#atoms&molecules
Explore tagged Tumblr posts
Text
Difference between atomic mass and molecular mass class 9-Science
In Class 9 science, understanding the difference between atomic mass and molecular mass is fundamental in the context of atoms and molecules. Let's explore these concepts:
Atomic Mass: -Definition: Atomic mass refers to the mass of an individual atom of an element, usually expressed in atomic mass units (amu) or unified atomic mass units (u). -Unit: Atomic mass is measured in atomic mass units (amu) or unified atomic mass units (u). -Calculation: The atomic mass of an element is determined by considering the weighted average of the masses of its isotopes, taking into account their abundance in nature. The atomic mass is typically found on the periodic table. -Example: The atomic mass of carbon (C) is approximately 12.01 u, indicating the average mass of a carbon atom.
2. Molecular Mass: -Definition: Molecular mass refers to the sum of the atomic masses of all the atoms present in a molecule. It is expressed in atomic mass units (amu) or unified atomic mass units (u). -Unit: Molecular mass is measured in atomic mass units (amu) or unified atomic mass units (u).
Calculation: To find the molecular mass, add up the atomic masses of all the atoms in the molecule, as indicated by its chemical formula. For example, the molecular mass of water (H₂O) is calculated by adding the atomic masses of two hydrogen atoms and one oxygen atom. -Example: The molecular mass of water (H₂O) is approximately 18.02 u, calculated as 2(1.01 u) + 16.00 u.
Key Differences:
-Focus:
Atomic mass is concerned with the mass of an individual atom of an element.
Molecular mass deals with the combined mass of all atoms in a molecule.
-Unit:
Both atomic and molecular masses are expressed in atomic mass units (amu) or unified atomic mass units (u).
-Calculation:
Atomic mass is determined by considering the weighted average of the masses of isotopes.
Molecular mass is calculated by adding the atomic masses of all atoms in a molecule.
Example:
Atomic mass is associated with individual elements, such as the atomic mass of carbon.
Molecular mass is associated with compounds or molecules, such as the molecular mass of water.
Understanding these concepts is crucial for further studies in chemistry and provides a foundation for comprehending the quantitative aspects of chemical reactions and reactions' stoichiometry.
#science#9thclass#chemistry#class 8#11th class#botany#biology#foundation#vavaclasses#11thclass#atomic mass and molecular mass#atoms&molecules#atoms
1 note
·
View note
Text

three atoms... a whole molecule
98 notes
·
View notes
Text
Sometimes I like to lock the door, turn off the light in the bathroom and lay down on the floor for a good few minutes just to pretend I don't exist. The cold tiles embrace me and the hum of the ventilator sernades me to oblivion <3
#I need to do it again tho. Haven't had a good bathroom sesh in a while#I like to pretend I'm nothing like when I wasn't born yet <3#Some people like to snuggle up to pretend like they're back in their mother's womb and I think that's weak shit. Bring it back FURTHER-#I wanna pretend I wasn't even CONCEIVED YET. My atoms and molecules not even FORMED. I wanna be NOTHINGNESS my dude#sput chatters
39 notes
·
View notes
Text
First time watching Invincible: A Thread
Episode 2: Eve is 1000% autistic-coded, I love her and if anything happens to her someone will die by my hands
#albin watches invincible#<- mute this tag if you're not interested in my live reactions#it might be a very stereotypical autistic rep but like#eve was considered a weirdo by everyone around her including her parents and had barely any friends in her life#and had a hyper-interest in science and molecules and atoms and shit from a very young age#like. ignoring the superhero context that's just autism#her parents should've just got her diagnosed. maybe they would've been kinder to her#invincible#atom eve#samantha eve wilkins#eve wilkins
13 notes
·
View notes
Text

A molecule is a group of atoms bonded together. Molecules make up nearly everything around you – your skin, your chair, even your food.
They vary in size, but are extremely small. You can’t see an individual molecule with your eyes or even a microscope. They are 100,000 times smaller than the width of a hair.
The smallest molecule is made of two atoms stuck together, while a large molecule can be a combination of 100,000 atoms or more. A molecule can be a repeat of the same atom, such as the oxygen molecules we breathe, or can be made up of a variety of atoms, such as a sugar molecule made of carbon, oxygen and hydrogen.
44 notes
·
View notes
Text
ik i said i was gonna sleep but then fanfic and my cat nemesis screaming. anyways thinking about how ever since i was a teen ive not wanted to have kids but wanted to foster teens cause id be too scared to fuck a kid up but my set of skills has always been on track to being that of someone good at fostering teens.
and like. idk being maggot granddyke has rlly scratched that itch? especially with the idea of maggot summer camp? i am so so so full of care. being able to teach and help and support. this is all stuff i always wanted to do. this is what i was trying to do school to. and im so grateful that i get to.
i think a lot about this elderly dyke when i worked at an old folks home who toasted me when i told her how honoured i was.
i think about the kids at my high school who tomorrow afternoon are having a st patricks day party with my mom because she is one of the adult supervision and how i started that pride club nine years ago and how having a legacy at 24 is beautiful and terrifying
i think about my roommates when i moved into my current place who were like seven and ten years older than me and declared themselves my parents, at a time when i was freshly out of inpatient and floating at best
i think about the actor at sleep no more, and me crying from the beauty of the connection of queerness
i think about a friend of mine who was a youth leader at my congregation when i was in high school who i thought was nonbinary when i first met them. they didnt realise until quite a bit later. they are one of my dearest friends now
i think about the only time i went to summer camp, a week of leadership camp. it was the first place nobody knew my birth name. where i used just they/them pronouns. it was the first place i learned of the beauty of physical platonic intimacy, where we would all cuddle, or be close while playing cards or reading my immortal
i think of all of us holding hands across the years and the time and the space. in my heart and my mind there is a hangmans tree, from peter pan. the inside is all hollow and infinitely large and there is space for all those i love.
in my soul we are at summer camp and i am yearning so deeply for that to be real in whatever way i can make it
#fuck theres an angels quote#the sould of these departed joined hands clasped ankles and formed a web a great net of sould#and the souls were three atom oxygen molecules of the stuff of ozone#and the outer rim absorbed them and was repaired#nothings lost forever#in this world theres a kid of painful progress#jehangel tag#maggot taggot#maggot summer camp
72 notes
·
View notes
Text
i love you if you are made of atoms and molecules
12 notes
·
View notes
Note
miss u bae <///3

#GOOD MORNING#we have been cooking up big things apologies for the disappearance#hope all is well with you tumblr user 0rphiichaze <3#dirkjohn#they are molecules. mere atoms intertwined#tideart
77 notes
·
View notes
Text
Researchers detect a new molecule in space
New Post has been published on https://thedigitalinsider.com/researchers-detect-a-new-molecule-in-space/
Researchers detect a new molecule in space
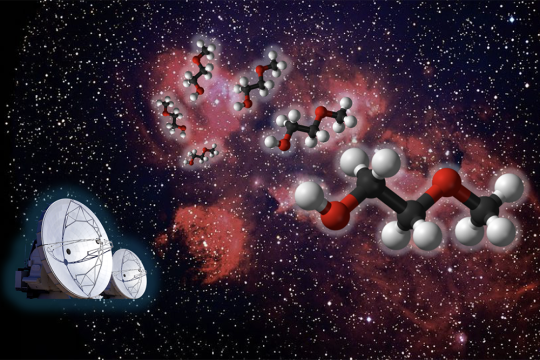

New research from the group of MIT Professor Brett McGuire has revealed the presence of a previously unknown molecule in space. The team’s open-access paper, “Rotational Spectrum and First Interstellar Detection of 2-Methoxyethanol Using ALMA Observations of NGC 6334I,” appears in April 12 issue of The Astrophysical Journal Letters.
Zachary T.P. Fried, a graduate student in the McGuire group and the lead author of the publication, worked to assemble a puzzle comprised of pieces collected from across the globe, extending beyond MIT to France, Florida, Virginia, and Copenhagen, to achieve this exciting discovery.
“Our group tries to understand what molecules are present in regions of space where stars and solar systems will eventually take shape,” explains Fried. “This allows us to piece together how chemistry evolves alongside the process of star and planet formation. We do this by looking at the rotational spectra of molecules, the unique patterns of light they give off as they tumble end-over-end in space. These patterns are fingerprints (barcodes) for molecules. To detect new molecules in space, we first must have an idea of what molecule we want to look for, then we can record its spectrum in the lab here on Earth, and then finally we look for that spectrum in space using telescopes.”
Searching for molecules in space
The McGuire Group has recently begun to utilize machine learning to suggest good target molecules to search for. In 2023, one of these machine learning models suggested the researchers target a molecule known as 2-methoxyethanol.
“There are a number of ‘methoxy’ molecules in space, like dimethyl ether, methoxymethanol, ethyl methyl ether, and methyl formate, but 2-methoxyethanol would be the largest and most complex ever seen,” says Fried. To detect this molecule using radiotelescope observations, the group first needed to measure and analyze its rotational spectrum on Earth. The researchers combined experiments from the University of Lille (Lille, France), the New College of Florida (Sarasota, Florida), and the McGuire lab at MIT to measure this spectrum over a broadband region of frequencies ranging from the microwave to sub-millimeter wave regimes (approximately 8 to 500 gigahertz).
The data gleaned from these measurements permitted a search for the molecule using Atacama Large Millimeter/submillimeter Array (ALMA) observations toward two separate star-forming regions: NGC 6334I and IRAS 16293-2422B. Members of the McGuire group analyzed these telescope observations alongside researchers at the National Radio Astronomy Observatory (Charlottesville, Virginia) and the University of Copenhagen, Denmark.
“Ultimately, we observed 25 rotational lines of 2-methoxyethanol that lined up with the molecular signal observed toward NGC 6334I (the barcode matched!), thus resulting in a secure detection of 2-methoxyethanol in this source,” says Fried. “This allowed us to then derive physical parameters of the molecule toward NGC 6334I, such as its abundance and excitation temperature. It also enabled an investigation of the possible chemical formation pathways from known interstellar precursors.”
Looking forward
Molecular discoveries like this one help the researchers to better understand the development of molecular complexity in space during the star formation process. 2-methoxyethanol, which contains 13 atoms, is quite large for interstellar standards — as of 2021, only six species larger than 13 atoms were detected outside the solar system, many by McGuire’s group, and all of them existing as ringed structures.
“Continued observations of large molecules and subsequent derivations of their abundances allows us to advance our knowledge of how efficiently large molecules can form and by which specific reactions they may be produced,” says Fried. “Additionally, since we detected this molecule in NGC 6334I but not in IRAS 16293-2422B, we were presented with a unique opportunity to look into how the differing physical conditions of these two sources may be affecting the chemistry that can occur.”
#2023#ALMA#Astronomy#Astrophysics#atoms#chemical#chemistry#college#complexity#data#Denmark#detection#development#Discoveries#earth#ether#fingerprints#form#France#how#interstellar#it#learning#Light#Machine Learning#measure#measurements#members#mit#molecules
30 notes
·
View notes
Text

hes just a little molecule of a man
#left to right:#katsuhiko akiyama#hikaru kotobuki#susumu hirasawa#hes literally a molecule. or perhaps an unbonded atom#into the hirasawa-verse#p-model
7 notes
·
View notes
Text
Atoms and Molecules Class 9 Science: Chapter-3 NCERT
Introduction:
In the realm of science, the study of atoms and molecules serves as the fundamental building block for understanding the nature of matter. Class 9 science introduces students to this fascinating world, unravelling the mysteries that lie within the microscopic realm. Let's delve into the basic concepts of atoms and molecules and explore how they form the basis of chemistry.
Atoms - The Building Blocks of Matter: At the heart of all matter, from the air we breathe to the food we eat, lies the atom. An atom is the smallest unit of an element that retains the chemical properties of that element. The concept of atoms dates back to ancient Greece, where philosophers such as Democritus first proposed the idea that matter is composed of indivisible particles.
Structure of an Atom: As class 9 science students, it's crucial to understand the structure of an atom. Atoms consist of three subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge, neutrons have no charge, and electrons carry a negative charge. The protons and neutrons reside in the nucleus at the centre of the atom, while electrons orbit the nucleus in energy levels or shells.
Atomic Number and Mass Number: Every element is characterized by its unique atomic number, representing the number of protons in its nucleus. The mass number, on the other hand, is the sum of protons and neutrons in an atom. Understanding these properties is essential for distinguishing one element from another.
Molecules - Combining Atoms: Molecules are formed when atoms combine through chemical bonds. Atoms can share, gain, or lose electrons to achieve a stable electron configuration, forming compounds with unique properties. The sharing of electrons leads to covalent bonds, while the transfer of electrons results in ionic bonds. Water (H₂O) and methane (CH₄) are examples of common molecules.
Chemical Formulas: Class 9 science explores the language of chemistry through chemical formulas. These notations represent the types and numbers of atoms present in a molecule or compound. For instance, the chemical formula for water is H₂O, indicating two hydrogen atoms and one oxygen atom in each molecule.
Laws of Chemical Combination: Students delve into the laws governing chemical combinations, such as the Law of Conservation of Mass and the Law of Definite Proportions. These laws provide a foundation for understanding the quantitative aspects of chemical reactions and the fixed ratios in which elements combine to form compounds. Some- Examples: Certainly! Here's a brief overview of the solutions for Class 9 Science Chapter 3 - Atoms and Molecules, based on the NCERT (National Council of Educational Research and Training) curriculum:
NCERT Solutions for Class 9 Science Chapter 3 - Atoms and Molecules Question 1: In a reaction, 5.3 g of sodium carbonate reacted with 6 g of acetic acid. The products were 2.2 g of carbon dioxide, 0.9 g water, and 8.2 g of sodium acetate. Show that these observations are in agreement with the law of conservation of mass.
Solution: To verify the law of conservation of mass, we need to compare the total mass of reactants with the total mass of products.
Mass of sodium carbonate (Na₂CO₃) = 5.3 g Mass of acetic acid (CH₃COOH) = 6 g
Total mass of reactants = 5.3 g + 6 g = 11.3 g
Mass of carbon dioxide (CO₂) + Mass of water (H₂O) + Mass of sodium acetate (CH₃COONa) = 2.2 g + 0.9 g + 8.2 g = 11.3 g
Since the total mass of products is equal to the total mass of reactants, the observations are in agreement with the law of conservation of mass.
Question 2: Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Solution: The given ratio is 1:8 for hydrogen and oxygen, respectively. Mass of hydrogen (H₂) = 3 g
According to the ratio, the mass of oxygen (O₂) required = 8 * Mass of hydrogen = 8 * 3 g = 24 g
Therefore, 24 g of oxygen gas would be required to react completely with 3 g of hydrogen gas. Question 3: What is the mass of one molecule of water?
Solution: The molecular formula of water is H₂O.
The molar mass of hydrogen (H) = 1 g/mol The molar mass of oxygen (O) = 16 g/mol
The molar mass of water (H₂O) = 2 * Molar mass of hydrogen + Molar mass of oxygen = (2 * 1 g/mol) + 16 g/mol = 18 g/mol
One mole of water contains Avogadro's number of molecules (6.022 × 10²³). Therefore, the mass of one molecule of water = Molar mass of water / Avogadro's number = 18 g/mol / (6.022 × 10²³ mol⁻¹)
This value is extremely small, indicating the tiny mass of a single water molecule. Question 4: If one mole of carbon atoms weighs 12 g, what is the mass (in grams) of 1 atom of carbon?
Solution: Given that one mole of carbon atoms weighs 12 g, which is equal to the molar mass of carbon.
The molar mass of carbon (C) = 12 g/mol
Now, to find the mass of 1 atom of carbon, divide the molar mass by Avogadro's number (6.022 × 10²³ mol⁻¹):
Mass of 1 atom of carbon = Molar mass of carbon / Avogadro's number = 12 g/mol / (6.022 × 10²³ mol⁻¹)
This value represents the mass of a single carbon atom.
These are just a few examples of the solutions found in Chapter 3 of Class 9 Science - Atoms and Molecules. It's essential to practice additional problems and exercises to strengthen your understanding of the concepts.
Conclusion: As students explore the world of atoms and molecules in class 9 science, they embark on a journey that unlocks the secrets of the microscopic realm. This knowledge not only forms the basis for understanding chemical reactions and properties but also lays the groundwork for advanced studies in chemistry. Embracing the wonder of the microscopic world, students gain valuable insights into the nature of matter that surrounds us every day.
#biology#botany#11thclass#science#9thclass#chemistry#class 8#foundation#vavaclasses#11th class#9th class#atoms&molecules#molecular mass#atoms
0 notes
Text
I'm as disappointed as anyone else that they cut out the reagent abuse subplot from the OG Re-Animator, but I do have to appreciate how now the audience is left to wonder whether Herbert is on speed or just an incredibly manic person
#plot twist: this is what he's like on prescription sedatives#herbert west needs to mainline seroquel or he'll vibrate at a frequency that tears molecules apart#he was expelled from his first medical school for causing an atomic antimatter explosion#herbert west#re-animator#cw drugs
20 notes
·
View notes
Text
everywhere I look.... chemistry is sneaking it's way into my other courses
#THE BRANCH OF SCIENCE IM THE WORST AT OFC IS THE ONE THAT IS LITERALLY EVERYTHING#text#how do i suck so bad at chem but am good with the others? no idea tbh its a mystery#when i say i find the microcosmos interesting im talking abt the organisms not atoms and molecules#please leave me alone chemistry u will never make sense to me#like i know the words i read and am told but i am unable to actually process and understand
7 notes
·
View notes
Text
some disney adult: "oh boy I wonder if I can spot a hidden mickey in my new science class!"
the humble water molecule:

#this came to me in a vision#it is inspired by god himself#i think i got too silly while working and it's starting to negatively impact my psyche#I NEED to drop out#I drew probably 30 of these through sn entire day of studying before this came to me#and I've been losing it since#genuinely i cannot stop thinking about it#everytime I do think about this it leaves me crippled and on the floor rolling#this is my exact brand of humour#it's so stupid and so#so good at the same time#atom#idk how to tag this bare with me#meme#molecules#adjective noun#adjective noun format#so fucking funny#I'm genuinely losing my mind#forgive me#adjective noun meme#memes#tumblr memes#this classifies as a shitpost right?#seagull becomes whimsy hours#shitpost
7 notes
·
View notes
Text
oh god i started thinking about it all and i feel weird
#i like to do this Thing. at night#where i start thinking about planets and soace and ahit#and then i start thinking aboutnlike atoms and molecules and stuff#and i start thinking about how im feeling the fabrics of my bed andhow im even thinking and how the words in my head mean something to me#and like . how im breathing#and that theres stuff i will never be able to see in the air all around#and how am i even thinking any of this right now and how does it mean anything? at all?? ti me?#and then i feel weird and fuzzy and distant and Tired
2 notes
·
View notes
Text
If u will allow me to say something elitist and dismissive and frankly a little mean…
#I don’t get people who don’t understand very very basic concepts of historical linguistics#like not even what PIE is or how anglo saxon became English after the norman conquest etc#just like. the fact that there are several families of languages that have nothing to do with each other#or that latin or greek isnt the language that every other language came from. how did u graduate HS w honors being so clueless#I understand that not everyone has the same interests as me but in terms of like. world history and basic cultural knowledge#that’s like the equivalent of me not knowing the difference between an atom and a molecule lmao. like HUH???
25 notes
·
View notes