#ethyl ethanoate
Explore tagged Tumblr posts
Text
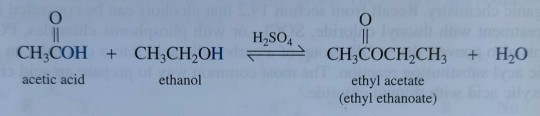
As an example of a Fischer esterification, treating acetic acid with ethanol in the presence of concentrated sulfuric acid gives ethyl acetate, a common solvent (figure 23.4), and water:


"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#fischer esterification#esterification#acetic acid#ethanol#sulfuric acid#ethyl acetate#solvent#water#ethyl ethanoate#chemical reactions
2 notes
·
View notes
Text
An example of a Fischer esterification, treating acetic acid with ethanol in the presence of concentrated sulfuric acid gives ethyl acetate, a common solvent (figure 23.4), and water:

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#fischer esterification#acetic acid#ethanol#sulfuric acid#ethyl acetate#ethyl ethanoate#water#chemical reactions#glue#nail polish remover
0 notes
Text
Acetic Acid Market - Forecast(2024 - 2030)
Acetic Acid Market Overview
Acetic Acid Market Size is forecast to reach $14978.6 Million by 2030, at a CAGR of 6.50% during forecast period 2024-2030. Acetic acid, also known as ethanoic acid, is a colorless organic liquid with a pungent odor. The functional group of acetic acid is methyl and it is the second simplest carboxylic acid. It is utilized as a chemical reagent in the production of many chemical compounds. The major use of acetic acid is in the manufacturing of vinyl acetate monomer, acetic anhydride, easter and vinegar. It is a significant industrial chemical and chemical reagent used in the production of photographic film, fabrics and synthetic fibers. According to the Ministry of Industry and Information Technology, from January to September 2021, the combined operating revenue of 12,557 major Chinese garment companies was US$163.9 billion, showing a 9% increase. Thus, the growth of the textile industry is propelling the market growth for Acetic Acid.
Report Coverage
The “Acetic Acid Market Report – Forecast (2024-2030)” by IndustryARC, covers an in-depth analysis of the following segments in the Acetic Acid industry.
By Form: Liquid and Solid.
By Grade: Food grade, Industrial grade, pharmaceutical grade and Others.
By Application: Vinyl Acetate Monomer, Purified Terephthalic Acid, Ethyl Acetate, Acetic Anhydride, Cellulose Acetate, Acetic Esters, Dyes, Vinegar, Photochemical and Others
By End-use Industry: Textile, Medical and Pharmaceutical, Oil and Gas, Food and Beverages, Agriculture, Household Cleaning Products, Plastics, Paints & Coating and Others.
By Geography: North America (the USA, Canada and Mexico), Europe (the UK, Germany, France, Italy, Netherlands, Spain, Russia, Belgium and the Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Australia and New Zealand, Indonesia, Taiwan, Malaysia and the Rest of APAC), South America (Brazil, Argentina, Colombia, Chile and the Rest of South America) and the Rest of the World (the Middle East and Africa).
Request Sample
Key Takeaways
The notable use of Acetic Acid in the food and beverages segment is expected to provide a significant growth opportunity to increase the Acetic Acid Market size in the coming years. As per the US Food and Agriculture Organization, world meat production reached 337 million tonnes in 2019, up by 44% from 2000.
The notable demand for vinyl acetate monomer in a range of industries such as textile finishes, plastics, paints and adhesives is driving the growth of the Acetic Acid Market.
Increase in demand for vinegar in the food industry is expected to provide substantial growth opportunities for the industry players in the near future in the Acetic Acid industry.
Acetic Acid Market Segment Analysis – by Application
The vinyl acetate monomer segment held a massive 44% share of the Acetic Acid Market share in 2021. Acetic acid is an important carboxylic acid and is utilized in the preparation of metal acetates and printing processes, industrially. For industrial purposes, acetic acid is manufactured by air oxidation of acetaldehyde with the oxidation of ethanol, butane and butene. Acetic acid is extensively used to produce vinyl acetate which is further used in formulating polyvinyl acetate. Polyvinyl acetate is employed in the manufacturing of plastics, paints, textile finishes and adhesives. Thus, several benefits associated with the use of vinyl acetate monomer is boosting the growth and is expected to account for a significant share of the Acetic Acid Market.
Inquiry Before Buying
Acetic Acid Market Segment Analysis – by End-use Industry
The food and beverages segment is expected to grow at the fastest CAGR of 7.5% during the forecast period in the Acetic Acid Market. Acetic Acid is also known as ethanoic acid and is most extensively used in the production of vinyl acetate monomer. Vinyl acetate is largely used in the production of cellulose acetate which is further used in several industrial usage such as textiles, photographic films, solvents for resins, paints and organic esters. PET bottles are manufactured using acetic acid and are further utilized as food containers and beverage bottles. In food processing plants, acetic acid is largely used as cleaning and disinfecting products. Acetic acid is extensively used in producing vinegar which is widely used as a food additive in condiments and the pickling of vegetables. According to National Restaurant Association, the foodservice industry is forecasted to reach US$898 billion by 2022. Thus, the advances in the food and beverages industry are boosting the growth of the Acetic Acid Market.
Acetic Acid Market Segment Analysis – by Geography
Asia-Pacific held a massive 41% share of the Acetic Acid Market in 2021. This growth is mainly attributed to the presence of numerous end-use industries such as textile, food and beverages, agriculture, household cleaning products, plastics and paints & coatings. Growth in urbanization and an increase in disposable income in this region have further boosted the industrial growth in this region. Acetic acid is extensively used in the production of metal acetates, vinyl acetate and vinegar which are further utilized in several end-use industries. Also, Asia-Pacific is one of the major regions in the domain of plastic production which provides substantial growth opportunities for the companies in the region. According to Plastic Europe, China accounted for 32% of the world's plastic production. Thus, the significant growth in several end-use industries in this region is also boosting the growth of the Acetic Acid Market.
Acetic Acid Market Drivers
Growth in the textile industry:
Acetic Acid, also known as ethanoic acid, is widely used in the production of metal acetate and vinyl acetate which are further used in the production of chemical reagents in textiles, photographic films, paints and volatile organic esters. In the textile industry, acetic acid is widely used in textile printing and dyes. According to China’s Ministry of Industry and Information Technology, in 2020, textile and garment exports from China increased by 9.6% to US$291.22 billion. Also, according to the U.S. Department of Commerce, from January to September 2021, apparel exports increased by 28.94% to US$4.385 billion, while textile mill products rose by 17.31% to US$12.365 billion. Vinyl acetate monomer is utilized in the textile industry to produce synthetic fibers. Thus, the global growth in demand for textiles is propelling the growth and is expected to account for a significant share of the Acetic Acid Market size.
Schedule a call
Surge in use of vinegar in the food industry:
The rapid surge in population along with the adoption of a healthy and sustainable diet has resulted in an increase in demand for food items, thereby increasing the global production level of food items. As per US Food and Agriculture Organization, in 2019, global fruit production went up to 883 million tonnes, showing an increase of 54% from 2000, while global vegetable production was 1128 million tonnes, showing an increase of 65%. Furthermore, world meat production reached 337 million tonnes in 2019, showing an increase of 44% from 2000. Acetic acid is majorly used in the preparation of vinegar which is further widely utilized as a food ingredient and in personal care products. Vinegar is used in pickling liquids, marinades and salad dressings. It also helps to reduce salmonella contamination in meat and poultry products. Furthermore, acetic acid and its sodium salts are used as a food preservative. Thus, the surge in the use of vinegar in the food industry is boosting the growth of the Acetic Acid Market.
Acetic Acid Market Challenge
Adverse impact of acetic acid on human health:
Acetic Acid is considered a strong irritant to the eye, skin and mucous membrane. Prolong exposure to and inhalation of acetic acid may cause irritation to the nose, eyes and throat and can also damage the lungs. The workers who are exposed to acetic acid for more than two or three years have witnessed upper respiratory tract irritation, conjunctival irritation and hyperkeratotic dermatitis. The Occupational Safety and Health Administration (OSHA) reveals that the standard exposure to airborne acetic acid is eight hours. Furthermore, a common product of acetic acid i.e., vinegar can cause gastrointestinal tract inflammatory conditions such as indigestion on excess consumption. Thus, the adverse impact of Acetic Acid may hamper the market growth.
Buy Now
Acetic Acid Industry Outlook
The top 10 companies in the Acetic Acid Market are:
Celanese Corporation
Eastman Chemical Company
LyondellBasell
British Petroleum
Helm AG
Pentoky Organy
Dow Chemicals
Indian Oil Corporation
Daicel Corporation
Jiangsu Sopo (Group) Co. Ltd.
Recent Developments
In March 2021, Celanese Corporation announced the investment to expand the production facility of vinyl portfolio for the company’s acetyl chain and derivatives in Europe and Asia.
In April 2020, Celanese Corporation delayed the construction of its new acetic acid plant and expansion of its methanol production by 18 months at the Clear Lake site in Texas.
In October 2019, BP and Chian’s Zhejiang Petroleum and Chemical Corporation signed MOU in order to create a joint venture to build a 1 million tonne per annum Acetic Acid plant in eastern China.
Key Market Players:
The Top 5 companies in the Acetic Acid Market are:
Celanese Corporation
Ineos Group Limited
Eastman Chemical Company
LyondellBasell Industries N.V.
Helm AG
For more Chemicals and Materials Market reports, please click here
#Acetic Acid Market#Acetic Acid Market Share#Acetic Acid Market Size#Acetic Acid Market Forecast#Acetic Acid Market Report#Acetic Acid Market Growth
2 notes
·
View notes
Text
Ethyl Acetate Market | Global Industry Size, Growth, Analysis & Forecast to 2030 | ChemAnalyst
According to ChemAnalyst report, “Ethyl Acetate Market Analysis: Plant Capacity, Production, Operating Efficiency, Demand & Supply, End Use, Distribution Channel, Region, Competition, Trade, Customer & Price Intelligence Market Analysis, 2015-2030”, Ethyl Acetate market has witnessed a considerable growth as it reached 3.2 million tonnes in 2020 and is expected to grow a healthy global CAGR of 4.50% in the forecast period. Continuously growing demand of Ethyl Acetate for the production of various coating formulations such as urethanes, epoxies, cellulosics, acrylics, vinyl, and others by the paints and coatings industry is likely to boost the global Ethyl Acetate market over the next ten years. The production of Ethyl Acetate is highly related with safety concerns on the human health before it can be further manufactured, which further enhances the compliance standards.
Ethyl Acetate is an organic ester compound having the molecular formula C4H8O2. It is a flammable and colorless liquid, which has the characteristic of a sweet fruity smell. Ethyl Acetate, as also known as Ethyl Ethanoate, is an important commodity chemical used in various industrial and commercial applications. Ethyl Ethanoate is primarily used as solvents and diluents for cleaning mixtures, paints, coatings, varnishes, adhesives, lacquers and perfumes. It is extensively used as a component of nail polish removers and lacquer thinners. In laboratories, solvent of Ethyl Acetate is commonly used in thin-layer and column chromatography. Ethyl Ethanoate is manufactured by two methods including esterification of Ethanol and Acetic acid, and catalytic condensation of Acetaldehyde with Alkoxides. Although, in fruits it occurs naturally, where it is responsible for the taste and smell of the fruit.
Read Full Report Here: https://www.chemanalyst.com/industry-report/ethyl-acetate-market-632
The global Ethyl Acetate market on the basis of end-use industries can be segmented into paints and coatings, food and beverages, automotive, construction, packaging, and others. Global demand of Ethyl Acetate is dominated by the paints and coatings industry as it is widely used in paints and coatings as an activator and hardener. Exponentially rising demand of Ethyl Acetate by the paints and coatings industry is likely to bolster in the forecast period as well due to increasing construction activities across the globe. Increasing demand of Ethyl Acetate in the food and beverage industry is expected to rise in the near future as it is acceptable for food applications like artificial flavor enhancer in confectionery items and decaffeinating tea and coffee. In emerging economies, the initiatives of government for the construction of commercial buildings, including hotels and resorts, are augmenting the demand for floor coatings, which will further boost the global market of Ethyl Acetate in upcoming years. Rising demand of Ethyl Acetate solvents by the packaging industries to manufacture flexographic and rotogravure inks, will fuel the global Ethyl Acetate market in the next few years. Owing to the robust demand of Ethyl Acetate by the pharmaceutical industry is expected to drive the global market of Ethyl Acetate in the future.
The outbreak of COVID-19 had a negative impact on the global Ethyl Acetate market. Several industries including paint and coatings, construction, printing, packaging, aerospace, and others were drastically affected due to interruptions in the global supply chain. There was a severe decline in the sales and demand of Ethyl Acetate solvents as many production units halted their operations during the first half of 2020. Once the restrictions imposed by the leading authorities are uplifted, major end-user industries including paints and coating and flexible packaging, will accelerate the demand of Ethyl Acetate around the world by the end of the year.
Read Free Sample Report Online: https://www.chemanalyst.com/ChemAnalyst/RequestForm
Among different regions, Asia Pacific region holds the major share of the global demand of Ethyl Acetate. Rapidly growing urbanization and income levels in populous countries like China and India, is driving the dominance of global Ethyl Acetate market in the Asia Pacific region and is forecasted to grow significantly until 2030. Rising construction activities in the Asia Pacific region will further augment the demand of Ethyl Acetate in the future. Expansion of major end-user industries including automotive, flexible packaging, paints and coatings is another factor driving the demand of Ethyl Acetate in the Asia Pacific region.
Some of the major players operating in Ethyl Acetate market include Celanese Corporation, Eastman Chemical Company, INEOS Capital Limited., Celanese Corporation, Jubilant Life Sciences Limited, Sipchem, Lonza, Sekab, PT. Indo Acidatama Tbk., Solvay, Merck KGaA, Shandong Jinyimeng Group Co. Ltd., DAICEL CORPORATION, KAI CO., LTD., and Others.
“Rapidly growing demand for Ethyl Acetate by the flourishing paints and coatings industry as well as flexible packaging industry across the globe is likely to boost the global Ethyl Acetate market in the forecast period until 2030. Initiatives of the government for the construction of commercial buildings and building of smart cities in the Asia Pacific region, will further augment the market growth of Ethyl Acetate in the next few years. As China is the world's largest producer of automobiles, rising demand of Ethyl Acetate solvents by the automotive industry will flourish the global Ethyl Acetate market in upcoming years.” said Mr. Karan Chechi, Research Director with TechSci Research, a research-based management consulting firm promoting ChemAnalyst worldwide.
About Us
ChemAnalyst is a subsidiary of Techsci Research, which was established in 2008, and has been providing exceptional management consulting to its clients across the globe for over a decade now. For the past four years, ChemAnalyst has been a prominent provider of Chemical commodity prices in more than 15 countries. We are a team of more than 100 Chemical Analysts who are committed to provide in-depth market insights and real-time price movement for 300+ chemical and petrochemical products. ChemAnalyst has reverberated as a preferred pricing supplier among Procurement managers and Strategy professionals worldwide. On our platform, we provide an algorithm-based subscription where users can track and compare years of historical data and prices based on grades and incoterms (CIF, CFR, FOB, & EX-Works) in just one go.
The ChemAnalyst team also assists clients with Market Analysis for over 1200 chemicals including assessing demand & supply gaps, locating verified suppliers, choosing whether to trade or manufacture, developing Procurement Strategies, monitoring imports and exports of Chemicals, and much more. The users will not only be able to analyze historical data for past years but will also get to inspect detailed forecasts for the upcoming years. With access to local field teams, the company provides high-quality, reliable market analysis data for more than 40 countries.
ChemAnalyst is your one-stop solution for all data-related needs. We at ChemAnalyst are dedicated to accommodate all of our world-class clients with their data and insights needs via our comprehensive online platform.
Contact Us:
ChemAnalyst
420 Lexington Avenue,
Suite 300, New York,
NY, United States, 10170
Call:- +1 3322586602
Email: [email protected]
Website: https://www.chemanalyst.com/
0 notes
Text
Artificial grape isn't even all that artificial either, it's derived from methyl 2-aminobenzoate (MA) in the Vitis labrusca hybrid known as the "Concord grape", which was originally developed in Concord, Massachusetts. Funny enough, MA from MA is also a core ingredient in apple flavoring as well (along with ethyl ethanoate and ethyl butanoate). It's also an important grape for the creation of wine that is produced in accordance with halakha, and kashrut.
If you want actual artificial, look at banana flavoring, which is made using isopentyl acetate, a chemical that was more common in the "Big Mike" variety of banana, vis-a-vis Cavendish (the one you normally buy at the store today) which is now functionally extinct thanks to the Fusarium oxysporum f. sp. cubense fungus. That's why anything that's banana flavored doesn't really taste like banana. It tastes like an almost extinct form of banana that you can't usually buy today.
Grape is the yellow of flavours. You know how your brain just makes up yellow because it needs something to fill in the gap between red and green? Grape is the same thing. You eat something purple and artificially flavoured and your brain fills in Grape. I don't care if it's berry or plum or blackcurrant or a mixture of blue raspberry and cherry or any other purple you can imagine. Even straight up chemical nonsense. Unless you're actively searching for something else, if you eat something purple, you taste Grape. I don't make the rules.
8 notes
·
View notes
Text
Why Ethyl Acetate Is Used In Rotogravure Inks?
Rotogravure is an intaglio printing procedure in which the image is engraved on the image carrier. Ethyl acetate manufacturers use a rotary printing press, and the image is to be engraved on a cylinder for printing. There are also drawbacks of using engraving printing, such as the rapid production of high-quality printing. The cylinders last a very long time and create countless sensations. Rotogravure Ink is put directly into the cylinder; after that, the ink passes on to the substrate.
Ethyl acetate is used in different coating forms such as epoxy, urethane, cellulose, acrylic, and vinyl. Applications for these coatings are various, including wood furniture and fixtures, agricultural, building, mining machinery, auto-refining and repair, and marine applications.
Ethyl acetate acts as a solvent
Ethyl acetate is used as a solvent in rotogravure printing inks. It is used to produce medicinal and agricultural products as an extraction solvent and as a carrier solvent for herbicides. High-purity materials may be used as viscose reducers for resins used as photoresist formulations in the electronics industry.
Low-Toxicity
Due to its low toxicity and pleasant odour, Ethyl Acetate is used as a solvent for rotogravure printing inks. Its key purpose is to melt the resin, monitor the viscosity, and change the drying rate. In the flexible packaging industry in screen printing, ethyl acetate is used in polyester film and film aluminium because of its fast evaporation properties and as an auxiliary in the manufacture of glazed and translucent paper.
Ink Saving
Consumption savings of 20% in ink on average and 30% in solvents on average without any improvements to the printing infrastructure and, on the technological side, increased printing efficiency, more reliable printing methods, faster printing speeds, and a more stable and sustainable printing process can be accomplished overall.
Quite often, printers are not conscious that they can refine and troubleshoot printing processes with little effort because they keep the equation solvent. In the past, because of the lack of substitutes, their use of conventional solvents has seldom been called into question; ethyl ethanoate can address multiple issues and lead to higher process stability. Furthermore, the direct substitution of Ethyls with Propyls is very easy and practically straightforward.

Reduces Plugging
Propyls eliminate the so-called plugging drying or clogging of the printing cylinder or anilox cells. As a medium evaporating solvent, Rotogravure evaporates more slowly than ethyl acetate but ensures that the ink dries consistently during the phase. As a result, with Propyls, fewer solvent fillers are used to preserve the target viscosity throughout the printing process. It leads to a substantial reduction in solvent intake and generally lower emissions of hazardous substances, so-called volatile organic compound Tocsin the Propyls solvent method, the printing ink's pigments may be dispersed more homogeneously to the film during application due to a slightly slower drying process.
This results in a greater density of ink with the same amount of pigment. In this way, the printer produces the same print output with considerably less dye, saving costly base ink.
Optimum Ink Transfer
Improved emptying and pick-up properties of both the engraving cylinder and the anilox ensure the printing ink's optimal transition to the ground. Thanks to the higher evaporation rate of Rotogravure, the printing press's speed can be increased in many situations without losing print quality. Finally, Propyls show less foam-forming than Ethyls in Rotogravure printing, particularly when it comes to warm and humid atmospheric weather conditions. Propyls outperform traditional solvents such as ethyl acetate and ethanol in engraving or flexographic applications without further adaptation.
0 notes
Text
Lupine Publishers | Phytochemical and Antimicrobial Screening of the Leaves of Crotalaria Lachnosema Against Staphylococcus Aureus, Salmonella Typhi, Escherichia Coli and Klebsiella Pneumoniae
Lupine Publishers | An archive of organic and inorganic chemical sciences

Abstract
The leaves of Crotalaria lachnosema were freshly collected, dried under-shade and ground into powder. The ethanolic extract of the sample was obtained by cold extraction and was fractionated with solvent of varied polarity. The fractions were analyzed for their phytochemicals and screened antimicrobial against Staphylococcus aureus, Salmonella typhi and Escherichia coli. The phytochemicals were distributed among the test fractions. Tannins were found to be present in all the fractions and methanol fraction contains all the other tested phytochemicals except alkaloids and cardiac glucosides. The activities of the fractions were found to be more pronounced against E. coli than against the other test organisms.
Keywords: Phytochemical Screening; Antimicrobial; Crotalaria lachnosema; Staphylococcus aureus; Salmonella aureus; Salmonella typhi; Escherichia coli; Klebsiella pneumoniae
Introduction
For many centuries, man explores and utilizes the natural endowment offered by both the species of flora and fauna to provide the basic necessity of life such as clothing, shelter, food and indeed health care. Medicinal plants are the richest and commonest natural resource used in traditional medicine. Of the 250, 000 higher plant species on earth, more than 80,000 are medicinal [1]. Although plants had been priced for their medicine, flavoring effect and aromatic qualities for centuries, but the synthetic products of the modern age had for some time surpassed their importance. However, the blind dependence on synthetics is over and people are returning to the naturals with hope of safety and security [1]. The development of drug resistance in human pathogens against commonly used antibiotics has necessitated a search for new antimicrobial substances from other sources including plants [2]. Many reports have attested the efficacy of herbs against microorganisms, as a result, plant is one of the bedrocks of modern medicine to attain new principles [3]. The therapeutic properties of plants may not be unconnected to the variety of chemical substances biosynthesized by the plants as “secondary metabolites’’ that bring about definite physiological action in the human body. The most important of these bioactive constituents of plants are alkaloids, tannins, flavonoids, saponins and etc. [4]. Presently many governments and major health institutions including the World Health Organization [5] have recognized, pharmacologically validated and improved many traditional herbal medicines and eventually integrated them in formal health care system [1]. Thus, in light of the evidence of rapid global spread of resistant clinical isolates, the need to find new antimicrobial agent is of paramount importance. However, the past record of rapid, widespread emergence of resistance to newly introduced antimicrobial agents, indicates that even new families of antimicrobial agents will have a short life expectancy [6]. For this reason, researchers are increasingly turning their attention to herbal products, looking for new leads to develop better drugs against MDR microbe strains [7].
Crotalaria lachnosema belongs to the family Fabaceace (Leguminoseae), sub-family Papilionoideae. It is a woody plant with a height of about 2 cm high. The plant is known as ‘Fara birana’ in Hausa, ‘komp’ in Yoruba, ‘Ake dinwo’ in Ibo and Birjibei in Fulani [8]. The genus Crotalaria is widespread in the tropics and subtropical region and has about 550 species [9]. C. lachnosema was found to be important in the treatment of scabies. The whole plant grounded and mixed with water are fed to animals to treat liver disease [8]. The presence of resins and balsams might support the use of the plant as emollient as well as for treatment of sore throat, rheumatism, wounds and burns. Since some basalms and resins has antiseptic properties [3]. Few species of Crotalaria have been assessed against some pests. For example, under greenhouse condition, C. retusa and C. juncea have been found to be resistant to attack by the nematode, Pratyylenchus zeae and also that C. retusa has shown a higher degree of resistance to attack by the nematode, Rotylenchus rnifirmis Linford and Olivera. It was also reported that, the non-polar extract of C. retusa contain some active ingredients for controlling flea beetle a pest on okro plant. So, could be useful in pest management [10].
Materials and Methods
Sampling and Sampling Sites
The leaves of Crotalaria lachnosema were freshly collected on 4th July 2011 at an uncultivated land in Damanko village about 9km west of Zaria main town, Zaria Local Government, Kaduna State. The plants were identified and authenticated by Mallam Umar Shehu Galla of the Herbarium unit, biological science, Ahmadu Bello Univesity, Zaria. The leaves of the plant were dried under-shade for seven days and ground into powder using clean pestle and mortar.
Extraction and Fractionation of Plant Materials
Cold extraction (Percolation) was adopted in this research, this is part of the appropriate measure to preserve constituents that may potentially be active and retain their original identities in the course of preparing the extract [11]. 200g of the powdered plant sample was weighed and sucked in1000cm3 of ethanol for 14 days. The crude extract was prepared by decantation, filtration and concentration of the filtrate using Rota vapor machine (RVO) at 400C and finally by drying the concentrated crude ethanol extract. Fractions of various degrees of polarities were obtained from ethanol extract by macerating the ethanol extract with different solvents in sequence starting with solvent of least polarity to the one of highest polarity [12]. For the fractionation, 30cm3 of n-hexane was poured into the beaker that contained the dried and gummy ethanol extract and stirred for 5minutes and the liquid portion was then drained into another cleaned and empty beaker. This process was repeated until a clear solution was obtained at the end. The entire procedure was repeated with other solvents in the series; chloroform, ethyl acetate and methanol. Four fractions were thus obtained from the exercise and were labeled as followed: n-hexane fraction, chloroform fraction, ethyl acetate fraction and methanol fraction.
Phytochemical Screening of Plant Sample
The phytochemical analyses of the fractions were conducted by subjecting the fractions to different standard confirmatory tests. This is to determine the presence of certain phytochemical classes.
Test for Alkaloids: Each fraction (0.5g) was stirred with 5ml of 1 percent aqeous hydrochloric acid on a steam bath; 1ml of the filtrate was treated with a few drops of Mayer’s reagent and a second 1ml portion was treated similarly with Dragendoff’s reagent. Turbidity or precipitation with either of these reagents was taken as evidence for the presence of alkaloids in the extract being evaluated [13].
Test for Saponins: Each fraction (0.5g) was shaken with water in a test tube. Frothing which persists on warning confirmed the presence of saponins [14].
Test for Tannins: Each fraction (0.5g) was stirred with 10ml of water. This was filtered, and ferric chloride reagent was added to the filtrate, a blue-black precipitate indicated the presence of tannins [15].
Test for Flavonoids: A portion of each fraction was heated with 10ml of ethylacetate over a steam bath for 3mins. The mixture was filtered and 4ml of the filtrate was shaken with 1ml of dilute ammonia solution. A yellow colouration indicated the presence of flavonoid.
Test for Reducing Sugar: 1ml of each fraction was taken in five separate test tubes. These were diluted with 2ml of distilled water followed by addition of Fehling’s solution (A+B) and the mixtures were warmed. Brick red precipitate at the bottom of the test tube indicated the presence of reducing sugar [16].
Test for Cardiac Glycosides: 2ml of each fraction was placed in a sterile test tube. This was followed by adding 3ml of 3.5% iron III chloride (FeCI3), then 3ml ethanoic acid. This gave a green precipitate and a dark colored solution respectively. Finally, concentrated H2SO4 was carefully poured down the side of the test tub e which resulted in the formation of brownish red layer, at the interface. This confirms the presence of cardiac glycosides.
Antimicrobial Activity Test
Agar disc diffusion technique was adopted for the sensitivity test as described by [17].
Preparation of Test Fractions’ Concentration: Discs of about 6mm diameter were punched from Whatman’s No 1 filter paper using a paper puncher. Batches of 10 of the paper discs were transferred into vial bottles and sterilized in an oven at 1400C for 60 minutes. Stock solutions of 100mg/ml of the fractions were prepared by dissolving 200mg of each fraction in 2ml of DMSO (Dimethyl sulphoxide). By means of 1ml sterile syringe, 0.1ml, 0.2ml, 0.5ml and 1.0ml were transferred into labeled vial bottles preoccupied with 10 paper discs from a stock solution of each fraction and the solution were subsequently diluted with 0.9ml, 0.8ml, 0.5mland 0.0ml (i.e. without dilution) of DMSO that correspondingly resulted to 1mg/disc, 2mg/disc, 5mg/disc and 10mg/disc concentration. The prepared concentrations of the test fractions in the labeled bottles were kept in refrigerator until required for use.
Preparation of Inoculum from the Test Micro-Organisms: Staphylococcus aureus, Salmonella typhi, Escherichia coli and Klebsiella pneumoniae that were sourced from Microbiology unit of Aminu Kano Teaching Hospital (AKTH) Kano, were the microorganisms used for the research. The identities of the microorganisms were confirmed by standard biochemical test [18]. The test organism was cultured and maintained in a nutrient agar slant at 40C. The organism was then inoculated into nutrient broth and incubated overnight at 370C for 24 hrs. They were then diluted with normal saline until they give concentration of bacterial cells equivalent to 0.5 McFarland standard of Barium sulphate solution (1% v/v) [19].
Antibacterial Susceptibility Test (Bio Assay)
A suspension of nutrient agar (28g in 1000ml of distilled water) was prepared and autoclaved at 1210C for 15mins according to the manufacturers’ instruction. It was then carefully poured into sterile petri-dishes and allowed to solidify. The standardized inoculums of the bacteria were swabbed on the surface of the solid nutrient agar plates by means of sterile wire loop for the confluent growth of the bacteria. Four paper discs of 10mg/disc, 5mg/disc, 2mg/ disc, 1mg/disc concentrations were taken from the prepared test fraction solutions and were carefully and aseptically placed on the inoculated surface of the nutrient agar and a positive control disc (Tetracycline 1mg/disc) was placed at the centre of the plate. The plates were incubated inverted at 370C for 18 hours. The diameters of clear areas surrounding the discs where growths of the organisms were impeded (Zone of inhibition) were measured in millimeter and recorded. The assay was repeated two more times. The mean and the standard deviation (±SD) for the triplicate values were then calculated.
Results and Discussion
Tables 1-3 Mean of the triplicates ± S.D (standard deviation). A total ethanolic extract of 16.05g was produced from the 200g powdered plant sample. The highest percentage mass (63.05%) of the total mass macerated was methanol fraction and the least percentage mass (0.56g) was the pet. ether fraction. The result of phytochemical analysis revealed the availability of some secondary metabolites in the fractions of the plant sample. The presence of these secondary metabolite’s accounts for the activities of the plants. This complied with several reports by researchers that plants contain bioactive substances. Tannins were detected in all the fractions of the plant sample and tannins were reported to have various physiological effects like anti-irritant, anti secretolytic, antiphlogistic, antimicrobial and antiparasitic effect. Phytotherapeutically, tannins containing plants are used to treat non-specific diarrhea, inflammations of mouth and throat and slightly injured skins [20-22]. While cardiac glucosides which are used as lexative and carthatic drugs were confirmed in chloroform and ethyl acetate fractions. Alkaloids that were present in n-hexane and chloroform fractions act as antimalarial and anti-amoebic agents [22]. The antimicrobial sensitivity test result revealed a varied degree of activities exhibited by the fractions of the plant against the test organisms. Although, the plant sample exhibited low activities when compare to the control, the results show that activity of the different fractions may increase further if the concentrations of the fractions were to be increased. The result also showed that the activities of the plant fractions were comparatively more pronounced against E. coli than against S. aureus, S. typhi. and K. pneumoniae. With the exception of chloroform fraction that demonstrated some activities against S. aureus with zone of inhibition of 12mm at1000ug/disc all other fractions were inactive against S. aureus. However, n-hexane and ethyl acetate fractions exhibited low activities against S. typhi.
Conclusion
The activities of the fractions of the plant sample are more pronounced against E. coli than against the other test organisms. E. coli can cause diarrhea, urinary tract infections, respiratory illness, bloodstream infections and other illness. So, the plant leaves can be used in the treatment of the aforementioned illnesses. However, the relative low activities of the plant sample fractions against S. typhi and K. pneumoniae revealed its un-befitting nature as an antityphoid and anti-pnuemoniie drug.
Recommendation
The other parts of the plant should also be exploited. To harness its full medicinal potential, the plant sample fractions should be tested against other bacteria isolates and further research should be carried out to isolate and characterize the active compounds in the plant.
https://lupinepublishers.com/chemistry-journal/pdf/AOICS.MS.ID.000173.pdf
https://lupinepublishers.com/chemistry-journal/fulltext/phytochemical-and-antimicrobial-screening-of-the-leaves-of-crotalaria-lachnosema-against-staphylococcus-aureus.ID.000173.php
For more Lupine Publishers Open Access Journals Please visit our website: https://lupinepublishersgroup.com/
For more Open Access Journal on Chemistry articles Please Click Here: https://lupinepublishers.com/chemistry-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers Follow on Twitter : https://twitter.com/lupine_online
1 note
·
View note
Text










Molymod Inorganic/Organic Student Set
Organic Compounds pt.1
Ethane, Ethene, Ethyne, Ethanol, Butanone, Ethanoic Acid, Ethyl Ethanoate, Dimethy Ether, Aminoethane, and Butane
2 notes
·
View notes
Text
some things we can do with ethanol
first, we need to make some ethanol by hydrating some ethane! of course, we need a strong acid to be present to catalyze the reaction XD CH3-CH3 + HOH + H+ --> CH3CH2OH ethane + water (in the presence of a strong acid) --> ethanol
we can oxidize the ethanol to become ethanal (AKA acetaldehyde)... CH3-CH2-OH + (O) --> CH3-CH=O ethanol + (oxidizing agent) --> ethanal
...and if we continue to oxidize the ethanal, we will make vinegar: CH3-CH=O + (O) --> CH3-COOH ethanal + (oxidizing agent) --> acetic (ethanoic) acid
now we can mix this vinegar with some more ethanol to form an ester in what we call an “esterification” reaction! CH3-COOH + CH3-CH2-OH + H+ + heat --> CH3-COO-CH2CH3 + HOH ethanoic acid + ethanol (in the presence of a strong acid and heat) --> ethyl ethanoate + water
but what if i don’t like the ester i’ve formed...? that’s okay because we can perform a saponification reaction to get back the acid and alcohol! CH3-COO-CH2CH3 + OH- + heat --> CH3-COOH + CH3-CH2-OH ethyl ethanoate (in the presence of a strong base and heat) --> ethanoic acid + ethanol
a cool little byproduct of the saponification reaction is the creation of soap! i didn’t show it in my example, but normally, there’s a cation from the base that reacts to form soap along with the major reactants listed above^ XD so cute!
u can do all sorts of stuff with ethanol! it is not only for drinking!
2 notes
·
View notes
Text
Ethyl Acetate Market is Expected to Grow at a CAGR of 4.50% by 2030

According to ChemAnalyst report, “Ethyl Acetate Market Analysis: Plant Capacity, Production, Operating Efficiency, Demand & Supply, End Use, Distribution Channel, Region, Competition, Trade, Customer & Price Intelligence Market Analysis, 2015-2030”, Ethyl Acetate market has witnessed a considerable growth as it reached 3.2 million tonnes in 2020 and is expected to grow a healthy global CAGR of 4.50% in the forecast period. Continuously growing demand of Ethyl Acetate for the production of various coating formulations such as urethanes, epoxies, cellulosics, acrylics, vinyl, and others by the paints and coatings industry is likely to boost the global Ethyl Acetate market over the next ten years. The production of Ethyl Acetate is highly related with safety concerns on the human health before it can be further manufactured, which further enhances the compliance standards.
Read Full Report Here: https://www.chemanalyst.com/industry-report/ethyl-acetate-market-632
Ethyl Acetate is an organic ester compound having the molecular formula C4H8O2. It is a flammable and colorless liquid, which has the characteristic of a sweet fruity smell. Ethyl Acetate, as also known as Ethyl Ethanoate, is an important commodity chemical used in various industrial and commercial applications. Ethyl Ethanoate is primarily used as solvents and diluents for cleaning mixtures, paints, coatings, varnishes, adhesives, lacquers and perfumes. It is extensively used as a component of nail polish removers and lacquer thinners. In laboratories, solvent of Ethyl Acetate is commonly used in thin-layer and column chromatography. Ethyl Ethanoate is manufactured by two methods including esterification of Ethanol and Acetic acid, and catalytic condensation of Acetaldehyde with Alkoxides. Although, in fruits it occurs naturally, where it is responsible for the taste and smell of the fruit.
Read Free Sample Report Online: https://www.chemanalyst.com/ChemAnalyst/RequestForm
The global Ethyl Acetate market on the basis of end-use industries can be segmented into paints and coatings, food and beverages, automotive, construction, packaging, and others. Global demand of Ethyl Acetate is dominated by the paints and coatings industry as it is widely used in paints and coatings as an activator and hardener. Exponentially rising demand of Ethyl Acetate by the paints and coatings industry is likely to bolster in the forecast period as well due to increasing construction activities across the globe. Increasing demand of Ethyl Acetate in the food and beverage industry is expected to rise in the near future as it is acceptable for food applications like artificial flavor enhancer in confectionery items and decaffeinating tea and coffee. In emerging economies, the initiatives of government for the construction of commercial buildings, including hotels and resorts, are augmenting the demand for floor coatings, which will further boost the global market of Ethyl Acetate in upcoming years. Rising demand of Ethyl Acetate solvents by the packaging industries to manufacture flexographic and rotogravure inks, will fuel the global Ethyl Acetate market in the next few years. Owing to the robust demand of Ethyl Acetate by the pharmaceutical industry is expected to drive the global market of Ethyl Acetate in the future.
The outbreak of COVID-19 had a negative impact on the global Ethyl Acetate market. Several industries including paint and coatings, construction, printing, packaging, aerospace, and others were drastically affected due to interruptions in the global supply chain. There was a severe decline in the sales and demand of Ethyl Acetate solvents as many production units halted their operations during the first half of 2020. Once the restrictions imposed by the leading authorities are uplifted, major end-user industries including paints and coating and flexible packaging, will accelerate the demand of Ethyl Acetate around the world by the end of the year.
Among different regions, Asia Pacific region holds the major share of the global demand of Ethyl Acetate. Rapidly growing urbanization and income levels in populous countries like China and India, is driving the dominance of global Ethyl Acetate market in the Asia Pacific region and is forecasted to grow significantly until 2030. Rising construction activities in the Asia Pacific region will further augment the demand of Ethyl Acetate in the future. Expansion of major end-user industries including automotive, flexible packaging, paints and coatings is another factor driving the demand of Ethyl Acetate in the Asia Pacific region.
Ethyl Acetate Market Analysis: Plant Capacity, Production, Operating Efficiency, Demand & Supply, Application, End Use, Distribution Channel, Region, Competition, Trade, Customer & Price Intelligence Market Analysis, 2015-2030”, some of the major players operating in Ethyl Acetate market include Celanese Corporation, Eastman Chemical Company, INEOS Capital Limited., Celanese Corporation, Jubilant Life Sciences Limited, Sipchem, Lonza, Sekab, PT. Indo Acidatama Tbk., Solvay, Merck KGaA, Shandong Jinyimeng Group Co. Ltd., DAICEL CORPORATION, KAI CO., LTD., and Others.
“Rapidly growing demand for Ethyl Acetate by the flourishing paints and coatings industry as well as flexible packaging industry across the globe is likely to boost the global Ethyl Acetate market in the forecast period until 2030. Initiatives of the government for the construction of commercial buildings and building of smart cities in the Asia Pacific region, will further augment the market growth of Ethyl Acetate in the next few years. As China is the world's largest producer of automobiles, rising demand of Ethyl Acetate solvents by the automotive industry will flourish the global Ethyl Acetate market in upcoming years.” said Mr. Karan Chechi, Research Director with TechSci Research, a research-based management consulting firm promoting ChemAnalyst worldwide.
Browse Related Reports
Adhesives Market: Plant Capacity, Production, Operating Efficiency, Demand & Supply, Form, Type, Application, Distribution Channel, Region, Competition, Trade, Customer & Price Intelligence Market Analysis, 2015-2030
https://www.chemanalyst.com/industry-report/adhesives-market-105
Coating Additives Market Analysis: Plant Capacity, Production, Operating Efficiency, Technology, Demand & Supply, End-User Industries, Distribution Channel, Regional Demand, 2015-2030
https://www.chemanalyst.com/industry-report/coating-additives-market-562
About Us
ChemAnalyst is a leading provider of chemical commodity prices in more than 12 countries since from last 4 Years. The company has emerged as a preferred pricing supplier amongst Procurement Managers and Strategy Professionals globally who wants to track near real time prices of chemicals on its interactive dashboard. Unlike most of its competitors such as ICIS, IHS & S&P Platts the company doesn’t believe in delivering prices in PDF reports. The company has developed proprietary algorithm based online subscription platform in which users can track years of historical prices of more than 250 chemical commodities. In addition, since it’s all online, the users cannot just compare prices across multiple countries but also with other commodities and play with the data by generating multiple graphs to find out amazing insights. The users get access to grade wise CIF, CFR & Ex Works prices at multiple ports in each country.
ChemAnalyst also provides market analysis for more than 1000+ chemical commodities such as Production, Demand, Supply, Plant Operating Rate, Imports, Exports, Suppliers, Customers and much more. The company has created online interactive dashboard in which customers can access all this data instantly with a click of a button. The users will not only be able to analyse historical data for past years but will also get to analyse short term and long-term forecasts for coming years. With the access to local field teams, the company can provide high quality reliable market analysis data for more than 20 countries.
ChemAnalyst is a one stop solution for all the data related needs. We at ChemAnalyst are committed to assist customers worldwide with their data and insights needs using our comprehensive online platform.
For more information, please visit us at www.chemanalyst.com
Contact Us:
Nilesh Vishwakarma
B-44 Sector-57 Noida,
National Capital Region
Tel: 0120-4523948
Mob: +91-8882336899
Email: [email protected]
#ethylacetate#ethylacetatemarket#ethylacetatereport#ethylacetateindustry#ethylacetatemarketsize#ethylacetatemarketshare#ethylacetatemarkettrends#ethylacetatemarketgrowth#ethylacetatedemand
0 notes
Text
“polypeptide” “production of ester ethyl ethanoate” “condensation polymerisation”

#chemistry paper two fight me till one of us dies challenge#its so insulting because the entire section on organic compounds and hydrocarbons n functional groups sucks ass#but then its like a topic edicated to rates of reactions which is not that deepiudfsgjkhdrng
1 note
·
View note
Text
How Increasing Demand for Adhesives is Contributing to Acetyls Market Growth?
For any product to reach its final stage, it has to go through the whole process of being extracted from raw materials, further refining or combing with other materials, and then further processing. Adhesives, are an essential component in the manufacturing of numerous products, be it any industry, such as electronics, wood/timber, packaging, leather goods, or furnishing. This is where acetyls come in; they are an essential component of adhesives themselves.
Acetyls, such as vinyl acetate and ethyl acetate monomer, are significant in the adhesives industry. Ethyl acetate solvent-based adhesives have a better bonding strength than equivalent toluene-based adhesives. Poly vinyl acetate (PVA or PVAc), commonly known as wood glue or carpenter’s glue, extensively utilizes vinyl acetate monomer for its manufacturing. When it is emulsified in water, it is used as an adhesive for porous materials, such as cloth, wood, paper, and sandstone. Therefore, acetyls are in high demand due to the flourishing end-use industries where adhesives are used.
In a P&S Intelligence study, it was reported that the acetyls market would accumulate $34,283.2 million in the coming years, advancing at a 6.9% CAGR from 2015, when it garnered $22,004.7 million. An acetyl group contains a methyl and a carbonyl group. From a small molecule, such as an OH group, to those with long chains of carbon atoms, this group can be attached to any molecule. The acetyl group is used in the manufacturing of various products, such as acetic anhydride, acetic acid, ethyl acetate, and vinyl acetate monomer.
Know the market worth & business opportunities at: http://bit.ly/2QeNiPe

In 2015, among all the products derived from those with an acetyl group, acetic acid was consumed the most. Acetic acid, also known as methanecarboxylic acid and ethanoic acid, is a colorless liquid with a pungent smell, which is used as a raw material in many industries. Even in the coming time, acetic acid would continue to be in the highest demand.
Among North America, Asia-Pacific, Europe, and other parts of the world, the highest demand for acetyl and its derivatives was generated in the Asia-Pacific region. This can be attributed to the various flourishing end-use industries, such as packaging and textiles and paints and coatings and the growing requirement for adhesives in construction and furniture here.
The improving gross domestic product of many countries, such as India and China, has resulted in the increasing spending power of customers as well as money inflow in industries, thereby resulting in a high demand for finished products. This region is expected to generate the highest demand for acetyls in the coming years as well as the requirement for products manufactured by numerous industries would be the maximum here.
A shift toward renewable energy from non-renewable sources is being observed as the latter are leading to high levels of pollution. Photo voltaic (PV) modules are used for storing energy generated from the sun. These modules have a glass-top surface, an outer frame, a rear layer, and an encapsulant, which is mostly made of ethylene-vinyl acetate (EVA).
In the solar power industry, EVA is gaining popularity due to its superior optical properties, cross-linking bonding abilities, and high durability. One of the major challenges that solar module manufacturers face is ensuring that in harsh outdoor environments, PV modules last long. EVA, a copolymer of vinyl acetate and ethylene, is used to make the encapsulant, which provides better protection against delamination and corrosion and has remarkable durability.
#Acetyls Market#Acetyls Market Growth#Acetyls Market Size#Acetyls Market Share#Acetyls Market Demand#Acetyls Market Opportunities#Global Acetyls Market#Acetyls Market Future#Acetyls Market Trends
0 notes
Photo

#AceticAcid VS Organic Vinegar #ReadSomeFacts "Acetic VS Vinegar #Vinegar is 5% acetic acid. Acetic acid is a compound that, when pure, is water clear and freezes just above room temperature. Pureacetic acid will seriously burn you if it contacts the skin or eyes, but when diluted to 5% with water becomes a tasty condiment. So vinegar is dilute acetic acid. #Acetic acid (CH3COOH), also called ethanoic acid, the most important of the carboxylic acids. A dilute (approximately 5 percent by volume) solution of acetic acid produced by fermentation and oxidation of natural carbohydrates is called vinegar; a salt, ester, or acylal of acetic acid is called acetate. Industrially, acetic acid is used in the preparation of metalacetates, used in some printing processes; vinyl acetate, employed in the production of plastics; cellulose acetate, used in making photographic films and textiles; and volatile organic esters (such as ethyl and butyl acetates), widely used as solvents for resins, paints, and lacquers. Biologically, acetic acid is an important metabolic intermediate, and it occurs naturally in body fluids and in plant juices. Acetic acid has been prepared on an industrial scale by air oxidation of acetaldehyde, by oxidation of ethanol (ethyl alcohol), and by oxidation of butane and butene. Today acetic acid is manufactured by a process developed by the chemical company Monsanto in the 1960s; it involves a rhodium-iodine catalyzed carbonylation of methanol (methyl alcohol). What Foods Use Acetic Acid as an Ingredient? As a food ingredient, it is found commonly in marinades, mustard, catsup, vinaigrettes and salad dressings, sauces, canned fruits, and mayonnaise. It may also be found in pickledproducts such as pickled sausages and pigs feet.Jul 17, 2015 FDA to update list of vinegar brands with synthetic acetic acid weekly Published June 5, 2019 5:18pm By JON VIKTOR D. CABUENAS, GMA News The Food and Drug Administration (FDA) on Wednesday said it set to https://www.gmanetwork.com/news/money/companies/696757/fda-to-update-list-of-vinegar-brands-with-synthetic-acetic-acid-weekly/story/?related https://www.instagram.com/p/ByVdrJdFpOQMbtXQ5XRWFEqmpTkAEoXdBSa-ME0/?igshid=14wr22isxctuk
0 notes