#US similar biotherapeutics products market
Explore tagged Tumblr posts
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Global Oncology Biosimilar Market
Global Oncology Biosimilar Treatment Market – Past, present, and way forward
Biosimilar or biogenerics are biotherapeutic products with large and complex structures that are similar to their innovator product in all aspects including their safety, efficacy, and quality.

There is a palpable sense of urgency among researchers and pharmaceutical industries to develop biosimilar for treatment, with its popularity expected to rise in the forthcoming years especially in developing low-cost biosimilar that is easily accessible and affordable for patients.
Market trends show that North America continues to dominate the global oncology biosimilar market followed by Europe, Asia Pacific, Latin America, Middle East, and Africa. The market is purported to reach approximately USD 45 Billion in the year 2026 from the current market of approximately USD 6 Billion.
Understanding the local and global market demand is necessary for companies, research institutes, and investors to study the changing dynamics in the biosimilar market. Segmentation of biosimilar by product, type of cancer, as well as targeted end-user such as hospital/online/retail pharmacies is particularly useful in empowering industries to make informed business decisions. Product segmentation also helps gauge the project’s feasibility so that the pharmaceutical industry can focus on developing safe and commercially viable products. Comprehensive analyses, robust legal and regulatory frameworks, continuing medical education for health care professionals and increased awareness among the public will undoubtedly increase the uptake of biosimilar and help the biosimilar market move ahead competitively.
#Oncology CRO in India#Oncology CRO#Biosimilar Studies in India#Oncology Biosimilar#Biopharmaceutics Services India
3 notes
·
View notes
Text
Biosimilars Market Demand, Size, Share, Scope & Forecast to 2030
The Global Biosimilar Market is expected to be valued at USD 159.02 Billion by 2030 from USD 15.6 Billion in 2021, at a CAGR of 23.5% during the forecast period of 2021 to 2030.
Medicines known as biologics or biological products are created using living organisms through extremely intricate production procedures. They must be handled and administered under strict supervision. The term "biologics" refers to a broad range of goods, including vaccines, therapeutic proteins, monoclonal antibodies, and cell and gene therapies. Biologics are used to prevent, treat, or cure a number of illnesses, such as cancer, diabetes, cystic fibrosis, chronic kidney disease, and autoimmune disorders. Biologics are at the cutting edge of scientific and biological research and may be able to treat a variety of illnesses for which there are no other effective treatments.

A biotherapeutic product that is extremely similar to a reference biologic medication is referred to as a biosimilar, also known as a follow-on biologic. It has a complicated molecular structure and is made from living things or cells. Manufacturers rely on regulatory authority authorisation to launch the production of biosimilars when the patent on a biologic medication expires. A biological medication must be demonstrated to be identical in terms of quality, safety, and efficacy in order to be labelled as a biosimilar. Biosimilars are more expensive than generic medications because their production needs greater investment in R&D and is more complicated.
Get Free Sample:- https://wemarketresearch.com/sample-request/biosimilars-market/5/
Global Biosimilar Market: Segment Analysis
Biosimilar Market segment based on Molecule
• Filgrastim
• Rituximab
Filgrastim injection is for the treatment of Neutropenia, that is low white blood cells in the body, which is caused by cancer medicines. It is a synthetic form of substance that is produced naturally in your body known as colony stimulating factor. Filgrastim helps the bone marrow in the body to make new white blood cells itself.
Rituximab is used to treat certain types of Cancer such as Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Lukemia. It slows or stops the growth of cancer cells. Some products of Rituximab are also used to treat the pain of Rheumatoid Arthritis and can also decrease swelling and pain of the joints.
Segmentation based on Indication Insights
Oncology
Female Infertility
Oncology is the largest segment in this market due to the availability of Biosimilar at a lower price than Innovation Biologics and a large number of cancer patients. The availability of Biosimilar in Oncology has reduced the prices and made cancer treatment more accessible and affordable. Due to the high prevalence of cancer, healthcare systems across the globe are more concentrated on reducing the burden of cancer by adopting cost effective treatment options.
Biologics are too expensive and cannot be afforded by everyone. Therefore, cost-effective and new Biosimilars are being manufactured in the market. In January 2016, Teva Pharmaceutical Industries Ltd. introduced Ovaleap, a biosimilar to GONAL-f (follitropin alfa) from Merck KGaA. The low cost of Biosimilars promotes patient trust and boosts the market growth.
Segmentation based on Manufacturing
Contract Manufacturing
In- house Manufacturing
Based on the type of manufacturing, the market is classified into in-house manufacturing and contract manufacturing. Amongst these, in-house manufacturing holds the majority of the market share.
Top Key Players:-
LG Life Sciences
Fresenius SE & Co. KGaA
Pfizer, Inc.
Hospira
STADA Arzneimittel AG
Dr. Reddy’s Laboratories Ltd.
Eli Lilly and Company
Genentech (Roche Group).
Synthon Pharmaceuticals, Inc.
Celltrion
Boehringer Ingelheim
Amgen, Inc.
Gedeon Richter PLC
Merck Serono (Merck Group)
Teva Pharmaceutical Industries Ltd.
Biocon Limited
Samsung Biologics
Biogen idec, Inc.
Coherus BioSciences
Viatris, Inc
Fujifilm Kyowa Kirin Biologics Co., Ltd.
Interested in purchasing this Report? Click here:- https://wemarketresearch.com/purchase/biosimilars-market/5/?license=single
Frequently Asked Questions (FAQ):
What is the impact of COVID-19 on the biosimilars market?
Who are the key players in the biosimilars market?
Which product segment dominates in the biosimilars market?
Which indication segment of the global biosimilars market is expected to witness the highest growth?
What is the market size for biosimilars market?
About We Market Research
WE MARKET RESEARCH is an established market analytics and research firm with a domain experience sprawling across different industries. We have been working on multi-county market studies right from our inception. Over the time, from our existence, we have gained laurels for our deep rooted market studies and insightful analysis of different markets.
Our strategic market analysis and capability to comprehend deep cultural, conceptual and social aspects of various tangled markets has helped us make a mark for ourselves in the industry. WE MARKET RESEARCH is a frontrunner in helping numerous companies; both regional and international to successfully achieve their business goals based on our in-depth market analysis. Moreover, we are also capable of devising market strategies that ensure guaranteed customer bases for our clients.
Contact Us:
We Market Research
Phone: +1(929)-450-2887
Email: [email protected]
Web: https://wemarketresearch.com/
0 notes
Text
Global Oncology Biosimilar Market
Global Oncology Biosimilar Treatment Market – Past, present, and way forward
Biosimilar or biogenerics are biotherapeutic products with large and complex structures that are similar to their innovator product in all aspects including their safety, efficacy, and quality.
There is a palpable sense of urgency among researchers and pharmaceutical industries to develop biosimilar for treatment, with its popularity expected to rise in the forthcoming years especially in developing low-cost biosimilar that is easily accessible and affordable for patients.
Market trends show that North America continues to dominate the global oncology biosimilar market followed by Europe, Asia Pacific, Latin America, Middle East, and Africa. The market is purported to reach approximately USD 45 Billion in the year 2026 from the current market of approximately USD 6 Billion.
Understanding the local and global market demand is necessary for companies, research institutes, and investors to study the changing dynamics in the biosimilar market. Segmentation of biosimilar by product, type of cancer, as well as targeted end-user such as hospital/online/retail pharmacies is particularly useful in empowering industries to make informed business decisions. Product segmentation also helps gauge the project’s feasibility so that the pharmaceutical industry can focus on developing safe and commercially viable products. Comprehensive analyses, robust legal and regulatory frameworks, continuing medical education for health care professionals and increased awareness among the public will undoubtedly increase the uptake of biosimilar and help the biosimilar market move ahead competitively
0 notes
Text
Chromatography Resins Market (2020-2027) - Growth, Trends, COVID-19 Impact, and Forecasts
The global chromatography resins market is projected to be worth USD 3,269.9 Million by 2027, according to a current analysis by Emergen Research. The chromatography resin market observes high demand attributed to its rising application in the drug development process as biotherapeutic development advances have produced a wide range of complex molecules, posing complex purification challenges. Affinity or liquid chromatographic technology has garnered immense significance as a preferred method of separation in pharmaceutical, biotechnology, biochemistry, and environmental science. The technology is primarily a more precise and efficient method for protein purification.
The evaluation of the companies involves assessment of production and consumption ratio, supply and demand, import/export, market share and size, revenue contribution, gross revenue, profit margins, and key business expansion strategies. Other vital information such as mergers and acquisitions, collaborations, joint ventures, government and corporate deals, agreements, and product launches, and brand promotional activities are also assessed in the report. Moreover, it also offers strategic recommendations to the new entrants on navigating the entry-level barriers along with recommendations for established players to help them fortify their positions in the industry.
Request for a PDF sample of this report: https://www.emergenresearch.com/request-sample/194
Key Highlights From The Report
· In October 2019, Danaher entered into an agreement with Sartorius Stedim Biotech to divest its three life sciences tools businesses for worth about USD 750.0 million. As per the agreement, Sartorius would acquire chromatography hardware & resins, label-free biomolecular characterization, and microcarriers & particle validation standards businesses of Danaher.
· The market in the Asia Pacific region is projected to grow at the fastest rate of 8.6% in the forecast period attributed to a growing emphasis on developing generic drugs and the growth of the food & beverage industry.
· Key participants include Thermo Fisher Scientific Inc., WR Grace & Co., Danaher Corporation, Merck KGaA, GE Healthcare, Bio-Rad Laboratories Inc., Purolite Corporation, Tosoh Corporation, Mitsubishi Chemical Corporation, and Kaneka Corporation, among others.
Emergen Research has segmented the global chromatography resins market on the basis of type, technology, application, and region:
· Type Outlook (Revenue, USD Billion; 2017-2027)
o Natural
o Synthetic
o Inorganic Media
· Technology Outlook (Revenue, USD Billion; 2017-2027)
o Ion Exchange
o Hydrophobic Interaction
o Affinity
o Size Exclusion
o Multimodal
o Others
· Application Outlook (Revenue, USD Billion; 2017-2027)
o Pharmaceutical
1. Biotechnology
2. Drug Discovery
3. Drug Production
o Food & Beverage
o Water & Environmental Agencies
o Others
Regional Bifurcation includes:
• North America(U.S., Canada)
• Europe(U.K., Italy, Germany, France, Rest of EU)
• AsiaPacific(India, Japan, China, South Korea, Australia, Rest of APAC)
• Latin America(Chile, Brazil, Argentina, Rest of Latin America)
• Middle East & Africa(Saudi Arabia, U.A.E., South Africa, Rest of MEA)
Request for Report Customization: https://www.emergenresearch.com/request-for-customization/194
Key inferences influencing the revenue patterns of the market:
· The study offers an in-depth analysis of the product outlook, which depicts the latest production growth trends and profit valuation. It further fragments the global Chromatography Resins market into a wide product spectrum.
· The study covers essential data pertaining to the application landscape of these products, the demand for and market share held by each application type, along with their growth rate analysis over the estimated period.
· A meticulous description of the distribution channels, including distributors, producers, and buyers, is one of the key highlights of the global Chromatography Resins market report.
Table of Content:
Chapter 1. Methodology & Sources 1.1. Market Definition 1.2. Research Scope 1.3. Methodology 1.4. Research Sources 1.4.1. Primary 1.4.2. Secondary 1.4.3. Paid Sources 1.5. Market Estimation Technique Chapter 2. Executive Summary 2.1. Summary Snapshot, 2019-2027 Chapter 3. Key Insights Chapter 4. Chromatography Resins Market Segmentation & Impact Analysis 4.1. Chromatography Resins Market Material Segmentation Analysis 4.2. Industrial Outlook 4.2.1. Market indicators analysis 4.2.2. Market drivers analysis 4.2.2.1. Surging demand in drug development process 4.2.2.2. Growing demand for green chromatography 4.2.2.3. Growing usage of separation methods in the food & beverage industry 4.2.2.4. Increased investment in R&D 4.2.3. Market restraints analysis 4.2.3.1. Lack of skilled personnel 4.3. Technological Insights 4.4. Regulatory Framework 4.5. Porter’s Five Forces Analysis 4.6. Competitive Metric Space Analysis 4.7. Price trend Analysis 4.8. Covid-19 Impact Analysis
READ MORE…!
To know more about the report, visit @ https://www.emergenresearch.com/industry-report/chromatography-resins-market
Thank you for reading our report. For further inquiry about customization, kindly get in touch with us, and our team will make sure the report is best suited for your needs.
Read similar reports by Emergen Research:
Marble Market@ https://www.emergenresearch.com/industry-report/marble-market
Industrial Lubricants Market@ https://www.emergenresearch.com/industry-report/industrial-lubricants-market
Greenhouse Film Market@ https://www.emergenresearch.com/industry-report/greenhouse-film-market
Plastic Waste Management Market@ https://www.emergenresearch.com/industry-report/plastic-waste-management-market
Activated Carbon Market@ https://www.emergenresearch.com/industry-report/activated-carbon-market
About Emergen Research
Emergen Research is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyze consumer behavior shifts across demographics, across industries, and help clients make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Types, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Emergen Research has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
Contact Us:
Eric Lee
Corporate Sales Specialist
Emergen Research | Web: www.emergenresearch.com
Direct Line: +1 (604) 757-9756
E-mail: [email protected]
Facebook | LinkdIn | Twitter | Blogs
0 notes
Text
ANTIBODIES MARKET ANALYSIS - (2019-2027)
Antibodies are used in therapeutic application for the treatment of diseases such as cancer, autoimmune diseases, hematological diseases, infectious diseases, and others. Increasing prevalence of cancer is expected to create lucrative opportunities for growth of manufacturers in the market. For instance, according to the National Cancer Institute, 2016, the number of new cancer cases per year is expected to increase to 23.6 million by 2030, worldwide. Antibodies products come under biologic therapeutic products. Biologic manufacturers require approval (similar to pharma drug approval) from the U.S. Food & Drug Administration prior to bringing the product in the market. However, unlike drugs, biologics require Biologics License Applications (BLA) to be filed with the Center for Biologics Evaluation and Research (CBER). Moreover, various government authorities are focused on controlling/lowering drug prices by adding reforms. For instance, the U.S. government is seeking to lower patients out-of-pocket spending in Medicare by curbing deals that drug manufacturers make with its distribution channel partners such as pharmacy benefit managers, February 2019 (these deals are responsible for increasing list price of drugs).
Request Sample Free Copy of Report here: https://www.coherentmarketinsights.com/insight/request-sample/2629
Download PDF Brochure:https://www.coherentmarketinsights.com/insight/request-pdf/2629
Restraints of the Global Antibodies Market
High cost of monoclonal antibodies therapies makes it unaffordable for the population in the low- and middle-income group to opt for these therapies, which in turn restrains growth of the market. For instance, Keytruda (Pembrolizumab), a monoclonal antibody for the treatment of various types of cancer, costs around US$ 2,250 for a vial of 50 mg.
Key features of the study:
This report provides in-depth analysis of the global Antibodies Market and provides market size (US$ Million) and compound annual growth rate (CAGR %) for the forecast period (2019–2027), considering 2018, as the base year
It elucidates potential revenue opportunity across different segments and explains attractive investment proposition matrix for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, regional outlook, and competitive strategy adopted by leading players
It profiles leading players in the global antibodies market based on the following parameters – company overview, financial performance, product portfolio, geographical presence, distribution strategies, key developments, strategies, and future plans
Key companies covered as a part of this study include Novartis AG, F. Hoffmann-La Roche Ltd., Johnson & Johnson Services, Inc., Takeda Pharmaceutical Company Limited, Amgen Inc., Biogen Inc., Bristol-Myers Squibb Company, AbbVie Inc., Sanofi, Eli Lilly and Co., Iovance Biotherapeutics, Inc., Ultragenyx Pharmaceutical Inc. and Kyowa Kirin Co., Ltd.
Insights from this report would allow marketers and the management authorities of companies to make informed decision regarding future product launches, technology up-gradation, market expansion, and marketing tactics
The global Antibodies Market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts.
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the market.
Detailed Segmentation
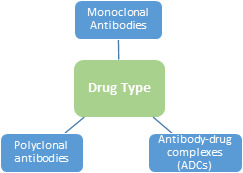
Global Antibodies Market, By Product Type:
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Global Antibodies Market, By Disease Indication:
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
Global Antibodies Market, By End User:
Hospitals
Long-term Care Facilities
Research institutes
Global Antibodies Market, By Geography:
North America
By Product Type
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Disease Indication
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
By End User
Hospitals
Long-term Care Facilities
Research institutes
By Country:
U.S.
Canada
Europe
By Product Type
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Disease Indication
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
By End User
Hospitals
Long-term Care Facilities
Research institutes
By Country:
U.K.
Germany
Italy
France
Spain
Russia
Rest of Europe
Asia Pacific
By Product Type
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Disease Indication
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
By End User
Hospitals
Long-term Care Facilities
Research institutes
By Country:
China
India
Japan
ASEAN
Australia
South Korea
Rest of Asia Pacific
Latin America
By Product Type
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Disease Indication
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
By End User
Hospitals
Long-term Care Facilities
Research institutes
By Country:
Brazil
Mexico
Argentina
Rest of Latin America
Middle East:
By Product Type
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Disease Indication
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
By End User
Hospitals
Long-term Care Facilities
Research institutes
By Country:
GCC
Israel
Rest of Middle East
Africa
By Product Type
Monoclonal antibodies
Polyclonal antibodies
Antibody-drug complexes (ADCs)
Disease Indication
CNS Disorders
Cardiovascular Diseases
Cancer
Autoimmune Disorders
By End User
Hospitals
Long-term Care Facilities
Research institutes
By Country/Region:
Central Africa
South Africa
North Africa
Company Profiles
Novartis International AG*
Company Overview
Product Portfolio
Financial Performance
Key Strategies
Recent Developments
F. Hoffmann-La Roche Ltd.
Johnson & Johnson Services, Inc.
Takeda Pharmaceutical Company Limited
Amgen Inc.
Biogen Inc.
Bristol-Myers Squibb Company
AbbVie Inc.
Sanofi S.A.
Eli Lilly and Co.
Iovance Biotherapeutics, Inc.
Ultragenyx Pharmaceutical Inc.
Kyowa Kirin Co., Ltd.
“*” marked represents similar segmentation in other categories in the respective section.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Explore CMI Services here
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/antibodies-market-2629
0 notes
Text
0 notes
Text
ANTIBODIES MARKET ANALYSIS by Coherent Market Insights
Antibodies Market To Surpass US$ 384.0 Billion By 2027 - Coherent Market Insights
PDF COPY:
https://www.coherentmarketinsights.com/insight/request-pdf/2629
Antibody, also called as immunoglobulin, is a protein produced by plasma cells in response to specific antigens. These antibodies can be used as therapeutic as well as diagnostic purposes for several indications, including cancers, autoimmune disorders, inflammatory & infectious diseases and others.
The global antibodies market is estimated to account for US$ 147,385.1 Mn in terms of value and is expected to reach US$ 384,011.6 Mn by the end of 2027.
Global Antibodies Market: Drivers
Therapeutic monoclonal antibodies find wide application in oncology, neurobiology, autoimmunology, and cardiology. Increasing prevalence of chronic disease is expected to propel growth of the global antibodies market. For instance, according to the World Health Organization, around 18.1 million new cases and 9.6 million deaths were registered due to cancer worldwide in 2018.
Moreover, availability of inexpensive biosimilar antibody therapeutics is also expected to aid in growth of the market. For instance, in December 2019, the World Health Organization (WHO) prequalified its first biosimilar medicine – trastuzumab –– a monoclonal antibody for the treatment of early stage breast cancer. On an average trastuzumab costs US$ 20,000. However, the biosimilar version of trastuzumab is generally 65% cheaper than the originator.
North America region held dominant position in the global antibodies market in 2018, accounting for 44.5% share in terms of value, followed by Europe.

Global Antibodies Market: Restraints
High cost of therapeutic antibodies is expected to hamper growth of the global antibodies market. For instance, in the U.S., the monthly treatment cost for rheumatoid arthritis with Humira is over US$ 5,500.
Use of antibodies may lead to some side effects such as serum sickness, acute anaphylaxis, and specific target-related adverse effects, which is also expected to hinder the market growth.
Global Antibodies Market: Opportunities

The patent of several blockbuster antibody products is set to expire in the near future. Therefore, key players in the market can focus on launching biosimilar antibodies to enhance their market share.
Moreover, evolution of clear regulatory approval process for biosimilars in emerging markets represents additional opportunity for antibody-based products. For instance, in 2012, Central Drugs Standard Control Organization, Gov. of India released regulatory requirements for marketing approval of similar biologics in India, which was revised in 2016 for more clarity. According to the revision, the reference biologic may be approved or marketed either in India or any other International Council for Harmonization countries.
Drug type segment in the global antibodies market was valued at US$ 131,472.4 in 2018 and is expected to reach US$ 384,011.6 by 2027 at a CAGR of 12.5% during the forecast period.
Market Trends/Key Takeaways
The adoption of antibody-based drugs for the treatment of cancer has significantly increased. Monoclonal antibody therapeutics offer high specificity, activity, favorable pharmacokinetics, and standardized manufacturing processes. Moreover, antibodies can increase immune response against cancer cells through complement-dependent cytotoxicity or antibody dependent cellular cytotoxicity.
The use of antibodies as a diagnostic reagents for the identification of disease markers or proteins based on immunoassays has decreased. This is attributed to increasing accuracy of molecular diagnostic tools.
Regulations:
North America
U.S.
Therapeutic antibodies are the biological products which are regulated by both the FDA’s Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER)
Therapeutic antibody products (including monoclonal antibody, antibody drug conjugates, Fc fragment proteins) are subject to submission of biologics license application (BLA)
Manufacturers of therapeutic antibody products thus needed to submit BLA to CBER or CDER under 21 CFR 601.2, which contains safety, purity and potency data from clinical and non-clinical laboratory studies and other manufacturing information
Whereas, diagnostic antibodies are classified under in vitro diagnostic device (IVD) as Analyte Specific Reagents (ASR) and thus subject to regulations under 21CFR864.4020
Diagnostic antibodies which are used in immunological testing of infectious diseases are classified as Class II IVDs whereas antibodies used for diagnosis of highly contagious diseases such as HIV, TB are classified as Class III IVDs, both of which require to obtain premarket approval under section 510 (k) of S. federal food drug and cosmetics (FD&C) act
In U.S., biosimilar biologics product approval is submitted to FDA under section 351(k) of the Public Health Service Act
Global Antibodies Market: Competitive Landscape
Major players operating in the global antibodies market include, Novartis AG, F. Hoffmann-La Roche Ltd., Johnson & Johnson Services, Inc., Takeda Pharmaceutical Company Limited, Amgen Inc., Biogen Inc., Bristol-Myers Squibb Company, AbbVie Inc., Sanofi, Eli Lilly and Co., Iovance Biotherapeutics, Inc., Ultragenyx Pharmaceutical Inc. and Kyowa Kirin Co., Ltd.
Global Antibodies Market: Key Developments
Key players in the market are focused on adopting partnership and collaboration strategies to expand their product portfolio. For instance, in January 2020, MorphoSys AG collaborated with Incyte Corporation for development and commercialization of MorphoSys’ tafasitamab, a humanized Fc-engineered monoclonal antibody against CD19.
Similarly, in January 2020, Ligand Pharmaceuticals Inc. entered into a license agreement with Pandion Therapeutics, under which the later will use Ligand’s OmniAb antibody discovery platform in exchange of an up-front platform access fee, development and regulatory milestone payments, and potential royalties on sales of marketed products.
Buy Now:
https://www.coherentmarketinsights.com/insight/buy-now/2629
“*” marked represents similar segmentation in other categories in the respective section.
About Us: Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. We are headquartered in India, having office at global financial capital in the U.S. Our client base includes players from across all business verticals in over 150 countries worldwide. We do offer wide range of services such as Industry analysis, Consulting services, Market Intelligence, Customized research services and much more. We have expertise in many fields such as healthcare, chemicals and materials, Automation, semiconductors, electronics, energy, food and beverage, packaging and many more. Visit our website to know more.
Buy Report Here:
https://www.coherentmarketinsights.com/insight/buy-now/2631
Contact Us: Mr. Shah Coherent Market Insights 1001 4th Ave. #3200 Seattle, WA 98154 Tel: +1-206-701-6702 Email: [email protected]
0 notes
Text
Report points to bright future for biologics and biosimilars
New Post has been published on https://biotechtimes.org/2019/11/22/report-points-to-bright-future-for-biologics-and-biosimilars/
Report points to bright future for biologics and biosimilars

By Sunderarajan Padmanabhan
New Delhi, November 22: Biologics and biosimilars could be the next rising stars in biotechnology with a new review by the Department of Biotechnology finding that the market size of the segment could grow at a compounded annual rate (CAGR) of 22 percent in the coming years to reach US dollars 12 billion by 2025.
The pharmaceutical industry is currently facing many changes both within India and globally. Small molecule therapeutics had till recently dominated research and accounted for a major share of the pharma market as they had several inherent advantages such as a tendency to be chemically and thermally stable, ability to access targets in intracellular regions and a simpler drug discovery and development process, besides being suitable for oral delivery.
However, biologics and biosimilars, which are larger molecule therapeutics have recently taken a lead over them as they were better in terms of targeted delivery and had substantially lower side effects in areas such as cancer treatments. They are increasingly becoming the treatment of choice.
Biologics are basically products that are produced from living organisms or contain components of living organisms. They include a wide variety of products derived from humans, animals or microorganisms using the tools of biotechnology. Follow-on biologics and biosimilars as a class of drugs have emerged as an effective alternative to reduce the cost of biologic therapies.
A biosimilar is a biotherapeutic product that is almost similar to an already licensed biotherapeutic in terms of quality, safety and efficacy, except for some minor differences modifications. Follow-on biologics, in turn, are nothing but biotherapeutics, whose patent has expired.
A report on the new review released as part of the ongoing international meet on biotechnology – Global Bio India 2019, noted that the Indian pharmaceutical industry in the country was well geared to take advantage of the emerging situation.
It has pointed out that while globally, 1015 biosimilars/ follow-up biologics are under development for various therapy areas such as rheumatoid arthritis, neutropenia and cancer by 475 companies, in India 201 active biosimilars were in the pipeline of 52 domestic pharmaceutical companies.
It also noted that product patents of more than 10 blockbuster biologics with total revenue of US dollar 60 billion are set to expire within two to three years thus offering a further potential boost to the biosimilars market.
The report was prepared in collaboration with the Department of Biotechnology’s Biotechnology Industry Research Assistance Council, Association of Biotechnology Led Enterprises, a not-for-profit pan-India forum that represents the Indian Biotechnology Sector, and Cortellis, a global firm that specializes in providing insights into various aspects of life science research. Union Minister for Science and Technology, Earth Sciences, and Health and Family Welfare, Dr. Harsh Vardhan, released it. (India Science Wire).
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
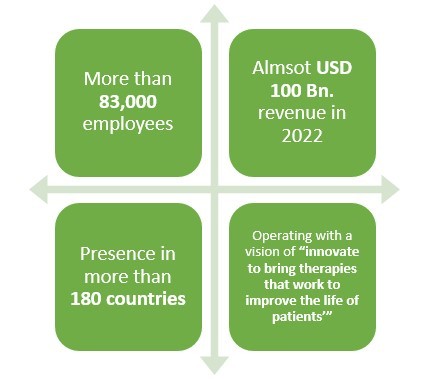
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
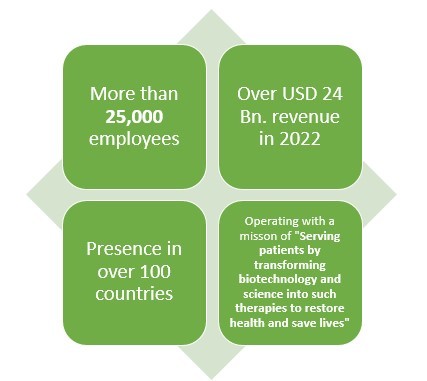
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
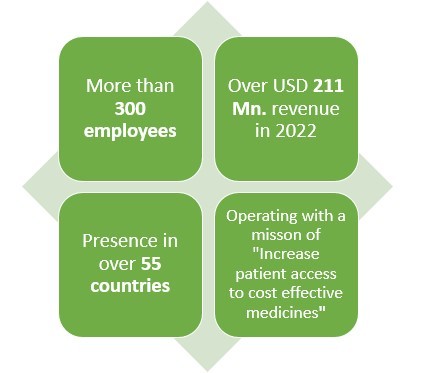
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
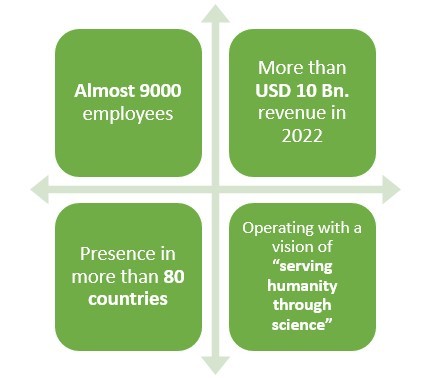
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Remicade Biosimilars Market Top-Vendor And Industry Analysis By End-User Segments Till 2026

Biosimilar refers to biotherapeutic product that is similar in terms of quality, efficacy, and safety to an existing licensed reference therapeutic drug. Biological products are the fastest growing class of therapeutic products, as they offer additional treatment options and help in lowering healthcare costs. Biosimilars are almost identical copies of the originally approved drugs and can be manufactured only when the patent for the original innovator drug expires. These products are highly sensitive to smallest changes in manufacturing procedure, as these drugs possess high molecular complexity. Remicade (Infliximab) refers to a chimeric monoclonal antibody biologic licensed by U.S. FDA in 1998, as an innovative product indicated for the treatment of Crohn’s disease in adults and children.
The monoclonal antibody drug was first developed in partnership by Janssen Biotech, Inc. and Merck & Co. The Remicade was later approved for its use in the treatment of ulcerative colitis, plague psoriasis, rheumatic arthritis, and spinal psoriatic arthritis in combination with methotrexate. Later, various pharmaceutical manufacturers developed biosimilars to infliximab, which lowered market share of Remicade due to cost-effective prices.
Download PDF Brochure Of This Research Report @ https://www.coherentmarketinsights.com/insight/request-pdf/1769
Regional Insights
On the basis of region, the global Remicade biosimilar market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Europe holds a dominant position in the global Remicade biosimilar market and is expected to retain its dominance over the forecast period, owing to the presence of top market players in the region, rapid entry of biosimilars in the European market as well as high adoption rate due low price of the biosimilars. For instance, in 2018, Sandoz, a Novartis division received European Commission (EC) approval for Zessly (infliximab) a remicade biosimilar for use in Europe. The successive research and speedy approvals by the U.S. regulatory authorities for market entry of biosimilars in North America is expected to drive growth of the market over the forecast period. For instance, in December 2017, Pfizer, Inc. received the U.S. FDA approval for second biosimilar, Ifixi to Janssen’s blockbuster drug Remicade to treat rheumatoid arthritis.
Furthermore, Asia Pacific is expected to witness significant growth in the market over the forecast period, owing to developments in healthcare infrastructure and U.S. FDA approval of Remicade (infliximab) biosimilars by regional players to market the products internationally. For instance, in April 2016, Celltrion- a South Korea-based manufacturing company received the U.S. FDA approval for intravenously administered version of the rheumatoid arthritis drug sold under the name Infllectra. Moreover, major investments by regional players for research and development of biosimilar production is expected to drive growth of the market in Asia. For instance, in October 2016, Cipla, Inc. invested US$ 8 million to set up a manufacturing plant for biosimilars in South Africa.
Competitive Landscape
Key players operating in the global remicade biosimilar market include Janssen Biotech, Inc., Merck and Company, Inc., Alvogen, Pfizer, Inc., Celltrion, Nippon Kayaku, Napp Pharmaceuticals, and others.
0 notes
Text
Remicade Biosimilars Market is forecasted to witness a thriving growth by 2026

Biosimilar refers to biotherapeutic product that is similar in terms of quality, efficacy, and safety to an existing licensed reference therapeutic drug. Biological products are the fastest growing class of therapeutic products, as they offer additional treatment options and help in lowering healthcare costs. Biosimilars are almost identical copies of the originally approved drugs and can be manufactured only when the patent for the original innovator drug expires. These products are highly sensitive to smallest changes in manufacturing procedure, as these drugs possess high molecular complexity. Remicade (Infliximab) refers to a chimeric monoclonal antibody biologic licensed by U.S. FDA in 1998, as an innovative product indicated for the treatment of Crohn’s disease in adults and children.
The monoclonal antibody drug was first developed in partnership by Janssen Biotech, Inc. and Merck & Co. The Remicade was later approved for its use in the treatment of ulcerative colitis, plague psoriasis, rheumatic arthritis, and spinal psoriatic arthritis in combination with methotrexate. Later, various pharmaceutical manufacturers developed biosimilars to infliximab, which lowered market share of Remicade due to cost-effective prices.
Market Dynamics:
Increasing incidence of autoimmune diseases such as plaque psoriasis and rheumatoid arthritis are expected to drive growth of the Remicade biosimilar market size. According to the American Autoimmune Related Disease Association, around 50 million American suffered from autoimmune diseases in the U.S. in 2017. Moreover, faster reaction rates of these biosimilars due to their availability in the form of intravenous mode of administration is further expected to increase the adoption of Remicade biosimilar over the forecast period. Furthermore, patent expiry of the branded versions is expected to increase the number of biosimilars for its branded counterparts thereby increasing the demand for Remicade biosimilar. However, stringent regulatory guidelines for development of these biosimilars as well as side effects of these drugs leading to risk of hospitalization are expected to restrain growth of the global Remicade biosimilar market.
Regional Insights
On the basis of region, the global Remicade biosimilar market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Europe holds a dominant position in the global Remicade biosimilar market and is expected to retain its dominance over the forecast period, owing to the presence of top market players in the region, rapid entry of biosimilars in the European market as well as high adoption rate due low price of the biosimilars. For instance, in 2018, Sandoz, a Novartis division received European Commission (EC) approval for Zessly (infliximab) a remicade biosimilar for use in Europe. The successive research and speedy approvals by the U.S. regulatory authorities for market entry of biosimilars in North America is expected to drive growth of the market over the forecast period. For instance, in December 2017, Pfizer, Inc. received the U.S. FDA approval for second biosimilar, Ifixi to Janssen’s blockbuster drug Remicade to treat rheumatoid arthritis.
Furthermore, Asia Pacific is expected to witness significant growth in the market over the forecast period, owing to developments in healthcare infrastructure and U.S. FDA approval of Remicade (infliximab) biosimilars by regional players to market the products internationally. For instance, in April 2016, Celltrion- a South Korea-based manufacturing company received the U.S. FDA approval for intravenously administered version of the rheumatoid arthritis drug sold under the name Infllectra. Moreover, major investments by regional players for research and development of biosimilar production is expected to drive growth of the market in Asia. For instance, in October 2016, Cipla, Inc. invested US$ 8 million to set up a manufacturing plant for biosimilars in South Africa.
Competitive Landscape
Key players operating in the global remicade biosimilar market include Janssen Biotech, Inc., Merck and Company, Inc., Alvogen, Pfizer, Inc., Celltrion, Nippon Kayaku, Napp Pharmaceuticals, and others. Market players are focused on introducing maximum number of biosimilar for multiple indications to retain their position in the global market. For instance, in July 2017, Merck & Company, Inc. in collaboration with Samsung Bioepis introduced Renflexis (infliximab-abda), a biosimilar to Remicade for the treatment of moderate to severe Crohn’s disease, active ulcerative colitis, rheumatoid arthritis, and other few disease indications.
Request For Customization of Research Report @ https://www.coherentmarketinsights.com/insight/request-customization/1769
Market Taxonomy
On the basis of disease indication, the global Remicade biosimilars market is segmented into:
Ulcerative Colitis
Rheumatoid Arthritis
Ankylosing Spondylitis
Crohn’s Disease
Psoriatic Arthritis
Plaque Psoriasis
On the basis of geography, the global Remicade biosimilars market is segmented into:
North America
Latin America
Europe
Asia Pacific
Middle East
Africa
About Coherent Market Insights
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
Remicade Biosimilars Market - Size, Share, Growth and Outlook, Analysis, 2018-2026
Biosimilar refers to biotherapeutic product that is similar in terms of quality, efficacy, and safety to an existing licensed reference therapeutic drug. Biological products are the fastest growing class of therapeutic products, as they offer additional treatment options and help in lowering healthcare costs. Biosimilars are almost identical copies of the originally approved drugs and can be manufactured only when the patent for the original innovator drug expires. These products are highly sensitive to smallest changes in manufacturing procedure, as these drugs possess high molecular complexity. Remicade (Infliximab) refers to a chimeric monoclonal antibody biologic licensed by U.S. FDA in 1998, as an innovative product indicated for the treatment of Crohn’s disease in adults and children. The monoclonal antibody drug was first developed in partnership by Janssen Biotech, Inc. and Merck & Co. The Remicade was later approved for its use in the treatment of ulcerative colitis, plague psoriasis, rheumatic arthritis, and spinal psoriatic arthritis in combination with methotrexate. Later, various pharmaceutical manufacturers developed biosimilars to infliximab, which lowered market share of Remicade due to cost-effective prices.
Complete Report Details @ https://www.coherentmarketinsights.com/ongoing-insight/remicade-biosimilars-market-1769
Market Dynamics
Increasing incidence of autoimmune diseases such as plaque psoriasis and rheumatoid arthritis are expected to drive growth of the Remicade biosimilar market size. According to the American Autoimmune Related Disease Association, around 50 million American suffered from autoimmune diseases in the U.S. in 2017. Moreover, faster reaction rates of these biosimilars due to their availability in the form of intravenous mode of administration is further expected to increase the adoption of Remicade biosimilar over the forecast period. Furthermore, patent expiry of the branded versions is expected to increase the number of biosimilars for its branded counterparts thereby increasing the demand for Remicade biosimilar. However, stringent regulatory guidelines for development of these biosimilars as well as side effects of these drugs leading to risk of hospitalization are expected to restrain growth of the global Remicade biosimilar market.
Regional Insights
On the basis of region, the global Remicade biosimilar market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Europe holds a dominant position in the global Remicade biosimilar market and is expected to retain its dominance over the forecast period, owing to the presence of top market players in the region, rapid entry of biosimilars in the European market as well as high adoption rate due low price of the biosimilars. For instance, in 2018, Sandoz, a Novartis division received European Commission (EC) approval for Zessly (infliximab) a remicade biosimilar for use in Europe. The successive research and speedy approvals by the U.S. regulatory authorities for market entry of biosimilars in North America is expected to drive growth of the market over the forecast period. For instance, in December 2017, Pfizer, Inc. received the U.S. FDA approval for second biosimilar, Ifixi to Janssen’s blockbuster drug Remicade to treat rheumatoid arthritis.
Request Sample Copy Of This Report: https://www.coherentmarketinsights.com/insight/request-sample/1769
Competitive Landscape
Key players operating in the global remicade biosimilar market include Janssen Biotech, Inc., Merck and Company, Inc., Alvogen, Pfizer, Inc., Celltrion, Nippon Kayaku, Napp Pharmaceuticals, and others. Market players are focused on introducing maximum number of biosimilar for multiple indications to retain their position in the global market. For instance, in July 2017, Merck & Company, Inc. in collaboration with Samsung Bioepis introduced Renflexis (infliximab-abda), a biosimilar to Remicade for the treatment of moderate to severe Crohn’s disease, active ulcerative colitis, rheumatoid arthritis, and other few disease indications.
Market Taxonomy
On the basis of disease indication, the global Remicade biosimilars market is segmented into: Ulcerative Colitis, Rheumatoid Arthritis, Ankylosing Spondylitis, Crohn’s Disease, Psoriatic Arthritis, Plaque Psoriasis,.
On the basis of geography, the global Remicade biosimilars market is segmented into: North America, Latin America, Europe, Asia Pacific, Middle East, Africa,.
Request TOC of the Report @ https://www.coherentmarketinsights.com/ongoing-insight/toc/1769
About Coherent Market Insights:
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us:
Mr. Shah Coherent Market Insights 1001 4th Ave, #3200 Seattle, WA 98154 Tel: +1-206-701-6702 Email: [email protected]
Visit our news Website: https://www.coherenttimes.org
0 notes
Text
Global Oncology Biosimilars Market with Rising Demand & Huge Application Potential from Industry by 2026
A new business intelligence report released by Data Bridge Market Research with title Global Oncology Biosimilars Market are taken from trustworthy sources such as websites, annual reports of the companies, journals, and others and were checked and validated by the market experts. Oncology Biosimilars market research report provides estimation and analysis of the rising trends along with major drivers, restraints, challenges and opportunities in the industry. Besides, Oncology Biosimilars report systematically gathers the information about influencing factors for the industry which contains customer behavior, emerging trends, product usage, and brand positioning. This Oncology Biosimilars report brings into light several information about the industry that display important facts and figures, expert opinions, and the most recent developments across the sphere. By applying business intelligence, the report is organized which provides thorough and extensive market insights. Some of the key players profiled in the study Amgen Inc, Pfizer Inc, Eli Lilly and Company, Novartis AG, F. Hoffmann-La Roche Ltd, Eisai Co., Ltd, Allergan, Teva Pharmaceutical Industries Ltd, Bristol-Myers Squibb Company and more.
Global oncology biosimilars market is expected to gain market growth in the forecast period of 2020 to 2027. Data Bridge Market Research analyses the market is growing at a healthy CAGR in the above-mentioned research forecast period. Emerging markets and huge investment in research and development are the factors responsible for the growth of this market.
Get Latest Sample for Global Oncology Biosimilars Market Report @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-oncology-biosimilars-market
Unlock new opportunities in Oncology Biosimilars Market; the latest release from Data Bridge Market Research highlights the key market trends significant to the growth prospects, Let us know if any specific players or list of players needs to consider to gain better insights.
Competition Analysis:
The major players covered in the global oncology biosimilars market are Amgen Inc, Pfizer Inc, Eli Lilly and Company, Novartis AG, F. Hoffmann-La Roche Ltd, Eisai Co., Ltd, Allergan, Teva Pharmaceutical Industries Ltd, Bristol-Myers Squibb Company, Takeda Pharmaceutical Company Limited, Endo International plc, Sun Pharmaceutical Industries Ltd, Mylan N.V., Apotex Inc, Biocad, and others.
Research Methodology
This research study involves the extensive usage of secondary sources, directories, and databases (such as Hoovers, Bloomberg, Businessweek, Factiva, and OneSource) to identify and collect information useful for this technical, market-oriented, and commercial study of the global Oncology Biosimilars market. In-depth interviews were conducted with various primary respondents, which include key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information, and assess future market prospects. The following figure shows the market research methodology applied in making this report on the global Oncology Biosimilars market.
Global Oncology Biosimilars Market Scope and Market Size
Oncology biosimilars market is segmented on the basis of indication, drug class, route of administration end-users and distribution channel.
On the basis of indication, the global oncology biosimilars market is segmented into breast cancer, lung cancer, colorectal cancer, cervical cancer, blood cancer and others.
Based on drug class, the global oncology biosimilars market is segmented into monoclonal antibodies, granulocyte colony-stimulating factor and others.
Route of administration segment for global oncology biosimilars market is categorized into intravenous, subcutaneous and others.
On the basis of end-users, the global oncology biosimilars market is segmented into hospitals, homecare, specialty clinics and others.
On the basis of distribution channel, the global oncology biosimilars market has been bifurcated into hospital pharmacy, online pharmacy and retail pharmacy.
Early buyers will receive 20% customization on reports. Read Detailed Index of full Research Study at @ https://www.databridgemarketresearch.com/toc/?dbmr=global-oncology-biosimilars-market
Global Oncology Biosimilars Market Dynamics:
Global Oncology Biosimilars Market, By Indication (Breast Cancer, Lung Cancer, Colorectal Cancer, Cervical Cancer, Blood Cancer, Others), Drug Class (Monoclonal Antibodies, Granulocyte Colony-Stimulating Factor, Others), Route of Administration (Intravenous, Subcutaneous, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy), Country (U.S., Canada, Mexico, Brazil, Argentina, Peru, Rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific, Saudi Arabia, U.A.E, Egypt, Israel, Kuwait, South Africa, Rest of Middle East and Africa) Industry Trends and Forecast to 2027
Market Drivers:
The growth of oncology biosimilars market enhanced by the growing cases of cancer such as lung cancer and breast cancer, vulnerable aging population and rise in research and development activities conducted by many pharmaceuticals’ companies. In addition, increase patient awareness level, advances in the treatment options and high cost of biologics drugs are some of the impacting factors for the demand of oncology biosimilars. Nevertheless, product recalls coupled with stringent regulations are the factors that hinder the growth of this market.
A biosimilar product describes as biotherapeutics that’s highly similar to the biological product or reference product. Also, biosimilars product has to be no clinically meaningful differences between the two products. The biosimilars which are employed for the treatment of cancer is termed as oncology biosimilars.
The Oncology Biosimilars report puts light on the change in the market which is taking place due to the moves of key players and brands such as product launches, joint ventures, mergers and acquisitions that in turn modifies the view of the global face of industry. This market report takes into account myriad of aspects of the market analysis which today’s businesses call for. To make the report outstanding, most up-to-date and advanced tools and techniques are used so that client achieves maximum benefits. The Oncology Biosimilars report also includes the market drivers and market restraints that are derived from SWOT analysis.
Chapters to deeply display the Global Oncology Biosimilars market.
Introduction about Oncology Biosimilars
Oncology Biosimilars Market Size (Sales) Market Share by Type (Product Category) in 2019
Oncology Biosimilars Market by Application/End Users
Oncology Biosimilars Sales (Volume) and Market Share Comparison by Applications
(2020-2027 ) table defined for each application/end-users
Oncology Biosimilars Sales and Growth Rate (2020-2027)
Oncology Biosimilars Competition by Players/Suppliers, Region, Type and Application
Oncology Biosimilars (Volume, Value and Sales Price) table defined for each geographic region defined.
Oncology Biosimilars Players/Suppliers Profiles and Sales Data
Additionally Company Basic Information, Manufacturing Base and Competitors list is being provided for each listed manufacturers
Market Sales, Revenue, Price and Gross Margin table for each product type which include , Product Type I, Product Type II & Product Type III
Oncology Biosimilars Manufacturing Cost Analysis
Oncology Biosimilars Key Raw Materials Analysis
Oncology Biosimilars Chain, Sourcing Strategy and Downstream Buyers, Industrial Chain Analysis
Market Forecast (2020-2027)
……..and more in complete table of Contents
Key questions answered in this report
What will the market size be in 2027 and what will the growth rate be
What are the key market trends?
What is driving Oncology Biosimilars Market?
What are the challenges to market growth?
Who are the key vendors in Market space?
What are the key market trends impacting the growth of the Oncology Biosimilars Market ?
What are the key outcomes of the five forces analysis of the Oncology Biosimilars Market?
What are the market opportunities and threats faced by the vendors in the Oncology Biosimilars market? Get in-depth details about factors influencing the market shares of the Americas, APAC, and EMEA?
About Data Bridge Market Research:
An absolute way to forecast what future holds is to comprehend the trend today! Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market.
Contact:
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
0 notes
Text
Understanding the Fundamentals of Analytical Method Validation Services
Analytical method development and validation are the inter-dependent and perpetual tasks linked to R&D, quality assurance and quality control departments. Analytical methods play a key role in risk assessment and equivalence management. It guarantees the stability of results and the formation of product-specific acceptance criteria.

Validation must establish that the analytical process is appropriate for its envisioned purpose. Design of experiment is a potent tool for validation and method characterization. Analytical experts must be comfortable while using it to optimize and characterize analytical methods. A powerful validation and analytical method development process can offer major enhancements in accuracy and a drop in bias errors. It can also help avoid time-consuming and expensive exercises.
Analytical Method Validation
Analytical method validation services and transfer are the fundamentals of a pharmaceutical development package. These are the crucial ways that ensure biotherapeutics, drugs, and other products made by firms in the biotechnological and pharmaceutical sector fulfil acceptance criteria. Habitually considered repetitive, the advantage that a properly developed analytical method can provide to general cost efficiency and developmental time for a program is underrated.
Acceptance criteria are built on the test process being used and the kind of product being examined. Analytical methods are carried out to test the purity, efficacy, potency, identity, and concentration of finished products and in-process samples.
Method-related events are interconnected as well as cyclic mainly during the initial drug development stage. Parts of each procedure might happen simultaneously or be refined at different stages of the drug development process. Modifications to one technique during the drug development process may entail changes to a current analytical method. These changes, in turn, may necessitate further transfer activities or validation.
Analytical Method Qualification
Analytical method qualification is closely linked to analytical method validation. In fact, the terms analytical method qualification and analytical method validation are essentially identical within the sector. Analytical method validation’s purpose is to authorize and record whether the method is working as intended. Regardless of any previous qualification or validation work done for potential methods, each time a method is installed, created or transferred on an existing system, it must be authorized. Comprehensive validation packages will be formed and implemented.
Elements of Validation
Analytical method validation establishes the scientific dependability of characterization and is needed during the process of regulatory submission. The validation exercise authenticates that an analytical method measures the right ingredient, in the right quantity, and in the proper range for intended samples. It allows the expert to comprehend the behaviour of the technique and establish the method’s performance limits.
To carry out method validation, the lab must follow a mandated Standard Operating Procedure that defines the method validation process. The lab must use calibrated and qualified instrumentation with an equivalent SOP.
A documented and well-developed test method should be put in place and permitted guidelines must be followed before carrying out validation experiments. The guidelines must define the method by which performance parameters will be verified, how the parameters will be evaluated, and the applied acceptance criteria. Lastly, samples of drug product or API, reference standards and placebos are required to accomplish validation tests.
Approaches to Validation Experiments
Accuracy will be maintained by spiking placebo with API for a drug or sample quantitation against a reference standard for API. It can also be decided by comparing results from different measurement procedures.
Accuracy can be decided by several measurements on a homogeneous and reliable set of samples. Samples can be evaluated by different analysts, on different days, on different apparatuses, or in different labs. There are 3 levels of precision validation assessments – intermediate precision, reproducibility, and repeatability.
Intermediate precision is a measure of accuracy within the same lab by different workers, using different apparatuses, and taking measurements on different days. Repeatability is a measure of accuracy under similar conditions over a short period. Reproducibility measures precision between two or more labs.
The variables mixed up in analytical method development are as distinctive as the abundant biotherapeutics and drug products we see in the market. Analytical method development is defined by the product being created, which is then examined. Based on the product, phased method validation strategy or early-phase method validation may be employed.
#Biologics Process#Biologics Quality Control#Analytical Method Validation#drug development#drug discovery
0 notes
Text
Peripheral Artery Disease Market Analysis, Cost, Production Value, Price, Gross Margin And Competition Forecast To 2025
Global Peripheral Artery Disease Market Research Report: By Disease Type (Organic and Functional), Treatment Type (Medications, Angioplasty, Bypass Grafting, Atherectomy, Thrombolytic Therapy and others), End User (Hospitals & Clinics, Research & Academic Institutes and others) and Region (Americas, Europe, Asia-Pacific and Middle East & Africa) - Forecast till 2025
Peripheral Artery Disease Market Scenario
Following a careful analysis of the global peripheral artery disease market, Market Research Future (MRFR) concludes that it will reach the valuation of USD 3.47 billion by 2023. The market is set to rise from its previous value of USD 2.23 billion in 2016, at a CAGR of 6.5% during the forecast period (2017-2023).
Growth Factors and Key Restraints
Rise in Geriatric Population
There has been a dramatic surge in the number of aging people worldwide. As per a WHO report, at least 524 million people were above the age of 65 in the year 2010, and the prediction is that number will be reaching 1.5 billion by 2050. This figure is expected to constitute almost 16% of the total global population. Furthermore, the number of elderly individuals is directly proportional to the number of disorders amongst them, like hypertension and diabetes, increasing the risk for peripheral artery disease. Thus, the expanding geriatric population will leave a positive impact on the growth of the global market in the years to come.
Get a FREE Sample Copy of Report with Complete TOC @ https://www.marketresearchfuture.com/sample_request/1540
Advanced Treatment Options
The peripheral artery disease market size is all set to expand significantly from 2017 to 2023, with a key reason being the increasing advanced treatment options as well as tools like atherectomy devices. These kinds of tools are used for debulking the plaque before using a stent for treatment. Thus, with the surging prevalence of calcified and hard lessions coupled with the upgraded atherectomy procedure, the overall growth of the market is going to be highly promising in the forthcoming years. Apart from this, other latest interventional products like drug eluting stents and drug coated balloon stents will also prove to be instrumental in the lucrative growth of the market during the conjectured time frame.
Increase in Research and Development Expenditure
Adding on to the list of growth drivers, one factor that will give a substantial boost to the market growth is the rising research and development expenditure. As per reports by European Federation of Pharmaceutical Industries and Association, in 2016, the R&D expenditure in the Pharmaceutical Industry was estimated at a whopping value of EUR 35000 million. Moreover, as per the Centers for Disease Control and Prevention, in 2015, the per capita national health expenditure was approximately USD 9,990, with the total national health expenditure being USD 3.2 trillion. Also, few reports suggest that out of total national health expenditures in the United States (US), the prescription drugs accounted for 10.1% of the total share.
Rising Trends in Peripheral Artery Disease Market
At present, the peripheral artery disease market is governed by numerous players. Also, as a result of the increasing research and development expenditure, a number of existing as well as new marketers are progressively developing advanced drugs that can control this specific condition.
For instance, Medtronic is one such company in the peripheral artery disease market, that is one of the largest standalone medical equipment development company in the world. The company boasts of a diverse portfolio of a range of products like NC Sprinter RX/ OTW Noncompliant Balloon Dilatation Catheter, NC Euphora Noncompliant Balloon Dilatation Catheter, Euphora Semicompliant Balloon Dilatation Catheter, among others.
Peripheral Artery Disease Market Segmentation
The market for peripheral artery disease is segmented on the basis of devices, drugs and types of diseases.
The global peripheral artery disease market, by the types of devices, is segmented into stents, angioplasty balloon catheters, drug-eluting balloons, atherectomy devices and others. Stents is sub-segmented into balloon-expandable and self-expandable, whereas the atherectomy devices are sub-segmented into rotational, orbital, laser and directional.
The types of drugs used for treating peripheral artery disease include lipid-lowering drugs, blood pressure lowering drug, glucose regulating drug, clot preventing drug and others.
The types of diseases mentioned in the report are blood clotting disorders, lymphedema, atherosclerosis and others.
Regional Insight
The regional segments in the global peripheral artery disease market are Asia Pacific, Europe, North America, and the Middle East & Africa.
With the largest share in the global market, North America seems to be on an upward trajectory due to the increasing number of people suffering from major or minor peripheral diseases. Moreover, the increasing obese and diabetic patients in the region has accelerated the demand for the drugs needed for the treatment of peripheral artery diseases. Other factors working in favor of the regional market include the growing awareness regarding the disease, favorable reimbursement policies, as well as expanding geriatric population.
On the other side, the growing population in the Asia Pacific region coupled with the speedy rate of development is luring numerous manufacturers to the region. This will be a key reason for the lucrative growth rate of the regional market in the coming years. Apart from this, Asia Pacific also presents lucrative opportunities to the market, in the form of surging awareness among patients about the latest interventional treatment options.
The growth drivers of the peripheral artery disease market in Europe include the improved screening and detection of the disease, the rising geriatric population, and the continuous advancements in technology. Of course, the strikingly improving healthcare infrastructure and the healthcare industry has also been one of the most important factors in the market growth over the years.
The Middle East and Africa peripheral artery disease market will also be making somewhat similar strides as other regional markets, backed by the growing interest for minimally invasive strategies, evolving lifestyle, maturing population and the upsurge of peripheral artery diseases.
Peripheral Artery Disease Market Key Vendors
Bayer HealthCare Pharmaceuticals (Germany), TheraVasc Inc. (US), Abbott Laboratories (U.S), AstraZeneca Plc. (UK), Boston Scientific Corporation (U.S), Johnson & Johnson (U.S), Medtronic (Ireland), Betagenon AB (Sweden), nGes MG, Inc. (Japan), Proteon Therapeutics, Inc. (US), Merck & Co., Inc. (US), Angioscore, Inc. (U.S), Symic Bio, Inc. (US), CardioVascular BioTherapeutics, Inc. (US), Bristol-Myers Squibb Company (US), Sanofi S.A. (France), are among some of the top players in the global peripheral artery disease market.
Get More Information on Peripheral Artery Disease Market Research Report - Global Forecast till 2025 @ https://www.marketresearchfuture.com/reports/peripheral-artery-disease-market-1540
Industry Developments
February 2019 - Mercy Hospital (US) is all set to offer a supervised exercise therapy program for those afflicted with symptomatic Peripheral Artery Disease (PAD). The 12-week supervised exercise therapy program will involve treadmill walking, along with various other exercise modalities, supervised by exercise physiologists, who will ensure the safety of patients.
#Peripheral Artery Disease Market#Peripheral Artery Disease Market Size#Peripheral Artery Disease Market Analysis#Peripheral Artery Disease Market Growth
0 notes