#b-312
Explore tagged Tumblr posts
Note
M!Noble Six with a cuddly s/o?
Ah, been a while since I wrote for Noble Six. Hope these aren't too late for you, anon.
Noble Six would be all for a cuddly S/O, despite his reservations about his armor being removed. To him, physical affection reassures him that his partner is real and safe, and the amount of dopamine that swirls through his veins is enough to send him off to Cloud Nine.
Just in general though, Six wouldn't mind PDA; in fact, I imagine he actively encourages it. He is a rare instance where despite him being a Spartan, he is quite open to the idea of physical touch in a relationship and isn't afraid to initiate it with his S/O if they'll allow him too. Be it hand-holding, an arm draped across his S/O's shoulders, he's all for it.
When the revelation is revealed to Noble Team about his S/O and how he can be quite affectionate with his partner (in a non-sexual way), it is something they didn't expect from someone who was formerly considered a lone wolf. Carter is the one who took the most issues with it; seeing it as they're active military, he believes that Six should keep physical contact with his S/O in public to a minimum and not preform it at all in front of superiors, but Six was never one to follow the rules too closely. The only part of the lecture he'll agree with is no PDA in front of his superiors, since he doesn't want his S/O be forced to split from him.
I follow my old set of HCs from quite a bit ago in the fact that Six would cuddle his S/O on the floor of their apartment when he's able to take some leave-time from his duties. Being a Spartan has some benefits, so Six would help his S/O buy many weighted blankets and pillows to help cushion the more rigged parts of his armor, along with buying stash of snacks and random movie flicks.
Prefers being the big spoon for obvious reasons - he's incredibly protective of his partner and has seen pieces of war that he would've never wanted to see, so holding them in his arms grants him some form of comfort. Doesn't mean he'll always want to be the big spoon though; after a nightmare or a rough week of jobs from Noble, he'd enjoy being held, or at least the best his S/O can hold him.
During his temporary leave-time from active duty, Six would likely carry his S/O on his back while he carries out the needed chores for the day. Quite quickly he'd become known through his S/O's little community - S/O's Spartan in shining armor, ever protective of his love, but radiates a soothing presence and carries a heart of glimmering gold. Six helps out whenever he's able to, but tries not to stay out too long as it make sure the sun doesn't agitate his S/O too much.
There have been a few occasions where Jorge and Jun visited Six during his leave, and the sight they got to witness Six catching a break under some shade, his S/O's head laying against his armored thigh and in a pleasant slumber; Six's gloved hands would be tenderly brushing against their scalp while his gaze was drawn to their sleeping form, love guiding all of his delicate actions. Jorge chuckled softly as Jun gave him a look - the mysterious Noble Six was absolutely love-struck.
#halo reach x reader#halo x reader#noble team#halo reach#halo ce x reader#halo#noble team x reader#x reader#reader insert#spartan b-312#b-312#noble six#noble six x reader#male noble six#six x reader
97 notes
·
View notes
Text

Elliot is yummy as fuck. - Anon @elliotbooker
0 notes
Text
Chapter 312 Subchapter B | O'Connor
O'Connor provides a brief summary of Chapter 312 Subchapter B of the tax code. Get in touch with O'Connor today! Visit https://www.poconnor.com/texas-property-code-2021-chapter-312-subchapter-b/
0 notes
Text
Navigating 21 CFR 312: A Comprehensive Guide
Keeping Up with FDA Regulations and Requirements
What is 21 CFR 312?
21 CFR 312 is a set of regulations and requirements set out by the Food and Drug Administration (FDA). It details the procedures and processes that clinical trials must follow in order to be approved by the FDA. These regulations must be followed in order to ensure that the clinical trial being conducted is safe and beneficial for the participants.
21 CFR 312 is a federal regulation from the Code of Federal Regulations that outlines the requirements for Investigational New Drugs (INDs). This section of the code provides guidance to drug manufacturers and sponsors on what is required to have an IND approved by the Food and Drug Administration (FDA). It also addresses topics such as requirements for preclinical safety testing, clinical trials protocol design, and data collection.
The regulation begins by defining the meaning of investigational new drugs and explains how these drugs differ from other types of pharmaceutical products. It then outlines the requirements for initiation and oversight of clinical trials, including protocols for filing a Form 1571 with FDA prior to commencing any trial. 21 CFR 312 also requires sponsors to submit detailed information about their proposed clinical trials, including a description of study objectives, expected endpoints, investigator qualifications and selection criteria, as well as a description of any potential risks associated with participation in the study. Additionally, sponsors must provide evidence that the proposed protocol has been reviewed and approved by an Institutional Review Board (IRB) prior to submitting it to FDA.
21 CFR 312 further addresses essential documentation that must be included in any submission to FDA requesting approval for an IND. This includes an investigator's brochure containing information regarding safety data collected during previous studies conducted using similar compounds or agents; reports summarizing preclinical studies performed such as toxicity testing; as well as all reports prepared by investigators involved in conducting the clinical trial. The regulation also sets forth requirements regarding monitoring ongoing clinical trials and reporting any adverse events or serious unexpected events that occur during the course of a trial.
21 CFR 312 plays a critical role in providing drug manufacturers and sponsors with necessary guidance on how to develop safe drugs while adhering to important ethical considerations related to human research protection standards. As such, it serves an essential function in ensuring that new drugs are developed safely before they can be released into the market for public use.
Who Does 21 CFR 312 Affect?
21 CFR 312 affects a wide range of individuals, including physicians, researchers, sponsors, and institutional review boards (IRBs). All of these individuals must comply with 21 CFR 312 in order to ensure that the clinical trials they are conducting are conducted in a safe and ethical manner.
How Can I Stay Up to Date With 21 CFR 312?
Staying up to date with 21 CFR 312 is essential for those conducting clinical trials. The best way to stay up to date is to regularly review the 21 CFR 312 regulations and requirements, as well as any changes or updates that have been made. Additionally, you should regularly consult with an expert on 21 CFR 312 to ensure that you are following the regulations correctly and to answer any questions or concerns you may have.
21 CFR 312 is a subchapter of the Code of Federal Regulations (CFR) that establishes the requirements for human drug clinical trials in the United States. It covers the regulations for drug safety and efficacy studies, which can include both non-clinical and clinical trials. The regulations are designed to protect human subjects while ensuring the accuracy, integrity, and reliability of data used to support applications seeking approval from the Food and Drug Administration (FDA). The scope of 21 CFR 312 encompasses all phases of drug development, including pre-clinical research, clinical trials, post-marketing studies, adverse event reporting, and manufacturing.
Under this regulation, sponsors must submit an Investigational New Drug (IND) application to FDA before beginning any human drug trial in order to obtain permission to use an unapproved investigational drug or biologic product in a clinical trial. Sponsors must provide detailed information on the manufacturing process, composition, pharmacology/toxicology data from animal models, chemistry data from nonclinical laboratory tests, previous clinical experience with similar drugs or biologics products as well as an assessment of potential risks associated with use of the investigational product.
21 CFR 312 also requires sponsors to develop comprehensive protocols for each study or trial conducted under its jurisdiction. These protocols must specify objectives for each study and define what measurements need to be taken during each stage in order to ensure that appropriate safety measures are taken throughout the entire process. Additionally, protocols should be clearly written so that consistent results are obtained across multiple sites conducting trials with similar investigational products.
Finally 21 CFR 312 ensures that adequate provisions exist for informed consent forms given to participants in clinical trials so they understand their rights as subjects and any potential risks associated with participating in a particular study or trial.
Study Guide for 21 CFR 312
1. Overview: 21 CFR 312 is a part of Title 21 of the Code of Federal Regulations (CFR). It details the regulations, procedures, and requirements that must be met in order to conduct clinical investigations involving drugs and medical devices. These regulations are designed to ensure patient safety and protection during such testing.
2. Investigational New Drug Application (IND): Subpart A outlines an application process for any new drug intended for use in a clinical investigation. The IND must include information about the preclinical pharmacology, toxicology, and other activities related to the development of the drug as well as proposed protocols for clinical testing.
3. Investigator Responsibilities: Subpart B outlines the responsibilities of investigators conducting clinical trials with investigational drugs or devices. This includes obtaining informed consent from subjects, submitting reports on progress and adverse effects experienced by subjects, maintaining accurate records, and reporting any unanticipated problems or serious adverse events that occur during a trial.
4. Sponsor Responsibilities: Subpart C outlines the responsibilities of sponsors who are responsible for conducting or sponsoring clinical trials with investigational drugs or devices. This includes monitoring study sites to ensure compliance with good clinical practice standards and providing investigators with necessary safety information regarding any investigational products they may be using in their trials.
5. Institutional Review Boards (IRBs): Subpart D outlines guidelines for IRBs which are responsible for ensuring that all research involving human subjects is conducted ethically and according to FDA regulations. This includes reviewing protocols for clinical trials before they can begin and providing ongoing oversight throughout the course of a study so that patient rights are protected throughout the duration of a trial.
6. Termination or Suspension: Subpart E outlines provisions allowing FDA to terminate or suspend ongoing investigations if any safety concerns arise during a trial that could threaten subject safety or render data generated from a trial unreliable or invalid due to protocol violations or unethical practices by investigators or sponsors involved in an experiment
What is 21 CFR 312?
21 CFR 312 is a set of regulations issued by the US Food and Drug Administration (FDA) to establish good clinical practice (GCP) standards for conducting clinical trials. It covers the design, conduct, performance, monitoring, auditing, recording, analysis and reporting of clinical investigations conducted under FDA regulations.
What types of studies does 21 CFR 312 cover?
21 CFR 312 covers interventional studies that involve human participants or data from human participants used to determine the safety or effectiveness of a drug product. These studies may include phase I through IV clinical trials for new drug products as well as bioavailability/bioequivalence studies and post-marketing surveillance activities.
Who must comply with 21 CFR 312?
All sponsors and investigators who are involved in conducting clinical investigations subject to FDA jurisdiction must comply with 21 CFR 312. This includes all sponsors and investigators who submit an Investigational New Drug application (IND) to the FDA or an Abbreviated New Drug Application (ANDA).
What are the key requirements of 21 CFR 312?
The key requirements outlined in 21 CFR 312 include obtaining informed consent from study participants; providing accurate records; establishing quality assurance procedures; protecting the rights and welfare of study subjects; ensuring appropriate data collection and analyses techniques; evaluating data integrity; maintaining confidentiality of subjects and their information; training personnel involved in the trial on GCP protocols; preparing detailed reports of findings; establishing audit trails; gaining approval from an Institutional Review Board before beginning any trial activity and many more important elements.
How does 21 CFR 312 affect research ethics?
By complying with 21 CFR 312, researchers ensure that human subjects are treated ethically during clinical trials. Key ethical considerations that must be met include obtaining informed consent from study participants; protecting patient privacy; minimizing risk to patients participating in trials and ensuring proper oversight throughout the duration of the trial.
What is a sponsor's responsibility when conducting a clinical investigation covered under 21 CFR 312?
A sponsor’s responsibility under 21 CFR 312 includes developing adequate protocols for each investigation, selecting qualified investigators to ensure effective oversight, obtaining informed consent forms from all patients involved in any investigational activities, providing adequate safeguards regarding patient confidentiality, informing participants about potential risks associated with any investigational activities they may be engaged in and ensuring compliance with all applicable laws related to GCP related activities.
What role does an Institutional Review Board play when conducting a clinical investigation covered under 21CFR312?
An Institutional Review Board (IRB) has a critical role when conducting investigations subject to FDA jurisdiction as outlined in21CFR312. An IRB is responsible for reviewing protocols submitted by sponsors prior to commencing any investigational activity involving human subjects. The IRB also provides ongoing review and monitoring throughout the course of an investigation to ensure continued adherence to all applicable FDA regulations related to GCP standards outlined in 21CFR312.
What type of documentation must be maintained according to 21CFR312?
According to 21CFR312 sponsors must maintain documentation outlining the duties performed by each individual involved in any investigational activity related tot he trial including protocol development activities, informed consent process details etc.. In addition sponsors must keep thorough records detailing all data collected during each stage off the trial as well as facilitate audit trails so that investigators can easily trace back any changes made during analysis or reporting stages off thee trial process..
How often should audits take place according for 21CFR312 ?
Sponsors must perform audits at least annually according twenty one C F R three twelve The objective off these audits is two ensure compliance within applicable regulatory standards In some cases additional audits may be necessary depending on complexity off thee protocol being investigated or if unusual deviations occur during thee course off thee trial ..
When should sponsors provide reports to FDA based on their findings ?
Sponsors should provide reports too FDA based on their findings no later than thirty days after completion off thee investigation . If necessary , requesting additional time due two extenuating circumstances can bee performed before submitting report results .
Review Questions for 21 CFR 312
MCQ 1: What is the purpose of 21 CFR 312?
A. To establish rules and regulations for the production and sale of drugs
B. To protect public health by ensuring drug safety
C. To reduce the cost of pharmaceuticals
D. To create standards for food safety
Answer: B. To protect public health by ensuring drug safety. 21 CFR 312 is a section of federal regulations that are designed to ensure drug safety through the establishment of rules and regulations for their production, distribution, labeling, quality control and advertising. The primary goal of this regulation is to protect public health by guaranteeing that all pharmaceutical products meet minimum standards for efficacy, potency, purity and quality.
MCQ 2: What type of information must be included on a label in accordance with 21 CFR 312?
A.Ingredient list
B.Expiration date
C.Instructions for use
D.Nutritional value
Answer: A. Ingredient list. According to 21 CFR 312, all pharmaceutical labels must include an ingredient list containing information about all active ingredients used in the product as well as any inactive ingredients that make up more than 2% of the total weight or volume of the product. Additionally, labels must also include information about any colorants used as well as impurities present in trace amounts that could adversely affect users if consumed in large quantities over time.
MCQ 3: How often must pharmaceutical companies submit manufacturing records to the FDA?
A. Monthly
B. Annually
C. Quarterly
D. Biannually
Answer: C Quarterly. Pharmaceutical companies must submit detailed manufacturing records to the Food and Drug Administration (FDA) on a quarterly basis when filing reports required by 21 CFR 312, Subpart G-Requirements for Registration of Manufacturers/Processors/Packers/Holders (§312). This includes comprehensive records regarding quality control systems testing procedures, manufacturing facilities, operations specifications, equipment maintenance schedules and more that demonstrate compliance with FDA regulations for safe production practices and product quality assurance purposes
MCQ 4: What role does advertising play within 21 CFR 312?
A .It is permitted but heavily restricted
B .It is not mentioned at all
C .It is prohibited altogether
D .It is unrestricted
Answer : A It is permitted but heavily restricted. The advertising and promotion requirements outlined in 21 CFR 312 provide comprehensive guidance on how firms should market their products while remaining compliant with regulatory requirements set forth by the Food & Drug Administration (FDA). While these regulations do permit firms to advertise their products under certain conditions it also places several restrictions such as prohibiting false or misleading claims or engaging in deceptive practices when promoting their products
MCQ 5 : Which type of information can be shared between manufacturers when trading biological materials according to §312.50(a)(1)?
A .Confidential trade secrets
B .Patent information
C .Test results
D .Facility locations
Answer : C Test results. Section 312.50(a)(1) outlines which types of information can be shared between manufacturers when trading biological materials such as microorganisms or raw materials intended for use in animal feed or fertilizer applications, medicinal drugs or food additives etc.. Test results from safety analysis performed on such materials may be shared between manufacturers provided they have been adequately validated for accuracy prior to disclosure
MCQ 6: Which factors are taken into account when determining whether a clinical trial should be conducted?
A. The predicted risk or benefit associated with a drug's use
B. The number of participants needed in a study to obtain valid results
C. The cost associated with conducting the trial
D. All of the above
Answer: D. All of the above. 21 CFR 312 states that clinical trials must be conducted in order to determine whether a drug is safe and effective and that considerations such as potential risks, benefits, number of participants needed for valid results, and cost must all be taken into account when making this determination.
MCQ 7: When must an investigational new drug application (IND) be submitted according to 21 CFR 312?
A. When initiating any clinical investigations involving a new drug
B. When submitting new marketing applications for a drug product
C .When introducing any changes to an approved drug product
D .All of the above
Answer: A .When initiating any clinical investigations involving a new drug . According to 21 CFR 312, an IND must be submitted prior to initiating any studies or trials involving an investigational new drug or biologic agent in humans in order to ensure patient safety and health protection standards are met prior to initiation of these activities.
MCQ 8: What type of data is required when submitting an investigational new drug application (IND)? A . Clinical data from past trials involving similar products
B .Data on animal testing conducted using the proposed product
C .Data on manufacturing processes used during development
D .All of the above
Answer: D .All of the above . In order for an IND to be approved by FDA regulatory authorities all available information regarding preclinical studies, pharmacology/toxicology studies, chemistry manufacturing controls, previous clinical experience with similar products, proposed protocol(s), investigator qualifications must all be submitted alongside supporting documents outlining these details so that decisions can be made based on available data points provided in these documents.
MCQ 9: What type of review process takes place after submission of an investigational new drug application (IND)?
A . Statistical analysis using collected clinical data
B .A comprehensive evaluation by FDA regulatory experts
C .Approval from Institutional Review Board (IRB)
D .All of the above
Answer: D .All After submission of an IND both statistical analysis using collected clinical data as well as comprehensive evaluations by FDA regulatory experts take place in order for decisions about approval or rejection to be made; additionally Institutional Review Boards review all materials before approving studies or trials for conduct with human participants per FDA guidelines outlined in 21 CFR 312
CCRPS offers certification with 21 CFR 312 review in depth. Demo our courses today to learn more.
#21 cfr 312#21 cfr part 312#cfr 21 part 312#21 cfr 312.32#21 cfr 312.23#21 cfr 312.62#21 cfr 312.40#21 cfr 312.60#21 cfr 312.120#21 cfr 312 investigator responsibilities#21 cfr 312 pdf#21 cfr 312 subpart d#21 cfr 312.110#21 cfr 312.160#21 cfr 312.2#21 cfr 312.2 b#21 cfr 312.2 b 1#21 cfr 312.20#21 cfr 312.21#21 cfr 312.21 a#21 cfr 312.21 b#21 cfr 312.22#21 cfr 312.23 a 10#21 cfr 312.23 a 2#21 cfr 312.23 a 3#21 cfr 312.23 a 5#21 cfr 312.23 a 6#21 cfr 312.23 a 6 iii b#21 cfr 312.23 a 7#21 cfr 312.23 a 7 iv e
0 notes
Text
Being Big Red
Rise Ramblings #312
In “What Was Meant To Be” and “What They Became,” I discuss how the turtles were created by Draxum to be weapons and then how the boys were embraced by Splinter to be a part of the Hamato clan.
I also discussed how Splinter viewed television as a window into his former life. He used television as a means to drown himself in a never-ending cycle of reminiscing the past and mourning his former self.

Splinter’s crushing depression, though never voiced, impacted the turtles’ emotional growth and development. As a result, all four brothers had to cope with their father’s lack of attention and his expectations for their lives in their own way…
However, I believe that no one had more pressure placed on them than Raphael Hamato.

Raphael is naturally easy-going, sweet, fun-loving, and supportive. But, as the oldest/biggest turtle, he became the impromptu leader of their little team by default. Consequently, he takes on several different roles for the sake and well-being of his family.
Their day-to-day training regimen is directed completely by him.

He is the boys' moral compass and who they go to for guidance.

He's the team’s backbone, support, and backup, which often cumulates in him acting as a physical shield when things get rough.
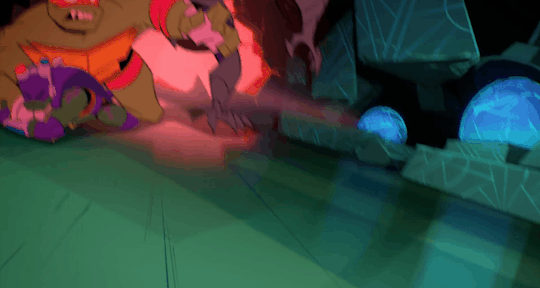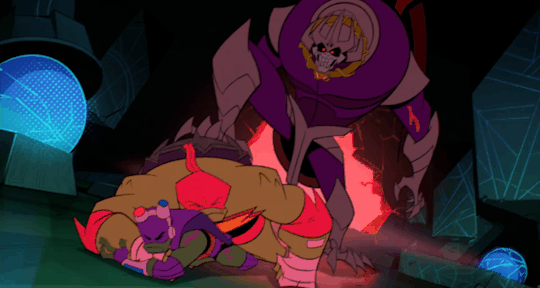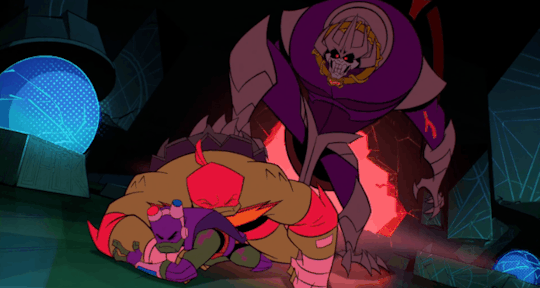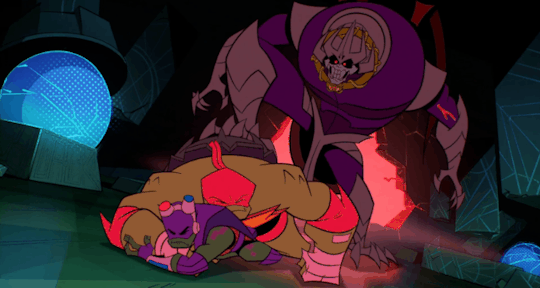
And, most significantly, Raph is the leader even when he himself wants nothing more than to crumble to pieces.

Raph is so physically imposing, strong-willed, and devastatingly kind-hearted that it’s easy to expect these roles from him.
But, Raph is also just a child.
In reality, these roles should never have been Raph’s to bear…
Parentification is a process in which a child or adolescent is forced to act as a parent to their siblings (or to their actual parent) through providing emotional support (Emotional Parentification) or physical support (Instrumental Parentification) in order to maintain the household.
I believe that Raphael was subjected to both, but was especially subjected to the former.
All of the roles described above are the roles of a supportive parent to their children (or Sensei to their students.) To verify this claim, you needn’t look further than the roles that Splinter encompassed in any other iteration.
With Raph, none of this responsibility comes naturally. He has to work hard to live up to the pressures and expectations placed onto him, resulting in a dissonance between his responsibilities and his true nature.
I believe that you can see the evidence of this dissonance in his chosen form of dress.
Have you noticed that when Raph casually dresses himself, he mostly wears white?



Even Donnie picked up on this trend when he chose outfits for his brother in "The Clothes Don't Make The Turtle." (See "The Fashionista" for a full breakdown on Donnie's impeccable fashion sense.✨)


Yet, when Raphael is filling a role, or dressing to impress others, Red is his automatic go-to.


It’s almost as if the title of “The Red One” was not one that he chose, but one that was merely placed onto him.
But I digress...
Raph is able to be a pseudo-parent to his brothers and serves to fill in the gaps that their actual father could not fill. However, with no outlet for his own insecurities, all of that pressure had no relief.
And, if you understand chemistry, pressure, with no release, creates an explosion.

“Acting out” is an unhealthy defense mechanism in which one expresses their unacceptable feelings through physical actions.
In this case, the "unacceptable feeling" is disappointment, not at his brothers, or with his father, or with any external force, but with himself. And with no outlet and with no one to turn to for support, that disappointment turns into red hot anger.
He’s so disappointed with himself, in fact, that he reaches his breaking point.

Then finally, finally, he opens up.

And at long last, he gets the support he so desperately needed.
Thus, he is able to ultimately let it all go...

It's so lovely to see that his family does not disappoint.

○○○○
Next | Being Baby Blue • Being Purple ○ Part One • Being Purple ○ Part Two • Orange, Baby!
Finale | Being Hamato Yoshi
#he is the best of boys#he tries so hard#and I love him for that#research resources provided upon request#starkiss ramblings#rise analysis#rottmnt analysis#character analysis#Raphael Ramblings#rise raph#rise raphael#raphael#rottmnt raphael#raphael hamato#rottmnt#tmnt#teenage mutant ninja turtles#rise of the teenage mutant ninja turtles#rise of the tmnt#tmnt2018#tmnt 2k18#tmnt 2018#save rottmnt#unpause rottmnt#unpause rise of the tmnt#save rise of the tmnt#save rise of the teenage mutant ninja turtles
2K notes
·
View notes
Text
Kiss - Arthur Leclerc
Words: 312 Prompt: "You're making it so hard to concentrate right now." "Good, pay attention to me."

Masterlist | Support Me! | Part of Sin's 5k & B-Day Celebration
Arthur can feel her looking at him. Her eyes seemed to bore into him. It makes him want to stop watching this onboard, to laugh, to get up from the kitchen table and to join her. But he doesn’t. He keeps watching and tries his best to ignore his girlfriend who is silently begging for his attention.
She gives a quiet huff and his lips twitch up into a smile.
The apartment is filled with quietness, only the sound of their breathing, the quiet hum of the refrigerator, and the even quieter sounds of the car onboard in his ears. It’s peaceful in an odd way.
He watches intently as the car goes into turn seven, nearly losing the racing line. As it regains he hears the couch creek and another quiet huff before the sound of socked feet padding across the hardwood fills his ears.
“Amour,” he warns when he feels her hovering behind him. She isn’t even touching him, not even making any noise but already his concentration is broken. “You’re making it so hard to concentrate right now.” “Good, pay attention to me.” He lets out a laugh as she speaks, her arms wrapping around him as she bends to rest her chin on his shoulder. “Have I been ignoring you?” She nods and he ignores the slight dig of her chin in his shoulder at the movement. “You have. It’s been hours since you even looked at me.” He lets out a little laugh, that was more than false, but he still closes his laptop and relaxes back into the chair, into her.
“Hello, amour.” He murmurs, tipping his head back when she stands straight to see her face. She smiles at him. “Hello, Thur.” He grins at her before puckering his lips. “Kiss?” She giggles but bends again, pressing a sweet gentle kiss to his lips. “Kiss.”

@ironspdy @crashingwavesofeuphoria @poppyflower-22 @racingheartsposts @namgification @alessioayla @gemofthenight @peachiicherries @lpab @hiireadstuff @iloveyou3000morgan @boiohboii @bibliosaurous @skepvids @elliegrey2803 @tallrock35 @casperlikej @clowngirlsstuff
#arthur leclerc x reader#arthur leclerc imagine#... i don't know what else to tag for him#elms x reader#elms imagine#i guess???#sins 5k bday bash fics#sins fics
598 notes
·
View notes
Text
Reason #312 for why I fully believe Katniss was the instigator of "so after".
Peeta B. Mellark is an old-fashioned GOOD BOY.
Man had to invent a whole fake wedding before bringing his fake baby into this.
He said "WED-LOCKED"
After "so after" he's gonna be like "so when are you going to make an honest man out of me?"
#peeta mellark#katniss everdeen#everlark#the hunger games#thg series#thg#hunger games headcanon#thg headcanons#so after#growing back together#if it weren't for the baby#put a ring on it
176 notes
·
View notes
Text

WTH House has been removed from the market. Built in 1932 in Westmont, Illinois, it's been renovated, but whoever did the reno must've been on shrooms at the time. 4bds, 4ba, and they're asking $849,900, which is probably why it's been taken off the market, b/c no one would pay that much for it, and it can't possibly pass a home inspection. Take a look.

Weird entrance. I don't even know if the door clears that first step, but there's this ersatz elevated platform w/a small door and angled stairs so you can display a lovely collection of large vases. (Also note the off-center pot light shining brightly.) That abstract painting is a prophesy of things to come.

The platform continues on around to the side entrance. Why do they need that thing at all? Unusual features: Light fixtures under the window sills and another one under the stair railing.

I don't know if this is the family room or main living room, but it's normal.

Observe the obstacles in the hall to get to the kitchen steps. The frame around the windows doesn't look right.

You can see it better from here. They made an elaborate kitchen entrance, there's some room off the stairs, maybe a toilet, and the fridge is too close to the edge.

Double doors in the dining area open to a bedroom.

Floor tile on the bathroom walls.

By the wild wood grain on the door to the primary suite, it looks like they used plywood to make the doors. There's a simple homemade kitchenette behind the door.

PeeWee's Playhouse doorway to the en-suite shows the shower in full view. (Nope.)

The mirror continues the theme of the ceiling angles and there's a long window seat.

Okay, this deck off the primary bedroom is nice.

This is another large bedroom.

These angle-lovin' folks must've had all the mirrors cut to order.

A monolithic column with niches stands in the middle of this bedroom.


The best part is the basement, though, where there's a castle-like feel. Love the walls and the fireplace. I must say that the mason they hired was superior to the carpenter.

This bath is nice and carries through with the castle theme.

The levels and angles continue on the exterior of the home.

Nice big yard. The house is on a .49 acre lot.
to
192 notes
·
View notes
Text

Todoroki Shoto (20):
Key: angst -💔; fluff -🤍; comfort -❤️🩹; angst to fluff -🧡; crack -💜; slight angst -🩵; Fan fav -❤️🔥
Fanfictions
...
Drabbles
Early Mornings 🤍
Just some fluffy snuggles with your hubby and son.
Night Terrors ❤️🩹🤍
Shoto has a nightmare, and you're quick to comfort him.
Morning Kisses 🤍🤍
You wake up from feather like kisses from your husband; Shoto. The following moments are filled with more kisses, fluff, and some bantering. | 315 words + gn reader
The Last Straw 💔🧡
You and Shoto get into a heated argument | 1,497 words | fluff ending
'Us Time' 🤍💜
Shoto is tired of barely having any time with you, so he proposes an idea to get you for himself for an entire week. | 854 words \\ posted: 9/26/23
Home 🤍❤️🩹 (requested)
Yandere Shoto. He takes you home after you get kidnapped. | 2,199 words \\ posted: 11/08/23
M'love 🤍
You come home from work to your alpha mate, who seems anxious about something. | 312 words \\ posted: 03/25/24 \\ A/B/O dynamics
Headcannons/Scenarios
MHA boys react to you torturing an Endeavor plushy🤍💜
Simply that my friends. Enjoy the chaos.
You don't say I love you back 💜🩵
You're gonna be murdered by some, and you'll break the others. Choose your poison.
How they would react to you going to therapy for past abuse 🤍❤️🩹 (request)
Self explanatory<3
You flinch during an argument 🧡❤️🩹
Self explanatory<3
Comfort headcannons 🤍❤️🩹 (request)
Self explanatory <3
You sing "Soft Kitty" to them when they're sick 🤍❤️🩹 (request)
Self explanatory<3 | posted: 9/4/23
Self conscious reader 🤍❤️🩹 (request)
Self explanatory<3
First Kiss 🤍 (requested)
Self explanatory<3 | 1,005 words \\ posted: 12/05/2023 \\ requested
Pregnant Reader Part 2 🤍 (requested)
Self explanatory<3 | 537 words \\ posted: 12/06/2023 \\ requested
Comforting Claustrophobia Reader Part Two 🤍❤️🩹 (requested)
Self explanatory<3 | 555 words \\ posted: 12/07/2023 \\ requested
Liking the Same Heroine as Dabi 🤍 (requested)
Self explanatory<3 | 711 words \\ posted: 12/19/2023 \\ two different endings \\ requested
Christmas Headcannons 🤍
Self explanatory<3 | posted: 12/25/2023
MHA Men When You're Sick 🤍❤️🩹
Self explanatory<3 | posted: 03/27/24 \\ 303 words
And more yet to come! <3
Last added to: 3/27/2024
Main masterlist | Requesting Rules
Do not copy, repost, nor plagiarize my work. Ask before you translate or use my work in any way, minus reblogging.
#mha#bnha#mha x reader#bnha x reader#mha fanfiction#thehusbandoden#fluff#mha fluff#angst#mha angst#bnha shoto#mha shoto#shoto x reader#todoroki shoto#shoto todoroki#shoto x reader angst#shoto torodoki#shoto x reader fluff#yandere shoto x reader#shoto x you#shoto x y/n#mha x reader comfort#mha x reader platonic#mha x reader angst#mha x reader fluff#bnha x reader comfort#bnha x reader cuddles#bnha x reader fluff#bnha x reader angst#bnha x you
243 notes
·
View notes
Text

2024/9/23 Mon. RJOI
USMC VMFA-312 Checkerboards
F/A-18C b/n.164950 "DR312"
FIGHT'S ON🔥
@CN_heroshi via X
22 notes
·
View notes
Text
ok, putting my thoughts in order
I get the why. youtube sucks and each year that passes, it sucks more. they have a company and employees, and they've said before that watcher wasn't actually making a profit. it is way too harsh to say they're too greedy or "just like buzzfeed" or other stuff I've seen thrown around. they deserve to get money for their work, we are not entitled to free content, etc! I agree with all that.
the thing is though... I don't see how this could feasibly work? like, putting aside how most people are fed up with the sheer amount of streaming platforms popping up lately, the way I see it, their content does not have enough variety to warrant a paid subscription. and if it were to become varied enough, it would probably need a bigger cast and shows run by different people. and the problem with that is that we can't deny that the main appeal of watcher is how much people care about shane and ryan and (it pains me to say this, you all know he's my favorite but, to a lesser extent) steven. a ton of us are here because we wanted to keep watching them. for the people, not the shows, essentially. that is very clear when you look at the views of their shows.
idk, what I mean to say is, I don't see how they could have a catalog of content that justifies paying a monthly subscription if you're not a very avid fan willing to support them just because they're them, and even then those avid fans might end up dissatisfied because either a) a lot of the content will not include the people they want to see or b) the content will not be frequent enough. maybe I'm wrong and there is a third option here but, let's be real, there's gotta be a limit to how many different shows they can put shane and ryan in to have a varied catalogue and frequent upload schedule. and if it's not them in those shows, we bump into problem a.
I know the topic of whether or not $5.99 is a lot of money also became a reason for fights around here. this is what I have to say, as an international fan: depending on what country you're from, it's the sort of expense you just can't justify. like, the sort of money you shouldn't even spend on netflix with its very extensive selection of content. the sort of money you could use to pay a whole bill, buy groceries for a week, a month even! as it stands, here in brazil, for now, it's not really feasible. R$312 a year is a ton of money for me and I can't even say I'm struggling financially.
still on this topic, it is really hard not to take this "affordable to anyone and everyone" thing to heart being someone outside of the US, because it is the sort of thing that happens again and again, this sort of americentrism the internet at large seems to be stuck in. when they outright say they view this price as affordable to everyone it's very clear they have not taken international fans into consideration or they just don't really care. if they hadn't said that with so much certainty, maybe I'd feel a little less hurt. and you know, whatever, it's my feelings vs the needs of a company, and companies are not your friends but! ever since the beginning, the relationship between us and them has been very parasocial. lol it's like a good friend of mine said something that hurt my feelings. although maybe that's my own fault for placing them in that role in my head in the first place.
anyway, idk if this makes sense, the goal here isn't even to pick a side or tell anyone they're wrong... as with most things this is just too complicated for that. what I can say is that the way they went about this could have been a lot better. and for now what I am feeling is that this is eventually going to crash and burn but well, I just really hope I'm wrong. they deserve good things.
#i also wanna say thank you to those who offered to pay for me you guys are so cool#but I'm not even sure I'll have time or motivation to blog about travel season or create any content related to it#and if i let any of you pay I'd feel so bad if I ended up not talking about the show here#idk! maybe i sound dumb#please don't come for me i really hope i don't make anyone mad here#watcher
49 notes
·
View notes
Text
AlterMidya on Twitter @altermidya:
DONATION DRIVE para sa mga apektado ng bagyong #CarinaPH [Donation drive for those affected by Typhoon Carina]
Nanawagan ng donasyon para sa relief goods ang iba't ibang grupo para umagapay sa mga apektado ng malakas na ulat at pagbaha na dulot ng bagyong Carina at malakas na habagat. [Various groups are calling for donations for relief goods to support those affected by the strong rains and flooding caused by Typhoon Carina and the strong southwest monsoon.]
Para sa mga dagdag na detalye, i-click ang mga poster sa thread na ito: [For additional details, click the posters under this thread:]
2024 Jul. 24
(1/2)

CALL FOR RELIEF DONATIONS | #TyphoonCarina
Patuloy na tumataas ang baha kasabay ng pagbuhos ng ulan sa Metro Manila at iba pang mga kalapit na lugar. Nananawagan ang mga apektadong residente sa iba't ibang lugar sa Quezon City, Rizal (Rodriguez at Antipolo). Sa nakalap na report ng Bayan Muna Partylist at Bayang Matulungin, ilan sa mga apektadong lugar ay ang mga sumusunod:
Brgy. Tatalon, District 4, QC
Brgy. Talayan, District 1, QC
Brgy. Cupang, Tawid-Ilog area, Antipolo City
NIA Road, Brgy. Pinyahan, District 4, QC
Brgy. 8, District 2, Caloocan City
Kasiglahan Village, Brgy. San Jose
Rodriguez, Rizal Brgy. Maysilo, Malabon City
Tumatanggap po ng donasyon na food packs, mga tinapay at biskwit, at tubig, ganundin ang pinansyal na tulong para sa community kitchen. Pauna na ang pasasalamat para sa mga magbabahagi ng tulong.
For more information, please contact: Ms. Vhienna or Ms. Sarah 09321175586
For GCash donations 09235354319
---

Isinailalim na sa RED RAINFALL WARNING ng PAGASA ang buong Kamaynilaan kaninang umaga. Maraming lugar na ang lubog sa baha at nagsilikas na sa mga evacuation center.
Sa mga nais tumulong sa mga kababayan natin mula sa mga pinakaapektadong komunidad, ang mga sumusunod ang pinakakinakailangan:
Para sa cash donations:
Chinabank
Account name: Lingap Gabriela, Inc.
Account no.: 105002008935
Swift code: CHBKPHMM
GCash
Jo*n Ma*c C. 09157687114
Para sa in-kind donations, ang drop off point ay sa
#35 Scout Delgado, Brgy. Laging Handa, QC
We are accepting the following in-kind donations:
Rice
Canned goods
Vitamins, medicine, and first aid equipment
---


🚨 URGENT CALL FOR DONATIONS 🚨
Tulong Kabataan PH Disaster Response Center, Inc., in partnership with United Marikina Community Fire and Rescue Volunteer, calls for your support. Several parts of Metro Manila, including Barangay Tumana, Malanday, and Sto. Niño in Marikina City and areas in Quezon City and Manila have been severely flooded due to non-stop rains since Tuesday.
We are accepting both cash and in-kind donations to aid these affected communities. Your generous contributions can help provide essential supplies, rescue operations, and immediate relief.
Bank Details:
UnionBank
Account Number - 1096 6083 8825
Account Name - Earl Benz Molina
GCash
Account Number - 0921 889 1348
Account Name - Anna Kristina M.
Drop-off Center and Coordinator:
MANILA: UP Manila USC Office, 4F Joaquin Gonzales Bldg., Padre Faura St., Ermita, Manila
Coordinator: 09164150726 Kyla Sofia Benedicto
QUEZON CITY: Leong Hall, Ateneo de Manila University (Near Gate 3)
Coordinator: 09151237788 Kenneth Amores
Let's come together to help our fellow Filipinos in this time of need.
---

#CarinaPH | TULONG GURO for Typhoon Carina victims
Many families and individuals are in urgent need of help and rescue. Donate now!
For cash donations: GCash 0951 312 1148 MH****E B.
For in-kind donations: You may contact 0951 312 1148 for details.
Thank you in advance!
---

MAULANG ARAW, MGA KA-MANGGAGAWA!
Kami ay nananawagan para sa inyong donasyon upang makapagbigay ng agarang tulong sa mga manggagawa at kanilang pamilyang naapektuhan ng Bagyong Butchoy at Carina na patuloy na nagdudulot ng malakas na ulan sa malaking bahagi ng bansa.
FOR CASH DONATIONS:
EASTWEST BANK
Balai Obrero Foundation Inc.
200057848889
GCASH
Emelinda Sagcal 09561594733
IN-KIND DONATIONS DROP-OFF POINT:
Balai Obrero Foundation
#63 Narra Street, Project 3, Quezon City
Sama-samang pagkilos para sa sama-samang pagbangon.
For those in need of immediate help, you may use the contact details below:
TYPHOON EMERGENCY HOTLINE
𝗡𝗔𝗧𝗜𝗢𝗡𝗔𝗟 𝗘𝗠𝗘𝗥𝗚𝗘𝗡𝗖𝗬 𝗛𝗢𝗧𝗟𝗜𝗡𝗘 - 911
𝗣𝗛𝗜𝗟𝗜𝗣𝗣𝗜𝗡𝗘 𝗥𝗘𝗗 𝗖𝗥𝗢𝗦𝗦
Hotline: 143, (02) 527-0000, (02) 527-8385 to 95
Disaster Management Office: 143(Staff), 132(Manager),133(Radio Room)
𝗡𝗔𝗧𝗜𝗢𝗡𝗔𝗟 𝗗𝗜𝗦𝗔𝗦𝗧𝗘𝗥 𝗥𝗜𝗦𝗞 𝗥𝗘𝗗𝗨𝗖𝗧𝗜𝗢𝗡 𝗔𝗡𝗗 𝗠𝗔𝗡𝗔𝗚𝗘𝗠𝗘𝗡𝗧 𝗖𝗢𝗨𝗡𝗖𝗜𝗟 (𝗡𝗗𝗥𝗥𝗠𝗖)
Trunkline: 911-5061 to 65
Phone Number: (+632) 91114016, (+632) 9122665
𝗣𝗔𝗚-𝗔𝗦𝗔
Hotline: (02) 824-0800
𝗗𝗘𝗣𝗔𝗥𝗧𝗠𝗘𝗡𝗧 𝗢𝗙 𝗦𝗢𝗖𝗜𝗔𝗟 𝗪𝗘𝗟𝗙𝗔𝗥𝗘 𝗔𝗡𝗗 𝗗𝗘𝗩𝗘𝗟𝗢𝗣𝗠𝗘𝗡𝗧
Text Hotline: 0918-912-2813
Trunkline: (02) 931-81-01
Disaster Response Unit: 856-3665, 852-8081
𝗣𝗛𝗜𝗟𝗜𝗣𝗣𝗜𝗡𝗘 𝗡𝗔𝗧𝗜𝗢𝗡𝗔𝗟 𝗣𝗢𝗟𝗜𝗖𝗘 𝗛𝗢𝗧𝗟𝗜𝗡𝗘 𝗣𝗔𝗧𝗥𝗢𝗟
Hotline: 117, 722-0650
Text Hotline: 0917-847-5757
𝗕𝗨𝗥𝗘𝗔𝗨 𝗢𝗙 𝗙𝗜𝗥𝗘 𝗣𝗥𝗢𝗧𝗘𝗧𝗜𝗢𝗡 (𝗡𝗖𝗥)
Direct Hotline: (02) 426-0219, (02)426-3812, (02) 426-0246
𝗣𝗛𝗜𝗟𝗜𝗣𝗣𝗜𝗡𝗘 𝗖𝗢𝗔𝗦𝗧 𝗚𝗨𝗔𝗥𝗗
Trunkline: (02) 527-8481 to 89
Action Center: (02) 527-3877
0917-PCG-DOTC 0917-724-3682(Globe)
0918-967-4697
𝗠𝗘𝗧𝗥𝗢 𝗠𝗔𝗡𝗜𝗟𝗔 𝗗𝗘𝗩𝗘𝗟𝗢𝗣𝗠𝗘𝗡𝗧 𝗔𝗨𝗧𝗛𝗢𝗥𝗜𝗧𝗬
Hotline:136
Trunkline: (02) 882-4150-77
loc. 337 (Rescue)
255 (Metrobase)
319 (Road Safety)
374 (Public Safety)
320 (Road Emergency)
(02) 882-0925 (Flood Control)
𝗗𝗘𝗣𝗔𝗥𝗧𝗠𝗘𝗡𝗧 𝗢𝗙 𝗖𝗢𝗠𝗠𝗨𝗡𝗜𝗖𝗔𝗧𝗜𝗢𝗡
(DOTC) Central Hotline
Public Assiatnce Center: 7890
𝗠𝗔𝗡𝗜𝗟𝗔 𝗜𝗡𝗧𝗘𝗥𝗡𝗔𝗧𝗜𝗢𝗡𝗔𝗟 𝗔𝗜𝗥𝗣𝗢𝗥𝗧 𝗔𝗨𝗧𝗛𝗢𝗥𝗜𝗧𝗬
Text Hotline: 0917-839-6462 (TEXTNAIA)
Terminals 1,2,and 4: 877-1109 and loc. 2444
Terminal 3: 887-7888 loc. 8046
𝗠𝗔𝗡𝗜𝗟𝗔 𝗪𝗔𝗧𝗘𝗥
Hotline: 162
---

SAGIP KANAYUNAN | CALL FOR DONATIONS for FARMERS & FISHERFOLK affected by Typhoon Carina
Heavy and continuous rainfall caused by Typhoon Carina resulted to severe flooding in different farming and coastal communities in the country. Many infrastructures and equipment used for their livelihood are affected.
NNARA Youth, in partnership with the areas, is opening a monetary and in-kind donation drive to help the communities with their urgent needs. We humbly ask for your support especially with the onslaught of the typhoon.
In-kind donations could be canned goods, noodles, or coffee. Message our FB page for details on where they can be sent. For monetary support, you may send it to the QR code attached to this post.
---

📣 CALL FOR DONATIONS 📣
Serve the People Corps is accepting donations for disaster-prone areas in CALABARZON heavily affected by #CarinaPH.
Cash donations may be coursed through:
💰GCASH:
Fr*****e A****a F.
09178348950
🥫We also accept in-kind donations such as:
Drinking water
ready-to-eat or canned goods
food packs
clean clothes, beddings, blankets
medicine kits
toiletries
other immediate needs.
Please send us a message if you have any queries.
---


CALL FOR DONATIONS PARA SA MGA KOMUNIDAD NA APEKTADO NG #CarinaPH
Ang National Office ng College Editors' Guild of the Philippines (CEGP) ay naglulunsad ng isang call for donations sa gitna ng hagupit ng Bagyong Carina at Southwest Monsoon o Habagat.
Nananawagan tayo sa lahat ng nais na tumulong at magbigay ng mga donasyon para sa mga komunidad na apektado ng kalamidad.
Para sa Monetary Donations:
Gcash:
09154457991 John Ray L.
Add note: CARINA PH DONATIONS
Maaring i-scan ang QR code sa baba. Maaring i-send ang proof of transaction sa aming Facebook page.
Para naman sa in-kind donations, narito ang maaring ipadala:
Canned goods
Noodles
Rice
Water
Good clothes
Hygiene kit
Blankets
Plastic bag
Medicine/First aid Kit
Contact: Maricho Tagailo, CEGP National Coordinator
Mobile: 09660033150
Telephone: 8-28751661
Drop off location: CEGP National Office
31 Narra St., Violago Homes Phase III, Pasong Tamo Quezon City
Bilang ibayong pag-iingat, narito rin ang kompilasyon ng mga emergency hotlines at emergency operations center na maaring ugnayan.
Katuwang ninyo ang National Office ng College Editors' Guild of the Philippines sa panahong ito. Hangad namin ang kaligtasan ng bawat isa.
---
21 notes
·
View notes
Text
~przepis na omlet piernikowy~
•3 białka jajek
•1 żółtko
•40g budyniu dyniowo piernikowego
•20g erytrolu
• 75 g skyru naturalnego
•25 ml mleka bądź napoju roślinnego
•szczypta soli
~krem~
75g skyru
5 g kremu lotus
15 g erytrolu
Makro omleta z kremem: kcal 389/b 33g/tł 8g/ węgl 45g
Makro omleta bez kremu: kcal 312/b 24g/tł 7g/ węgl 39g
Białka ubijamy z odrobiną soli aż powstanie gęsta piana, mieszamy skyr,budyń i erytrytol z żółtkiem jajka i dodajemy do piany. Omlet smażymy pod przykryciem przez ok. 2 min,następnie przewracamy.
Na gotowy omlet wykładamy wcześniej przygotowany krem i opcjonalnie owoce
Omlet powinien wyjść gruby i puszysty polecam smażyć na mniejszej patelni!
Smacznego 🎀

#zdrowe odżywianie#zdrowe przepisy#Przepisy#jesień#redukcja#niskokaloryczne#Bezcukru#wysokobiałkowe#zdrowadieta#zdrowejedzenie#zdrowie
14 notes
·
View notes
Text

Ignazio Giunti (Ferrari 312 B) devant Jack Brabham (Brabham BT33 Ford-Cosworth) Grand Prix d'Autriche - Zeltweg 1970. © Rainer Schlegelmilch / Motorsport. - source Carros e Pilotos.
28 notes
·
View notes
Text
Al-Marrudhi said, "I asked Ahmad b. Hanbal how he showed gratitude for the recognition Allah had blessed him with." "I pray that I never do anything for the sake of appearances," he replied.
Dhahabi, Siyar aʿlam al-nubalaʾ, xi. 312.
#islam#quote#allah#hijab#knowledge#inspirational quotes#islam4 life#islamicadvice#jilbab#la ilaha illa allah#islamicreminder#islamicart#islamicquotes#islamic
29 notes
·
View notes
Note
bee movie
According to all known laws of aviation, there is no way a bee should be able to fly.
Its wings are too small to get its fat little body off the ground.
The bee, of course, flies anyway because bees don't care what humans think is impossible.
Yellow, black. Yellow, black. Yellow, black. Yellow, black.
Ooh, black and yellow!
Let's shake it up a little.
Barry! Breakfast is ready!
Coming!
Hang on a second.
Hello?
Barry?
Adam?
Can you believe this is happening?
I can't.
I'll pick you up.
Looking sharp.
Use the stairs, Your father paid good money for those.
Sorry. I'm excited.
Here's the graduate.
We're very proud of you, son.
A perfect report card, all B's.
Very proud.
Ma! I got a thing going here.
You got lint on your fuzz.
Ow! That's me!
Wave to us! We'll be in row 118,000.
Bye!
Barry, I told you, stop flying in the house!
Hey, Adam.
Hey, Barry.
Is that fuzz gel?
A little. Special day, graduation.
Never thought I'd make it.
Three days grade school, three days high school.
Those were awkward.
Three days college. I'm glad I took a day and hitchhiked around The Hive.
You did come back different.
Hi, Barry. Artie, growing a mustache? Looks good.
Hear about Frankie?
Yeah.
You going to the funeral?
No, I'm not going.
Everybody knows, sting someone, you die.
Don't waste it on a squirrel.
Such a hothead.
I guess he could have just gotten out of the way.
I love this incorporating an amusement park into our day.
That's why we don't need vacations.
Boy, quite a bit of pomp under the circumstances.
Well, Adam, today we are men.
We are!
Bee-men.
Amen!
Hallelujah!
Students, faculty, distinguished bees,
please welcome Dean Buzzwell.
Welcome, New Hive City graduating class of 9:15.
That concludes our ceremonies And begins your career at Honex Industries!
Will we pick our job today?
I heard it's just orientation.
Heads up! Here we go.
Keep your hands and antennas inside the tram at all times.
Wonder what it'll be like?
A little scary.
Welcome to Honex, a division of Honesco and a part of the Hexagon Group.
This is it!
Wow.
Wow.
We know that you, as a bee, have worked your whole life to get to the point where you can work for your whole life.
Honey begins when our valiant Pollen Jocks bring the nectar to The Hive.
Our top-secret formula is automatically color-corrected, scent-adjusted and bubble-contoured into this soothing sweet syrup with its distinctive golden glow you know as... Honey!
That girl was hot.
She's my cousin!
She is?
Yes, we're all cousins.
Right. You're right.
At Honex, we constantly strive to improve every aspect of bee existence.
These bees are stress-testing a new helmet technology.
What do you think he makes?
Not enough.
Here we have our latest advancement, the Krelman.
What does that do?
Catches that little strand of honey that hangs after you pour it.
Saves us millions.
Can anyone work on the Krelman?
Of course. Most bee jobs are small ones.
But bees know that every small job, if it's done well, means a lot.
But choose carefully because you'll stay in the job you pick for the rest of your life.
The same job the rest of your life? I didn't know that.
What's the difference?
You'll be happy to know that bees, as a species, haven't had one day off in 27 million years.
So you'll just work us to death?
We'll sure try.
Wow! That blew my mind!
"What's the difference?"
How can you say that?

sans #312
sans is trying to follow the instructions.
#art#sans#btw this is the only ask of this kind ill answer. this is one of those jokes where...its funny the first time.
12 notes
·
View notes