#US Biosimilar Sector
Explore tagged Tumblr posts
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Why Indian pharmaceutical companies are among the world’s best
India has emerged as a global leader in the pharmaceutical industry, making significant contributions to healthcare worldwide. With its vast network of pharma manufacturing companies in India, world-class infrastructure, and highly skilled workforce, the country has established itself as a major hub for drug production and innovation. The pharmaceutical industry in India plays a vital role in ensuring affordable and high-quality medicines reach patients across the globe. But what makes pharmaceutical companies in India stand out from the rest? Let us explore the factors that contribute to India's success in this sector.

1. Cost-Effective Manufacturing and High Production Capacity
One of the biggest reasons why pharma companies in India are highly regarded is their cost-effective manufacturing processes. Indian companies have mastered the art of producing high-quality medicines at a fraction of the cost compared to other countries. The medicine manufacturing company in India benefits from lower labor costs, efficient supply chain management, and advanced technological integration.
India is also known for its massive production capacity. Whether it is generic medicines, vaccines, or complex biologics, pharmaceutical companies in India produce a significant percentage of the world’s pharmaceutical products. This high-volume production capability allows India to meet global healthcare demands efficiently.
2. Strong Research and Development (R&D) Capabilities
India is home to some of the top pharmaceutical companies in India that invest heavily in research and development. The country has numerous state-of-the-art research facilities that focus on drug discovery, formulation development, and biosimilars.
Moreover, Indian pharma firms collaborate with global research institutions to develop innovative treatments for life-threatening diseases. This commitment to innovation has helped India stay ahead in the competitive pharmaceutical landscape.
3. Leadership in Generic Medicines
India is often referred to as the “Pharmacy of the World” due to its dominance in the generic drug market. The best Indian pharma industry supplies more than 50% of the global demand for generic medicines. Generic drugs offer the same therapeutic benefits as branded medications but at much lower prices, making healthcare more affordable for millions worldwide.
The ability of pharma manufacturing companies in India to produce high-quality generics at competitive prices has helped many developing and underdeveloped nations improve their healthcare systems. This stronghold in the generics market has cemented India’s position as a leading player in the pharmaceutical industry.
4. Regulatory Compliance and Global Certifications
Indian pharmaceutical companies adhere to stringent quality control standards and obtain regulatory approvals from leading global authorities such as the U.S. FDA, EMA (European Medicines Agency), and WHO-GMP. These certifications validate the high-quality standards maintained by medicine manufacturing company in India, enabling them to export medicines to over 200 countries.
The strict compliance with international regulations ensures that Indian pharmaceutical products meet global safety and efficacy standards. This commitment to quality has helped Indian pharma companies build trust among healthcare professionals and patients worldwide.
5. Growing Biopharmaceutical and Vaccine Industry
While India is well known for generic drugs, it has also made significant advancements in biopharmaceuticals and vaccine production. The country is home to leading vaccine manufacturers like Serum Institute of India, which played a crucial role in supplying COVID-19 vaccines globally.
The best pharmaceutical industry in India continues to invest in biopharmaceutical research, aiming to develop advanced biologics and biosimilars. With increasing government support and private sector investments, India's biopharma industry is set to achieve new milestones in the coming years.
6. Availability of Skilled Workforce and Advanced Infrastructure
India’s pharmaceutical success is backed by a vast pool of skilled professionals, including scientists, pharmacists, engineers, and regulatory experts. The country produces thousands of pharmacy and life sciences graduates every year, ensuring a steady supply of talent for the industry.
Additionally, top pharmaceutical companies in India have invested in state-of-the-art manufacturing facilities that integrate automation, artificial intelligence, and data analytics to enhance production efficiency. The combination of skilled labor and modern infrastructure has helped Indian pharmaceutical companies maintain their global competitiveness.
7. Strong Export Market and Global Presence
Indian pharmaceutical companies have a significant global presence, exporting medicines to countries across North America, Europe, Africa, and Asia. The best pharma company in India contributes to over 20% of the global supply of generic drugs, making India the largest exporter of pharmaceutical products by volume.
The country’s ability to provide affordable and effective medicines has strengthened its reputation as a trusted supplier in the global pharmaceutical industry. With continued investment in research, innovation, and quality control, top pharma companies in India are expected to expand their reach further.
8. Government Support and Policy Initiatives
The Indian government has played a crucial role in supporting the growth of the pharmaceutical sector. Various policy initiatives such as the Production Linked Incentive (PLI) Scheme and Make in India program have encouraged domestic manufacturing and research.
Moreover, the government has eased regulatory processes and provided financial incentives to boost pharmaceutical exports. These measures have helped pharma companies in India scale up their operations and compete with global pharmaceutical giants.
9. Focus on Emerging Therapeutic Areas
Indian pharmaceutical companies are increasingly focusing on emerging therapeutic areas such as oncology, neurology, and rare diseases. With the rise in chronic and lifestyle diseases, the demand for specialized treatments is growing. Many top pharma companies in the world are partnering with Indian firms to develop innovative therapies that address these healthcare challenges.
The shift towards high-value and niche segments showcases India's potential to lead in advanced drug discovery and personalized medicine.
Conclusion
The best pharmaceutical industry in India has earned its place among the top pharma companies in the world through its cost-effective manufacturing, strong research capabilities, regulatory compliance, and global reach. As the pharmaceutical industry in India continues to evolve, it is set to play an even more significant role in shaping the future of global healthcare.
With continuous innovation, government support, and a skilled workforce, pharmaceutical companies in India are well-positioned to remain at the forefront of the global pharmaceutical landscape. Whether it is generic medicines, vaccines, or cutting-edge biopharmaceuticals, India’s pharma industry is poised for continued growth and success in the years to come.
#Pharma manufacturing companies in India#Medicine manufacturing company#Top pharmaceutical companies in India#Pharma companies in India#Pharmaceutical companies in India#Top pharma companies in the world#Best pharmaceutical industry in India#Best pharma company in India#Pharmaceutical industry in India#Medicine manufacturing company in India#Best Indian pharma industry#Top pharma companies in India#Healthcare#Hospitals#Diagnostics#Pharmacy#Prescription Medications
3 notes
·
View notes
Text
Global Chromatography Resin Market Analysis: Opportunities & Challenges
Rising Biopharmaceutical Research and Drug Development Drive Growth in the Chromatography Resin Market.

The Chromatography Resin Market Size was USD 2.6 billion in 2023 and is expected to reach USD 5.1 billion by 2032 and grow at a CAGR of 7.4% over the forecast period of 2024-2032.
The Chromatography Resin Market is experiencing significant growth due to its critical role in separation and purification processes across various industries, including pharmaceuticals, biotechnology, food & beverage, and environmental testing. The increasing adoption of monoclonal antibodies, vaccines, and biosimilars has fueled the demand for chromatography resins in biopharmaceutical manufacturing. Additionally, advancements in protein purification techniques and drug discovery research are further driving market expansion.
Key Players in the Chromatography Resin Market
Merck KGaA (Sepharose, Eshmuno)
Bio-Rad Laboratories, Inc. (Nuvia, Bio-Scale)
WIPRO GE HEALTHCARE PVT LTD (MabSelect, MabSelect SuRe)
Purolite (Chromalite, ProSep)
GRACE (Matrix, Discovery)
Mitsubishi Chemical Holdings Corporation (Kromasil, HICresin)
Danaher (Cytiva, ÄKTA)
Tosoh Corporation (Tosoh Biosep, TSKgel)
GE Healthcare (MabSelect, Sepharose)
Avantor Performance Materials Inc. (VWR, J.T. Baker)
Future Scope of the Market
The Chromatography Resin Market is expected to grow due to:
Increasing demand for biopharmaceuticals, including monoclonal antibodies and gene therapy products.
Rising investment in drug discovery, proteomics, and genomics research.
Expanding applications in food safety testing and environmental monitoring.
Technological advancements in resin manufacturing for enhanced separation efficiency.
Growth in personalized medicine and biotechnology-driven therapies.
Emerging Trends in the Chromatography Resin Market
The chromatography resin industry is undergoing rapid advancements driven by the need for high-purity separations and cost-effective purification solutions. The shift toward single-use chromatography systems is gaining traction in the biopharmaceutical sector due to its ability to enhance productivity and reduce contamination risks. Additionally, affinity chromatography resins are witnessing increased demand for their role in high-selectivity protein purification. The integration of nanotechnology and hybrid chromatography resins is further enhancing the efficiency of separation techniques. Sustainability efforts are also influencing the market, with manufacturers focusing on biodegradable and recyclable resins to reduce environmental impact.
Key Points:
Rising demand for chromatography resins in biopharmaceutical and life sciences research.
Increasing adoption of monoclonal antibody purification techniques.
Growth in single-use chromatography technologies for efficient drug manufacturing.
Advancements in hybrid and affinity chromatography resins for high-selectivity separation.
Sustainability initiatives promoting the development of eco-friendly resins.
Conclusion
The Chromatography Resin Market is set for substantial expansion, driven by biopharmaceutical innovations, technological advancements, and growing research activities. With increasing investments in drug discovery, precision medicine, and protein purification technologies, the demand for high-performance chromatography resins is expected to rise. As the industry moves toward sustainable and efficient purification solutions, manufacturers are focusing on innovation and product development to meet the evolving needs of pharmaceutical, food safety, and environment.
Read Full Report: https://www.snsinsider.com/reports/chromatography-resin-market-1693
Contact Us:
Jagney Dave — Vice President of Client Engagement
Phone: +1–315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Chromatography Resin Market#Chromatography Resin Market Size#Chromatography Resin Market Share#Chromatography Resin Market Report#Chromatography Resin Market Forecast
0 notes
Text
Powerhouses of Ofatumumab: Countries Dominating the Market
According to a recent research, Industry revenue for Ofatumumab is expected to rise to $1634.6 million by 2035 from $680.0 million of 2024. U.S., UK, Germany, Japan and Canada are the top 5 markets and combinely holds substantial demand share. The revenue growth of market players in these countries is expected to range between 6.1% and 8.7% annually for period 2025 to 2035.
Industry transition including impact of technological advancements and influence of regulatory measures, are transforming the supply chain of Ofatumumab market. The field of pharmaceuticals has experienced new and groundbreaking technological advances that have revolutionized how products such as ofatumumab are used and how well they work. The progress made in biotechnology and biosimilar research has played a role in creating this medication by Genmab and Novartis which provides an efficient treatment for individuals dealing with diseases like chronic lymphocytic leukemia and multiple sclerosis. These improvements have led to effectiveness in pharmaceuticals, reduced adverse effects and improved results, for patients. Henceforth the advancing technology in the field supports the shift towards more effective medications like ofatumumab. Enhanced comprehension of biosimilars has also boosted the sectors ability to develop tailored treatments thus solidifying ofatumumabs distinct potential, in the market.
Potential Application Areas
Multiple Sclerosis Management: Ofatumumab has proven to be particularly effective in the treatment of sclerosis . This treatment involves using a form of ofatumumab that targets B cell lymphocytes and is known for its ability to slow down the progression of relapsings forms of MS. Big players in the industry such as Genmab A/S and GlaxoSmithKline have taken advantage of the benefits offered by ofatumumab and have secured strong positions, in managing MS.
Rheumatoid Arthritis Therapy: Ofatumumab is also used to treat conditions such as rheumatoid arthritis . Its role in this context involves reducing inflammation and slowing down the progression of the disease by lowering B cell counts which aids in regulating the bodys response. Companies like Janssen Pharmaceuticals and Bristol Myer Squibb are, at the forefront of utilizing ofatumumab strategically to enhance treatment results for patients.
Industry Leadership and Strategies
The Ofatumumab market is characterized by intense competition, with a number of leading players such as Novartis AG, GlaxoSmithKline plc, Roche Holding AG, Amgen Inc., Bristol-Myers Squibb Company, Merck & Co. Inc., Eli Lilly and Company, Celgene Corporation, Johnson & Johnson, Sanofi, AstraZeneca plc and Pfizer Inc.. These players are pushing the boundaries of innovation & technological advancements and forging strategic partnerships to expand the existing reach of the market. Below table briefs about adopted market strategies by leading players.
Access detailed report insights here - https://datastringconsulting.com/industry-analysis/ofatumumab-market-research-report
About DataString Consulting
DataString Consulting assist companies in strategy formulations & roadmap creation including TAM expansion, revenue diversification strategies and venturing into new markets; by offering in depth insights into developing trends and competitor landscapes as well as customer demographics. Our customized & direct strategies, filters industry noises into new opportunities; and reduces the effective connect time between products and its market niche.
0 notes
Text
The Role of Contract Manufacturers in the Pharmaceutical Industry

Introduction
The pharmaceutical industry is constantly evolving, with increasing demands for efficiency, cost-effectiveness, and regulatory compliance. One of the key players driving this evolution is contract manufacturing organizations (CMOs). These third-party service providers enable pharmaceutical companies to outsource drug production, allowing them to focus on research, development, and marketing.
What Are Contract Manufacturers in Pharmaceuticals?
Contract manufacturers in the pharmaceutical industry specialize in producing drugs for pharmaceutical companies that may not have the necessary infrastructure or capacity to manufacture products in-house. They provide a range of services, including formulation development, production, packaging, and even regulatory support.
Benefits of Contract Manufacturing
Cost Savings: Establishing a manufacturing facility requires significant capital investment. CMOs help companies reduce these expenses by providing ready-to-use facilities and expertise.
Expertise & Compliance: CMOs have extensive experience in adhering to Good Manufacturing Practices (GMP) and regulatory guidelines from agencies like the FDA and EMA.
Scalability & Flexibility: Companies can adjust production volumes based on market demand without making heavy infrastructure investments.
Faster Time-to-Market: Leveraging a CMO’s expertise can help companies accelerate the production process and meet market demands efficiently.
Focus on Core Competencies: By outsourcing manufacturing, pharmaceutical firms can concentrate on research, innovation, and commercialization.
Key Services Offered by Contract Manufacturers
Drug Development and Formulation: CMOs assist in developing formulations that meet regulatory and commercial requirements.
Bulk Manufacturing: Large-scale production of Active Pharmaceutical Ingredients (APIs) and finished dosage forms.
Analytical and Quality Testing: Ensuring that drugs meet quality, safety, and efficacy standards.
Regulatory Assistance: Helping companies navigate complex regulatory frameworks and obtain necessary approvals.
Packaging and Labeling: Providing compliant and attractive packaging solutions for global markets.
Challenges Faced by Contract Manufacturers
Despite the numerous advantages, CMOs face challenges such as:
Regulatory Compliance: Adhering to different regulatory requirements across various markets can be complex and resource-intensive.
Intellectual Property Protection: Ensuring confidentiality of proprietary formulations and technologies is crucial.
Supply Chain Management: Managing raw material procurement, logistics, and inventory can be challenging, especially in times of global disruptions.
Quality Control Issues: Maintaining consistent quality across different production batches is critical for regulatory approval and brand reputation.
Choosing the Right Contract Manufacturer
When selecting a CMO, pharmaceutical companies should consider factors such as:
Regulatory Track Record: A history of compliance with regulatory agencies ensures a smooth manufacturing process.
Technical Capabilities: The ability to manufacture different dosage forms and drug formulations.
Scalability and Capacity: The CMO should be able to scale production as per demand.
Quality Assurance Systems: Strong quality control measures help maintain consistency and compliance.
Financial Stability: A financially stable CMO ensures long-term reliability and partnership.
The Future of Contract Manufacturing in Pharmaceuticals
The contract manufacturing industry is expected to grow significantly due to increased outsourcing trends, demand for biosimilars, and technological advancements. Innovations such as AI-driven quality control, continuous manufacturing, and personalized medicine are set to revolutionize the sector.
Conclusion
Contract manufacturers play a crucial role in the pharmaceutical industry by providing cost-effective, high-quality, and scalable production solutions. By partnering with the right CMO, pharmaceutical companies can enhance efficiency, ensure regulatory compliance, and bring life-saving drugs to market faster.
0 notes
Text
The Unsung Heroes of Bioprocessing: Diving into the Chromatography Resin Market
Chromatography resins are the quiet workhorses of biopharmaceutical production, responsible for purifying and isolating precious biomolecules such as proteins, antibodies, and vaccines. These unique materials are critical to guaranteeing the quality, safety, and efficacy of biotherapeutics, fueling a strong and dynamic market.
Market Growth and Key Drivers
The worldwide chromatography resin market is seeing tremendous growth. Although exact market valuations are subject to source and scope variations, the overall trend indicates steady growth. The following are a number of reasons for this growth:
��Rising biopharmaceutical sector: The explosive expansion of the biopharmaceutical sector, coupled with rising demand for protein therapeutics, monoclonal antibodies, and vaccines, is the key driver for the chromatography resin market.
• Technological innovations: Advances in resin chemistry, particle technology, and process manufacturing are driving the creation of more selective and efficient chromatography resins, fueling market growth.
• Increasing demand for ultra-high-purity products: The stringent regulatory environment for biopharmaceuticals demands the application of high-grade chromatography resins to obtain the required purity and safety levels.
•Steep increase in the use of advanced chromatography techniques: Techniques like high-performance liquid chromatography (HPLC) and ultra-high-performance liquid chromatography (UHPLC) are developed using patented resins, thus driving the market further.
•Higher R&D spending: The pharmaceutical and biotechnology companies are investing heavily in research and development to discover and develop new biopharmaceuticals, leading to higher demand for chromatography resins.
Key Types and Applications
The market for chromatography resins can be divided on the basis of several factors, including:
•Resin type: Ion exchange resins, affinity resins, size exclusion resins, hydrophobic interaction resins, mixed-mode resins.
•Applications: Protein purification, antibodies, vaccines, nucleic acids, and other biomolecules purification.
These resins are utilized in various areas:
•Manufacturing biopharmaceuticals: Puriying and separating therapeutic proteins, antibodies, vaccines, and other biologics.
•Research and development: Purification and analysis of biomolecules in research institutions.
•Food and beverages: Purification and separation of food ingredients and contaminants removal.
Challenges and Opportunities
Though it has great potential, the chromatography resin market is subject to some challenges:
•Cost: High-performance chromatography resins are costly, especially for large-scale biopharmaceutical production.
•Selectivity and capacity: It is difficult to develop resins with high selectivity and capacity for target molecules.
•Scalability: Scaling up chromatography processes from laboratory to industrial scale is a challenge that needs careful optimization and resin selection.
The future of the chromatography resin market is bright. There are ongoing R&D activities aimed at creating more cost-efficient, very selective, and scalable resins. The high usage of biosimilars and the implementation of continuous process chromatography technology are also creating new opportunities for the resin manufacturers. Additionally, new resin chemistries and particle technologies are expanding the applications of chromatography resins. With the ongoing growth and development of the biopharmaceutical industry, chromatography resins will remain a primary tool for ensuring the quality and purity of life-saving drugs.
Author's Bio:
Ric P.
Senior Market Research expert at The Insight Partners
0 notes
Text
Growth and Challenges of Pharmaceutical Manufacturers in India
The pharmaceutical industry in India is one of the largest in the world. With a strong manufacturing base, skilled workforce, and cost-effective production, India has become a global leader in pharmaceutical exports. The country is home to many pharmaceutical manufacturers in India, which produce high-quality medicines at affordable prices. However, despite the remarkable growth, the industry also faces several challenges. In this article, we will explore the growth and challenges of the pharmaceutical sector in India.
Growth of Pharmaceutical Manufacturers in India
Global Leader in Generic Medicine Production India is the world's largest supplier of generic medicines, contributing to about 20% of the global supply. The affordability and availability of Indian generic drugs make them highly popular in international markets. Many top Indian pharma export companies in India supply medicines to the United States, Europe, and other parts of the world.
Increasing Demand for Indian Medicines The demand for Indian pharmaceutical products has been growing steadily due to their quality and cost-effectiveness. The COVID-19 pandemic further boosted this demand, as Indian companies played a crucial role in supplying vaccines and essential medicines worldwide.
Government Support and Policies The Indian government has implemented several policies to support the growth of the pharmaceutical industry. Initiatives like the "Production Linked Incentive (PLI) Scheme" encourage investments in the sector. Moreover, schemes like "Make in India" promote local manufacturing, helping medicine manufacturing companies in India expand their production capacity.
Strong R&D and Innovation Indian pharmaceutical manufacturers are investing heavily in research and development (R&D) to create innovative medicines. Many companies are focusing on biosimilars, specialty drugs, and new formulations to meet global healthcare demands.
Rising Pharmaceutical Exports India exports medicines to over 200 countries, including highly regulated markets like the USA, UK, and Canada. The top Indian pharma export companies in India include Sun Pharma, Dr. Reddy’s Laboratories, Cipla, Lupin, and Aurobindo Pharma. These companies play a key role in making Indian pharmaceutical products widely accessible worldwide.
Challenges Faced by Pharmaceutical Manufacturers in India
Stringent Regulatory Requirements The pharmaceutical industry must comply with strict regulatory standards in both domestic and international markets. Different countries have different compliance norms, making it challenging for Indian manufacturers to meet all requirements. Obtaining approvals from regulatory bodies like the US FDA and EMA can be time-consuming and expensive.
Rising Raw Material Costs India relies heavily on China for Active Pharmaceutical Ingredients (APIs), which are essential raw materials for medicine production. Any disruption in the supply chain or increase in API prices directly affects the profitability of medicine manufacturing companies in India.
Intellectual Property Rights (IPR) Issues Patent-related disputes and intellectual property rights issues often create obstacles for pharmaceutical manufacturers. While India follows a strict patent law, international pharmaceutical giants sometimes challenge Indian drug patents, leading to legal battles.
Increasing Competition With the growth of the pharmaceutical sector, competition among pharmaceutical manufacturers in India has also increased. Small and medium-sized companies find it difficult to compete with large corporations that have better financial and technological resources.
Quality Control and Compliance Maintaining high-quality standards is crucial for pharmaceutical companies. Even a minor deviation in drug quality can lead to product recalls, financial losses, and damage to a company’s reputation. Indian manufacturers must invest in advanced technology and quality control systems to ensure global compliance.
Skilled Workforce Shortage While India has a large pool of skilled professionals, the pharmaceutical industry still faces a shortage of highly trained scientists and regulatory experts. Continuous training and skill development programs are needed to bridge this gap.
The Future of Indian Pharmaceutical Industry
Despite these challenges, the future of the Indian pharmaceutical industry remains bright. With continuous investments in research, government support, and a focus on self-reliance, India is expected to strengthen its position as a global pharmaceutical hub. The industry is likely to witness further growth in biopharmaceuticals, personalized medicine, and digital healthcare solutions.
Conclusion
The pharmaceutical industry in India has achieved significant growth over the years, making the country one of the world's largest medicine suppliers. Many pharmaceutical manufacturers in India are expanding their global reach, supplying affordable and high-quality medicines. However, the industry must overcome challenges such as regulatory compliance, rising raw material costs, and increasing competition. By addressing these challenges and investing in innovation, medicine manufacturing companies in India can continue to thrive in the global market. Additionally, the top Indian pharma export companies in India will play a crucial role in maintaining India’s leadership in the pharmaceutical sector.
0 notes
Text
AI-Powered Drug Discovery in Dermatology Market Forecast, Size, Share by 2025-2033

The Reports and Insights, a leading market research company, has recently releases report titled “AI-Powered Drug Discovery in Dermatology Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033.” The study provides a detailed analysis of the industry, including the global AI-Powered Drug Discovery in Dermatology Market share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the AI-Powered Drug Discovery in Dermatology Market?
The global AI-powered drug discovery in dermatology market was valued at US$ 246.4 Million in 2024 and is expected to register a CAGR of 24.7% over the forecast period and reach US$ 1,796.6 Mn in 2033.
What are AI-Powered Drug Discovery in Dermatology?
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/2568
What are the growth prospects and trends in the AI-Powered Drug Discovery in Dermatology industry?
What is included in market segmentation?
The report has segmented the market into the following categories:
By Drug Type
Small Molecules
Biologics
Biosimilars
Natural Compounds
RNA-Based Therapeutics
Others
By AI Model
Machine Learning (ML)
Supervised Learning
Unsupervised Learning
Reinforcement Learning
Others
Deep Learning
Neural Networks
Convolutional Neural Networks (CNNs)
Others
Natural Language Processing (NLP)
Generative Models
GANs (Generative Adversarial Networks)
Transformer Models
Others
Others
By Indication
Psoriasis
Atopic Dermatitis (Eczema)
Acne
Skin Cancer (e.g., Melanoma, Basal Cell Carcinoma)
Vitiligo
Wound Healing
Rare Dermatological Disorders
Others
By End User
Pharmaceutical Companies
Biotechnology Firms
Academic and Research Institutes
Contract Research Organizations (CROs)
Healthcare Providers
North America
United States
Canada
Europe
Germany
United Kingdom
France
Italy
Spain
Russia
Poland
Benelux
Nordic
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
ASEAN
Australia & New Zealand
Rest of Asia Pacific
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
Saudi Arabia
South Africa
United Arab Emirates
Israel
Rest of MEA
Who are the key players operating in the industry?
The report covers the major market players including:
Almirall
SCARLETRED
QIMA Monasterium
Quantificare
Google
Microsoft
CytoReason LTD
Atomwise Inc.
View Full Report: https://www.reportsandinsights.com/report/AI-Powered Drug Discovery in Dermatology-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd. 1820 Avenue M, Brooklyn, NY, 11230, United States Contact No: +1-(347)-748-1518 Email: [email protected] Website: https://www.reportsandinsights.com/ Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/ Follow us on twitter: https://twitter.com/ReportsandInsi1
#AI-Powered Drug Discovery in Dermatology Market share#AI-Powered Drug Discovery in Dermatology Market size#AI-Powered Drug Discovery in Dermatology Market trends
0 notes
Text
The amount Does Rituximab Cost Universally?
rituximab price and other marked items which incorporate rituximab are applied usually for non Hodgkin's lymphomas and CLL and rheumatoid joint pain . In any case, the value of its item may not be fixed and may contrast starting with one country then onto the next. This blog plans to discuss the costs of rituximab treatment, particularly in USA, UAE and UK and what makes these costs thusly.
Rituximab Cost in the USA The US has the most elevated drug cost rates internationally, and Rituximab was not excluded from the exorbitant costs. The expense for a solitary Rituxan mixture in the USA can be from $5,000 to $7,000 for normal portion. Rituximab cost changes from $10,000 to $15,000 per implantation whenever treated as a short term, while for a full treatment cycle the expense could run somewhere in the range of $20,000 and $30,000 relying on the quantity of mixtures required. Factors adding to these expenses include: Other comparable worries, for example, are the idea of machining processes that don't profit from government-directed estimating. Article expenses that were ascribed to projects in innovative work. Hardly any opponent items since marked medications can't appreciate rivalry as a result of patent privileges. Albeit most patients have health care coverage, the person in question might spend less cash yet high deductibles as well as co-pay makes it troublesome.
Rituximab Cost in the UAE Just like with a ton of things in the UAE, there is both a public side and a confidential side with regards to medical services, and the costs of medications are clear of this too. The expense of the rituximab in confidential offices in UAE differs from 4 000 to 6 000 US rupees for every organization. There are public choices accessible for Emirati nationals where they get some kind of an arrangement that makes the costs essentially better. In any case the expenses are imagined to remain moderately high because of the country's dependence on imports for prescriptions especially since it adjusts to the worldwide costs.
Rituximab Cost in the UK The UK offers one of the least expensive public passage focuses to Rituximab through the NHS. To this end, the portrayed treatment utilizing Rituximab is practically free for qualified patients looking for care under the Public Wellbeing Administration. They figured out that in confidential medical care setting, rituximab cost shifts somewhere in the range of £2,500 and £5,000 per imbuement. Factors impacting these lower costs include: Vertical discussions by state run administrations with the makers of drugs. Medical care appropriations toward fair usage of clinical offices. To numerous patients, the NHS will constantly be their essential wellspring of reasonable treatment.
Key Variables Impacting Rituximab Estimating Medical care Framework Designs Created nations with free state medical care (as in the UK) can foresee lower costs on drugs. Wellbeing Frameworks that are overwhelmed by confidential wellbeing areas have greater expenses for instance America and UAE. Licenses and Biosimilars Brand name Rituximab actually keeps up with exorbitant costs in view of licenses. The separation of biosimilars is currently conceivable, and they appreciate cost benefits in certain business sectors. Expenses of assembling and bringing in comprise of the uses brought about in assembling the items as well as the expense of bringing in/sembly of the items The consumptions incorporate pay rates of representatives if the firm fabricates the items and the expense of shipping imported/sembly of the items. Contrasting and neighborhood creation, bringing in drugs raises costs, as it has been seen in the UAE. Protection and Sponsorships Albeit in the USA and UAE patients pay a specific measure of cash using cash on hand and protection makes up for some of it, Rituximab is substantially more reasonable in the UK, because of endowments.
Ways to decrease Rituximab Expenses Investigate Biosimilar Choices Biosimilars can decrease treatment costs by up to 30-half, contingent upon the market. Influence Monetary Help Projects Numerous drug organizations offer expense decrease programs for qualified patients. Go for Treatment For patients in significant expense districts like the USA, looking for treatment in nations like the UK can bring about huge reserve funds. Protection Advancement Guarantee thorough inclusion and survey choices for specialty drug plans.
Conclusion
Valuing of Rituximab contrasts altogether in the three nations in view of contrasts in framework structure, estimating strategies and powers of interest and supply. The USA has the absolute greatest costs around the world, the UK offers more open access with the NHS, and the UAE with its blended framework, falls some place in the center. Thusly, a patient must realize the elements impacting rituximab costs and the chance of the utilization of biosimilar and monetary help programs. Rituximab and comparative medications ought not be a question of money similar to the creation of an educated choice.
0 notes
Text
The Future of Healthcare: Brain-Computer Interface Technology
Brain-Computer Interface (BCI) are innovative systems that directly link the brain to external devices, converting neural activity into actionable commands. By utilizing sensors, electrodes, or imaging technologies, BCIs decode brain signals and transmit them to operate medical tools, robotic systems, or computers. This groundbreaking technology is revolutionizing healthcare by offering novel solutions for treating neurological diseases and driving forward new healthcare advancements on a global scale.
How Can BCIs Assist Individuals with Neurological Disorders?
BCIs offer groundbreaking treatments for people with neurological disorders such as ALS, Parkinson's disease, and spinal cord injuries. For instance, bci medical applications allow individuals with mobility challenges to control prosthetics or wheelchairs through thought alone. They are also helping restore communication abilities in non-verbal patients and improving the management of conditions like epilepsy and stroke recovery.
What Are the Different Types of Brain-Computer Interfaces?
BCIs are primary methods of Brain-Computer Interface categorized into three primary types:
Invasive BCIs: These devices are surgically implanted into the brain and provide high accuracy, particularly for those with severe disabilities.
Non-invasive BCIs: These use external sensors, such as EEG caps, and are commonly used in mental health and rehabilitation settings.
Partially invasive BCIs: These devices are placed on the surface of the brain, offering a balance between precision and invasiveness.
Benefits and Challenges of Brain-Computer Interfaces
BCIs offer a wide range of benefits of BCI in healthcare, including improved mobility, better mental health treatment, and real-time brain activity monitoring. However, challenges such as high costs, data privacy issues, and technical complexity continue to hinder their widespread use.
Leading Companies in the Brain-Computer Interface (BCI) Market
Several key players are driving Brain-Computer Interface companies , including Advanced Brain Monitoring, Inc., Cadwell Industries, Inc., Cortech Solutions, Inc., Emotiv, G.Tec Medical Engineering GmbH, Integra Lifesciences, Natus Medical Incorporated, Neurosky, Nihon Kohden Corporation, OpenBCI, Medtronic, Compumedics Neuroscan, Brain Products GmbH, Interaxon, Inc., ANT Neuro, Neuroelectrics, Ripple Neuro, NIRx Medical Technologies, LLC, CGX (A Cognionics Company), NextMind SAS, Blackrock Neurotech, among others.
The Future of Brain-Computer Interfaces
The future of brain-computer interfacing is incredibly promising, with expanding applications in personalized medicine, mental health, and remote care. As the MedTech industry continues to evolve, BCIs will redefine healthcare by offering transformative solutions for patients and shaping the future of medical technology.
Latest Healthcare Market Research Reports:
Lipodystrophy Market | Nasolabial Fold Market | Natural Killer T Cell Lymphoma Market | Nrg1 Fusion Cancer Market | Osteochondrodysplasias Market | Pachyonychia Congenita Market | Pegfilgrastim Biosimilar Insight | Salivary Gland Infection Market | Scedosporium Infection Market | Shoulder Replacement Devices Market | Single Ventricle Heart Disease Market | Spinal Decompression/traction Devices Market | Stuttering Market | Tenosynovitis Market | Treatment-resistant Hypertension Market
About DelveInsight
DelveInsight is a prominent market research and consulting firm specializing in the life sciences and healthcare sectors. Through its actionable insights, DelveInsight empowers pharmaceutical, biotech, and medical device companies to make informed decisions in a rapidly evolving marketplace.
Contact Information
Kanishk
Email: [email protected]
0 notes
Text
Breaking Ground in Pharma: The 15th Digital Pharmaceutical Innovations Exhibition & Congress
Breaking Ground in Pharma: The 15th Digital Pharmaceutical Innovations Exhibition & Congress
Introduction: The pharmaceutical industry is rapidly evolving, driven by cutting-edge technologies and breakthrough innovations that are transforming healthcare. One of the key events that highlight these advancements is the 15th Digital Pharmaceutical Innovations Exhibition & Congress. This prestigious event gathers thought leaders, industry experts, and innovators from across the globe to discuss the latest trends, research, and technologies in the field of biopharmaceuticals and biosimilars. The growing importance of digital technologies and innovative solutions in the biopharmaceutical sector is at the heart of this event, which showcases how these advances are making healthcare more accessible, efficient, and personalized.
The 15th Digital Pharmaceutical Innovations Exhibition & Congress: A Spotlight on Biopharmaceuticals and Biosimilars
The world of pharmaceuticals is evolving rapidly, and one of the most important and exciting sectors within it is biopharmaceuticals and biosimilars. These cutting-edge treatments are reshaping healthcare and providing new opportunities to treat diseases in ways that were once thought impossible. The 15th Digital Pharmaceutical Innovations Exhibition & Congress offers an incredible opportunity for professionals, experts, and innovators to come together and explore the future of these transformative technologies.
What is the 15th Digital Pharmaceutical Innovations Exhibition & Congress?
The 15th Digital Pharmaceutical Innovations Exhibition & Congress is an annual event that brings together stakeholders from across the pharmaceutical and biotechnology industries. This year’s congress is entirely digital, offering attendees the flexibility to participate from anywhere in the world, interact with key industry players, and access valuable insights from thought leaders and researchers.
The event will focus on the latest advancements in biopharmaceuticals and biosimilars, which are at the forefront of the pharmaceutical landscape. With the ongoing rise in demand for biologic treatments, the discussion around their production, regulation, and accessibility has never been more relevant.
What Are Biopharmaceuticals and Biosimilars?
Before diving into the specifics of the exhibition, it’s important to define biopharmaceuticals and biosimilars:
Biopharmaceuticals: These are drugs that are produced using living organisms or contain components from living organisms. Unlike traditional chemical drugs, biopharmaceuticals are complex molecules such as proteins, vaccines, and monoclonal antibodies. They are used to treat a wide range of conditions, including cancers, autoimmune diseases, and rare genetic disorders.
Biosimilars: A biosimilar is a biologic medical product that is highly similar to an already approved reference product. While they are not identical to their reference products, biosimilars are proven to have no clinically meaningful differences in terms of safety, efficacy, and quality. The advent of biosimilars has opened up new opportunities for patients to access life-saving medications at a more affordable cost.
Key Topics at the Exhibition & Congress
Innovative Biopharmaceutical Research and Development: The event will feature the latest breakthroughs in biopharmaceutical R&D, focusing on the design, discovery, and optimization of biologic drugs. Experts will share insights into how new technologies like gene editing, artificial intelligence, and advanced analytics are accelerating the development of next-generation biologics.
Regulatory Landscape for Biosimilars: As the market for biosimilars continues to grow, regulatory frameworks are evolving. The congress will explore the regulatory hurdles that need to be overcome to bring biosimilars to market and how agencies around the world are addressing these challenges.
Manufacturing of Biopharmaceuticals: The complexity of biologic production and the need for highly specialized manufacturing processes is another area that will be explored at the event. Topics will include innovations in biomanufacturing technologies, quality control, and strategies for scaling production while maintaining product consistency.
Patient Access and Market Access for Biopharmaceuticals and Biosimilars: One of the biggest challenges in the pharmaceutical industry is ensuring that patients have access to the medications they need. The congress will examine the role of policy, insurance, and pricing models in improving access to biopharmaceuticals and biosimilars, ensuring that these treatments are available to those who need them most.
The Future of Personalized Medicine: Personalized medicine is an emerging field that aims to tailor treatment to an individual’s genetic profile. Biopharmaceuticals, particularly gene therapies, are at the forefront of this revolution. Attendees will learn about the latest developments in personalized medicine and its potential to revolutionize the way diseases are treated.
Networking Opportunities
In addition to the informative sessions, the 15th Digital Pharmaceutical Innovations Exhibition & Congress provides numerous networking opportunities. Attendees can connect with industry leaders, researchers, and peers through virtual networking sessions, roundtable discussions, and one-on-one meetings. Whether you are looking to collaborate on a research project, discuss potential partnerships, or simply exchange ideas, this event will facilitate valuable connections.
Who Should Attend?
The congress is open to a wide range of professionals, including:
Pharmaceutical Companies: Executives and managers looking to stay at the forefront of biopharmaceutical innovation.
Researchers and Scientists: Those engaged in cutting-edge research in the fields of biopharmaceuticals, biosimilars, and related disciplines.
Regulatory Experts: Professionals involved in the approval and regulation of biologic products.
Manufacturers and Suppliers: Companies involved in the production and supply chain of biopharmaceuticals and biosimilars.
Investors: Those interested in the investment opportunities within the biopharmaceutical and biosimilar sectors.
Why Attend?
By attending the 15th Digital Pharmaceutical Innovations Exhibition & Congress, you’ll gain access to:
In-depth insights from industry leaders and experts.
Cutting-edge research and trends in biopharmaceuticals and biosimilars.
Networking opportunities with peers, potential collaborators, and thought leaders.
A front-row seat to the future of pharmaceuticals.
Benefits of Attending the Digital Pharmaceutical Innovations Exhibition & Congress: Biopharmaceuticals and Biosimilars
Cutting-Edge Insights: Stay ahead of the curve with the latest research, developments, and trends in biopharmaceuticals and biosimilars.
Networking Opportunities: Connect with industry leaders, innovators, and peers from around the globe.
Access to Experts: Gain valuable knowledge from leading experts through presentations, discussions, and panels.
Market Access Strategies: Learn about new strategies for improving access to biopharmaceuticals and biosimilars.
Regulatory Insights: Stay informed on the evolving regulatory landscape surrounding biologics and biosimilars.
Investment Potential: Explore the investment opportunities in the biopharmaceutical and biosimilar sectors.
Personalized Medicine: Dive into the future of personalized medicine powered by biopharmaceuticals and gene therapies.
Conclusion
As the pharmaceutical industry continues to evolve, events like the 15th Digital Pharmaceutical Innovations Exhibition & Congress play a crucial role in shaping the future. By focusing on biopharmaceuticals and biosimilars, this year’s event will provide attendees with the knowledge and connections needed to stay ahead in the ever-changing landscape of medicine.
Don’t miss out on this incredible opportunity to learn, network, and engage with the leaders of tomorrow’s pharmaceutical innovations. Register today and be part of the digital revolution in healthcare!
More Information
To learn more about the 15th Digital Pharmaceutical Innovations Exhibition & Congress, visit our website or connect with us on social media:
#Biopharmaceuticals #Biosimilars #Biotech #PharmaceuticalInnovation #BiologicTherapies #BiosimilarDevelopment #FutureOfMedicine #DrugDevelopment #MedicalInnovation #BioTechRevolution #PharmaResearch #AffordableHealthcare #Biopharma #BiosimilarTherapies #PrecisionMedicine
👉 Register here: -https://pharmacy.utilitarianconferences.com/registration
Website: https://utilitarianconferences.com/
Twitter: @UCGConferences
Facebook: Utilitarian Conferences Gathering
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
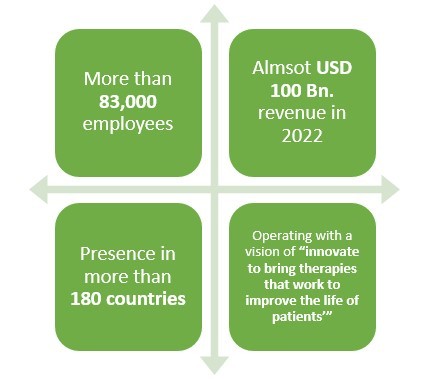
1.Pfizer
Click to read more about Pfizer
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
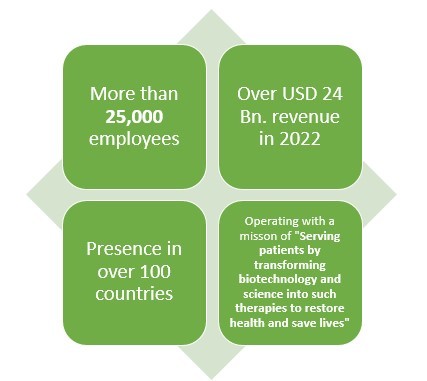
2.Amgen
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
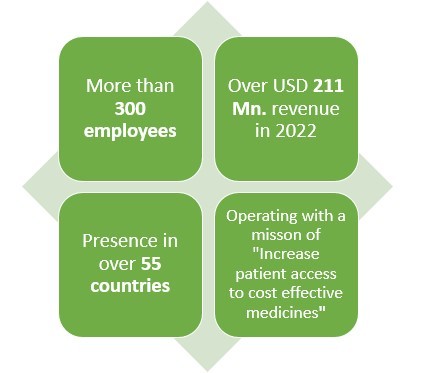
4.Coherus Biosciences:
Click here to Ask for a Custom Report
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
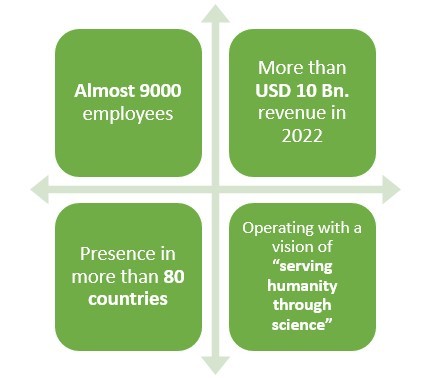
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
The Prokaryotic Recombinant Protein Market is projected to grow from USD 2725.2 million in 2024 to an estimated USD 4278.42 million by 2032, with a compound annual growth rate (CAGR) of 5.8% from 2024 to 2032.The Prokaryotic Recombinant Protein Market has been experiencing significant growth, driven by advancements in biotechnology, expanding research in protein therapeutics, and increasing demand for cost-effective biologics production. Prokaryotic systems, particularly Escherichia coli (E. coli), have emerged as a preferred host for recombinant protein expression due to their simplicity, rapid growth, and ability to produce high yields. This article explores the key factors driving the market, challenges, applications, and future prospects.
Browse the full report at https://www.credenceresearch.com/report/prokaryotic-recombinant-protein-market
Market Drivers and Dynamics
Rising Demand for Biologics and Biosimilars Biologics, including monoclonal antibodies, vaccines, and enzymes, are critical in treating chronic diseases like cancer, diabetes, and autoimmune disorders. The production of recombinant proteins using prokaryotic systems is cost-effective and scalable, making it an attractive option for biosimilar development.
Technological Advancements in Recombinant Protein Production Continuous innovations in genetic engineering, such as CRISPR-Cas9 and synthetic biology, have improved the precision and efficiency of prokaryotic expression systems. Advanced tools for optimizing codon usage, promoters, and plasmids have significantly enhanced the expression of complex proteins.
Growing Biopharmaceutical Research and Development (R&D) The surge in R&D investments by pharmaceutical and biotech companies to develop novel therapies has fueled the demand for prokaryotic recombinant proteins. Research initiatives aimed at understanding disease pathways, drug discovery, and protein-protein interactions rely heavily on these proteins.
Applications in Diverse Sectors
Pharmaceutical and Therapeutics Prokaryotic recombinant proteins are widely used to produce therapeutic proteins such as insulin, growth hormones, and clotting factors. The affordability and scalability of prokaryotic systems make them indispensable for meeting the global demand for life-saving biologics.
Diagnostics The diagnostic industry uses recombinant proteins to develop enzyme-linked immunosorbent assays (ELISA), Western blotting, and other diagnostic tools. These proteins are essential for detecting infectious diseases, autoimmune disorders, and cancers.
Agriculture and Industrial Applications In agriculture, recombinant proteins are used to develop genetically modified crops with enhanced resistance to pests and diseases. Industrial enzymes produced in prokaryotic systems are employed in various industries, including food and beverage, textiles, and biofuels.
Challenges in the Market
Limitations in Post-Translational Modifications Prokaryotic systems lack the machinery for post-translational modifications, such as glycosylation, which are essential for the biological activity of certain therapeutic proteins. This limitation has restricted the use of prokaryotic systems for complex protein production.
Protein Misfolding and Aggregation High expression levels in prokaryotic systems can lead to misfolded or aggregated proteins, affecting their functionality. Overcoming these challenges requires optimizing culture conditions and using molecular chaperones.
Regulatory and Ethical Considerations The production of recombinant proteins must comply with stringent regulatory standards to ensure safety and efficacy. The ethical implications of genetic engineering also continue to be a topic of debate.
Future Prospects
The Prokaryotic Recombinant Protein Market is poised for continued growth, supported by advancements in synthetic biology, the integration of AI in protein design, and the development of hybrid systems that combine the strengths of prokaryotic and eukaryotic hosts. Moreover, the increasing focus on personalized medicine and precision therapies is likely to expand the market's applications.
Sustainability in protein production will also play a critical role. Efforts to reduce environmental impact, such as using renewable feedstocks and optimizing bioprocesses, will shape the market's trajectory.
Key Player Analysis:
Abnova Corporation
Batavia Biosciences
Bioclone
Cayman Chemical Company
Cusabio Technology
Eli Lilly and Company
Geltor IndieBio
Geno Technology
Kaneka and Eurogentec
Merck
Prospec Tany Technogene
Randox Laboratories
Roche
Segmentation:
By Product Type:
Hormones
Interferons
Interleukins
Others
By End-User/Application:
Biotechnology Companies
Research institutes
Contract Research organizations
Hospital
Laboratories
Others
By Region
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/prokaryotic-recombinant-protein-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
0 notes
Text
The Albumin Industry: A Vital Component in Healthcare and Biopharmaceuticals
Albumin, a vital protein found in blood plasma, has become an indispensable resource in the healthcare and biopharmaceutical industries. Known for its versatility and wide-ranging applications, albumin serves critical roles in medical treatments, drug delivery systems, and diagnostics. Its increasing demand across healthcare systems worldwide underscores its importance in addressing diverse clinical needs, from treating life-threatening conditions like hypovolemia and shock to enabling advanced drug formulations in biopharmaceuticals.
The albumin market is projected to reach a value of USD 7.27 billion in 2024 and is anticipated to grow to USD 10.56 billion by 2029, exhibiting a compound annual growth rate (CAGR) of 7.76% over the forecast period from 2024 to 2029.
Understanding Albumin and Its Importance
Albumin, primarily derived from human plasma, is a key protein responsible for maintaining oncotic pressure and fluid balance in the body. It also serves as a carrier for hormones, enzymes, drugs, and other vital substances. In the medical field, albumin is used to treat various conditions, including:
Hypovolemia: Restoring blood volume in cases of severe fluid loss.
Hypoproteinemia: Managing low protein levels due to liver or kidney diseases.
Burns and Trauma: Providing nutritional and osmotic support during recovery.
Neonatal Conditions: Addressing complications in premature infants.
Beyond its therapeutic use, albumin is widely employed in research and drug formulation as a stabilizer and carrier. Its ability to bind with various molecules makes it an ideal component for drug delivery systems, especially for targeting challenging diseases like cancer and autoimmune disorders.
Key Drivers of Growth in the Albumin Industry
1. Rising Incidence of Chronic Diseases and Critical Conditions
The growing prevalence of chronic diseases such as liver cirrhosis, nephrotic syndrome, and cancer has significantly increased the demand for albumin. These conditions often lead to complications requiring albumin-based therapies. Additionally, the rising number of surgeries, trauma cases, and burn injuries has further fueled the need for albumin in critical care settings.
2. Expansion of Biopharmaceutical Applications
Albumin is gaining prominence in the biopharmaceutical sector as a stabilizer in drug formulations and as a carrier for targeted drug delivery. With the increasing development of biologics and biosimilars, albumin's role in enhancing the stability and efficacy of therapeutic agents is driving its adoption. Technologies like albumin-fusion platforms are enabling the creation of long-acting drugs, further expanding its applications in cutting-edge treatments.
3. Growth in Plasma Fractionation
Advancements in plasma fractionation technology have improved the efficiency of albumin production, allowing for greater yields and quality. Plasma fractionation remains the primary method for extracting albumin from blood plasma, and innovations in this area have made albumin more accessible to meet growing demand.
4. Increasing Awareness and Adoption in Emerging Markets
Developing regions, particularly in Asia-Pacific and Latin America, are witnessing increased adoption of albumin due to rising awareness about its therapeutic benefits. Governments and healthcare organizations in these regions are investing in improved healthcare infrastructure, driving demand for albumin-based treatments.
5. Regulatory Support and Strategic Partnerships
Government initiatives and regulatory frameworks supporting plasma-derived therapies have encouraged investments in the albumin market. Furthermore, collaborations between pharmaceutical companies and research institutions are accelerating the development of novel albumin-based products, contributing to market growth.
Challenges in the Albumin Industry
1. Limited Plasma Supply
Albumin production relies heavily on human plasma donations, which are subject to strict regulations and limited availability. The supply-demand gap poses a significant challenge, particularly as global demand for plasma-derived therapies continues to rise.
2. High Production Costs
The extraction and purification of albumin through plasma fractionation are resource-intensive processes, contributing to high production costs. These costs can limit the affordability of albumin-based therapies, particularly in low-income regions.
3. Competition from Synthetic Alternatives
The development of synthetic or recombinant albumin as an alternative to plasma-derived albumin is gaining traction. While these alternatives offer advantages such as reduced dependency on plasma donations, they also introduce competition that could impact the traditional albumin market.
4. Stringent Regulatory Requirements
Albumin production and distribution are subject to rigorous regulatory standards to ensure safety and efficacy. Navigating these regulations can be time-consuming and costly for manufacturers, delaying product approvals and market entry.
5. Ethical and Supply Chain Concerns
The ethical considerations surrounding plasma donation, coupled with complex supply chain logistics, present additional challenges. Ensuring a sustainable and ethical plasma supply chain is critical for the long-term growth of the albumin industry.
Emerging Trends Shaping the Albumin Industry
1. Recombinant Albumin Development
Recombinant albumin, produced through genetic engineering, is gaining attention as a sustainable alternative to plasma-derived albumin. It offers advantages such as consistent quality, reduced risk of contamination, and scalability in production.
2. Integration with Advanced Drug Delivery Systems
Albumin is increasingly being integrated with nanoparticle-based drug delivery systems to enhance targeted drug delivery. This innovation is particularly relevant in oncology, where albumin-bound drugs like paclitaxel (marketed as Abraxane) have demonstrated improved efficacy and reduced side effects.
3. Expansion of Albumin in Diagnostics
In addition to its therapeutic applications, albumin is being explored for use in diagnostic tools, such as in vitro assays and biomarkers for detecting diseases. Its role in diagnostics is expected to expand as research uncovers new applications.
4. Sustainability and Ethical Plasma Sourcing
The industry is focusing on creating more sustainable plasma collection practices and ensuring ethical sourcing to meet growing demand. Initiatives to increase plasma donation awareness and streamline collection processes are gaining momentum.
5. Personalized Medicine and Albumin Fusion Technology
Albumin fusion technology is enabling the development of personalized therapies by extending the half-life of drugs and optimizing their pharmacokinetics. This trend aligns with the broader movement toward personalized medicine, offering tailored treatment options for patients.
Conclusion
The albumin industry is an essential pillar of modern healthcare, addressing critical medical needs while supporting advancements in biopharmaceuticals and diagnostics. As demand for albumin continues to grow, driven by rising chronic disease prevalence, expanding biopharmaceutical applications, and technological innovations, the industry is poised for sustained growth.
While challenges such as plasma supply limitations and high production costs persist, emerging trends like recombinant albumin and advanced drug delivery systems offer promising solutions. With a focus on sustainability, innovation, and accessibility, the albumin industry is set to play a transformative role in shaping the future of healthcare, ensuring better outcomes for patients worldwide. For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence: https://www.mordorintelligence.com/industry-reports/albumin-market
#Albumin Market#Albumin Market Size#Albumin Market Share#Albumin Market Analysis#Albumin Market Report
0 notes
Text
Global Golimumab Powerhouses: Top 5 Countries Driving Growth
According to a recent research, Industry revenue for Golimumab is expected to rise to $26.7 billion by 2035 from $16.8 billion of 2024. U.S., Germany, France, Japan and UK are the top 5 markets and combinely holds substantial demand share. The revenue growth of market players in these countries is expected to range between 3% and 4% annually for period 2025 to 2035.
Industry transition including embracing biosimilars and technological innovation in drug administration, are transforming the supply chain of Golimumab market. In the changing landscape of the pharmaceutical sectors development there seems to be a move away from conventional medications towards biosimilars, which are akin to biological medical products. Golimumab—a biologic for autoimmune conditions—has felt the effects of this change. The emergence of biosimilars like Simponi has intensified competition in the market and led to price reductions providings patients with better access, to treatments. Furthermore the increased profitability linked with biosimilars is attracting a number of companies to venture into this field. This surge is fostering advancements and effectiveness, in golimumab therapies.
Potential Application Areas
Psoriatic Arthritis Management: From a perspective the use of golimumab is crucial in treating Psoriatic Arthritis. Subcutaneous administration of golimumab is the method in this context owing to its effectiveness and positive outcomes. The medication has demonstrated results in alleviating symptoms and slowing down the advancement of Psoriatic Arthritis. Significantly companies such as Johnson & Johnson have embraced this approach successfully strengthen their position, in the market space.
Ankylosing Spondylitis Therapy: The use of golimumab represents an advancement in the treatment of Ankylosing Spondylitis as healthcare providers frequently suggest intravenous golimumab due to its quick effectiveness and advantages, in managing this condition.
Industry Leadership and Strategies
The Golimumab market is characterized by intense competition, with a number of leading players such as Johnson & Johnson, Merck & Co. Inc, Pfizer Inc, AbbVie Inc, Eli Lilly and Company, Amgen Inc, Bristol-Myers Squibb, Celltrion Inc, Gilead Sciences, Biocon Ltd, Momenta Pharmaceuticals and Mylan N.V.. These players are pushing the boundaries of innovation & technological advancements and forging strategic partnerships to expand the existing reach of the market. Below table briefs about adopted market strategies by leading players.Access detailed report insights here - https://datastringconsulting.com/industry-analysis/golimumab-market-research-report
About DataString Consulting
DataString Consulting assist companies in strategy formulations & roadmap creation including TAM expansion, revenue diversification strategies and venturing into new markets; by offering in depth insights into developing trends and competitor landscapes as well as customer demographics. Our customized & direct strategies, filters industry noises into new opportunities; and reduces the effective connect time between products and its market niche.
DataString Consulting is a professional market research company which aims at providing all the market & business research solutions under one roof. Get the right insights for your goals with our unique approach to market research and precisely tailored solutions. We offer services in strategy consulting, comprehensive opportunity assessment across various sectors, and solution-oriented approaches to solve business problems.
0 notes
Text
The Rise of CMOs: How Outsourcing is Transforming Pharma Manufacturing
https://jpcdn.it/img/e030d2be4cc14e79bf66420ff3b18cbe.jpg
In recent years, the pharmaceutical industry has undergone a significant transformation, with Contract Manufacturing Organizations (CMOs) playing a pivotal role in this evolution. As companies face increasing pressure to reduce costs, accelerate time-to-market, and navigate complex regulatory landscapes, CMOs have emerged as strategic partners, reshaping the way drugs are developed and manufactured. This blog explores the rise of CMOs and how outsourcing is driving innovation and efficiency in the pharmaceutical sector.
The Role of CMOs in Pharma Manufacturing
CMOs are third-party organizations that provide manufacturing services to pharmaceutical and biotechnology companies. These services range from drug development and formulation to large-scale commercial production. By outsourcing manufacturing to CMOs, pharmaceutical companies can focus on their core competencies, such as drug discovery and marketing, while leveraging the specialized expertise and advanced infrastructure of their CMO partners.
Key Drivers of CMO Growth
Cost Efficiency:
Establishing and maintaining in-house manufacturing facilities is capital-intensive. CMOs allow companies to avoid these upfront costs by providing access to state-of-the-art facilities and equipment.
Outsourcing also helps companies optimize operational expenses, as CMOs often operate at scale, reducing per-unit production costs.
Accelerated Time-to-Market:
Speed is critical in the competitive pharmaceutical landscape. CMOs have the expertise and resources to streamline production processes, enabling faster development and commercialization of drugs.
Their experience in navigating regulatory approvals ensures compliance without unnecessary delays.
Flexibility and Scalability:
CMOs offer flexible solutions that adapt to changing market demands. Companies can scale production up or down without the need for significant internal adjustments.
This flexibility is especially valuable for smaller biotech firms that may not have the resources to build their own facilities.
Access to Expertise:
CMOs specialize in specific areas, such as biologics, small molecules, or advanced drug delivery systems. Partnering with them provides access to cutting-edge technology and technical know-how.
Many CMOs invest heavily in R&D, which translates into innovative solutions for their clients.
Global Reach:
With a network of facilities across multiple regions, CMOs enable pharmaceutical companies to access global markets efficiently. This is particularly important for meeting regulatory requirements in different countries.
How CMOs Are Transforming the Industry
Advancing Biologics Manufacturing:
The rise of biologics and biosimilars has created a demand for specialized manufacturing capabilities. CMOs with expertise in cell and gene therapies, monoclonal antibodies, and vaccines are leading this transformation.
Driving Digitalization:
Many CMOs are adopting digital technologies, such as automation, artificial intelligence, and IoT, to enhance production efficiency and quality control. These advancements ensure consistency and reduce errors in manufacturing.
Fostering Innovation:
CMOs often act as innovation hubs, collaborating with pharmaceutical companies to develop novel formulations and delivery systems. This collaboration accelerates the introduction of new therapies to the market.
Promoting Sustainability:
Environmental concerns are driving CMOs to adopt sustainable manufacturing practices. From reducing waste to using renewable energy, CMOs are contributing to a greener pharmaceutical industry.
Challenges and Considerations in Outsourcing
While CMOs offer numerous benefits, outsourcing also comes with its challenges:
Quality Control: Ensuring consistent product quality across different CMO facilities can be complex.
Regulatory Compliance: Pharmaceutical companies must ensure their CMOs adhere to stringent regulatory standards.
Intellectual Property (IP) Protection: Safeguarding proprietary information and formulations is critical when working with external partners.
Dependence on Partners: Relying heavily on a single CMO can pose risks if there are disruptions in their operations.
To mitigate these challenges, companies must establish robust contractual agreements, conduct thorough due diligence, and maintain strong communication with their CMO partners.
The Future of CMOs in Pharma
As the pharmaceutical industry continues to evolve, the role of CMOs is expected to grow even further. Key trends shaping the future include:
Increased Focus on Personalized Medicine: CMOs will play a crucial role in manufacturing small-batch, customized therapies for individual patients.
Expansion of Emerging Markets: The demand for affordable medicines in developing countries will drive CMO growth in these regions.
Integration of AI and Automation: Advanced technologies will enable CMOs to deliver even greater efficiency, quality, and speed.
Conclusion
The rise of CMOs has transformed pharmaceutical manufacturing, enabling companies to innovate, scale, and compete in an increasingly complex market. By leveraging the expertise and capabilities of CMOs, pharmaceutical firms can focus on their strengths while delivering high-quality medicines to patients around the world. As the industry continues to embrace outsourcing, CMOs will remain at the forefront of this transformation, driving progress and redefining the future of pharma manufacturing.
0 notes