#Antigen Processing and Presentation
Explore tagged Tumblr posts
Text
Structure/Function Studies of Antigen Processing and Presentation
Structure/Function Studies of Antigen Processing and Presentation The intricate process of antigen processing and presentation is fundamental to the immune response, enabling the recognition of a vast array of pathogens by T cells. This process is primarily mediated by major histocompatibility complex (MHC) molecules, which present peptide antigens on the surfaces of cells. Structure/function…
#academic research#Antigen#Antigen Processing and Presentation#Antigen-presenting cells#immunity#immunology#Major Histocompatibility Complex#MHC#protocol#research#science
0 notes
Text
Human Cell Tournament Round 2
Propaganda!
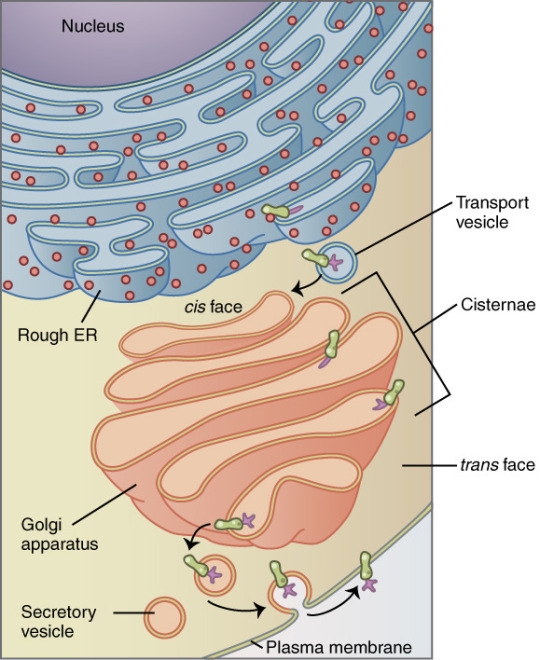
The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
A dendritic cell (DC) is an antigen-presenting cell (also known as an accessory cell) of the mammalian immune system. A DC's main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and adaptive immune systems.
#Golgi apparatus#Dendritic cells#poll#polls#tumblr poll#tumblr polls#tournament poll#wikipedia#cells of the human body#science tournament#biochemistry
16 notes
·
View notes
Text
SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes - Published May 23, 2024
Abstract RNA processing is a highly conserved mechanism that serves as a pivotal regulator of gene expression. Alternative processing generates transcripts that can still be translated but lead to potentially nonfunctional proteins. A plethora of respiratory viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), strategically manipulate the host’s RNA processing machinery to circumvent antiviral responses. We integrated publicly available omics datasets to systematically analyze isoform-level expression and delineate the nascent peptide landscape of SARS-CoV-2-infected human cells. Our findings explore a suggested but uncharacterized mechanism, whereby SARS-CoV-2 infection induces the predominant expression of unproductive splicing isoforms in key IFN signaling, interferon-stimulated (ISGs), class I MHC, and splicing machinery genes, including IRF7, HLA-B, and HNRNPH1. In stark contrast, cytokine and chemokine genes, such as IL6 and TNF, predominantly express productive (protein-coding) splicing isoforms in response to SARS-CoV-2 infection. We postulate that SARS-CoV-2 employs an unreported tactic of exploiting the host splicing machinery to bolster viral replication and subvert the immune response by selectively upregulating unproductive splicing isoforms from antigen presentation and antiviral response genes. Our study sheds new light on the molecular interplay between SARS-CoV-2 and the host immune system, offering a foundation for the development of novel therapeutic strategies to combat COVID-19.
#covid#mask up#pandemic#covid 19#wear a mask#coronavirus#sars cov 2#still coviding#wear a respirator#public health#long covid
6 notes
·
View notes
Text
The T Cell Landscape
T cells, a critical component of the adaptive immune system, stand as the body's elite force in combatting infections and diseases. These specialized lymphocytes boast remarkable diversity, each type playing a distinct role in orchestrating a targeted and effective immune response.
T cells, like all blood cells, originate from hematopoietic stem cells residing in the bone marrow. However, their training ground lies within the thymus, a specialized organ located in the chest. Here, they undergo a rigorous selection process known as thymocyte education. During this process, immature T cells, called thymocytes, are presented with self-antigens (molecules unique to the body) by special cells. Thymocytes that bind too strongly to these self-antigens are eliminated, preventing them from attacking healthy tissues later. Only thymocytes that demonstrate the ability to recognize foreign invaders while exhibiting tolerance to self are released into the bloodstream as mature T cells.
Following this rigorous training, mature T cells exit the thymus and embark on their patrol, circulating throughout the bloodstream and lymphatic system. They remain vigilant, constantly scanning for their specific targets – antigens. Antigens are foreign molecules, such as fragments of viruses, bacteria, or even cancerous cells, that trigger the immune response.
The hallmark of a T cell is its T cell receptor (TCR), a highly specialized protein complex embedded on its surface. This receptor acts like a lock, uniquely shaped to fit a specific antigen, the "key." Each T cell develops a unique TCR capable of recognizing only a single antigen, enabling a highly specific immune response.
But how do T cells encounter these hidden antigens lurking within infected or cancerous cells? This critical role is played by antigen-presenting cells (APCs). APCs, such as macrophages and dendritic cells, engulf pathogens or abnormal cells, break them down into smaller fragments (peptides), and present them on their surface complexed with major histocompatibility complex (MHC) molecules. MHC molecules act as identification tags, allowing T cells to distinguish between "self" and "non-self." When a T cell's TCR encounters its specific antigen bound to an MHC molecule on an APC, a dance of activation begins. The T cell becomes stimulated, and a cascade of signaling events is triggered. This leads to the T cell's proliferation, producing an army of clones specifically tailored to combat the recognized threat.
T cells are not a single, monolithic entity. They comprise a diverse population, each type with a specialized function:
Helper T Cells (Th Cells):
Helper T cells, often abbreviated as Th cells, play a central role in coordinating immune responses. They express the CD4 surface marker and can recognize antigens presented by major histocompatibility complex class II (MHC-II) molecules. Subtypes of helper T cells include Th1, Th2, Th17, and regulatory T cells (Tregs), each with distinct functions and cytokine profiles.
Th1 cells mediate cellular immunity by activating macrophages and cytotoxic T cells, crucial for defense against intracellular pathogens.
Th2 cells are involved in humoral immunity, promoting B cell activation and antibody production, thus aiding in defense against extracellular parasites.
Th17 cells contribute to the immune response against extracellular bacteria and fungi, producing pro-inflammatory cytokines. Regulatory T cells (Tregs) maintain immune tolerance and prevent autoimmunity by suppressing excessive immune responses.
Cytotoxic T Cells (Tc Cells):
Cytotoxic T cells, also known as Tc cells or CD8+ T cells, are effector cells responsible for directly killing infected or aberrant cells. They recognize antigens presented by MHC class I molecules on the surface of target cells. Upon activation, cytotoxic T cells release perforin and granzymes, inducing apoptosis in target cells and eliminating the threat.
Memory T Cells:
Memory T cells are a long-lived subset of T cells that persist after the clearance of an infection. They provide rapid and enhanced immune responses upon re-exposure to the same antigen, conferring immunological memory. Memory T cells can be either central memory T cells (TCM), residing in lymphoid organs, or effector memory T cells (TEM), circulating in peripheral tissues.
γδ T Cells:
Unlike conventional αβ T cells, γδ T cells express a distinct T cell receptor (TCR) composed of γ and δ chains. They recognize non-peptide antigens, such as lipids and metabolites, and are involved in immune surveillance at epithelial barriers and responses to stress signals.
Beyond the Battlefield: The Expanding Roles of T Cells: The remarkable capabilities of T cells have opened doors for several groundbreaking applications in medicine:
Vaccines: By presenting weakened or inactivated forms of pathogens, vaccines "train" the immune system to generate memory T cells. This prepares the body to recognize and rapidly eliminate the real pathogen upon future exposure, preventing disease.
Cancer immunotherapy: CAR T-cell therapy, a revolutionary approach, genetically engineers a patient's own T cells to express chimeric antigen receptors (CARs) that recognize and target specific cancer cells. These "supercharged" T cells are then reintroduced into the patient, unleashing a potent attack against the tumor.
Autoimmune disease treatment: Researchers are exploring ways to manipulate T cells to suppress harmful immune responses that underlie autoimmune diseases like rheumatoid arthritis and multiple sclerosis.
The diverse array of T cells underscores the immune system's complexity and adaptability in mounting tailored responses against a myriad of threats. From orchestrating immune reactions to maintaining tolerance and establishing long-term immunity, T cells play multifaceted roles in safeguarding the body's health. Understanding the intricacies of T cell biology not only sheds light on immune-mediated diseases but also paves the way for developing novel therapeutic strategies harnessing the power of the immune system.
T cells represent a fascinating aspect of immunology, with their diversity and specificity driving the complexity of immune responses. As research advances, further insights into T cell biology promise to revolutionize immunotherapy and enhance our ability to combat diseases ranging from infections to cancer. By understanding and harnessing their power, we can unlock new avenues for protecting and improving human health.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#t cells#T helper cells#autoimmune#autoimmunity#helathcare#immunology#immunotherapy#medical care#cancer#human health#research#scientific research#the glass scientists#scientific illustration#research scientist
11 notes
·
View notes
Text
WE HAVE SOLVED THE MYSTERY OF WHY CELLS HATE NEUTROPHILS SO MUCH IN CAW
So, last night, my boyfriend and I got into some random discussion about programmed cell death and some new breakthroughs in medicine, you know, the usual things you talk about with your boyfriend at 1 AM. It is well known that leftover bodies of dead cells are phagocytosed (literally consumed) by macrophages. And that’s why I always wondered why aren’t cells scared of macrophages as much as they are of neutrophils, since neutrophils don’t consume the dead cells. With my limited understanding of immunity (which we technically don’t learn a lot about in biology) I thought that neutrophils only consumed invader bacteria and fungi.
And OH BOY was I wrong about that.
Because (and yes I have spent whole night researching this, I’ll provide the links to papers in the end lol) neutrophils are little freaks and not only do they phagocytose leftovers of cells they actually cause them to die in the first place. This happens during infections, especially with viruses that cause the excess release of cytokines (like Coronaviridae). Cytokines activate neutrophils who basically just follow the signal towards the infection site and there all hell breaks loose. Neutrophils phagocytose bacteria and virions (those are viruses that haven’t infected a cell yet) which is fine, but they also degranulate and NETose. I’ll explain this in simple terms to my best ability.
Degranulation is when granulocytes (neutrophils, eosinophils, basophils and mastocytes are all different granulocytes) release their granules which are kind of like little sacks inside their cytoplasm which contain various chemicals. Releasing these chemicals happens when the cell receives appropriate stimulus, the little granules expel their contents out of the cell’s interior. In the case of neutrophils, granules contain very toxic compounds that cause the formation of free radicals which damage DNA and proteins of the surrounding cells, as well as granules filled with digestive enzymes which, well, digest the surrounding tissues.
NETosis is a special type of cell death specific to neutrophils in which they literally degranulate pieces of their own, or their mitochondrial DNA together with more toxic compounds. This creates a net of DNA strands called chromatin which entangles invading bacteria and severely damages them and also marks them for phagocytosis by macrophages. But this process is not well controlled and some of that chromatin and toxic compounds can land onto neighboring cells which is, as you can conclude, very bad for them.
With these two abilities at hand, neutrophils are very well equipped to kill cells and destroy tissue. Which is good in cases when the cells are infected and the tissue is damaged, but their quite aggressive methods can damage healthy cells in the area as well, some of them will die and neutrophils will phagocytose their dead particles.
Basically, to neutrophils every infection is a huge kill and eat all you can buffet. They literally phagocytose until they physically cannot anymore and then go to the spleen or bone marrow to die. They also allow macrophages to consume them and thus pass on the antigens for antigen presentation which influences further immune response. But they can also cause a lot of damage, especially if cytokine storm happens and they completely lose control. This is what causes SARS and it can kill you if it’s severe enough.
Biologically speaking, neutrophils are very important because they are the first ones to come to the sight of infection and their crazy methods usually finish the things before they get too severe. They themselves produce cytokines that mobilize macrophages and dendritic cells so that more immune cells can join and help them. They also have a role in repairing the tissues they damaged.
However, other immune cells, including macrophages and killer T cells, simply don’t cause as much damage. Neutrophils just go all out, which is why they live for such a short period of time compared to their colleagues (they live for only few days, compared to macrophages who can live up to a month and lymphocytes who can live for months, even years).
So, yeah, my boyfriend and I have concluded (at 4AM this morning) that neutrophils are so feared because they damage tissue, go crazy and violently kill healthy cells by accident, then consume them and that’s not by accident, it’s a mechanism to repair tissues.
I can’t believe I wasted whole night just for this. My boyfriend is also disappointed. But I hope that we finally have an explanation for this mystery. Tell me what you think lol.
References:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8589350/
https://www.nature.com/articles/nri.2017.105
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5820392/#:~:text=Neutrophils%20contribute%20to%20tissue%20injury,detail%20here%20(Kruger%20et%20al.
#cells at work#hataraku saibou#neutrophils#immunology#medicine#science#biology#i actually need help#it’s 4am
66 notes
·
View notes
Text
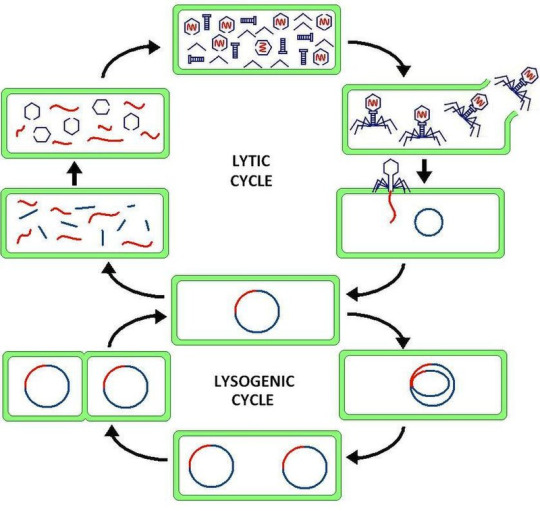
Virus latency (or viral latency) is the ability of a pathogenic virus to lie dormant (latent) within a cell, denoted as the lysogenic part of the viral life cycle.[1] A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny (the lytic part of the viral life cycle) without the host becoming reinfected by new outside virus, and stays within the host indefinitely.[2]
Episomal latency refers to the use of genetic episomes during latency. In this latency type, viral genes are stabilized, floating in the cytoplasm or nucleus as distinct objects, either as linear or lariat structures. Episomal latency is more vulnerable to ribozymes or host foreign gene degradation than proviral latency (see below).
Advantages of episomal latency include the fact that the virus may not need to enter the cell nucleus, and hence may avoid nuclear domain 10 (ND10) from activating interferon via that pathway. Disadvantages include more exposure to cellular defenses, leading to possible degradation of viral gene via cellular enzymes.[12]
Proviral latency: A provirus is a virus genome that is integrated into the DNA of a host cell
All interferons share several common effects: they are antiviral agents and they modulate functions of the immune system. Administration of Type I IFN has been shown experimentally to inhibit tumor growth in animals, but the beneficial action in human tumors has not been widely documented. A virus-infected cell releases viral particles that can infect nearby cells. However, the infected cell can protect neighboring cells against a potential infection of the virus by releasing interferons. In response to interferon, cells produce large amounts of an enzyme known as protein kinase R (PKR). This enzyme phosphorylates a protein known as eIF-2 in response to new viral infections; the phosphorylated eIF-2 forms an inactive complex with another protein, called eIF2B, to reduce protein synthesis within the cell. Another cellular enzyme, RNAse L—also induced by interferon action—destroys RNA within the cells to further reduce protein synthesis of both viral and host genes. Inhibited protein synthesis impairs both virus replication and infected host cells. In addition, interferons induce production of hundreds of other proteins—known collectively as interferon-stimulated genes (ISGs)—that have roles in combating viruses and other actions produced by interferon.[13][14] They also limit viral spread by increasing p53 activity, which kills virus-infected cells by promoting apoptosis.[15][16] The effect of IFN on p53 is also linked to its protective role against certain cancers.[15]
Another function of interferons is to up-regulate major histocompatibility complex molecules, MHC I and MHC II, and increase immunoproteasome activity. All interferons significantly enhance the presentation of MHC I dependent antigens. Interferon gamma (IFN-gamma) also significantly stimulates the MHC II-dependent presentation of antigens. Higher MHC I expression increases presentation of viral and abnormal peptides from cancer cells to cytotoxic T cells, while the immunoproteasome processes these peptides for loading onto the MHC I molecule, thereby increasing the recognition and killing of infected or malignant cells. Higher MHC II expression increases presentation of these peptides to helper T cells; these cells release cytokines (such as more interferons and interleukins, among others) that signal to and co-ordinate the activity of other immune cells.[17][18][19]
Epstein–Barr virus lytic reactivation (which can be due to chemotherapy or radiation) can result in genome instability and cancer.[5]
HSV reactivates upon even minor chromatin loosening with stress,[7] although the chromatin compacts (becomes latent) upon oxygen and nutrient deprivation.[8]
Cytomegalovirus (CMV) establishes latency in myeloid progenitor cells, and is reactivated by inflammation.[9] Immunosuppression and critical illness (sepsis in particular) often results in CMV reactivation.[10] CMV reactivation is commonly seen in patients with severe colitis.[11]
viral latency is so fucked up
11 notes
·
View notes
Text
OH ALSO macrophages. remember them? the hungry blokes? when they eat pathogens, they process them, and then present the pathogens antigen on their cell membrane.
then the helper t cell will bind to that pathogen. then the macrophage releases chemicals, the t helper cell then releases chemicals, and then that stimulates the cytotoxic t cells, also known as killer t cells.
helper t cells are very cool, they arent just in the cell mediated response but also in the humoral response
6 notes
·
View notes
Text
NP plays a central role in viral replication [18]. As a structural protein with no intrinsic enzymatic activity [19], it is the most abundant viral protein in infected cells.
In an influenza infection, nucleoprotein is the first viral protein to replicate, so the infected cell quickly presents the nucleoprotein antigens, provoking a strong early immune response, they explained.
"Another aspect worth pointing out is that protection against currently circulating influenza viruses has been reached even with a very old variant of the nucleoprotein protein," they continued.
"The OVX836 vaccine is based on the full-length nucleoprotein of the influenza A virus A/WSN/1933 (H1N1), and, despite the fact that this antigen is highly conserved and has been through over 90 years of evolution, this protein has undergone some changes...
Of the 11 RSV-encoded proteins, N is one of the most conserved structural proteins and is essential for virus encapsidation by coating the entire viral RNA genome to form the ribonucleoprotein (RNP).
These data suggest that the antigenic repertoire of T cells in IS subjects is skewed compared to HSV-2+ subjects and that IS subjects had more frequent T cells responses to IE proteins and infrequent T cell responses to virion components.

The preponderance of T cell responses directed at IE proteins in IS subjects suggests that IS subjects have been exposed to replicating virus since IE proteins are the first proteins made during the virus infectious life cycle and are not present in infectious virions. T cells directed at IE proteins would be engaged early in the infectious life cycle and may be able to kill the virally-infected cell before the production of infectious progeny and thus advantageous to the host. If some of the IS subjects are infected with HSV-2 in the absence of seroconversion, the presence of T cells directed at IE proteins at the neural-epidermal junction would provide the quickest defense against the virus spreading to the periphery and may explain why we did not detect any HSV DNA at mucosal sites in IS subjects (4).
The HPV genome contains an early expressed region containing the ORF (Open reading frames) of E1 to E7 genes which are necessary for viral replication and transcriptional regulation6. The E6, E7 and E5 proteins are able to interact with many cell targets, promoting cellular transformation7,8. The E1 protein is encoded within the early expressed region and it is localized in nuclear and cytoplasmic fractions. This protein is highly conserved among different HPV types and is the unique HPV protein with enzymatic activity.
The initiation of the HPV infection is from the basal layer of the squamous epithelium. The viral replication process and transcription of other E proteins are regulated by E1 and E2 proteins...
E1 protein has been reported highly conserved among the types of HPV and commonly decoded during the early expression of HPV infection...
E1 and E2 proteins play crucial role in the initiation and regulation of HPV replication as illustrated in Fig. 1. Initially, E2 proteins bind to their binding sites (E2BS11 and E2BS12) at the origin of replication, which recruits a pair of E1 proteins to form a complex.
2 notes
·
View notes
Text
Understanding Rapid COVID Tests: How They Work and When to Use Them

The COVID-19 pandemic has brought about many changes in our daily lives, and one of them is the need for frequent COVID testing. Rapid COVID tests have become a popular choice for quick and easy testing, but many people still have questions about how they work and when they should be used. In this blog, we will explore rapid COVID tests Austin and provide information to help you make informed decisions about testing.
What are Rapid COVID Tests?
Rapid COVID tests are a type of COVID test that can produce results within minutes. Unlike PCR tests, which require laboratory processing and can take several days to produce results, rapid tests can be done on-site and provide results in a matter of minutes.
There are two types of rapid COVID tests currently available: antigen tests and antibody tests. Antigen tests detect the presence of the virus by identifying specific proteins on the surface of the virus. Antibody tests, on the other hand, detect the presence of antibodies produced by the body in response to the virus.
How do Rapid COVID Tests Work?
Antigen tests work by using a nasal or throat swab to collect a sample from the patient. The sample is then mixed with a solution that contains antibodies that bind to the virus if it is present. If the virus is present, the antibodies will bind to it and produce a visible reaction, indicating a positive result.
Antibody tests, on the other hand, work by using a blood sample to detect the presence of COVID antibodies. The test looks for two types of antibodies: IgM antibodies, which are produced in the early stages of infection, and IgG antibodies, which are produced later and can remain in the body for months.
When to Use Rapid COVID Tests?
Rapid COVID tests are most useful in situations where quick results are needed, such as in hospitals, schools, and workplaces. They can be used to screen people for the virus, especially in high-risk settings, such as nursing homes or prisons. Rapid tests can also be used to test people who have symptoms of COVID-19 or have been exposed to someone with the virus.
It's important to note that rapid COVID tests are not as accurate as PCR tests, especially in people who are asymptomatic or have a low viral load. Therefore, if you have symptoms or have been exposed to someone with the virus, it's recommended to get a PCR test to confirm the result.
In conclusion, rapid PCR test Austin can be a useful tool in the fight against COVID-19, providing quick and easy testing in high-risk settings or for people .
2 notes
·
View notes
Text
Antigen Presenting Cells
Antigen Presenting Cells Antigen-presenting cells (APCs) play a crucial role in the immune system, bridging innate and adaptive immunity. They are responsible for capturing, processing, and presenting antigens to T cells, thus enabling the body to recognize and respond to pathogens, cancerous cells, and other foreign substances. The primary professional APCs are dendritic cells, macrophages, and…
#academic research#Antigen#APCs#B-Cell#Dendritic Cells#immunity#immunology#Macrophages#Major Histocompatibility Complex#MHC#protocol#research#science
0 notes
Text

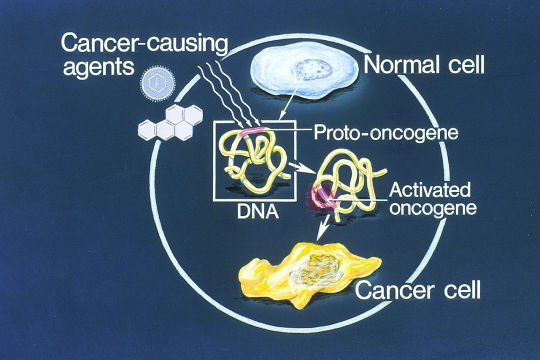
Propaganda!
A dendritic cell (DC) is an antigen-presenting cell (also known as an accessory cell) of the mammalian immune system. A DC's main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and adaptive immune systems.
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels. Most normal cells will undergo a programmed form of rapid cell death (apoptosis) when critical functions are altered and malfunctioning. Activated oncogenes can cause those cells designated for apoptosis to survive and proliferate instead.
#tournament poll#polls#wikipedia#cells of the human body#science tournament#dendritic cell#oncogene#image descriptions in alt text
11 notes
·
View notes
Text
Colorectal Cancer Treatment in Chennai
Colorectal cancer, which includes cancers of the colon and rectum, is one of the most common types of cancer worldwide. In India, colorectal cancer is becoming increasingly prevalent, especially among urban populations. Chennai, known for its world-class medical infrastructure, offers a variety of treatment options for colorectal cancer. Among the leading healthcare facilities specializing in colorectal cancer treatment is The Arc Gut.
The Arc Gut is known for its dedicated team of gastroenterologists, oncologists, and colorectal surgeons who provide comprehensive care, from early diagnosis to advanced treatments. This article will explore the causes, symptoms, diagnostic techniques, treatment options, and the role of The Arc Gut in the management of colorectal cancer in Chennai.
Understanding Colorectal Cancer
Colorectal cancer originates in the colon or rectum, which are parts of the large intestine. The large intestine is responsible for absorbing water and salts from the food we digest. Colorectal cancer typically begins as polyps (small growths) on the inner lining of the colon or rectum. Over time, some of these polyps can become cancerous.
The exact cause of colorectal cancer is unknown, but several risk factors have been identified, including:
Age: Most colorectal cancers are diagnosed in individuals over the age of 50.
Family History: A family history of colorectal cancer or other genetic conditions can increase the risk.
Lifestyle Factors: Diets high in processed foods, red meat, and low in fiber are linked to higher risks.
Chronic Inflammatory Conditions: Conditions like ulcerative colitis or Crohn’s disease increase the risk of colorectal cancer.
Symptoms of Colorectal Cancer
Early-stage colorectal cancer may not present noticeable symptoms. However, as the disease progresses, several signs and symptoms may appear, including:
Changes in bowel habits (e.g., diarrhea, constipation)
Rectal bleeding or blood in stool
Unexplained weight loss
Persistent abdominal discomfort (e.g., cramps, bloating)
Fatigue or weakness
Iron-deficiency anemia (due to internal bleeding)
If you experience any of these symptoms, it is crucial to seek medical advice promptly, as early detection significantly improves treatment outcomes.
Diagnosis of Colorectal Cancer
Early diagnosis of colorectal cancer is critical for successful treatment. Several diagnostic techniques are used to detect colorectal cancer, and at The Arc Gut, the latest technology and techniques are employed for precise diagnosis. These methods include:
1. Colonoscopy
A colonoscopy is the most common procedure for diagnosing colorectal cancer. During the procedure, a long, flexible tube with a camera is inserted through the rectum to examine the inner lining of the colon and rectum. If any abnormal growths or polyps are detected, they can be removed and sent for biopsy to determine if they are cancerous.
2. Biopsy
A biopsy involves removing a small sample of tissue from the colon or rectum to be examined under a microscope. This is often done during a colonoscopy.
3. Imaging Tests
Imaging tests such as CT scans, MRIs, and PET scans are used to determine the stage of cancer, evaluate the spread (metastasis), and plan treatment.
4. Blood Tests
Blood tests may be used to check for signs of colorectal cancer, such as elevated levels of certain tumor markers, including carcinoembryonic antigen (CEA).
Treatment of Colorectal Cancer at The Arc Gut
At The Arc Gut, a multidisciplinary team works together to provide a personalized treatment plan for each patient, based on the stage and location of the cancer, as well as the patient’s overall health. The main treatment modalities for colorectal cancer include:
1. Surgery
Surgery is the most common and effective treatment for localized colorectal cancer. It involves removing the tumor and a surrounding portion of healthy tissue. If cancer has spread to nearby lymph nodes, they may also be removed. There are different types of surgeries used, including:
Local excision: Removing small polyps or early-stage cancers from the lining of the colon or rectum.
Colectomy: Removal of a part of the colon or the entire colon.
Colostomy or ileostomy: In some cases, part of the colon is removed, and an opening (stoma) is created in the abdomen to allow waste to exit the body.
At The Arc Gut, surgeons perform these procedures with advanced laparoscopic techniques, which result in smaller incisions, faster recovery times, and less postoperative pain.
2. Chemotherapy
Chemotherapy is often used after surgery to kill any remaining cancer cells or to shrink tumors before surgery. It can also be used as a primary treatment for advanced or metastatic cancer. Chemotherapy drugs are usually administered intravenously or orally, and they target rapidly dividing cancer cells.
At The Arc Gut, chemotherapy regimens are tailored to the patient’s specific condition, ensuring the most effective treatment with minimal side effects.
3. Radiation Therapy
Radiation therapy uses high-energy rays to kill cancer cells or shrink tumors. It is often used for rectal cancer or when surgery cannot completely remove the tumor. Radiation therapy can also be used in conjunction with chemotherapy to enhance the effects of treatment.
4. Targeted Therapy
Targeted therapy involves drugs that specifically target cancer cells without harming healthy cells. This treatment is often used in advanced stages of colorectal cancer when the cancer has spread. It is used in combination with chemotherapy or radiation therapy.
5. Immunotherapy
Immunotherapy is an emerging treatment for colorectal cancer, particularly for cancers with specific genetic mutations. This treatment boosts the immune system’s ability to recognize and fight cancer cells.
Post-Treatment Care and Rehabilitation
After completing the main treatment for colorectal cancer, ongoing care is essential. At The Arc Gut, patients are provided with comprehensive post-treatment care, including:
Follow-up appointments: Regular check-ups to monitor recovery and detect any signs of recurrence.
Dietary advice: Nutritionists guide patients on maintaining a healthy diet to promote healing and improve overall health.
Rehabilitation services: Physical therapy helps patients regain strength and mobility after surgery or treatment.
Psychological support: Cancer treatments can be emotionally challenging, and The Arc Gut offers counseling services to support mental health during recovery.
Why Choose The Arc Gut for Colorectal Cancer Treatment in Chennai?
Experienced Team: The Arc Gut has a team of highly skilled gastroenterologists, oncologists, and colorectal surgeons with vast experience in treating colorectal cancer.
State-of-the-Art Technology: The center uses the latest diagnostic tools, including advanced imaging and minimally invasive surgical techniques.
Comprehensive Care: The Arc Gut offers a holistic approach, combining surgery, chemotherapy, radiation, and supportive therapies to ensure the best outcomes for patients.
Patient-Centric Approach: Personalized care plans are created based on each patient’s needs, ensuring the highest level of care and attention.
Conclusion
Colorectal cancer is a serious condition, but with early detection and timely treatment, the prognosis can be significantly improved. The Arc Gut in Chennai offers comprehensive, cutting-edge treatments for colorectal cancer, supported by a team of specialists dedicated to providing the best care possible. Whether through surgery, chemotherapy, radiation therapy, or advanced immunotherapies, The Arc Gut is committed to improving the quality of life for patients with colorectal cancer, offering hope and healing during challenging times. For more details visit https://thearcgut.clinic/digestive-and-gut-health-test-in-chennai/
0 notes
Text
The Immune System's All-Star Team: The Mighty Cells That Protect You
Your body is like a bustling city, constantly facing threats from outside invaders like viruses and bacteria. Thankfully, you have a team of dedicated defenders keeping you safe: your immune cells! Our immune system is a marvel of biological defense, tirelessly safeguarding our bodies from harmful invaders like bacteria, viruses, and parasites. At the forefront of this defense are numerous types of immune cells, each with its unique functions and capabilities. Did you know the average adult has about 2 trillion white blood cells, which contain most immune cells? That's more people than live in China! These tiny warriors come in different shapes and sizes, each with unique superpowers to protect you. Let's meet some of the key players:
The Innate Force: First up, we have the innate immune system. This frontline defense acts fast and nonspecifically, providing immediate protection against any threat. The key players: 1. Neutrophils: Think of these guys as the city's SWAT team. They're the first responders, rushing to attack invaders with toxic chemicals and swallowing them whole with their arsenal of enzymes! These are the most abundant immune cells, are short-lived but highly effective. Unfortunately, they die in the fight, leaving behind a green gooey mess (pus) that signals infection.
2. Macrophages: These are the veterans, the wise generals of the immune system. They go beyond mere engulfing, processing antigens (foreign molecules) and presenting them to other immune cells for recognition and attack. They also act as scavengers, cleaning up debris and orchestrating healing. These are the cleaners and recyclers. They gobble up dead neutrophils, debris, and even worn-out cells, keeping your city sparkling clean.
3. Natural Killer (NK) Cells: These are the ninjas of the immune system. They silently patrol, sniffing out suspicious cells infected with viruses or even cancer and eliminating them with a swift punch. The Adaptive Arsenal:
The Adaptive Arsenal: If the innate system fails, the adaptive immune system steps in. This highly specific defense remembers past encounters and tailors its response to each unique threat. The cells of adaptive immune system are:
B Cells: These are the antibody factories, producing highly specific proteins called antibodies that neutralize pathogens and toxins. Each B cell produces a unique antibody, like a lock and key, targeting specific invaders. They whip up special proteins called antibodies that lock onto specific invaders, like sticky notes, marking them for destruction.
T Cells: These are the generals, coordinating the entire defense. There are different types of T cells:
Helper T Cells: These are the commanders, directing and coordinating the immune response through chemical signals. They activate B cells, macrophages, and other immune cells, orchestrating a multi-pronged attack.
Cytotoxic T Cells: These are the elite soldiers, directly targeting and eliminating infected cells or cancer cells. They recognize and bind to specific enemy markers, unleashing a lethal attack.
Memory T Cells: These are the veterans, remembering past encounters with invaders and helping the immune system respond faster next time.
The Unsung Heroes: Beyond these main players, numerous other immune cells contribute to our defense. These include:
Dendritic cells: These antigen-presenting cells capture and process pathogens, presenting their fragments to T cells for activation. They're like the scouts, gathering enemy intel and relaying it to the command center.
Mast cells: These cells reside in tissues and release inflammatory chemicals in response to allergens or parasites. They're like the alarm system, alerting the immune system to local threats.
Eosinophils: These specialize in fighting parasitic infections, releasing toxic chemicals to neutralize them.
Basophils: These are involved in allergic reactions and contribute to wound healing.
The beauty of the immune system lies in its intricate collaboration. These diverse cell types work together in a complex and beautiful dance, each playing a specific role to achieve a common goal: protecting our health. They communicate extensively through chemical signals, creating a complex network of interactions. Imagine B cells producing antibodies that bind to a pathogen, flagging it for destruction. Macrophages engulf and eliminate the tagged pathogen, while T cells coordinate the attack and eliminate any infected cells. Dendritic cells present captured fragments to T cells, priming them for future encounters. This seamless cooperation ensures a swift and effective response to any threat.
Your immune system is constantly learning. Each time you get a vaccine or fight off an infection, your immune cells create memory T cells, making you more resistant to future attacks. Understanding these cellular heroes can help us appreciate the incredible machinery that keeps us healthy and appreciate the importance of maintaining a strong immune system. It also allows us to make informed decisions about supporting our immune system. Maintaining a healthy lifestyle, getting adequate sleep, managing stress, and consuming a balanced diet can all contribute to a robust immune response.
#science sculpt#life science#science#biology#biotechnology#artists on tumblr#immunology#immunotherapy#immune cells#b cells#t cells#neutrophil#macrophages#immunity#health and wellness#medicine#healthcare#illustration#illustrative art
9 notes
·
View notes
Text
10 Essential Phlebotomy Facts Every Aspiring Technician Should Know
10 Essential Phlebotomy Facts Every Aspiring Technician Should Know
10 Essential Phlebotomy Facts Every Aspiring Technician Should Know
Phlebotomy is a crucial aspect of the healthcare industry, often serving as the frist step in the laboratory testing process. Whether you are an aspiring technician or considering a career in healthcare, understanding the fundamentals of phlebotomy is essential. In this article, we will explore ten essential facts that every budding phlebotomist should be aware of.
1. What is phlebotomy?
Phlebotomy is the art and science of drawing blood from a patient for various medical purposes, including diagnostics, research, and transfusions. Phlebotomists are skilled healthcare professionals responsible for ensuring this procedure is performed safely and effectively.
2. The Importance of Phlebotomy in Healthcare
Phlebotomy is essential for disease diagnosis and monitoring.
It plays a vital role in blood banking processes.
Phlebotomy helps in the research and development of new therapies.
3. Key Skills Required for a Phlebotomist
To become an effective phlebotomist, certain skills and attributes are essential:
Attention to Detail: accuracy is crucial when drawing blood and labeling samples.
Interaction Skills: You must communicate effectively with patients and healthcare teams.
Compassion and Empathy: Many patients may feel anxious about the procedure; being supportive is vital.
Technical Proficiency: Familiarity with various blood collection techniques and equipment is a must.
4. Educational Requirements and Certification
Becoming a certified phlebotomist typically requires the following educational steps:
Complete a phlebotomy training programme, which can be found at community colleges or vocational schools.
Gain hands-on experience through clinical practice.
Pass a nationally recognized certification exam.
5. Common Blood Collection Techniques
Understanding the different methods of blood collection is crucial for aspiring phlebotomists. Here are some common techniques:
Venipuncture: This is the moast common method and involves drawing blood from a vein, typically in the arm.
Capillary Puncture: This method uses a small prick to collect blood, often from a fingertip. It’s useful for smaller blood samples.
Arterial Puncture: This technique involves accessing an artery, usually performed by trained professionals in specific situations.
6. Safety Precautions in Phlebotomy
Safety is a top priority in phlebotomy. Here are some essential safety precautions:
Always wear gloves to protect yourself and the patient.
Dispose of needles properly in designated sharps containers.
keep equipment sterile to prevent infections.
Follow infection control protocols at all times.
7. Understanding Blood Types and Their Importance
Knowledge of blood types is critical for various reasons,including transfusions and organ donations. Here’s a simple table to summarize blood types:
Blood Type
Antigen Present
Can Donate To
can Receive From
A
A
A, AB
A, O
B
B
B, AB
B, O
AB
A, B
AB
A, B, AB, O
O
None
A, B, AB, O
O
8. The Role of Technology in Phlebotomy
Modern phlebotomy has evolved significantly with technology. Key advancements include:
Automatic Blood Draw Devices: These devices reduce the time and discomfort during blood collection.
Electronic Medical Records (EMRs): EMRs improve accuracy and accessibility of patient data.
Sample Tracking Systems: These systems ensure better management of specimens for accurate testing.
9. Continuing Education and Career Advancement
Phlebotomy offers various career advancement opportunities,including:
Specializing in areas such as pediatrics or oncology.
Pursuing further education in laboratory sciences.
Transitioning into roles such as lab technician, research assistant, or even managerial positions.
10. Real-World Experiences and Case Studies
Many phlebotomists find their roles rewarding and impactful. Such as,one phlebotomist shared how a simple blood test helped diagnose a patient’s rare condition,leading to life-saving treatment. Such experiences underscore the importance of compassion, accuracy, and professionalism in this field.
Conclusion
Phlebotomy is a vital aspect of healthcare that requires a solid foundation of knowledge and skills. From understanding blood collection techniques to emphasizing safety precautions, these ten essential facts provide a complete overview for aspiring technicians. As you embark on your phlebotomy journey, remember that continuous learning and adaptation to advancements in technology will be key to your success.
Call to Action
Are you ready to start your training in phlebotomy? Explore local programs, gain hands-on experience, and take the first step towards a rewarding career in this essential healthcare field!
youtube
https://phlebotomyschoolsonline.org/10-essential-phlebotomy-facts-every-aspiring-technician-should-know/
0 notes
Text
Rheumatic Fever And Rheumatic Heart Disease
Rheumatic Fever and Rheumatic Heart Disease
#Rheumatic 💛
#Fever 🤎
#RheumaticFever 💜
#RheumaticHeartDisease 💚
#Worldwide ❤️
#Cardiac 🩷
#Disease 🩵
Acute rheumatic fever and rheumatic heart disease are leading causes of acquired cardiac disease in developing countries, with between 5 and 30 million children and young adults affected worldwide and a mortality rate of 1% to 10%.94 .
In the United States, the availability of antibiotics to treat streptococcal pharyngitis has markedly reduced the incidence of this disease but sporadic cases still occur.95 In children, the peak incidence occurs between 5 and 14 years of age.
Rheumatic fever results from infection by particular strains of group A β-hemolytic Streptococcus or Streptococcus pyogenes. Both cellular and humoral immune responses to the bacterial antigens cross react with native tissues to result in a multisystemic infl ammatory disorder.
The incubation period for most strains of group A β-hemolytic Streptococcus is typically 3 to 5 days, although some children will present with a more remote history of pharyngitis.
The diagnosis of rheumatic fever is made clinically based on the modifi ed Jones criteria.96 Major criteria include carditis, polyarthritis, chorea, subcutaneous nodules, and erythema marginatum. Evidence of a prior streptococcal infection along with two major or one major and two minor criteria are required to make the diagnosis in children without prior history of rheumatic fever or echocardiographic evidence of typical valvular involvement. Fever and arthritis are common symptoms.
The polyarthritis has a migratory pattern, typically aff ecting large joints. Cardiac involvement or carditis occurs in nearly 50% of children with their fi rst attack of rheumatic fever.
Rheumatic heart disease represents a sequela of the acute process, aff ecting the mitral and aortic valves most frequently. Primary prevention of rheumatic fever and rheumatic heart disease begins with prompt recognition and appropriate treatment of the initial streptococcal infection.97,98 Secondary prevention, with ongoing therapy in individuals with a known history of rheumatic fever, has been shown to be extremely effective at preventing recurrent attacks.
#DrBhishmaChowdary 🩺
#BestCardiologistInHyderabad 🥼

#cardiology#health#healthcare#doctor#health tips#anatomical heart#health and wellness#heart doctor#heart specialist#cardiovascularhealth#rheuamtic fever#rheumatic#rheumatology
0 notes