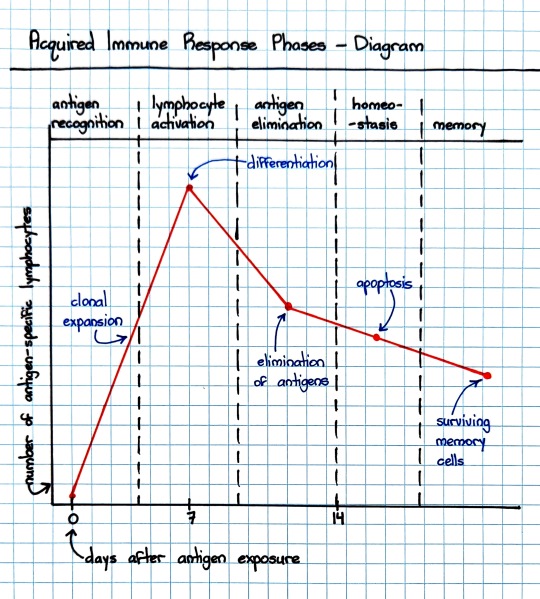
#antigen recognition
Explore tagged Tumblr posts
Text
Patreon
#studyblr#notes#medblr#medical notes#med notes#immunology#immunology notes#immune system#immune system notes#antigen recognition#antigen elimination#homeostasis#biology#life science#health science#medical science#science#scienceblr#sciblr#memory cells#apoptosis#lymphocytes#t cells#b cells
15 notes
·
View notes
Text
Fourth dose bivalent COVID-19 vaccines outperform monovalent boosters in eliciting cross-reactive memory B cells to Omicron subvariants - Published Aug 8, 2024
Remember when that kid called me an uneducated idiot when I suggested that monovalent vaccines provide less long-lasting broad protection from all covid strains? I present Scientific Catharsis
Abstract Bivalent COVID-19 vaccines comprising ancestral Wuhan-Hu-1 (WH1) and the Omicron BA.1 or BA.5 subvariant elicit enhanced serum antibody responses to emerging Omicron subvariants. Here, we characterized the RBD-specific memory B cell (Bmem) response following a fourth dose with a BA.1 or BA.5 bivalent vaccine, in direct comparison with a WH1 monovalent fourth dose. Healthcare workers previously immunized with mRNA or adenoviral vector monovalent vaccines were sampled before and one-month after a fourth dose with a monovalent or a BA.1 or BA.5 bivalent vaccine. Serum neutralizing antibodies (NAb) were quantified, as well as RBD-specific Bmem with an in-depth spectral flow cytometry panel including recombinant RBD proteins of the WH1, BA.1, BA.5, BQ.1.1, and XBB.1.5 variants. Both bivalent vaccines elicited higher NAb titers against Omicron subvariants compared to the monovalent vaccine. Following either vaccine type, recipients had slightly increased WH1 RBD-specific Bmem numbers. Both bivalent vaccines significantly increased WH1 RBD-specific Bmem binding of all Omicron subvariants tested by flow cytometry, while recognition of Omicron subvariants was not enhanced following monovalent vaccination. IgG1+ Bmem dominated the response, with substantial IgG4+ Bmem only detected in recipients of an mRNA vaccine for their primary dose. Thus, Omicron-based bivalent vaccines can significantly boost NAb and Bmem specific for ancestral WH1 and Omicron variants, and improve recognition of descendent subvariants by pre-existing, WH1-specific Bmem, beyond that of a conventional, monovalent vaccine. This provides new insights into the capacity of variant-based mRNA booster vaccines to improve immune memory against emerging SARS-CoV-2 variants and potentially protect against severe disease.
One-sentence summary Omicron BA.1 and BA.5 bivalent COVID-19 boosters, used as a fourth dose, increase RBD-specific Bmem cross-recognition of Omicron subvariants, both those encoded by the vaccines and antigenically distinct subvariants, further than a monovalent booster.
41 notes
·
View notes
Text
Will these vaccines provide immediate protection?
Vaccines contain an active ingredient used to trigger generation of immune memory. That active ingredient, the antigen, is what initiates the immune response, starting with innate immune recognition and ending with adaptive immunity generation (read here for a more detailed summary on viral immunology).
Adaptive immune response leads to antibody by plasma cells, activation of helper T cells and cytotoxic T cells, and generation of memory B and T cells.
This process takes time, roughly 2 weeks after we encounter the foreign invader (either the vaccine antigen or an actual pathogen). Once memory B and T cells are created, the response to a second encounter is much quicker because all the preparation is already done. The adaptive immune system is ready to go: B cells can start spitting out antibodies, helper T cells can augment the B cell responses, and cytotoxic T cells can directly kill infected cells to limit the spread of infection.
Vaccines train the immune system in advance of an infection.
The ability to immediately respond means that even if virus infects you after vaccination, your body can fight it more quickly, reducing viral load, lessening disease severity, and decreasing the likelihood of more serious illness or death.
#novavax#covid#covid 19#sars cov 2#vaccines#vaccine#vaccination#get vaccinated#long covid#tw death#illness#chronic illness#covid vaccine#mrna#jn.1#kp.2#sf9#Novavax vaccine#mrna vaccine
16 notes
·
View notes
Link
The launch of AlphaFold signaled a revolution in computational modeling: armed with new knowledge of protein structures and unencumbered by limitations on resources and time, scientists were able to use the tool to further their understanding of protein structure, functionality, and interactions. However, AlphaFold’s success in the realm of antibody-antigen modeling is limited. A new study from the University of Maryland evaluates its accuracy and provides new insights into the factors influencing protein modeling.
Antibodies are an important part of the immune system and defend the body from a host of pathogenic organisms due to their high specificity and affinity to antigens. Usually, these targets are engaged through the use of hypervariable CDR loops present in the variable domain. The high specificity of these interactions makes the development of antibodies an important consideration in fields like therapeutics and vaccine development.
The high-resolution structure development of antibody-antigen complexes has served to significantly enhance our understanding of immunity and its underlying mechanisms, allowing for the design of immunogens and antibodies. However, experimental methods for structure determination pose many challenges and constraints on resources and time, often making it infeasible to obtain experimentally derived characterizations for most complexes. In order to fill this need, an array of computational tools have been created – general methods for protein-protein docking have been utilized but haven’t been very successful due to their failure to consider the mobile nature of important CDR loops. Hence, tools have been developed specifically to model antibody-antigen complexes, but obtaining accurate predictions remains challenging.
Continue Reading
26 notes
·
View notes
Text
The Immune System's All-Star Team: The Mighty Cells That Protect You
Your body is like a bustling city, constantly facing threats from outside invaders like viruses and bacteria. Thankfully, you have a team of dedicated defenders keeping you safe: your immune cells! Our immune system is a marvel of biological defense, tirelessly safeguarding our bodies from harmful invaders like bacteria, viruses, and parasites. At the forefront of this defense are numerous types of immune cells, each with its unique functions and capabilities. Did you know the average adult has about 2 trillion white blood cells, which contain most immune cells? That's more people than live in China! These tiny warriors come in different shapes and sizes, each with unique superpowers to protect you. Let's meet some of the key players:
The Innate Force: First up, we have the innate immune system. This frontline defense acts fast and nonspecifically, providing immediate protection against any threat. The key players: 1. Neutrophils: Think of these guys as the city's SWAT team. They're the first responders, rushing to attack invaders with toxic chemicals and swallowing them whole with their arsenal of enzymes! These are the most abundant immune cells, are short-lived but highly effective. Unfortunately, they die in the fight, leaving behind a green gooey mess (pus) that signals infection.
2. Macrophages: These are the veterans, the wise generals of the immune system. They go beyond mere engulfing, processing antigens (foreign molecules) and presenting them to other immune cells for recognition and attack. They also act as scavengers, cleaning up debris and orchestrating healing. These are the cleaners and recyclers. They gobble up dead neutrophils, debris, and even worn-out cells, keeping your city sparkling clean.
3. Natural Killer (NK) Cells: These are the ninjas of the immune system. They silently patrol, sniffing out suspicious cells infected with viruses or even cancer and eliminating them with a swift punch. The Adaptive Arsenal:
The Adaptive Arsenal: If the innate system fails, the adaptive immune system steps in. This highly specific defense remembers past encounters and tailors its response to each unique threat. The cells of adaptive immune system are:
B Cells: These are the antibody factories, producing highly specific proteins called antibodies that neutralize pathogens and toxins. Each B cell produces a unique antibody, like a lock and key, targeting specific invaders. They whip up special proteins called antibodies that lock onto specific invaders, like sticky notes, marking them for destruction.
T Cells: These are the generals, coordinating the entire defense. There are different types of T cells:
Helper T Cells: These are the commanders, directing and coordinating the immune response through chemical signals. They activate B cells, macrophages, and other immune cells, orchestrating a multi-pronged attack.
Cytotoxic T Cells: These are the elite soldiers, directly targeting and eliminating infected cells or cancer cells. They recognize and bind to specific enemy markers, unleashing a lethal attack.
Memory T Cells: These are the veterans, remembering past encounters with invaders and helping the immune system respond faster next time.
The Unsung Heroes: Beyond these main players, numerous other immune cells contribute to our defense. These include:
Dendritic cells: These antigen-presenting cells capture and process pathogens, presenting their fragments to T cells for activation. They're like the scouts, gathering enemy intel and relaying it to the command center.
Mast cells: These cells reside in tissues and release inflammatory chemicals in response to allergens or parasites. They're like the alarm system, alerting the immune system to local threats.
Eosinophils: These specialize in fighting parasitic infections, releasing toxic chemicals to neutralize them.
Basophils: These are involved in allergic reactions and contribute to wound healing.
The beauty of the immune system lies in its intricate collaboration. These diverse cell types work together in a complex and beautiful dance, each playing a specific role to achieve a common goal: protecting our health. They communicate extensively through chemical signals, creating a complex network of interactions. Imagine B cells producing antibodies that bind to a pathogen, flagging it for destruction. Macrophages engulf and eliminate the tagged pathogen, while T cells coordinate the attack and eliminate any infected cells. Dendritic cells present captured fragments to T cells, priming them for future encounters. This seamless cooperation ensures a swift and effective response to any threat.
Your immune system is constantly learning. Each time you get a vaccine or fight off an infection, your immune cells create memory T cells, making you more resistant to future attacks. Understanding these cellular heroes can help us appreciate the incredible machinery that keeps us healthy and appreciate the importance of maintaining a strong immune system. It also allows us to make informed decisions about supporting our immune system. Maintaining a healthy lifestyle, getting adequate sleep, managing stress, and consuming a balanced diet can all contribute to a robust immune response.
#science sculpt#life science#science#biology#biotechnology#artists on tumblr#immunology#immunotherapy#immune cells#b cells#t cells#neutrophil#macrophages#immunity#health and wellness#medicine#healthcare#illustration#illustrative art
9 notes
·
View notes
Text
Gather Round
The immune system's B cells recognise their foe (antigens) with receptors (also known as immunoglobulin or antibody) on their cell surface. Here, super resolution microscopy combined with 4D image analysis reveals how these receptors on the cell surface localise into clusters as ridges and finger-like projections called microvilli to aid recognition of their targets
Read the published research paper here
Video adapted from work by Deniz Saltukoglu and colleagues
Department of Molecular Immunology, Biology III, Faculty of Biology, University of Freiburg, Freiburg, Germany
Video originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in The EMBO Journal, January 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#biology#immunology#immunoglobulin#B cells#immune system#antibody#antigen#lattice light-sheet microscopy#super resolution microscopy#4D imaging
11 notes
·
View notes
Text
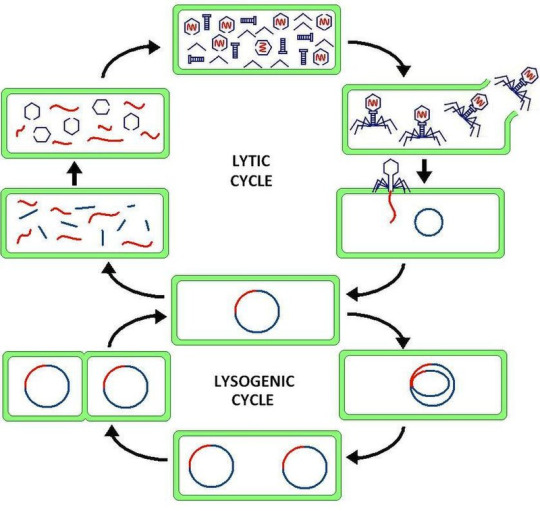
Virus latency (or viral latency) is the ability of a pathogenic virus to lie dormant (latent) within a cell, denoted as the lysogenic part of the viral life cycle.[1] A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny (the lytic part of the viral life cycle) without the host becoming reinfected by new outside virus, and stays within the host indefinitely.[2]
Episomal latency refers to the use of genetic episomes during latency. In this latency type, viral genes are stabilized, floating in the cytoplasm or nucleus as distinct objects, either as linear or lariat structures. Episomal latency is more vulnerable to ribozymes or host foreign gene degradation than proviral latency (see below).
Advantages of episomal latency include the fact that the virus may not need to enter the cell nucleus, and hence may avoid nuclear domain 10 (ND10) from activating interferon via that pathway. Disadvantages include more exposure to cellular defenses, leading to possible degradation of viral gene via cellular enzymes.[12]
Proviral latency: A provirus is a virus genome that is integrated into the DNA of a host cell
All interferons share several common effects: they are antiviral agents and they modulate functions of the immune system. Administration of Type I IFN has been shown experimentally to inhibit tumor growth in animals, but the beneficial action in human tumors has not been widely documented. A virus-infected cell releases viral particles that can infect nearby cells. However, the infected cell can protect neighboring cells against a potential infection of the virus by releasing interferons. In response to interferon, cells produce large amounts of an enzyme known as protein kinase R (PKR). This enzyme phosphorylates a protein known as eIF-2 in response to new viral infections; the phosphorylated eIF-2 forms an inactive complex with another protein, called eIF2B, to reduce protein synthesis within the cell. Another cellular enzyme, RNAse L—also induced by interferon action—destroys RNA within the cells to further reduce protein synthesis of both viral and host genes. Inhibited protein synthesis impairs both virus replication and infected host cells. In addition, interferons induce production of hundreds of other proteins—known collectively as interferon-stimulated genes (ISGs)—that have roles in combating viruses and other actions produced by interferon.[13][14] They also limit viral spread by increasing p53 activity, which kills virus-infected cells by promoting apoptosis.[15][16] The effect of IFN on p53 is also linked to its protective role against certain cancers.[15]
Another function of interferons is to up-regulate major histocompatibility complex molecules, MHC I and MHC II, and increase immunoproteasome activity. All interferons significantly enhance the presentation of MHC I dependent antigens. Interferon gamma (IFN-gamma) also significantly stimulates the MHC II-dependent presentation of antigens. Higher MHC I expression increases presentation of viral and abnormal peptides from cancer cells to cytotoxic T cells, while the immunoproteasome processes these peptides for loading onto the MHC I molecule, thereby increasing the recognition and killing of infected or malignant cells. Higher MHC II expression increases presentation of these peptides to helper T cells; these cells release cytokines (such as more interferons and interleukins, among others) that signal to and co-ordinate the activity of other immune cells.[17][18][19]
Epstein–Barr virus lytic reactivation (which can be due to chemotherapy or radiation) can result in genome instability and cancer.[5]
HSV reactivates upon even minor chromatin loosening with stress,[7] although the chromatin compacts (becomes latent) upon oxygen and nutrient deprivation.[8]
Cytomegalovirus (CMV) establishes latency in myeloid progenitor cells, and is reactivated by inflammation.[9] Immunosuppression and critical illness (sepsis in particular) often results in CMV reactivation.[10] CMV reactivation is commonly seen in patients with severe colitis.[11]
viral latency is so fucked up
11 notes
·
View notes
Text
Precision in Antibody Development: Advancing Research with Custom Monoclonal Solutions
In the ever-evolving landscape of scientific research, antibodies play a crucial role in diagnostics, therapeutics, and biomedical studies. Monoclonal Custom Antibodies have revolutionized the field by offering specificity and consistency, making them invaluable tools for researchers worldwide. Among the various antibody production methods, the development of Custom Mouse Monoclonal Antibody solutions remains one of the most effective approaches to ensuring high-affinity and highly specific antibodies tailored to unique research needs.
Understanding Monoclonal Custom Antibodies
Monoclonal antibodies are generated from a single B-cell clone, ensuring uniformity in antigen recognition. Unlike polyclonal antibodies, which are derived from multiple B-cell lineages, monoclonal antibodies provide unmatched specificity. This makes them ideal for applications such as immunohistochemistry, flow cytometry, and Western blotting. The ability to develop customized monoclonal antibodies allows scientists to target unique epitopes, enhancing the accuracy of their experiments.
The production of Custom Mouse Monoclonal Antibody solutions involves a well-established hybridoma technology. This process includes the immunization of mice, fusion of spleen cells with myeloma cells, and subsequent screening for high-affinity antibody-producing clones. Once the desired clone is identified, large-scale production can be achieved, providing consistent results for research and therapeutic applications.
The Significance of Customization
Customization in antibody development allows for the precise targeting of antigens that may not be effectively recognized by commercially available antibodies. Whether for drug discovery, biomarker identification, or therapeutic research, tailored antibodies ensure high specificity and reproducibility. Researchers can modify aspects such as isotype, affinity, and conjugation to match their experimental needs, making Monoclonal Custom Antibodies an essential tool in biomedical advancements.
Another advantage of customized monoclonal antibody development is batch-to-batch consistency. Standardized production methods ensure that every batch of antibodies performs identically, eliminating variability in experimental outcomes. This level of reliability is particularly crucial in clinical and diagnostic applications where reproducibility is paramount.
Applications in Biomedical Research
The use of monoclonal antibodies spans across various disciplines, including oncology, infectious disease research, and neuroscience. In cancer research, monoclonal antibodies help in detecting specific tumor markers, aiding in early diagnosis and targeted therapy development. In immunology, they play a pivotal role in understanding immune responses and developing vaccines.
Furthermore, Custom Mouse Monoclonal Antibody solutions are widely used in pharmaceutical development. They assist in drug screening processes, enabling researchers to identify potential therapeutic candidates with precision. Their role in personalized medicine is also expanding, as custom antibodies can be engineered to interact with patient-specific biomarkers, leading to more effective treatments.
Enhancing Diagnostic Accuracy
Diagnostic applications heavily rely on the specificity of monoclonal antibodies. From ELISA tests to lateral flow assays, these antibodies contribute to the rapid and accurate detection of diseases. Their high-affinity binding ensures minimal cross-reactivity, reducing the risk of false positives or negatives. With customized solutions, diagnostic manufacturers can develop assays that cater to emerging infectious diseases and novel biomarkers, enhancing public health response capabilities.
Overcoming Challenges in Antibody Production
Despite the numerous advantages of monoclonal antibody technology, challenges such as production costs and hybridoma stability remain. However, advancements in recombinant antibody technology and cell culture methods are helping to overcome these hurdles. Scientists now have access to advanced expression systems that allow for scalable and cost-effective antibody production without compromising quality.
The demand for high-quality monoclonal antibodies continues to grow, driving innovation in production techniques. Hybridoma engineering, CRISPR-based modifications, and novel expression platforms are paving the way for more efficient antibody development processes. These advancements are not only benefiting research institutions but also contributing to the development of next-generation therapeutics.
0 notes
Text
Role of Anti-Glycan Antibodies in Disease Diagnosis and Treatment
In the intricate realm of medical diagnostics and therapeutic advancements, antibodies targeting glycans have emerged as pivotal players. Glycans, complex sugar molecules decorating proteins and lipids, play crucial roles in cellular communication, immune responses, and disease pathogenesis. Harnessing antibodies specific to these glycans opens new avenues for diagnosis, treatment, and biomedical research.
Glycans are diverse carbohydrate structures attached to proteins and lipids, influencing their stability, function, and recognition by other biomolecules. These structures are essential in various physiological processes, including cell signaling, immune modulation, and pathogen recognition.
Antibodies, on the other hand, are immune proteins produced by B cells in response to specific antigens. They are key components of the immune system, binding to target molecules with remarkable specificity.
Glycan Antibody in Diagnostics
Anti-glycan antibodies serve as invaluable biomarkers in diagnosing a spectrum of diseases. For instance, in autoimmune disorders like rheumatoid arthritis and systemic lupus erythematosus (SLE), detection of anti-nuclear antibodies (ANA), which target glycans associated with nuclear components, aids in early disease identification and differentiation from other conditions.
Similarly, in infectious diseases, antibodies against bacterial or viral glycan antigens facilitate rapid and specific diagnosis. These antibodies are instrumental in serological tests used to detect pathogens like Streptococcus and influenza virus, enhancing diagnostic accuracy and treatment initiation.
Therapeutic Applications
Beyond diagnostics, anti-glycan antibodies are increasingly explored for therapeutic interventions. Monoclonal antibodies designed to target specific glycans on tumor cells, for instance, are revolutionizing cancer treatment. By binding to these glycans, therapeutic antibodies can block tumor growth signals, trigger immune responses against cancer cells, or deliver cytotoxic agents directly to malignant tissues, improving patient outcomes and survival rates.
In autoimmune diseases, therapeutic antibodies are engineered to modulate aberrant immune responses by targeting self-glycans involved in disease pathogenesis. This approach offers a promising alternative to conventional therapies, aiming for greater efficacy and reduced side effects.
Research Tools and Insights
Anti-glycan antibodies also serve as indispensable tools in biomedical research. Developing anti-glycan antibody facilitate detailed investigations into glycan structures, functions, and interactions within biological systems. Researchers utilize these antibodies in studies exploring glycan-mediated processes such as cell adhesion, viral entry mechanisms, and immune evasion strategies employed by pathogens.
Moreover, advancements in antibody engineering technologies and glycan antibody analysis have propelled the development of novel research methodologies. These methodologies enable deeper insights into disease mechanisms and aid in the discovery of new therapeutic targets.
0 notes
Text
Meridian Bioscience: Pioneering Innovations in Diagnostics and Life Sciences
Meridian Bioscience is a leading life science and diagnostic solutions company that develops, manufactures, and markets a broad range of innovative products. The company is dedicated to providing accurate, fast, and simple solutions that help in the early detection and diagnosis of diseases, ultimately improving patient outcomes and advancing global health.
Expertise in Diagnostics
Meridian Bioscience offers cutting-edge diagnostic solutions for a variety of medical conditions, including gastrointestinal and upper respiratory infections, as well as blood lead level testing. Its products are widely used in hospitals, laboratories, research centers, and biotech companies in over 70 countries. By focusing on user-friendly, rapid, and precise testing methods, the company continues to enhance the efficiency of medical diagnostics worldwide.
Contributions to Life Sciences
In addition to diagnostics, Meridian Bioscience is a key player in the life science industry, providing essential raw materials such as antibodies, antigens, and molecular reagents. These materials are used in immunological and molecular tests for human, animal, and environmental applications. The company’s high-quality components help researchers and manufacturers develop reliable testing solutions for various diseases and scientific studies.
Commitment to Innovation
Meridian Bioscience has a strong commitment to innovation, consistently developing new technologies and improving existing solutions. One of its notable achievements was winning a prestigious award from the Centers for Disease Control and Prevention (CDC) for advancements in blood lead testing. This recognition underscores the company’s leadership in public health and its dedication to providing impactful diagnostic tools.
Sustainable and Future-Ready Solutions
As a forward-thinking company, Meridian Bioscience is also invested in sustainable solutions for in vitro diagnostic manufacturers. By optimizing production processes and reducing environmental impact, the company ensures that its innovations align with the latest sustainability goals while maintaining high-quality standards.
A Leader in Global Health
Headquartered in Cincinnati, Ohio, Meridian Bioscience continues to shape the future of medical diagnostics and life sciences. With a mission to improve healthcare accessibility and efficiency, the company remains at the forefront of technological advancements, providing reliable and innovative solutions to meet the evolving needs of the healthcare industry.
0 notes
Text
Structure/Function Studies of Antigen Processing and Presentation
Structure/Function Studies of Antigen Processing and Presentation The intricate process of antigen processing and presentation is fundamental to the immune response, enabling the recognition of a vast array of pathogens by T cells. This process is primarily mediated by major histocompatibility complex (MHC) molecules, which present peptide antigens on the surfaces of cells. Structure/function…
#academic research#Antigen#Antigen Processing and Presentation#Antigen-presenting cells#immunity#immunology#Major Histocompatibility Complex#MHC#protocol#research#science
0 notes
Text
What’s the difference between Pfizer/BioNTech, Moderna, and Novavax COVID-19 vaccines?
The Pfizer-BioNTech and Moderna COVID-19 vaccines use mRNA as the active ingredient. The mRNA is converted by our cells into the antigen, in this case, the spike protein of SARS-CoV-2.
The vaccine contains the mRNA, which is synthesized in the lab using a DNA template, the building blocks of RNA, and the enzyme that puts those building blocks together into the right order. mRNA is the molecule template for every protein in every organism. The mRNA sequence is a code for our cells to link amino acids together into functional proteins
mRNA is very fragile, so it is encased in a lipid nanoparticle (LNP) that protects it until it gets into our cells. When the vaccine is administered, the mRNA is released and is used to synthesize the spike protein which is displayed by cells that produced it. That spike protein is recognized by our innate immune cells like dendritic cells and macrophages as well as B cells, which initiates immune response and generation of memory immunity.

In contrast, Novavax is a protein-based vaccine, which contains the prefabricated antigen - the spike protein - instead of the template for it. To make the antigen, we turn cells into protein-producing factories in the lab.
Novavax uses Sf9 cells (moth cells) infected with an insect-specific virus that has been genetically engineered to contain the gene for the spike protein of SARS-CoV-2. These viruses will hijack the cellular machinery of the Sf9 cells to produce lots of spike proteins and baby viruses. Those will continue to reproduce and produce proteins, which will be harvested, purified, and formulated with the other ingredients in the final vaccine.
When the vaccine is injected, the antigen will be recognized by the same innate immune cells listed above, which will trigger the same immune response pathway.

While the vaccines use different technologies, ingredients, and manufacturing processes, the immune responses center around recognition of the spike protein and generating adaptive immune responses targeting that antigen.
#novavax#covid#covid 19#sars cov 2#vaccines#vaccine#vaccination#get vaccinated#long covid#illness#chronic illness#covid vaccine#mrna#jn.1#kp.2#sf9#Novavax vaccine#mrna vaccine#Pfizer-BioNTech#Moderna#moderna vaccine#pfizer vaccine#pfizer
6 notes
·
View notes
Text
The Science Behind Immunotherapy: The Biotech Revolution in Cancer Treatment

The immune system is a remarkable defense mechanism designed to protect the body against threats. Yet, cancer often finds ways to bypass this system, allowing it to grow and spread unchecked. Immunotherapy has changed this reality by enabling the immune system to identify and destroy cancer cells effectively. This innovative approach leverages the body’s natural defenses, offering an alternative to traditional treatments like chemotherapy and radiation. By enhancing the immune system’s ability to recognize cancer, immunotherapy has become a powerful tool in the fight against this disease. In this article, I’ll explore how immunotherapy works, its types, recent advancements, and its impact on cancer treatment.
What Is Immunotherapy?
Immunotherapy uses biological methods to empower the immune system in its fight against cancer. Unlike treatments that directly attack cancer cells, immunotherapy strengthens or modifies immune responses to target tumors more effectively. It works by either boosting the activity of immune cells or providing artificial components, such as antibodies, to aid in the destruction of cancer cells. This method has shown success in treating various cancers, including melanoma, lung, and bladder cancers. By focusing on the immune system, immunotherapy provides a targeted approach that often reduces the side effects associated with traditional therapies.
Key Types of Immunotherapy
Several types of immunotherapy have been developed to tackle cancer in unique ways. Checkpoint inhibitors, for example, are designed to remove the brakes on the immune system. Cancer cells often exploit immune checkpoints—proteins that regulate immune activity—to evade detection. Drugs like pembrolizumab and nivolumab block these checkpoints, allowing T cells to attack cancer cells more aggressively. Another groundbreaking approach is CAR T-cell therapy, which involves modifying a patient’s T cells to recognize and destroy cancer. This personalized treatment has shown remarkable success in blood cancers like leukemia and lymphoma.
Monoclonal antibodies are another vital tool in the immunotherapy arsenal. These lab-engineered molecules bind to specific antigens on cancer cells, marking them for destruction by the immune system. Some monoclonal antibodies also deliver toxic substances directly to tumors, enhancing their effectiveness. Cancer vaccines represent yet another innovative approach, training the immune system to identify and attack specific cancer-associated antigens. Recent advancements in mRNA technology have significantly improved the development of these vaccines, making them more precise and effective.
How Immunotherapy Works
Immunotherapy addresses cancer’s ability to evade the immune system by enhancing the immune response in several ways. One key strategy is enhancing immune recognition, which involves exposing hidden cancer cells to immune surveillance. Tumors often disguise themselves to avoid detection, but immunotherapies can unmask these cells, prompting an immune response. Another critical mechanism involves modifying the tumor microenvironment. Tumors create an environment that suppresses immune activity, making it difficult for T cells to infiltrate and attack. Immunotherapy can alter this environment, making it more supportive of immune cell activity.
Checkpoint inhibitors, in particular, play a vital role by blocking proteins that deactivate T cells prematurely. This allows the immune system to sustain its attack on cancer cells without interruption. These combined mechanisms demonstrate the multifaceted ways in which immunotherapy enhances the body’s ability to fight cancer.
Recent Advances in Immunotherapy
The field of immunotherapy has seen rapid progress in recent years, driven by technological advancements. One of the most exciting developments is the integration of CRISPR technology with immunotherapy. CRISPR allows scientists to edit genes within T cells, enhancing their ability to target cancer cells more effectively. Early trials using CRISPR-modified T cells have shown promising results, paving the way for more precise and personalized treatments.
Combination therapies are another area of significant advancement. Researchers have found that combining immunotherapy with other treatments, such as chemotherapy or targeted therapy, can improve outcomes by addressing multiple pathways simultaneously. Personalized cancer vaccines are also gaining traction, with advancements in tumor profiling enabling the creation of vaccines tailored to individual patients. These innovations are helping to expand the reach and effectiveness of immunotherapy.
Benefits of Immunotherapy
Immunotherapy offers several distinct advantages over traditional cancer treatments. Its targeted nature ensures that only cancer cells are attacked, sparing healthy tissue and reducing side effects. This contrasts with chemotherapy and radiation, which can harm healthy cells and lead to significant complications. Additionally, immunotherapy has the potential to provide durable responses. Some patients experience long-term remission, with their immune systems continuing to protect against cancer even after treatment ends. The reduced side effects associated with immunotherapy make it a more tolerable option for many patients, improving their quality of life during treatment.
Challenges and Considerations
Despite its promise, immunotherapy is not without challenges. One of the most significant issues is variable effectiveness. Not all patients respond to immunotherapy, and researchers are still working to identify biomarkers that can predict response rates. Another challenge is the risk of immune-related side effects. Overactivation of the immune system can lead to inflammation or autoimmune-like symptoms, which require careful management. The cost of immunotherapy treatments also remains a barrier for many patients, highlighting the need for strategies to reduce expenses and expand accessibility. Addressing these challenges will be critical to ensuring that immunotherapy reaches its full potential.
Key Immunotherapy Techniques
Checkpoint inhibitors block proteins that suppress immune activity.
CAR T-cell therapy reprograms T cells to target cancer.
Monoclonal antibodies mark cancer cells for destruction.
Cancer vaccines teach the immune system to attack tumors.
The Future of Immunotherapy
Looking ahead, the potential of immunotherapy continues to grow. Advances in artificial intelligence and machine learning are helping researchers identify biomarkers that predict patient responses, enabling more personalized treatments. The use of CRISPR technology is likely to expand, offering even more precise ways to engineer immune cells for cancer therapy. Additionally, ongoing research aims to apply immunotherapy to cancers that have been resistant to treatment, broadening its scope. Combining immunotherapy with other emerging technologies, such as nanotechnology, could further enhance its effectiveness. As research progresses, immunotherapy is set to become a cornerstone of cancer care.
In Conclusion
Immunotherapy has redefined cancer treatment by harnessing the power of the immune system to target and destroy cancer cells. With its tailored approach, long-lasting effects, and reduced side effects, it has become an invaluable tool in oncology. Advancements in technology and personalized medicine are driving its evolution, offering new hope to patients worldwide. As we continue to explore and refine this biotech innovation, immunotherapy stands as a testament to the power of science in transforming lives.
0 notes
Text
Rheumatic Fever And Rheumatic Heart Disease
Rheumatic Fever and Rheumatic Heart Disease
#Rheumatic 💛
#Fever 🤎
#RheumaticFever 💜
#RheumaticHeartDisease 💚
#Worldwide ❤️
#Cardiac 🩷
#Disease 🩵
Acute rheumatic fever and rheumatic heart disease are leading causes of acquired cardiac disease in developing countries, with between 5 and 30 million children and young adults affected worldwide and a mortality rate of 1% to 10%.94 .
In the United States, the availability of antibiotics to treat streptococcal pharyngitis has markedly reduced the incidence of this disease but sporadic cases still occur.95 In children, the peak incidence occurs between 5 and 14 years of age.
Rheumatic fever results from infection by particular strains of group A β-hemolytic Streptococcus or Streptococcus pyogenes. Both cellular and humoral immune responses to the bacterial antigens cross react with native tissues to result in a multisystemic infl ammatory disorder.
The incubation period for most strains of group A β-hemolytic Streptococcus is typically 3 to 5 days, although some children will present with a more remote history of pharyngitis.
The diagnosis of rheumatic fever is made clinically based on the modifi ed Jones criteria.96 Major criteria include carditis, polyarthritis, chorea, subcutaneous nodules, and erythema marginatum. Evidence of a prior streptococcal infection along with two major or one major and two minor criteria are required to make the diagnosis in children without prior history of rheumatic fever or echocardiographic evidence of typical valvular involvement. Fever and arthritis are common symptoms.
The polyarthritis has a migratory pattern, typically aff ecting large joints. Cardiac involvement or carditis occurs in nearly 50% of children with their fi rst attack of rheumatic fever.
Rheumatic heart disease represents a sequela of the acute process, aff ecting the mitral and aortic valves most frequently. Primary prevention of rheumatic fever and rheumatic heart disease begins with prompt recognition and appropriate treatment of the initial streptococcal infection.97,98 Secondary prevention, with ongoing therapy in individuals with a known history of rheumatic fever, has been shown to be extremely effective at preventing recurrent attacks.
#DrBhishmaChowdary 🩺
#BestCardiologistInHyderabad 🥼

#cardiology#health#healthcare#doctor#health tips#anatomical heart#health and wellness#heart doctor#heart specialist#cardiovascularhealth#rheuamtic fever#rheumatic#rheumatology
0 notes
Text
Abstract
Human metapneumovirus (hMPV) belongs to the Pneumoviridae family and is closely related to respiratory syncytial virus (RSV). The surface fusion (F) glycoprotein mediates viral fusion and is the primary target of neutralizing antibodies against hMPV. Here we report 113 hMPV-F specific monoclonal antibodies (mAbs) isolated from memory B cells of human donors. We characterize the antibodies’ germline usage, epitopes, neutralization potencies, and binding specificities. We find that unlike RSV-F specific mAbs, antibody responses to hMPV F are less dominant against the apex of the antigen, and the majority of the potent neutralizing mAbs recognize epitopes on the side of hMPV F. Furthermore, neutralizing epitopes that differ from previously defined antigenic sites on RSV F are identified, and multiple binding modes of site V and II mAbs are discovered. Interestingly, mAbs that bind preferentially to the unprocessed prefusion F show poor neutralization potency. These results elucidate the immune recognition of hMPV infection and provide novel insights for future hMPV antibody and vaccine development.
0 notes