#phosphorylative
Explore tagged Tumblr posts
Text
i binged all of nqd recently but these are the doodles i sketched during season one. my designs def have changed since this but here’s what i originally imagined
#my art#my post#nqd#nqd pod#not quite dead#not quite dead podcast#i’m this is messier than usual but#i’ve had school#tagging this i realized i do not know these characters full names#in my defense#i listened while studying for bio#alfie’s last name is oxidative phosphorylation in my brain#queue#art#artists on tumblr
25 notes
·
View notes
Text

Man I love the Golgi apparatus.
Removal of Man? Addition of Gal?? Trans network??? Sign me up
#she phosphorylated on my oligosaccharides until i lysosomal proteined#golgi apparatus#shitpost#cell biology
11 notes
·
View notes
Text
The systemic production of proteinase inhibitors in young tomato plants is triggered by a complex sequence of events (Figure 23.20):
Wounded tomato leaves synthesize prosystemin, a large (200 amino acids) precursor protein.
Prosystemin is proteolytically processed to produce the short (18 amino acids) polypeptide DAMP called systemin.
Systemin is released from damaged cells into the apoplast.
In adjacent intact tissue (phloem parenchyma), systemin binds to a pattern recognition receptor on the plasma membrane.
The activated systemin receptor becomes phosphorylated and activates a phospholipase A2 (PLA2).
The activated PLA2 generates the signal that initiates JA biosynthesis.
JA is then transported through the phloem to systemic parts of the plant by an unknown mechanism.
JA is taken up by target tissues and activates the expression of genes that encourage proteinase inhibitors.

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#proteinase#tomato#solanum lycopersicum#prosystemin#amino acids#polypeptide#damage associated morning patterns#damp#apoplast#phloem#parenchyma#phosphorylation#pla2#phospholipase a2#ja#jasmonic acid#plant cells#vascular tissue#companion cells#plasmodesma
9 notes
·
View notes
Text
2 final exams day is OVER!! ended up with an A in chinese😋 and im reluctant to get my hopes up bc i felt great about the midterm and ended up just getting a 78 but secretly i feel like i mega slayed the signal transduction final
#adderall is a fucking miracle drug my memory/recall is back to how it used to be before i got all depressed#like i remembered the specific residues that got phosphorylated in different pathways and stuff like that#anyways i felt really good about it i hope i actually did good and im not just delusional about my own intelligence
3 notes
·
View notes
Text
bio class is just full of fucking words
4 notes
·
View notes
Text

TSRNOSS, p 810.
#shape of mitochondria#phosphorylation of glucose#half-life of B vitamins#Faraday Cage Effect#anaesthesia under pressure#Laplace's law#fat content of the mitochondrion#ascorbic acid#prime numbers#body temperature#satyendra sunkavally#theoretical biology#manuscript#cursive handwriting
0 notes
Text
So turns out that in ME/CFS all of your body’s cells are probably using the same energy production compensation methods as cancer cells. Fucking tracks lmao
#to clarify the evidence points to enhanced glycolysis due to#impairment of oxidative phosphorylation#it’s called the Warburg effect and tumor cells use it because they are in an oxygen deprived environment#glycolysis is much less efficient at ATP production so basically your cells can just barely manage to keep up#if you are at rest with no heightened energy demand whatsoever#but the minute you go beyond that threshold it’s uh oh scoob#I just thought it was poetic
0 notes
Text
lil rant in the tags
will delete later jsbdjs
ps. didnt know there were max 30 tags wow
#so i live in the netherlands right. best public transport (supposedly) in europe/the world even#and every day i need to go from one city to another. 1 hour with the train 30 mins with the bus#but this fuckass train is NEVER on time#the step over to this bus is always too late - so i miss it#and that happens twice every day#so my 1.5 hour commute easily goes to 3 hours. to AND fro#and it stresses me the FUCK out#my mental health has been in the fucking drain due to stress and i feel like breaking every day#and my stupid experiments suck ass because my supervisors wont supervise me#like. i have a professor and a phd student and the professor is my main supervisor but i did not know that until a few weeks ago????#like ive been here for 5 months and that guy said nothing#had maybe 2 small talks with him#and during the feedback moment with my supervisor from school has he the balls to say i am not independant enough and that i rely too much -#-on the phd student#because with every result from the lab i got i went to her because she asked me that#and i though SHE was my supervisor#and all my labwork has been on the maturation of proteins while it was supposed to be a light-induced on-off system of phosphorylation#of which i did basically NO work because i did not get any information nor the primers to even start#so i grew some sad colonies and did a pcr twice. which was negative because OF COURSE it was#so i am so fucking stressed#i still need to write the damn report too#the smallest mercy to the fact i have shitty ass almost no results#but i still have to present it to the group#itll be SO embarassing#at least the job applications this week led me to a new internship#the guy seemed chill and really nice#and i will get paid. which i do not now#im writing/typing this on the train home and the delay is already 13 minutes. how did we get here#im so fucking tired and stressed out#delete later
1 note
·
View note
Text
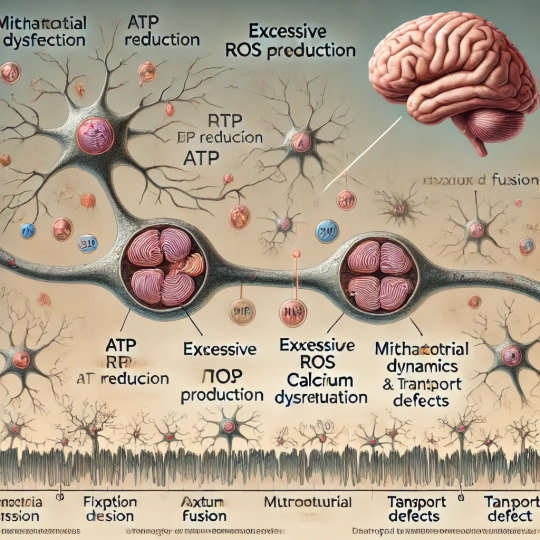
Mitochondrial Dysfunction Drives Cognitive Decline
Introduction
Mitochondria, often referred to as the powerhouses of the cell, are crucial organelles responsible for energy production through adenosine triphosphate (ATP) synthesis. Beyond their well-known role in energy metabolism, mitochondria regulate a wide range of cellular processes, including calcium homeostasis, reactive oxygen species (ROS) generation, and apoptosis. When mitochondria malfunction, the consequences can be far-reaching, especially for energy-intensive organs like the brain. Recent research highlights mitochondrial dysfunction as a central factor in cognitive decline, contributing to neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s disease. This article explores the mechanisms by which mitochondrial dysfunction impacts cognitive function and discusses potential therapeutic strategies.
The Brain's Energy Demands and Mitochondrial Function
The human brain, despite accounting for only about 2% of body weight, consumes approximately 20% of the body’s energy. Neurons, the primary cells of the nervous system, rely heavily on mitochondrial ATP to sustain synaptic activity, ion gradient maintenance, and neurotransmitter synthesis. Efficient mitochondrial function is critical for maintaining neuronal health and connectivity, which are foundational for learning, memory, and other cognitive processes.
Mechanisms of Mitochondrial Dysfunction in Cognitive Decline
Reduced ATP Production: Mitochondria produce ATP through oxidative phosphorylation (OXPHOS) in the electron transport chain (ETC). Damage to ETC components, often caused by genetic mutations or oxidative stress, can reduce ATP production. Energy-starved neurons may fail to maintain synaptic function, leading to cognitive impairments.
Excessive ROS Generation: While ROS are natural byproducts of mitochondrial activity and play roles in cell signaling, excessive ROS can damage mitochondrial DNA (mtDNA), proteins, and lipids. This oxidative damage exacerbates mitochondrial dysfunction, creating a vicious cycle that contributes to neuronal degeneration.
Impaired Calcium Regulation: Mitochondria help buffer intracellular calcium levels, which are critical for neurotransmitter release and synaptic plasticity. Dysfunctional mitochondria may fail to regulate calcium, leading to excitotoxicity—a condition where excessive calcium causes neuronal injury and death.
Mitochondrial Dynamics: Mitochondria constantly undergo fission (division) and fusion (joining) to adapt to cellular demands and maintain their integrity. Imbalances in these processes can result in fragmented or overly fused mitochondria, impairing their function and transport within neurons.
Mitochondrial Transport Defects: Neurons have long axons and dendrites that require efficient transport of mitochondria to regions of high energy demand, such as synaptic terminals. Dysfunction in mitochondrial transport mechanisms can disrupt synaptic activity and contribute to cognitive decline.
Mitochondrial Dysfunction in Neurodegenerative Diseases
Alzheimer’s Disease (AD): Mitochondrial dysfunction is a hallmark of AD. Amyloid-beta plaques and tau tangles, characteristic of AD, have been shown to impair mitochondrial function. Elevated ROS levels and reduced ATP production exacerbate neuronal loss and cognitive decline in AD.
Parkinson’s Disease (PD): PD is associated with mutations in genes like PINK1 and PARKIN, which regulate mitochondrial quality control. Impaired mitophagy—the process of removing damaged mitochondria���leads to their accumulation, contributing to dopaminergic neuron degeneration and motor as well as cognitive deficits.
Huntington’s Disease (HD): In HD, mutant huntingtin protein interferes with mitochondrial dynamics and function, resulting in energy deficits and increased oxidative stress. These mitochondrial abnormalities contribute to the progressive cognitive and motor decline observed in HD patients.
Diagnostic and Therapeutic Approaches
Biomarkers of Mitochondrial Dysfunction: Advances in molecular biology have identified potential biomarkers, such as altered mtDNA levels, ROS, and metabolites associated with mitochondrial pathways. These biomarkers can aid in early diagnosis and monitoring of neurodegenerative diseases.
Pharmacological Interventions:
Antioxidants: Compounds like coenzyme Q10, vitamin E, and MitoQ target mitochondrial ROS, reducing oxidative damage and preserving mitochondrial function.
Mitochondrial Biogenesis Enhancers: Agents like resveratrol and PGC-1α activators promote the production of new mitochondria and improve mitochondrial health.
Calcium Modulators: Drugs that stabilize calcium levels, such as memantine, may protect neurons from excitotoxicity.
Gene Therapy: Gene-editing tools like CRISPR/Cas9 offer potential to correct mtDNA mutations or enhance the expression of genes involved in mitochondrial quality control. For example, boosting PINK1 or PARKIN expression could improve mitophagy in PD.
Lifestyle Interventions:
Dietary Interventions: Ketogenic diets and intermittent fasting have been shown to enhance mitochondrial function by promoting efficient energy utilization and reducing ROS.
Exercise: Regular physical activity stimulates mitochondrial biogenesis and reduces oxidative stress, offering neuroprotective benefits.
Sleep Optimization: Adequate sleep is essential for mitochondrial repair and the clearance of damaged proteins, such as amyloid-beta.
Future Directions in Research
Understanding the interplay between mitochondrial dysfunction and cognitive decline opens new avenues for research and therapy. Emerging technologies, such as single-cell transcriptomics and advanced imaging, allow for detailed exploration of mitochondrial dynamics in neurons. Additionally, the development of mitochondria-targeted drugs and nanotechnologies holds promise for precise therapeutic interventions.
Conclusion
Mitochondrial dysfunction plays a pivotal role in driving cognitive decline and is implicated in the pathogenesis of various neurodegenerative diseases. Addressing mitochondrial health through targeted therapies, lifestyle modifications, and early diagnostic measures offers hope for mitigating cognitive impairments and improving quality of life. As our understanding of mitochondrial biology deepens, so too does the potential for innovative treatments that could transform the landscape of neurodegenerative disease management.
#Mitochondrial dysfunction#Cognitive decline#Neurodegenerative diseases#Alzheimer\u2019s disease (AD)#Parkinson\u2019s disease (PD)#Huntington\u2019s disease (HD)#ATP production#Oxidative phosphorylation (OXPHOS)#Reactive oxygen species (ROS)#Mitochondrial DNA (mtDNA)#Calcium homeostasis#Synaptic activity#Excitotoxicity#Mitochondrial dynamics#Mitochondrial fission and fusion#Mitophagy#Mitochondrial transport#Biomarkers#Antioxidants#Mitochondrial biogenesis#Gene therapy#CRISPR/Cas9#Lifestyle interventions#Ketogenic diet#Exercise#Sleep optimization#Neuronal health#Therapeutic strategies
0 notes
Text
Abstract Protein kinases represent one of the largest eukaryotic enzyme superfamilies. However, only a few can directly phosphorylate tubulin and contribute to the modulation of the “tubulin code.” The authors previously confirmed the structural and functional homology of the plant protein kinase IREH1 and members of the mammalian MAST kinase family. Their participation in the regulation of the microtubule system in plant and animal cells was also experimentally confirmed. At the same time, the direct contribution of MAST/IRE to the “tubulin code” remains unclear. In the current study, based on bioinformatical and structural biology methods, the possibility of such an interaction was evaluated. The target sites of MAST/IRE-phosphorylation of tubulin were predicted based on similarity to the generalized specific profiles. Two potential MAST/IRE specific sites, conserved in human and Arabidopsis tubulins were selected: Thr73 (80) exists in most isotypes of α-tubulin and Ser115 was found in the majority of human and plant isotypes of β-tubulin. It was predicted that phosphorylation of the first site can affect the assembly of α/β-tubulin heterodimer, and phosphorylation of the second may affect the interaction between neighboring protofilaments of microtubules. The last site Ser433, was found in both γ-tubulin isotypes of A. thaliana, but it was absent in mammals. The external position of Ser433 in plant γ-tubulin allows for suggesting that phosphorylation of this amino acid can affect the structure of the γTuRC complex but it does not affect inner contacts of γTuSC and their interaction in the ring.
0 notes
Text
youtube
#Metabolic regulation#stem cell function#leukemic stem cells#cancer metabolism#normal stem cells#self-renewal#differentiation#tissue homeostasis#glycolysis#oxidative phosphorylation#Warburg effect#reactive oxygen species#cell survival#metabolic pathways#biosynthesis#tumor microenvironment#cancer progression#metabolic flexibility#chemotherapy resistance#targeted therapy.#Youtube
0 notes
Text
do you think oxidative phosphorylation feels good for the adp
0 notes
Text
One of these experiments, carried out by Emerson, measured the quantum yield of photosynthesis as a function of wavelength and revealed an effect known as the red drop (Figure 7.12). (...) The observation that the overall quantum yield of photosynthesis is nearly independent of wavelength (see Figure 7.12) strongly suggests that such a mechanism exists.
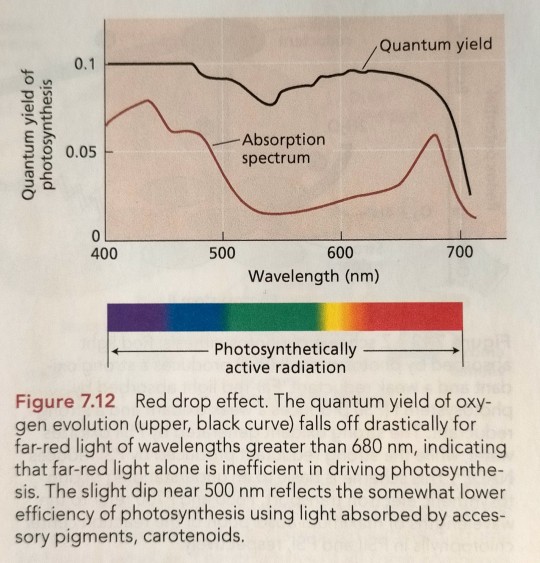
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#experimentation#experiment#photochemistry#red drop effect#thylakoid#kinase#phosphorylation#quantum yield#absorption spectrum#photosynthesis#carotenoids#wavelength#robert emerson#1950s#50s#20th century#plant cells
2 notes
·
View notes
Text
Hear me out:

Would (Graphed using Desmos, created by me)


1 note
·
View note
Text
It is the year of our lord twenty twenty-four, how has no one made a sick remix of Oxidative Phosphorylation (song by Science Groove, 2004)
1 note
·
View note
Text

TSRNOSS, p 619.
#hydrochloric acid#Faraday Cage Effect#aromatic rings#resonance stabilization#thyroid hormone#beta radiation#electron affnity of oxygen#oxidative phosphorylation#superoxide#Heisenberg's Uncertainty Principle#rotation of molecules#nitric oxide#velocity of oxygen molecules#ultraviolet absorption by the ozone layer#satyendra snkavally#manucript#calligraphy#diaries#handwriting
0 notes