#VX-β
Explore tagged Tumblr posts
Text
BREAKING: Yamaha Reveals Commercial Version VX-β Release Date!
After months of waiting, Yamaha has officially revealed the release date for the commercial version of the VX-β plugin which is planned to be included in an upcoming update for VOCALOID6! The Official VX-β Release Date Reveal trailer from the Official Vocaloid Twitter Account As announced on the Official Vocaloid Twitter account, the VX-β plug-in is set to release on November 27th, alongside…
9 notes
·
View notes
Text
Uge for VOCALOID6 Announced!

VOCALOID6 AI port of the VX-β voicebank And Ugo is set to release on January 15th! She will be available alone with VOCALOID6 Editor Lite or in a bundle with the full V6 editor and Cubase AI. She also has a demo song by Kashii Moimi out now!
youtube
7 notes
·
View notes
Text
ゲキヤクV調声メモ
VOCALOID6(以下V6)で発売されたゲキヤクVの調声メモ
ゲキヤクと言えばしゃくり、キャーやグワァーって感じなのですがそれをV6で再現することが難しかったので備忘録としてメモをしています
AIボイスの前提として以下の点を甘味してください
グロウルがない
ブレシネスなどのザラザラさせるパラメーターも無い
日本語、英語、中国語の発音がある
鼻濁音などの特殊な発音記号は未発見
(ほぼ苦味なんですけど…vx-βはよ戻ってきて…)
EXP:expressionを高く設定する
通常50なのですが70ぐらいからゲキヤクっぽいハキハキとした力強い歌声になります
記号を利用する
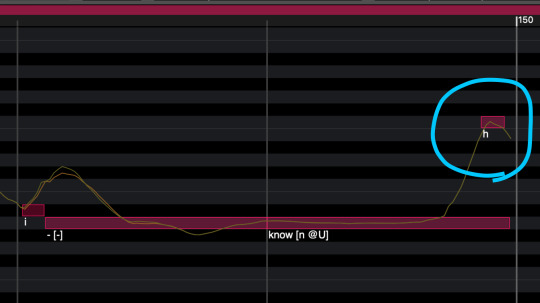
h
T
Z
UTAUゲキヤクで言う「息捨て」に近い記号ですが、UTAUよりもピッチの動きに影響が大きいです。音を発音させずに語尾だけしゃくらせたい時に使用すると効果的かも。前後の母音に引っ張られる傾向があるので、発音記号を[母音 h]とした方が綺麗に繋がる場合があります
h T Zのどれを使うのが一番いいのかは調査中ですが、hが汎用性が高かった印象。とまああれこれしたのですが、結局βの方が自然でした
英語でしゃくらせる
子音の発音が曖昧な英語でしゃくらせる方が自然に聞こえます。vx-βではピッチでしゃくりを再現出来たのですが、V6エディタでは大きくピッチを上げることができない。かつパラメーターを0に合わせることがこの上なくストレスなので、ノートを分けて英語で繋いだ方が良さそう
ちなみにエッジボイス、ボーカルフライに挑んだが挫折。上から下はいけるんですけど
中国語の可愛い発音
ma[m a] マォっぽい発音
ni[n i] 鼻にかかったニ"
kou[k_h @ U] 吐息多めのコ
dou[t @ U] 吐息多めのド
ga[k a] 鼻にかかったガ
英語のかっこいい発音
do[dh @ U] 力強いドゥ
le[l0 e] 甘めのれ
※@ Uは本来ツメなのですがTumblrのメンションになってしまうので意図的に離しています
vx-βとの差
vx-βとは以前YAMAHA様から使用期間中無料で利用可能だった合成音声作成ソフトです。軽くてリアルタイムの操作が可能
(↑あそんだやつ)🔈音量注意
これの発音の滑らかさが素晴らしくてベタ打ちでも問題ないくらい滑らか。やすりがけした工作の時間の木材や、油刺した後の自転車、鉛筆の芯を削って鍵穴に通した家の玄関ぐらい滑らか
当時はサポートが日本語だけだったのかな、口語の発音や舌使いは現段階でV6の方が自由度が高いけどそれは新しいだけの話。ボカロらしさ以前に滑らかに歌わせるだけでどれだけ難しいことか
ゲキカゼV6発売のタイミングでvx-βは復活するとアナウンスされていたので待ち遠しい。歌詞入力の使用が変わっていなければV6である程度発音を決めてvx-βに流すと思います
でもゲキカゼVも本当に本当にありがとう…😭
ちなみに購入される際はセットのこちらを強くオススメします(実質無料なので)
4 notes
·
View notes
Text
Ο ετεροθαλής αδελφός του ηγέτη της Β. Κορέας δολοφονήθηκε από το καθεστώς με τη χρήση VX
Η κυβέρνηση των ΗΠΑ κατέληξε στο συμπέρασμα ότι ο ετεροθαλής αδελφός του ηγέτη της Βόρειας Κορέας Κιμ Γιονγκ Ουν δολοφονήθηκε από το καθεστώς με τη χρήση του παράγοντα VX, μιας νευροτοξικής ουσίας, κι επέβαλε νέες κυρώσεις στην Πιονγκγιάνγκ, γνωστοποίησε την Τρίτη η εκπρόσωπος του Στέιτ Ντιπάρτμεντ με μια ανακοίνωση που έδωσε στη δημοσιότητα. Το συμπέρασμα ...
περισσότερα Ο ετεροθαλής αδελφός του ηγέτη της Β. Κορέας δολοφονήθηκε από το καθεστώς με τη χρήση VX at Φούιτ.gr.
περισσότερα στο fouit.gr
0 notes
Text
Merck: Driving Innovation in Cancer Care at ESMO 2017 With New Data in Hard-to-Treat Cancers
DARMSTADT, Germany, Aug. 31, 2017 /PRNewswire/ --
Not intended for U.K. or U.S. based media
ESMO 2017 abstract #
Erbitux®: 576P, 593P, 1068P, 1579P; avelumab: 1227P, 913P, 1377TiP, 882P, 856P; M6620 (ATR inhibitor): 242PD; M2698 (dual p70S6K/Akt inhibitor): 370PD, 393P; tepotinib (c-Met kinase inhibitor): 701P
Data to showcase Merck's strong and diverse pipeline ranging from immuno-oncology to DNA damage response
Avelumab data validate potential in hard-to-treat cancers and highlight progress of the JAVELIN clinical development program
First stand-alone data in mTNBC for ATR inhibitor (M6620) from Merck's comprehensive portfolio in DNA damage response
Merck, a leading science and technology company, today announced it will present data for a number of tumor types across its rapidly evolving pipeline. A total of 23 abstracts, representing five therapeutic agents, will highlight the company's expanding scientific expertise at this year's European Society for Medical Oncology congress (ESMO 2017; September 8-12, Madrid, Spain).
Data to be presented include continued reinforcement of the role of established brand Erbitux® (cetuximab) as a standard of care therapy, with quality of life (QoL) data in colorectal cancer (CRC) and real-world data in both CRC and squamous cell carcinoma of the head and neck (SCCHN); updated efficacy and safety data for avelumab in metastatic Merkel cell carcinoma (mMCC) and urothelial carcinoma (UC) among other cancers; and new data and updates from Merck's rapidly evolving pipeline, including first stand-alone data in metastatic triple negative breast cancer (mTNBC) from potential first-in-class ataxia telangiectasia and Rad3-related protein (ATR) inhibitor M6620* (also known as VX-970).
"The Merck Oncology Franchise has had a momentous year, particularly with the positive regulatory milestones achieved for avelumab. The story continues to evolve at ESMO 2017 from our legacy with Erbitux to our diverse and robust pipeline which has potential novel molecules that could become new standards of care," said Luciano Rossetti, Executive Vice President, Global Head of Research & Development at the biopharma business of Merck. "The data reinforce Merck's commitment to pursuing approaches that will bring important benefits to patients and transform the way cancer is treated."
Merck's innovative approach and strategic collaborations in oncology are exemplified through the ongoing partnership with Pfizer, and the significant progress of avelumab. Granted two accelerated approvals** by the U.S. Food and Drug Administration (FDA) this year, more recently the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending the approval of avelumab as monotherapy for the treatment of adult patients with mMCC. ESMO 2017 includes new data for avelumab in the treatment of mMCC, a rare and aggressive skin cancer, and 12-month follow-up data in pre-treated patients with locally advanced or metastatic UC. The progress of the broader JAVELIN clinical development program will also be highlighted, with updated data in hard-to-treat tumors such as metastatic adrenocortical carcinoma (mACC).
The addition of the recently acquired Vertex DNA damage response (DDR) portfolio to its own in-house DDR platform has positioned Merck as one of the key players in the DDR field. The company's broad DDR portfolio includes inhibitors for enzymes of major DDR pathways, such as ATR, DNA-PK and ATM. At ESMO 2017, first data will be presented for ATR inhibitor M6620 in metastatic triple-negative breast cancer (mTNBC). M6620 is currently being investigated in several ongoing Phase I trials across a variety of tumor types.
Other pipeline updates will include data on the potential first-in-class dual p70S6K/Atk inhibitor M2698*; and tepotinib***, a highly selective c-Met kinase inhibitor, in patients with advanced hepatocellular carcinoma (HCC).
Product related information contained herein is subject to local product approval and can therefore vary from country to country. For information relevant to your country, please check in with local regulatory authorities.
*M6620, M2698 and tepotinib are under clinical investigation and have not been proven to be safe and effective. There is no guarantee any product will be approved in the sought-after indication by any health authority worldwide.
***Tepotinib is the proposed International Non-proprietary Name (INN) for the c-Met kinase inhibitor (also known as MSC2156119J).
Notes to editors
Accepted Merck-supported key abstracts at ESMO 2017 are listed below. In addition, a number of abstracts with data from investigator-sponsored studies have been accepted, including abstracts related to Erbitux (not listed).
Title
Lead Author
Abstract #
Presentation Date / Time (CEST)
Location
Avelumab
Poster sessions
JAVELIN Lung 100: updated design of a phase 3 trial of avelumab vs platinum doublet chemotherapy as first-line (1L) treatment for metastatic or recurrent PD-L1+ non-small-cell lung cancer (NSCLC)
Reck M.
1377TiP
September 9
13:15 – 14:15
Hall 8
JAVELIN MERKEL 200: Avelumab treatment in chemotherapy-naïve patients with metastatic Merkel cell carcinoma (mMCC).
D'Angelo S.P.
1227P
September 10
13:15-14:15
Hall 8
Avelumab in patients with metastatic adrenocortical carcinoma (mACC): results from the JAVELIN Solid Tumor trial
Le Tourneau C.
913P
September 10
13:15-14:15
Hall 8
Potential impact of avelumab+axitinib (A+Ax) on tumor size (TS) compared with historical data of sunitinib (S) as evaluated by a modeling and simulation (MS) approach
Zheng J.
882P
September 10
13:15-14:15
Hall 8
Avelumab treatment of metastatic urothelial carcinoma (mUC) in the phase 1b JAVELIN Solid Tumor study: updated analysis with ≥6 months of follow-up in all patients
Apolo A.B.
856P
September 10
13:15-14:15
Hall 8
Title
Lead author
Abstract #
Presentation date/time (CEST)
Location
M6620 (VX-970)
Poster session
Initial results of a phase 1 dose expansion cohort of M6620 (VX-970), a first-in-class ATR inhibitor, in combination with
cisplatin (Cis) in patients (pts) with metastatic triple-negative breast cancer (mTNBC) (NCT02157792)
Telli M.L.
242PD
September 10
09:15 - 10:45
Sevilla auditorium
Title
Lead author
Abstract #
Presentation date/time (CEST)
Location
M2698
Poster session
Phase I dose escalation study of M2698, a p70S6K/AKT inhibitor, in patients with advanced cancer
Tsimberidou A.M.
370PD
September 9
16:30-18:00
Alicante auditorium
Pharmacodynamic (PD) biomarkers for the p70S6K/Akt inhibitor, M2698: translation from animal to human and its relevance for dosing rationale
Xiong W.
393P
September 11
13:15-14:15
Hall 8
Title
Lead author
Abstract #
Presentation date/time (CEST)
Location
Erbitux®:
Poster session
Biomarker testing practices in the SECURE (proSpective obsErvational clinical practiCe stUdy in the first-line management of metastatic colorectal cancer [mCRC] with eRbitux in combination with chemothErapy) study
Aravantinos G.
576P
September 9
13:15-14:15
Hall 8
Quality of life (QoL) analyses in patients with RAS wild-type (wt) metastatic colorectal cancer (mCRC) treated with first-line FOLFOX-4 ± cetuximab in the phase 3 TAILOR trial
Liu T.
593P
September 9
13:15-14:15
Hall 8
ENCORE: a phase 4 observational study of cetuximab and platinum-based therapy (PBT) for the first-line treatment of patients with recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN)
Le Tourneau C.
1068P
September 10
13:15-14:15
Hall 8
A survey of patient acceptance of skin toxicities from cetuximab-based therapy
Tischer B.
1579P
September 10
13:15-14:15
Hall 8
Title
Lead author
Abstract #
Presentation date/time (CEST)
Location
Tepotinib
Poster session
Final data from a phase Ib trial of tepotinib in Asian patients with advanced hepatocellular carcinoma (HCC)
Qin S.
701P
September 9
13:15-14:15
Hall 8
Title
Lead author
Abstract #
Presentation date/time (CEST)
Location
Anti-PD-L1/TGF-ß pathways
Poster session
Analysis of programmed death-ligand 1 (PD-L1) expression, transforming growth factor (TGF)-β gene expression signatures (GES) and tumor-infiltrating immune cells (IC) in hepatocellular carcinoma (HCC): rationale for targeting PD-L1- and TGF-β
Zhang Y.
1645P
September 11
13:15-14:15
Hall 8
About avelumab
Avelumab is a human antibody specific for a protein called PD-L1, or programmed death ligand-1. Avelumab is designed to potentially engage both the adaptive and innate immune systems. By binding to PD-L1, avelumab is thought to prevent tumor cells from using PD-L1 for protection against white blood cells, such as T-cells, exposing them to anti-tumor responses. Avelumab has been shown to induce antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro. In November 2014, Merck and Pfizer announced a strategic alliance to co-develop and co-commercialize avelumab.
**Indications in the US
The U.S. Food and Drug Administration (FDA) granted accelerated approval for avelumab (BAVENCIO®) for the treatment of (i) metastatic Merkel cell carcinoma (mMCC) in adults and pediatric patients 12 years and older and (ii) patients with locally advanced or metastatic urothelial carcinoma (UC) who have disease progression during or following platinum-containing chemotherapy, or who have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. These indications are approved under accelerated approval based on tumor response rate and duration of response. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials. Avelumab is not approved for any indication in any market outside the U.S.
Important Safety Information
The warnings and precautions for avelumab (BAVENCIO®) include immune-mediated adverse reactions (such as pneumonitis, hepatitis, colitis, endocrinopathies, nephritis and renal dysfunction and other adverse reactions), infusion-related reactions and embryo-fetal toxicity.
Common adverse reactions (reported in at least 20% of patients) in patients treated with avelumab for mMCC and patients with locally advanced or metastatic UC include fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, peripheral edema, decreased appetite/hypophagia, urinary tract infection and rash.
About Erbitux® (cetuximab)
Erbitux® is a highly active IgG1 monoclonal antibody targeting the epidermal growth factor receptor (EGFR). As a monoclonal antibody, the mode of action of Erbitux is distinct from standard non-selective chemotherapy treatments in that it specifically targets and binds to the EGFR. This binding inhibits the activation of the receptor and the subsequent signal-transduction pathway, which results in reducing both the invasion of normal tissues by tumor cells and the spread of tumors to new sites. It is also believed to inhibit the ability of tumor cells to repair the damage caused by chemotherapy and radiotherapy and to inhibit the formation of new blood vessels inside tumors, which appears to lead to an overall suppression of tumor growth. Erbitux also targets cytotoxic immune effector cells towards EGFR expressing tumor cells (antibody dependent cell-mediated cytotoxicity, ADCC).
The most commonly reported side effect with Erbitux is an acne-like skin rash. In approximately 5% of patients, hypersensitivity reactions may occur during treatment with Erbitux; about half of these reactions are severe.
Erbitux has already obtained market authorization in over 90 countries world-wide for the treatment of RAS wild-type metastatic colorectal cancer and for the treatment of squamous cell carcinoma of the head and neck (SCCHN). Merck licensed the right to market Erbitux, a registered trademark of ImClone LLC, outside the U.S. and Canada from ImClone LLC, a wholly-owned subsidiary of Eli Lilly and Company, in 1998.
About M6620
Also known as VX-970, M6620 is an investigational small-molecule inhibitor of ataxia telangiectasia and Rad3-related protein (ATR). ATR is a key sensor for DNA damage, activating the DNA damage checkpoint and leading to cell cycle arrest. It is thought that inhibition of ATR can enhance the efficacy of DNA-damaging agents, and could potentially also be efficacious as monotherapy against tumors with high levels of replication stress induced by overexpression of oncogenes. M6620 complements our strong DDR portfolio and is currently being investigated in Phase I and II trials.
About M2698
A potential first-in-class, investigational small-molecule that is designed to inhibit both p70S6K and Akt. Both targets are part of the phosphoinositide 3-kinase (PI3K) pathway, which is often dysregulated in solid tumors.
About tepotinib
Tepotinib is an investigational small-molecule inhibitor of the c-Met receptor tyrosine kinase. Alterations of the c-Met signaling pathway are found in various cancer types and correlate with aggressive tumor behavior and poor clinical prognosis. Tepotinib is being investigated in two Phase II studies in non-small cell lung cancer and hepatocellular carcinoma.
All Merck Press Releases are distributed by e-mail at the same time they become available on the Merck Website. Please go to http://ift.tt/P4xUC9 to register online, change your selection or discontinue this service.
About Merck
Merck is a leading science and technology company in healthcare, life science and performance materials. Around 50,000 employees work to further develop technologies that improve and enhance life - from biopharmaceutical therapies to treat cancer or multiple sclerosis, cutting-edge systems for scientific research and production, to liquid crystals for smartphones and LCD televisions. In 2016, Merck generated sales of € 15.0 billion in 66 countries.
Founded in 1668, Merck is the world's oldest pharmaceutical and chemical company. The founding family remains the majority owner of the publicly listed corporate group. Merck holds the global rights to the Merck name and brand. The only exceptions are the United States and Canada, where the company operates as EMD Serono, MilliporeSigma and EMD Performance Materials.
Your Contact: Martina Brunner, +49-6151-724-3959
Read this news on PR Newswire Asia website: Merck: Driving Innovation in Cancer Care at ESMO 2017 With New Data in Hard-to-Treat Cancers
0 notes
Text
Чем отравили брата Ким Чен ЫнаКим Чен Нама умер 13 февраля от приступа по дороге в больницу, успев пожаловаться, что в аэропорту Куала-Лумпур незнакомая женщина распылила ему в лицо какое-то вещество.В Малайзии специалисты уже определили это химическое вещество в качестве отравляющего вещества нервно-паралитического действия VX, или ��и-газ. Он представляет собой фосфорорганическое соединение, О-этил-S-β-диизопропиламиноэтилметилфосфонат, летальное химическое оружие, которое, несмотря на название, остается жидкими при комнатной температуре. Ви-газ был разработан в Великобритании в середине 1950-х годов. Как и все токсины нервно-паралитического действия, он блокирует биологическое действие фермента ацетилхолинэстеразы, который отвечает за метаболизм нейротрансмиттера ацетилхолина. Последний, в свою очередь, отвечает за
https://namzdorovje.ru/novosti-mediciny/1558-chem-otravili-brata-kim-chen-yna.html
0 notes
Text
VOCALOID's VX-β Plug-in Officially Releases Alongside VOCALOID6 Kanade! + Otomachi Una's SynthesizerV Officially Released!
VX-B and Kanade have officially released today and many users are already downloading them and trying them out! VX-β Overview Trailer The commercial version of the VX-B plugin has been highly anticipated since it was announced back this summer. Releasing as a 3.0 version, users can now use it for free if they already own VOCALOID6 and any of its voicebanks! VOCALOID6 Kanade Official Demo Song…
#Kanade#Kanade Kanon#Otomachi Una#SynthesizerV#SynthV#VOCALOID#VOCALOID beta#VOCALOID6#vx-b#VX-β#VXB
3 notes
·
View notes
Text
GekiyakuV & KazehikiV Release for VOCALOID6! Others from VX-B Announced for VOCALOID6!
In a slew of excitement and anticipation, Yamaha has made many announcements today relating to VX-B, VOCALOID6, and Kazehiki and Gekiyaku! Announcement Video Regarding the VX-B plugin and Voicebanks for VX-B In the announcement, it was revealed that alongside GekiyakuV’s and KazehikiV’s VOCALOID6 Release, the VX-B plug-in will eventually make it’s way to commercial release and will be included…
#Ci-chan#Gekiyaku#GekiyakuV#Kanade#Kanade Kanon#Kasukabe Tsumugi#Kazehiki#KazehikiV#Uge#Uge And#Vocalo no Ci-chan#VOCALOID#VOCALOID beta#VOCALOID β-STUDIO#VOCALOID6#vx-b#VXB
29 notes
·
View notes
Text
New Vocal Synth Label GEMVOX Bursts onto the Scene with SynthesizerV Topaz!
In some very surprising news today reported by SynthV News on Twitter, a new Vocal Synth Label known as GEMVOX has become the talk of the town with its upcoming trilingual vocal, SynthesizerV Topaz. 💎 GEMVOX Debuts with Topaz for Synthesizer VUnveil the dark and mysterious AI singer, Topaz, and the creative vision behind GEMVOX.🔗 Find out more in our article featuring an exclusive interview with…
#AI Resonate Society#ARS#beta#Beta-Studio#CeVIO#GEMVOX#GONZO#L#Studio ENTRE#SynthesizerV#SynthV#Topaz#VOCALOID#VOCALOID beta#VOCALOID β-STUDIO#VoiSona#vx-b#VXB
7 notes
·
View notes
Text
Yamaha Teases Gekiyaku and Kazehiki's Return to VOCALOID
After VOCALOID β-STUDIO’s program ended back in March, many users have been voicing how they’ve wanted to use many of the vocals and the vst “vx-b” itself. Early this morning Yamaha posted a teaser video along with a tweet saying “Until we meet again”. 「また会う日まで」 pic.twitter.com/717RRP3HaZ— ボーカロイド – VOCALOID6 (@vocaloid_yamaha) July 9, 2024 The teaser video flashes what looks like Gekiyaku and…
#beta#Beta-Studio#Gekiyaku#Kazehiki#UTAU#VOCALOID#VOCALOID beta#VOCALOID β-STUDIO#VOCALOID6#vx-b#VXB
6 notes
·
View notes
Text
Commercial Version of VX-β for Vocaloid6 Will Release on November 27th!
youtube
4 notes
·
View notes
Text
New VOCALOID β-STUDIO Vocals Revealed! + ST Media expresses interest in new Korean bank development and plans for final SeeU sales run + VOCALOID4 Stardust 100% Out of Print
After a short wait, the new vocals for the VX-β plug are now released and revealed! Kanade Kanonβ demo “��むれわたがし” The vocals were revealed today to be Kanade Kanonβ, Uge Andβ, Ci-chanβ, and Kasubake Tsumugiβ! Demo’s are still releasing for these 4 and many users are excited to update their version of the plug-in. For those interested in using the VX-β plug-in; You can find information on the…

View On WordPress
#Beijing Photek#Ci-chan#Kanade Kanon#Kasukabe Tsumugi#Quadimension#SBS Artech#SeeU#ST Media#Stardust#SynthesizerV#SynthV#Uge And#UNI#VOCALOID#VOCALOID beta#VOCALOID β-STUDIO#VOCALOID3#VOCALOID4#Xingchen
1 note
·
View note