnetic Resonance (1H NMR), Carbon-13 Nuclear Magnetic Resonance (13C NMR), and High Resolution Mass Spectrometry (HRMS) analyses. In our work, we obtained amide derivatives of DOX by reaction of the amino group with α-linolenic (LNA) and docosahexaenoic (DHA) acids ( 2, 3), as well as double-substituted derivatives via amide and ester linkages ( 4, 5). We are still looking for methods that will allow us to preserve the therapeutic effect against the tumor cells and reduce the toxicity to the normal cells. Doxorubicin (DOX) is a leading cytostatic drug with many adverse effects in use.

0 notes
Text
How much do you know about rosmarinic acid?
What is rosmarinic acid powder?
Rosmarinic acid is a water-soluble natural phenolic acid compound isolated from rosemary, a plant of the Lamiaceae family. Among the various plants of the Umbelliferae, the highest content is especially in the Lamiaceae and the comfrey.
Rosmarinic acid is a natural antioxidant with strong antioxidant activity. Its antioxidant activity is stronger than vitamin E, caffeic acid, chlorogenic acid, folic acid, etc. It helps prevent cell damage caused by free radicals, The risk of cancer and arteriosclerosis is thus reduced.
Rosmarinic acid extract effects
1. Antioxidant effect

2. Antidepressant effect
Rosmarinic acid has antidepressant effects in animal models of depression. Experiments were performed on cell proliferation in the hippocampus of mice, and bromodeoxyuridine antibody was used for immunohistochemical analysis to explore its mechanism of action. It was found that in the rosmarinic acid treatment group, the labeled proliferative cells increased, combined with the forced swimming of depression animal models. Experiments have shown that rosmarinic acid proliferates and produces an antidepressant-like effect at least in part through the proliferation of new cells in the hippocampus.
3. Antibacterial effect of rosmarinic acid
Rosmarinic acid has broad-spectrum antimicrobial activity against both bacteria and fungi. Rosmarinic acid has obvious inhibitory effect on bacteria such as Bacillus subtilis, Bacillus luteus, Escherichia coli, Staphylococcus aureus and Rhizococcus solani. It has been scientifically found that rosmarinic acid can inhibit the growth and biofilm formation of Streptococcus caries and Streptococcus mutans, and reduce their glucosyltransferase activity, indicating that rosmarinic acid can be used for the prevention and treatment of oral diseases. Research on the antibacterial mechanism of rosmarinic acid shows that: on the one hand, rosmarinic acid can increase the permeability of bacterial cell membranes, resulting in a large amount of sugar and protein leakage, affecting the normal metabolism of bacteria; on the other hand, it can also affect bacterial protein It also inhibits Taq DNA polymerase. Rosmarinic acid has antibacterial activity against Gram-negative bacteria Escherichia coli and Gram-positive bacteria Staphylococcus aureus, but Staphylococcus aureus is significantly more sensitive to rosmarinic acid than Escherichia coli.
(1) Inhibition of bacteria: Rosmarinic acid has a significant inhibitory effect on Bacillus subtilis, Luccinococcus and Escherichia coli.
(2) Inhibitory fungi: the inhibitory activity of rosmarinic acid on mycelial growth and spore germination of different phytopathogenic fungi.
(3) Inhibition of mold: Rosmarinic acid extracted from plants has an inhibitory effect on Phytophthora and its mold spores that cause soil-borne diseases, effectively reducing the germination of zoospores.
4. Anti-inflammatory effects of rosmarinic acid
(1) Anti-nephritis effect: Rosmarinic acid can inhibit the proliferation of mesangial cells and glomerular expansion.
(2) Anti-hepatitis effect: Rosmarinic acid can alleviate lipopolysaccharide (LPS)-induced liver injury and significantly inhibit the increase of plasma transaminase levels, which proves that the liver protective effect of rosmarinic acid is through the removal or reduction of superoxide. or oxidized nitrite rather than by inhibiting TNF-α.
(3) Anti-pneumonic effect: Rosmarinic acid inhibits diesel exhaust particulate-induced neutrophil retention and lung injury characterized by interstitial edema.
(4) Anti-arthritis effect: Rosmarinic acid can inhibit collagen-induced arthritis and significantly reduce the number of arthritis and affected joints.
(5) Anti-periodontitis effect: Rosmarinic acid can inhibit the formation of bacterial plaque, thereby preventing chronic gingivitis.
(6) Anti-dermatitis effect: Rosmarinic acid has a relieving effect on atopic dermatitis, also known as atopic eczema or atopic eczema.
5. Antiviral effects of rosmarinic acid
(1) Anti-herpes virus: rosmarinic acid shows special activity on type I and type II herpes simplex virus, and is an effective ingredient for controlling herpes disease. , but also rapidly combined with the viral coat protein, thereby inactivating the virus.
(2) Anti-HIV: Rosmarinic acid can inhibit the activity of HIV-1 (human immunodeficiency virus 1) integrase.
(3) Anti-encephalitis virus: Rosmarinic acid can reduce the mortality rate of mice with Japanese encephalitis virus, significantly reduce the viral load, and make the virus difficult to spread.
6. Anti-cancer and anti-tumor effects of rosmarinic acid: Rosmarinic acid inhibits the expression of CCL11 and CCR3 by inhibiting the activity of β-kinase and the activation of related genes by nuclear factor kappa B, so as to achieve the purpose of anti-tumor.
7. Anti-allergic effect of rosmarinic acid: Rosmarinic acid can inhibit allergic inflammation induced by microallergens.
8. Antioxidative effect of rosmarinic acid: Rosmarinic acid has good free radical scavenging and antioxidant effects.
9. Antithrombotic and antiplatelet aggregation effects of rosmarinic acid: Rosmarinic acid can inhibit the formation of malondialdehyde in human platelets in vitro, and its IC50 value is 3.37nmol/L, indicating that rosmarinic acid has antiplatelet aggregation activity.
10. Antidepressant effects of rosmarinic acid
11. Anti-radiation and UV-protection of rosmarinic acid: Rosmarinic acid can be used as a photoprotectant to protect against radiation and UV rays.
Rosemary rosmarinic acid application fields
Rosmarinic acid has strong anti-inflammatory activity, while rosmarinic acid also has antibacterial, antiviral, antitumor activities, and has the properties of inhibiting acute and chronic infections, anti-ultraviolet rays, and inhibiting elastin degradation. Acids become additives in cosmetics. At present, rosmarinic acid has shown its important application value in the fields of pharmacy, food, cosmetics and so on.
1. Food field
As a natural and efficient antioxidant, rosmarinic acid can replace BHA and BHT in animal and vegetable oils, dairy products, oil-rich foods, candy and baked foods; it can also be used as a spice in various soups and flavored foods; And both antiseptic and antibacterial effect. In Japan, rosmarinic acid-rich shiso extract is used as a garnish to improve the shelf life of fresh seafood.
2. Health care products
Anti-tumor, anti-hepatitis and protection of liver damage, anti-nephritis, anti-thrombotic and anti-platelet aggregation, as well as refreshing, enhancing memory, improving tension and drowsiness.
3. Cosmetics
It can be used in skin care products to remove freckles, anti-oxidation, increase skin elasticity, and delay aging; when used in shampoos and hair care products, it can promote scalp blood circulation, improve hair loss, and reduce the occurrence and irritation of dandruff Hair grows and moisturizes. Therefore, rosmarinic acid can be used as an excellent additive in cosmetics.
1 note
·
View note