#Oncology Biosimilars Market Growth
Explore tagged Tumblr posts
Text
The global oncology biosimilars market has grown to $4.7 Billion in 2023 and is set to reach $30.3 Billion by 2032, with a robust CAGR of 22.3%. This marks a major advancement in affordable cancer treatments.
0 notes
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
India's Pharma Industry – The Leading Companies You Need to Know
India's pharmaceutical industry stands as a global powerhouse, contributing significantly to the world's supply of medicines and pharmaceutical products. The country's ability to produce high-quality, affordable medicines has earned it the title of "Pharmacy of the World." As the industry continues to grow and innovate, several companies have emerged as leaders in the market. For Centurion HealthCare Pvt. Ltd., understanding the landscape of the top pharma companies in India provides insights into the key players driving the industry's success.

The Rise of the Pharmaceutical Industry in India
The pharmaceutical industry in India has seen exponential growth over the past few decades. From generic drug manufacturing to complex biotechnological innovations, Indian pharma companies have made substantial contributions to global healthcare. This growth can be attributed to several factors, including a skilled workforce, robust research and development infrastructure, and supportive government policies.
Key Players in India's Pharma Industry
The landscape of the pharmaceutical industry in India is populated by numerous companies, each contributing to various segments of the market. Here are some of the top pharmaceutical companies in India that are leading the charge:
1. Sun Pharmaceutical Industries Ltd.
As the largest pharmaceutical company in India, Sun Pharma is renowned for its diverse product portfolio, including generics, branded generics, specialty medicines, and active pharmaceutical ingredients (APIs). The company has a significant global presence and continues to expand its footprint through strategic acquisitions and partnerships.
2. Dr. Reddy's Laboratories
Dr. Reddy's is a major player in the global generic pharmaceutical market. Known for its strong focus on research and development, the company offers a wide range of pharmaceuticals and biotechnology products. Their commitment to quality and innovation has solidified their position as one of the best pharmaceutical companies in India.
3. Cipla Ltd.
Cipla has been at the forefront of providing affordable medicines for over eight decades. The company specializes in respiratory, cardiovascular, anti-retroviral, and anti-infective therapies. Cipla's dedication to healthcare accessibility and its significant contributions to global health initiatives make it a top pharmaceutical company in India.
4. Lupin Limited
Lupin is a leading pharmaceutical company known for its focus on complex generics and specialty drugs. The company's strong presence in both developed and emerging markets has earned it a place among the top 10 pharmaceutical companies in India. Lupin's investment in R&D and its broad therapeutic portfolio are key drivers of its success.
5. Aurobindo Pharma
Aurobindo Pharma is recognized for its extensive range of generic formulations and APIs. The company's robust manufacturing capabilities and strategic global presence have made it one of the top pharmaceutical companies in India. Aurobindo's commitment to innovation and quality continues to propel its growth.
6. Zydus Cadila
Zydus Cadila, a leading pharmaceutical company, offers a wide range of healthcare solutions, including small molecules, biologics, biosimilars, and vaccines. The company's integrated operations and strong research capabilities have established it as a key player in the pharma industry in India.
7. Glenmark Pharmaceuticals
Glenmark is a global research-led pharmaceutical company known for its focus on innovation in the fields of dermatology, respiratory, and oncology. The company's strong pipeline of new chemical entities and biosimilars underscores its position as one of the best pharmaceutical companies in India.
8. Torrent Pharmaceuticals
Torrent Pharma is a major player in the cardiovascular and central nervous system therapeutic areas. The company's strategic acquisitions and focus on niche segments have helped it become one of the top pharmaceutical companies in India. Torrent's commitment to quality and patient-centric approach is evident in its product offerings.
9. Biocon Ltd.
Biocon is India's largest biopharmaceutical company, specializing in biologics and biosimilars. The company's focus on affordable innovation and its significant contributions to chronic disease management make it a leader in the pharmaceutical industry in India. Biocon's global partnerships and strong R&D capabilities are key to its success.
10. Cadila Healthcare (Zydus)
Cadila Healthcare, also known as Zydus, is a prominent player in the Indian pharma industry, offering a wide range of healthcare solutions. The company's innovative approach and comprehensive product portfolio have positioned it among the top 10 pharmaceutical companies in India.
The Role of Pharma Manufacturing Companies in India
Pharma manufacturing companies in India play a crucial role in the global supply chain of medicines. These companies not only produce high-quality generics but also invest heavily in research and development to bring new and innovative drugs to the market. The efficiency and scale of Indian pharma manufacturing are key factors in the country's ability to provide affordable medicines worldwide.
Finding the Best Pharma Companies Near You
For those searching for "pharma companies near me," it's important to recognize the regional presence of leading pharmaceutical companies. Many top pharma companies in India have established manufacturing and research facilities in various parts of the country, ensuring widespread access to their products and services.
Centurion HealthCare Pvt. Ltd. – A Leading Player in the Industry
Centurion HealthCare Pvt. Ltd. is an emerging name in the Indian pharmaceutical landscape. As a medicine manufacturing company in India, Centurion HealthCare is dedicated to providing high-quality pharmaceutical products across various therapeutic categories. The company's commitment to innovation, quality, and patient care positions it among the best pharma companies in India.
The Future of the Pharmaceutical Industry in India
The future of the pharmaceutical industry in India looks promising, with continued growth driven by innovation, increasing healthcare needs, and expanding global reach. Indian pharma companies are expected to play a pivotal role in addressing global health challenges, developing new treatments, and ensuring the availability of affordable medicines.
Conclusion
India's pharmaceutical industry is a dynamic and rapidly evolving sector, with numerous companies leading the way in innovation, quality, and global healthcare contributions. From established giants like Sun Pharma and Dr. Reddy's to emerging leaders like Centurion HealthCare Pvt. Ltd., the top pharmaceutical companies in India are making significant strides in improving healthcare outcomes worldwide.
As the industry continues to grow, these companies will remain at the forefront of pharmaceutical advancements, ensuring that India retains its position as a global leader in medicine production and innovation. Whether you are looking for the best pharma company in India or seeking reliable pharmaceutical companies in India, the landscape is rich with options that exemplify excellence and commitment to health.
For Centurion HealthCare Pvt. Ltd., being part of this esteemed group of pharma companies in India is a testament to its dedication to quality, innovation, and patient care. As the industry moves forward, Centurion HealthCare is poised to continue its growth and contribute to the global healthcare landscape, solidifying its place among the best pharmaceutical companies in India.
#Pharma companies near me#Top pharma companies in India#Pharma industry in India#Pharmaceutical industry in India#Top 10 pharmaceutical companies in India#Best pharma company in India#Top pharmaceutical companies in India#best pharmaceutical companies in India#Pharma manufacturing companies in India#Medicine company in India#Pharma companies in India#Pharmaceutical companies in India#Medicine manufacturing company in India
3 notes
·
View notes
Text
CD19 Market Size, Target Population, Competitive Landscape, and Market Forecast to 2034
Introduction to CD19 Therapeutics
CD19, a surface protein expressed on B cells, has become a significant therapeutic target for treating B-cell malignancies such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL). Therapies targeting CD19, including monoclonal antibodies and CAR-T therapies, have demonstrated remarkable clinical outcomes, driving interest and investment in this space.
CD19 Market Size and Growth Projections
The CD19 therapy market is projected to experience substantial growth, with an estimated compound annual growth rate (CAGR) exceeding 20% through 2034. This expansion is driven by the increasing adoption of CAR-T cell therapies, monoclonal antibodies, and other targeted modalities addressing CD19-positive malignancies. The global market is expected to surpass several billion dollars in valuation by 2034, reflecting a strong pipeline of innovative therapies and expanding indications.
Download report @ https://www.delveinsight.com/report-store/cd19-market-forecast
Key factors contributing to the growth of the CD19 market include:
1. Rising Incidence of B-cell Malignancies: The increasing prevalence of ALL, NHL, and related disorders worldwide fuels demand for effective CD19-targeted therapies.
2. Expanding Indications: Beyond hematologic cancers, ongoing research explores the potential of CD19 therapies for autoimmune diseases and other immune disorders.
3. Innovation in Therapeutics: Technological advances, such as next-generation CAR-T cells and bispecific antibodies, improve safety and efficacy, widening the patient base.
CD19 Market Target Population
The target population for CD19 therapies includes patients with:
1. Relapsed/Refractory Hematologic Malignancies: Patients with limited options due to resistance to traditional treatments are primary candidates.
2. Pediatric and Adult Populations: CD19 therapies have demonstrated efficacy in both children (especially for ALL) and adults, ensuring broad applicability.
3. Emerging Areas: Research into autoimmune conditions, where CD19-expressing B cells play a role, could further expand the target demographic.
As the therapeutic landscape evolves, the patient pool is expected to grow, particularly with earlier-line approvals and the development of safer, more effective treatments.
CD19 Market Competitive Landscape
The CD19 market features a highly competitive environment with contributions from both large pharmaceutical companies and innovative biotech firms. Key players in this space include:
1. Novartis: Known for its CAR-T therapy Kymriah, the first FDA-approved CAR-T targeting CD19, Novartis has established itself as a market leader.
2. Gilead Sciences (Kite Pharma): With its Yescarta therapy, Gilead continues to dominate the treatment landscape for large B-cell lymphoma.
3. Bristol Myers Squibb: Through Breyanzi, BMS has entered the CD19 CAR-T space, expanding its oncology portfolio.
4. Emerging Biotechs: Companies such as Allogene and Cellectis are advancing allogeneic CAR-T therapies, focusing on improving accessibility and scalability.
Request for a sample report @ https://www.delveinsight.com/report-store/cd19-market-forecast
CD19 Market Strategic Developments:
- Biosimilar Competition: With patents on existing therapies expiring in the coming years, biosimilars are expected to play a significant role, enhancing competition and accessibility.
- Collaborations and Partnerships: Companies are increasingly collaborating to address manufacturing bottlenecks and regulatory challenges.
CD19 Market Trends and Innovations
1. Next-Generation Therapies: Dual-targeting CAR-T cells and engineered antibodies that improve specificity and reduce toxicity are gaining momentum.
2. Global Expansion: Approvals in emerging markets, including Asia-Pacific and Latin America, will drive market growth as healthcare systems improve access to advanced treatments.
3. Manufacturing Improvements: Efforts to streamline the production of autologous therapies and develop off-the-shelf options will enhance scalability and affordability.
CD19 Market Forecast to 2034
By 2034, the CD19 market is expected to:
- Exceed Multi-Billion Dollar Valuations: Driven by a robust pipeline and expanded indications, the market will continue its upward trajectory.
- Transform Treatment Paradigms: Improved technologies and broader indications will establish CD19-targeted therapies as standard care for many malignancies.
- Increase Global Accessibility: Collaborative efforts to address pricing and manufacturing challenges will make therapies available to a broader range of patients worldwide.
CD19 Market Challenges and Opportunities
Challenges:
- High Costs: Current therapies, particularly CAR-T, are expensive, posing affordability and reimbursement challenges.
- Complex Manufacturing: The personalized nature of autologous CAR-T production limits scalability and availability.
Opportunities:
- Emerging Markets: Expanding into underrepresented regions offers significant growth potential.
- Allogeneic CAR-T Therapies: Off-the-shelf solutions will address cost and manufacturing bottlenecks, transforming the market landscape.
The CD19 market is poised for substantial growth, driven by innovation, expanding indications, and improving accessibility. As therapies evolve to address challenges such as cost and toxicity, their potential to transform the oncology landscape remains unmatched. By 2034, CD19-targeted treatments will likely be at the forefront of precision medicine, offering hope to millions of patients worldwide.
For further insights, explore DelveInsight's [CD19 Market Forecast](https://www.delveinsight.com/report-store/cd19-market-forecast).
0 notes
Text
The Oncology Biosimilars Market is projected to grow from USD 4725 million in 2024 to an estimated USD 23341.41 million by 2032, with a compound annual growth rate (CAGR) of 22.1% from 2024 to 2032. The oncology biosimilars market is rapidly emerging as a key segment in the biopharmaceutical industry. With increasing cases of cancer globally and the rising costs of innovative biologics, oncology biosimilars offer a cost-effective alternative while ensuring similar safety and efficacy profiles. This article delves into the market dynamics, trends, challenges, and future prospects of oncology biosimilars.
Browse the full report https://www.credenceresearch.com/report/oncology-biosimilars-market
Understanding Oncology Biosimilars
Biosimilars are biologic medical products that are highly similar to an already approved reference product, with no clinically meaningful differences in terms of safety, efficacy, or quality. In oncology, biosimilars address various cancers such as breast cancer, colorectal cancer, non-small cell lung cancer, and lymphoma. They replicate biologics like monoclonal antibodies and growth factors used in cancer treatment and supportive care.
Market Growth Drivers
1. Rising Cancer Incidence: According to the World Health Organization (WHO), cancer is a leading cause of death worldwide, with an estimated 20 million new cases in 2022 alone. This growing disease burden amplifies the demand for cost-effective treatment options, making oncology biosimilars a critical component of cancer care.
2. Cost-Effectiveness: Biosimilars are priced approximately 15-30% lower than their reference biologics, offering significant savings for healthcare systems and patients. This affordability is particularly impactful in low- and middle-income countries where access to expensive biologics is limited.
3. Patent Expirations: Several blockbuster oncology biologics, including trastuzumab (Herceptin) and bevacizumab (Avastin), have lost patent protection in recent years. This has paved the way for the entry of biosimilars, driving market competition and adoption.
4. Regulatory Support: Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established clear guidelines for the approval of biosimilars, encouraging pharmaceutical companies to invest in their development.
Key Market Trends
1. Expanding Product Portfolio: The oncology biosimilars market is witnessing an expansion in product offerings. Biosimilars for blockbuster drugs such as rituximab, pegfilgrastim, and cetuximab are gaining traction, with multiple players entering the space.
2. Increasing Approvals and Launches: Over the past decade, the FDA and EMA have approved numerous oncology biosimilars, including biosimilars for trastuzumab (e.g., Ogivri, Herzuma) and bevacizumab (e.g., Zirabev, Mvasi). These approvals have bolstered the market and enhanced access to affordable cancer treatments.
3. Collaborations and Partnerships: Pharmaceutical companies are increasingly entering strategic collaborations to enhance biosimilar development and commercialization. Partnerships between biotech firms, contract research organizations, and healthcare providers are streamlining market entry and expanding distribution networks.
4. Rising Acceptance Among Physicians: Initial skepticism surrounding biosimilars is gradually fading as real-world evidence demonstrates their safety and efficacy. Educational initiatives and clinical data are fostering confidence among oncologists and patients.
Challenges in the Market
1. Regulatory and Development Complexity: Developing biosimilars involves sophisticated processes and significant investment. Ensuring similarity in structure, function, and clinical outcomes with the reference biologic is challenging and time-consuming.
2. Market Competition: While competition drives innovation, it also exerts pressure on pricing and profitability. Companies must adopt innovative pricing strategies and differentiation approaches to capture market share.
3. Physician and Patient Perception: Despite increasing acceptance, some healthcare providers and patients remain cautious about switching from biologics to biosimilars. Addressing these concerns through education and robust clinical evidence is crucial.
Future Outlook
The oncology biosimilars market is poised for exponential growth, driven by factors like rising cancer prevalence, supportive regulatory frameworks, and ongoing technological advancements. By 2030, the market is expected to reach significant valuations, with Asia-Pacific and emerging markets playing a pivotal role due to their large patient populations and cost-sensitive healthcare systems.
Moreover, advancements in biosimilar manufacturing, including the use of artificial intelligence and machine learning, promise to reduce development timelines and costs. Governments and healthcare organizations worldwide are also likely to continue promoting biosimilar adoption through favorable policies and reimbursement frameworks.
Key Player Analysis:
Allergan (Ireland)
Amneal Pharmaceuticals LLC. (U.S.)
Apotex Inc. (Canada)
Aurobindo Pharma (India)
BIOCAD (Russia)
Bristol-Myers Squibb Company (U.S.)
Cipla Inc. (U.S.)
Eli Lilly and Company (U.S.)
Endo International plc (Ireland)
Hoffmann-La Roche Ltd. (Switzerland)
GlaxoSmithKline plc (U.K.)
Glenmark Pharmaceuticals Limited (India)
Lupin (India)
Mylan N.V. (U.S.)
Novartis AG (Switzerland)
Pfizer Inc. (U.S.)
Sanofi (France)
Sun Pharmaceutical Industries Ltd. (India)
Takeda Pharmaceutical Company Limited (Japan)
Teva Pharmaceutical Industries Ltd.(Israel)
Zydus Cadila (India)
Segmentation:
By Drug
G-CSF
Monoclonal Antibody
Hematopoietic Agents
By Disease Indication
Breast Cancer
Non-Small Cell Lung Cancer
Colorectal Cancer
Neutropenia
Blood Cancer
Leukemia
Myeloid Leukemia
Chronic Lymphocytic Leukemia (CLL)
Non-Hodgkin Lymphoma
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
By Regional
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report https://www.credenceresearch.com/report/oncology-biosimilars-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Top 10 Pharma Franchise Companies in Bangalore
Top 10 Pharma Franchise Companies in Bangalore - Ryze Lifecare
Bangalore, a thriving metropolis and a major center for India’s pharmaceutical industry, is home to some of the country’s most reputable pharma franchise companies. Known for its strong healthcare infrastructure and robust industrial network, Bangalore is the preferred location for pharmaceutical investments, partnerships, and franchises. If you’re considering a partnership with a pharma franchise company, it’s crucial to explore the top players in the city for a reliable and rewarding experience. Here, we’ll discuss the top 10 pharma franchise companies in Bangalore, with a special highlight on Ryze Lifecare.
1. Ryze Lifecare
Ryze Lifecare stands at the forefront of the pharma franchise industry in Bangalore, celebrated for its commitment to quality, transparency, and a wide range of products that meet stringent international standards. Ryze Lifecare specializes in various therapeutic areas, including cardiology, neurology, gastroenterology, and dermatology. The company offers an attractive franchise model that includes support in marketing, product training, and distribution to ensure the success of its franchise partners. With a vision to deliver innovative and accessible healthcare solutions, Ryze Lifecare is a strong choice for franchise investors in Bangalore.
2. Biocon Limited
One of India’s largest biotech companies, Biocon Limited has made significant strides in biosimilars, generics, and novel drugs. Their franchise opportunities are centered around high-demand therapeutic areas such as oncology, diabetes, and autoimmune diseases. Biocon’s commitment to innovation and research makes it a preferred partner for those seeking long-term growth and stability in the pharmaceutical industry.
3. Medlife International Pvt. Ltd.
Medlife International Pvt. Ltd. is well-known for its extensive portfolio of pharmaceutical products and its user-friendly online platform. With an array of offerings in health supplements, skincare, and generic medicines, Medlife provides its franchise partners with ample resources for distribution and digital marketing. Medlife’s reputation for reliable delivery and customer service makes it a strong contender in the pharma franchise space.
4. Cipla Ltd.
A globally recognized name, Cipla Ltd. is highly regarded for its commitment to delivering high-quality, affordable healthcare. Cipla’s product portfolio spans respiratory care, HIV/AIDS treatment, and diabetes management, among others. The company’s franchise model is designed to support partners with marketing resources, distribution channels, and regulatory assistance, making it an ideal choice for new entrepreneurs in the pharma sector.
5. Cadila Pharmaceuticals
Cadila Pharmaceuticals is a well-established pharma company with a strong presence across India. Known for its advancements in antibiotics, cardiovascular medicines, and biotechnology, Cadila offers an exceptional franchise program with extensive product training and technical support. Its focus on R&D and innovation provides franchise partners with access to a broad product pipeline and market opportunities in specialized therapeutic areas.
6. Lupin Ltd.
A global player in the pharmaceutical industry, Lupin Ltd. specializes in a range of areas such as cardiovascular health, pediatrics, and neurology. Lupin’s franchise program is built to provide comprehensive business support, ensuring their partners have the necessary resources to succeed. Lupin’s extensive market reach and reputation for high-quality drugs make it a top choice for franchise opportunities in Bangalore.
7. GlaxoSmithKline Pharmaceuticals Ltd.
With a well-established global presence, GlaxoSmithKline (GSK) Pharmaceuticals Ltd. offers an extensive range of products in vaccines, oral health, and respiratory health. GSK’s franchise program includes robust support for partners in terms of training, marketing assistance, and a reliable supply chain network. Their cutting-edge R&D ensures that franchisees have access to a continuously updated portfolio of products.
8. Mankind Pharma
Mankind Pharma is a trusted name in the Indian pharmaceutical market, known for its cost-effective range of medicines across various therapeutic categories. With an impressive product portfolio that includes antibiotics, anti-allergics, and lifestyle products, Mankind Pharma offers franchise partners strong marketing support and competitive pricing models. Their widespread recognition in rural and urban markets provides franchise partners with a diverse customer base.
9. Dr. Reddy’s Laboratories
Dr. Reddy’s Laboratories is a multinational pharmaceutical company known for its innovations in generic formulations, biosimilars, and specialty drugs. The company offers an advanced franchise program with top-tier support in regulatory compliance, marketing, and logistics. Dr. Reddy’s Laboratories’ strong focus on ethical business practices and quality makes it a preferred choice for franchise opportunities in Bangalore.
10. Aurobindo Pharma Ltd.
Aurobindo Pharma Ltd. is renowned for its wide-ranging pharmaceutical offerings, including antiretrovirals, anti-infectives, and pain management products. Aurobindo’s franchise model offers partners robust marketing resources and a streamlined supply chain, ensuring a competitive edge in the market. The company’s extensive international presence also lends credibility and resources to its franchisees, making it a popular choice among Bangalore-based pharma investors.
Conclusion
In conclusion, the pharma franchise industry in Bangalore offers a multitude of opportunities for both seasoned and new entrepreneurs. Each company mentioned above, particularly Ryze Lifecare, provides franchisees with strong support and an excellent product portfolio, ensuring high returns on investment. When choosing a franchise partner, it’s essential to consider factors such as product range, market reputation, and support infrastructure. By aligning with one of these top companies, aspiring franchise owners can establish a profitable business in the ever-growing pharma sector of Bangalore.
0 notes
Text
Competitive Landscape and Innovations in Sterile Injectable Products
Sterile Injectable products are critical components of the pharmaceutical market, designed to be administered directly into the bloodstream, muscles, or tissues. These injectables, including drugs, vaccines, and biologics, undergo rigorous sterilization processes to ensure they meet stringent quality and safety standards, minimizing the risk of contamination. The need for Sterile Injectable solutions spans numerous therapeutic areas such as oncology, cardiology, and infectious diseases, making them indispensable in patient care. Due to their immediate bioavailability, Sterile Injectable medications are often the preferred option in emergencies or critical care settings where rapid therapeutic effects are needed.
According to MRFR analysis, the market size for sterile injectables was projected to reach USD 69.76 billion in 2022. The market for sterile injectables is anticipated to increase from 73.65 billion USD in 2023 to 120.0 billion USD in 2032. Over the course of the forecast period (2024–2032), the sterile injectable market is anticipated to increase at a CAGR of approximately 5.58%.
Sterile Injectable Market Analysis
The Sterile Injectable market analysis indicates robust growth, driven by the increasing demand for biologic drugs, which are primarily administered through sterile injections. The rise in chronic diseases, including cancer, diabetes, and cardiovascular conditions, has heightened the need for efficient drug delivery systems. Additionally, advancements in biotechnology have introduced a variety of monoclonal antibodies and immunotherapies that require sterile injection. Regulatory support for sterile manufacturing practices and the growing need for outsourced sterile injectables in pharmaceutical production have further strengthened the market. The Sterile Injectable market analysis also highlights the role of emerging economies where healthcare infrastructure is improving, driving growth in the demand for injectables.
Sterile Injectable Market Trends
Significant Sterile Injectable market trends include the rise of biosimilars and personalized medicine. With patents on many blockbuster biologics expiring, there’s an increased demand for biosimilar sterile injectables, which offer affordable alternatives. The rise of personalized medicine has also influenced Sterile Injectable market trends, as many of these treatments, such as gene and cell therapies, require sterile injection delivery. Additionally, innovative packaging, such as prefilled syringes and dual-chamber injectables, has gained traction due to ease of use, reduced dosing errors, and improved patient compliance. Furthermore, manufacturers are adopting eco-friendly production and packaging practices to meet the rising demand for sustainable solutions.
Reasons to Buy the Reports
In-depth Market Insights: Offers comprehensive analysis on the Sterile Injectable market, including emerging trends, growth drivers, and key challenges.
Competitive Landscape Overview: Assists in understanding the competitive environment, market positioning, and strategic moves of key players.
Regional Market Analysis: Provides breakdowns of regional Sterile Injectable markets, helping stakeholders identify growth opportunities in specific regions.
Product and Innovation Analysis: Offers details on new products and technological advancements, keeping investors informed about industry developments.
Future Market Forecasts: Predictive insights into the market’s growth trajectory help companies make data-driven investment decisions and align with future demand.
Recent Developments
Recent developments in the Sterile Injectable market highlight the industry’s focus on innovation and expansion. Pfizer, for instance, has expanded its portfolio of sterile injectables with new cancer therapeutics and antibiotics aimed at meeting the high demand in oncology and infection management. In 2023, Baxter International launched a line of prefilled syringe sterile injectables to improve dosing accuracy and patient safety. Additionally, new players have entered the market, particularly in Asia-Pacific, where favorable regulations and government initiatives are encouraging local manufacturing. Emerging technologies such as lyophilization and advanced sterilization methods are also being widely adopted to enhance shelf life and safety, representing key advancements in the Sterile Injectable market.
Related repots :
cellular nutrition market
cleaning chemicals healthcare market
dental contouring market
0 notes
Text
The Active Pharmaceutical Ingredients (API) Industry: Driving Growth and Innovation in Pharmaceuticals
The Active Pharmaceutical Ingredients (API) Market Size is projected to be valued at USD 216.5 billion in 2024, with expectations to grow to USD 306.90 billion by 2029, reflecting a CAGR of 7.22% over the forecast period (2024-2029).

Market Overview and Growth Factors
The global API market is experiencing robust expansion, driven by the rising prevalence of chronic and lifestyle-related diseases, advancements in biopharmaceuticals, and growing generic drug production. The demand for high-quality, affordable drugs is fueling the need for APIs, especially as healthcare accessibility improves worldwide. Additionally, the surge in demand for innovative drugs and targeted therapies, particularly for diseases like cancer, diabetes, and cardiovascular disorders, is propelling the API sector forward.
Key Trends in the API Market
Growing Demand for Specialty APIs Specialty APIs, such as high-potency active pharmaceutical ingredients (HPAPIs) and biologics, are seeing heightened demand due to their effectiveness in treating complex diseases. HPAPIs, particularly, are gaining traction in oncology treatments due to their targeted approach, which minimizes adverse effects and improves patient outcomes.
Focus on Quality and Compliance Regulatory standards for API manufacturing are becoming increasingly stringent, with a focus on Good Manufacturing Practices (GMP). This emphasis on compliance ensures product quality and safety, essential for patient health. Global regulatory bodies, such as the FDA and EMA, are imposing rigorous oversight, and compliance is now a competitive differentiator in the API industry.
Rise in Biotech and Biopharmaceuticals Biotechnology-based APIs are gaining ground in treating previously unmanageable conditions. Biologics, biosimilars, and vaccines are increasingly contributing to API demand. As biopharmaceutical R&D investments increase, the demand for biologic APIs is expected to climb, fueling industry growth.
API Manufacturing Outsourcing Many pharmaceutical companies are outsourcing API production to focus on core competencies. This trend reduces operational costs and taps into specialized expertise in regions like Asia-Pacific, where API manufacturing is more cost-effective and has well-established supply chains.
Innovation in Drug Manufacturing Processes Advanced technologies such as continuous manufacturing, green chemistry, and AI-driven optimization are reshaping the API landscape. These innovations enhance production efficiency, reduce costs, and minimize environmental impact, making API manufacturing more sustainable.
Conclusion
The Active Pharmaceutical Ingredients (API) industry is set for sustained growth, supported by rising healthcare needs, regulatory advancements, and innovations in drug manufacturing. As APIs remain integral to drug formulation and therapeutic efficacy, this market is crucial to advancements in global healthcare. Strategic outsourcing, enhanced quality standards, and technological progress in API production are likely to remain key drivers in the sector's development. In a dynamic pharmaceutical landscape, the API industry is well-positioned to support the demand for high-quality, effective medications that enhance patient outcomes worldwide.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/global-active-pharmaceutical-ingredients-api-market
#Active Pharmaceutical Ingredients (API) Market#Active Pharmaceutical Ingredients (API) Market Size#Active Pharmaceutical Ingredients (API) Market Share#Active Pharmaceutical Ingredients (API) Market Trends#Active Pharmaceutical Ingredients (API) Market Analysis#Active Pharmaceutical Ingredients (API) Market Forecast
0 notes
Link
0 notes
Text
Monoclonal Antibody Therapy Market Report, Consumer Insights, Growth Prospects, Industry Outlook 2024-2032
Monoclonal antibody therapy has emerged as a groundbreaking approach in the treatment of various diseases, including cancers, autoimmune disorders, and infectious diseases. These laboratory-engineered antibodies are designed to target specific antigens on cells, making them highly effective in modulating immune responses or directly attacking disease-causing cells. Monoclonal antibodies have shown remarkable success in conditions such as breast cancer, rheumatoid arthritis, and COVID-19, offering patients a targeted and personalized treatment option. The development of monoclonal antibody therapies marks a significant advancement in precision medicine, allowing for tailored approaches to treatment based on individual patient profiles.
The Monoclonal Antibody Therapy Market size was valued at USD 87.8 Bn in 2023 and is expected to reach USD 230.38 Bn by 2031 with a growing CAGR of 12.8% Over the Forecast Period of 2024-2031.
Future Scope
The future of monoclonal antibody therapy is full of potential as researchers continue to explore new targets and enhance the efficacy of existing treatments. Ongoing studies are investigating combination therapies that pair monoclonal antibodies with other treatment modalities, such as chemotherapy or immune checkpoint inhibitors, to improve overall effectiveness. Advances in genetic engineering are also paving the way for the development of bispecific antibodies, which can simultaneously target two different antigens, providing a more robust therapeutic effect. As the understanding of the immune system deepens, the potential applications of monoclonal antibody therapies will likely expand, offering new hope for patients with previously untreatable conditions.
Trends
Current trends in monoclonal antibody therapy include a shift towards personalized medicine and the exploration of new therapeutic targets. The development of companion diagnostics is becoming increasingly important, allowing for the identification of patients who are most likely to benefit from specific monoclonal antibodies. Additionally, the rise of biosimilars—biologically similar versions of existing monoclonal antibodies—offers cost-effective alternatives and increases patient access to these treatments. There is also a growing focus on developing therapies that can effectively cross the blood-brain barrier, targeting central nervous system disorders more efficiently.
Applications
Monoclonal antibody therapies are widely applied across various medical fields, including oncology, immunology, and infectious diseases. In oncology, monoclonal antibodies are utilized to target cancer cells, enhancing the immune response and blocking tumor growth. In immunology, these therapies are used to modulate the immune system in autoimmune diseases, helping to reduce inflammation and tissue damage. Recent applications have also emerged in the treatment of infectious diseases, notably the use of monoclonal antibodies against COVID-19 to neutralize the virus and prevent severe disease. As research continues, the range of applications for monoclonal antibody therapies is expected to expand significantly.
Key Points
Monoclonal antibody therapy represents a major advancement in targeted treatment for various diseases.
These therapies are used in oncology, immunology, and infectious diseases, providing personalized treatment options.
Future developments focus on combination therapies, bispecific antibodies, and expanding therapeutic targets.
Trends emphasize personalized medicine, companion diagnostics, and the rise of biosimilars to enhance accessibility.
Ongoing research aims to develop therapies that effectively target central nervous system disorders.
Conclusion
Monoclonal antibody therapy has transformed the landscape of modern medicine, offering targeted and effective treatment options for patients with a wide range of conditions. As research progresses and new technologies emerge, the potential for monoclonal antibodies to improve patient outcomes continues to grow. The integration of personalized medicine and the development of biosimilars will further enhance accessibility and effectiveness, making these therapies more widely available. With a focus on innovation and expanding applications, monoclonal antibody therapy is set to play a pivotal role in the future of healthcare, providing hope and improved quality of life for countless individuals facing serious health challenges.
Read More Details: https://www.snsinsider.com/reports/monoclonal-antibody-therapy-market-3393
Contact Us:
Akash Anand — Head of Business Development & Strategy
Email: [email protected]
Phone: +1–415–230–0044 (US) | +91–7798602273 (IND)
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
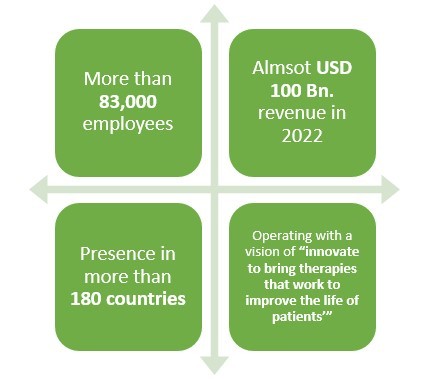
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
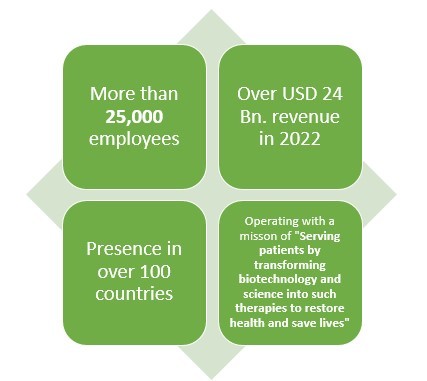
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
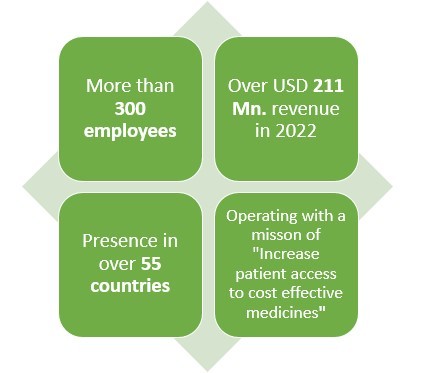
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
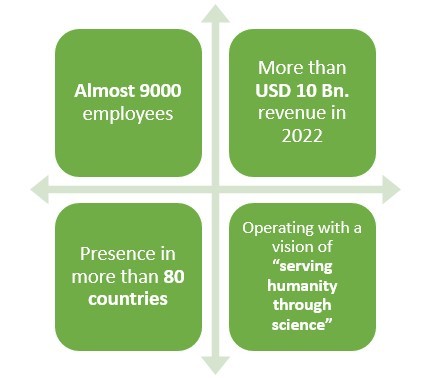
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Medication Delivery Systems Market Size, Share, Key Drivers, Trends, Challenges and Competitive Analysis
"Global Medication Delivery Systems Market – Industry Trends and Forecast to 2028
Global Medication Delivery Systems Market, By Type (Oral Drug Delivery System, Injection- Based Drug Delivery System, Inhalation/ Pulmonary Drug Delivery System, Transdermal Drug Delivery System, Trans Mucosal Drug Delivery System, Carrier- Based Drug Delivery System, Other Types), Technology (Prodrug, Implants and Intrauterine Devices, Targeted Drug Delivery, Polymeric Drug Delivery, Other Technologies), Carrier Type (Liposomes, Nanoparticles, Microspheres, Monoclonal Antibodies, Others), Application (Cardiovascular Diseases, Oncology, Urology, Diabetes, CNS, Ophthalmology, Inflammatory Diseases Infections, Other Applications), End-Users (Hospitals, Specialized Clinics, Clinical Research & Development Centers), Country (U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa) Industry Trends and Forecast to 2028
Access Full 350 Pages PDF Report @
The global Medication Delivery Systems Market is witnessing significant growth due to factors such as the increasing prevalence of chronic diseases, rising demand for self-administration devices, technological advancements in drug delivery systems, and the growing elderly population. The market is expected to showcase lucrative opportunities in the coming years, driven by the need for personalized and targeted drug delivery solutions, integration of digital health technologies, and the rising adoption of injectable biologics and biosimilars in healthcare practices. Moreover, the COVID-19 pandemic has further emphasized the importance of efficient medication delivery systems to ensure timely and accurate administration of therapies.
**Segments**
- By System Type: Prefilled syringes, autoinjectors, wearable injectors, injectable pens, infusion pumps, nasal sprays, nebulizers, and others. - By Application: Diabetes, oncology, autoimmune diseases, cardiovascular disorders, respiratory diseases, and others. - By End User: Hospitals & clinics, home care settings, ambulatory surgical centers, and others.
Considering the market players in the Medication Delivery Systems Market, several leading companies are actively participating in product innovation and strategic collaborations to strengthen their market position and cater to the evolving needs of healthcare providers and patients.
**Market Players**
- Becton, Dickinson and Company - Baxter International Inc. - Pfizer Inc. - Novartis AG - Gerresheimer AG - Johnson & Johnson Services, Inc. - GlaxoSmithKline plc - Novo Nordisk A/S - 3M - F. Hoffmann-La Roche Ltd
In conclusion, the Medication Delivery Systems Market is poised for substantial growth, fueled by the increasing demand for advanced drug delivery technologies, the rising burden of chronic diseases, and the shift towards patient-centric healthcare solutions. Market players are focusing on research and development initiatives to introduce innovative products and gain a competitive edge in the industry. With the integration of digital health solutions and emphasis on personalized medicine, the market is expectedThe Medication Delivery Systems market is experiencing remarkable growth, driven by various factors contributing to the expanding demand for efficient drug administration solutions. The surge in chronic diseases worldwide, coupled with the increasing elderly population, has necessitated the development of advanced medication delivery systems to ensure effective treatment outcomes. Moreover, the rising preference for self-administration devices and the advent of innovative technologies in drug delivery mechanisms have revolutionized the healthcare sector. The market is anticipated to witness significant opportunities in the foreseeable future, driven by the need for personalized drug delivery solutions and the integration of digital health technologies into medical practices.
Segmentation of the Medication Delivery Systems market based on system type includes prefilled syringes, autoinjectors, wearable injectors, injectable pens, infusion pumps, nasal sprays, nebulizers, among others. These diverse system types cater to a wide range of medical applications such as diabetes, oncology, autoimmune diseases, cardiovascular disorders, and respiratory conditions, among others. The market is also segmented by end-users, including hospitals & clinics, home care settings, and ambulatory surgical centers, reflecting the various settings where these medication delivery systems are utilized.
Key market players in the Medication Delivery Systems industry, such as Becton, Dickinson and Company, Pfizer Inc., Novartis AG, and Johnson & Johnson Services, Inc., are actively engaged in product innovations and strategic collaborations to enhance their market presence and meet the evolving needs of healthcare providers and patients. These companies are focusing on research and development efforts to introduce cutting-edge products that ensure efficient drug delivery and improve patient outcomes. The market landscape is characterized by intense competition, prompting companies to differentiate themselves through technological advancements and enhanced product offerings.
In conclusion, the Medication Delivery Systems market is set for substantial growth in the coming years, driven by the increasing demand for advanced drug delivery technologies and the escalating burden of chronic ailments globally. The industry's focus on patient-centric healthcare solutions, coupled with the integration of digital health solutions, is expected to propel market**Global Medication Delivery Systems Market**
- **Type:** Includes Oral Drug Delivery System, Injection-Based Drug Delivery System, Inhalation/Pulmonary Drug Delivery System, Transdermal Drug Delivery System, Transmucosal Drug Delivery System, Carrier-Based Drug Delivery System, and Other Types. - **Technology:** Encompasses Prodrug, Implants and Intrauterine Devices, Targeted Drug Delivery, Polymeric Drug Delivery, and Other Technologies. - **Carrier Type:** Comprises Liposomes, Nanoparticles, Microspheres, Monoclonal Antibodies, and Others. - **Application:** Targets Cardiovascular Diseases, Oncology, Urology, Diabetes, CNS, Ophthalmology, Inflammatory Diseases, Infections, and Other Applications. - **End-Users:** Includes Hospitals, Specialized Clinics, and Clinical Research & Development Centers.
The Global Medication Delivery Systems Market is witnessing robust growth and is poised for significant advancements in the forecast period. The proliferation of chronic diseases worldwide, coupled with the expanding elderly population, is fueling the demand for efficient drug delivery solutions. Technological innovations are revolutionizing the healthcare sector, with a focus on personalized and targeted drug delivery systems gaining traction. Integrating digital health technologies into medication delivery is a key trend shaping the market landscape, alongside the increasing adoption of injectable biologics and biosimilars in healthcare practices. The impact of the COVID-19 pandemic has underscored the critical need for precise and timely administration
Core Objective of Medication Delivery Systems Market:
Every firm in the Medication Delivery Systems Market has objectives but this market research report focus on the crucial objectives, so you can analysis about competition, future market, new products, and informative data that can raise your sales volume exponentially.
Size of the Medication Delivery Systems Market and growth rate factors.
Important changes in the future Medication Delivery Systems Market.
Top worldwide competitors of the Market.
Scope and product outlook of Medication Delivery Systems Market.
Developing regions with potential growth in the future.
Tough Challenges and risk faced in Market.
Global Medication Delivery Systems-top manufacturers profile and sales statistics.
Highlights of TOC:
Chapter 1: Market overview
Chapter 2: Global Medication Delivery Systems Market
Chapter 3: Regional analysis of the Global Medication Delivery Systems Market industry
Chapter 4: Medication Delivery Systems Market segmentation based on types and applications
Chapter 5: Revenue analysis based on types and applications
Chapter 6: Market share
Chapter 7: Competitive Landscape
Chapter 8: Drivers, Restraints, Challenges, and Opportunities
Chapter 9: Gross Margin and Price Analysis
How the Report Aids Your Business Discretion?
This section of this Market report highlights some of the most relevant factors and growth enablers that collectively ensure a high-end growth spurt
The report unravels details on pronounced share assessments across both country-wise as well as region-based segments
A leading synopsis of market share analysis of dynamic players inclusive of high-end industry veterans
New player entry analysis and their scope of new business models
The report includes strategic recommendations for new business veterans as well as established players seeking novel growth avenues
A detailed consultation services based on historical as well as current timelines to ensure feasible forecast predictions
A thorough evaluation and detailed study of various segments as well as sub-segments across regional and country-specific developments
Details on market estimations, market size, dimensions
A review of market competitors, their high-end product and service portfolios, dynamic trends, as well as technological advances that portray high end growth in this Market
Browse Trending Reports:
Asia Pacific Mainframe Market
Asia Pacific Menstrual Cramps Treatment Market
Europe Commercial Cleaning Equipment Market
Europe Mainframe Market
Europe Quantum Computing Market
Europe Tomatoes Market
Europe Utility Locator Market
India Blood Gas Analyzer Market
Middle East And Africa Commercial Cleaning Equipment Market
Middle East And Africa Neurosurgery Market
Middle East And Africa Sports Medicine Market
Middle East And Africa Vaccines Market
Nigeria Cassava Starch Market
Nigeria Starch Processing Market
North America Menstrual Cramps Treatment Market
North America Neurosurgery Market
West Africa Baby Food Market
Antenna Market
Baby Apparel Market
Cassava Starch Market
Castor Oil Market
Cenospheres Market
Coconut Water Market
Collagen Casings Market
Facility Management Market
Functional Confectionery Market
Hemp Seed Market
Infrared Imaging Market
Instant Noodles Market
Laundry Detergents Market
Menstrual Cramps Treatment Market
Micro And Nano Plc Market
Motorcycle Market
Neurosurgery Market
Nickel Alloy Market
Over The Counter Probiotic Supplements Market
Polycystic Kidney Disease Adpkd Market
Private Label Food And Beverage Market
Recreational Cannabis Market
Tofu And Tempeh Market
Tomatoes Market
Vaccines Market
Varnish Makers Market
Wireless Medical Device Connectivity Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
CD20 Market Size, Target Population, Competitive Landscape & Market Forecast - 2034
The CD20 market represents a vital segment in oncology and autoimmune disease therapeutics, driven by the role of CD20-targeting drugs in treating conditions like non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), and rheumatoid arthritis.
CD20 Market Size and Forecast
The CD20 drug market is projected to grow significantly, influenced by rising global cancer incidence, the expansion of biologics, and ongoing innovation in monoclonal antibody (mAb) therapies. Growth is particularly notable in regions like the U.S., the EU4 (France, Germany, Italy, Spain), the UK, and Japan. Increased awareness, enhanced diagnostics, and an aging population also contribute to the market’s expansion. This market is expected to achieve substantial valuation by 2034 due to upcoming therapeutic launches and improved access to treatments.
Read more @ https://www.delveinsight.com/report-store/cd20-market-forecast
CD20 Target Population
The primary target population includes individuals diagnosed with B-cell malignancies and autoimmune conditions. Non-Hodgkin lymphoma and chronic lymphocytic leukemia patients form the largest share of this population. Enhanced diagnostic methods are broadening the scope of the addressable market.
CD20 Market Competitive Landscape
The competitive landscape is robust, dominated by major players such as Roche (with Rituxan/MabThera and Gazyva/Gazyvaro), Novartis, and emerging biosimilars. Innovations include new monoclonal antibodies and combination regimens integrating CD20-targeting therapies with CAR-T cells or small-molecule inhibitors.
Additionally, the emergence of biosimilars is introducing competitive pricing dynamics, fostering accessibility in markets with constrained healthcare budgets.
CD20 Market Market Future Outlook
Ongoing clinical trials for novel CD20 inhibitors aim to address unmet needs, such as reducing side effects and increasing efficacy in refractory or relapsed cases. Combination therapies, particularly those integrating immune checkpoint inhibitors, represent a promising avenue. Regulatory approvals and adoption in emerging markets will also shape the market trajectory.
For an in-depth report, you can explore DelveInsight's dedicated resource on the [CD20 Market Forecast - 2034](https://www.delveinsight.com/report-store/cd20-market-forecast).
0 notes
Text
The MENA Biologics and Biosimilars Market is witnessing robust growth, fueled by increasing demand for innovative therapeutic solutions and the rising prevalence of chronic diseases. As of 2024, the market is valued at approximately USD 477.2 million and is projected to reach around USD 645.583 million by 2032, growing at a compound annual growth rate (CAGR) of 3.85% over the forecast period.The Middle East and North Africa (MENA) region is witnessing rapid growth in the biologics and biosimilars market, driven by increasing demand for advanced healthcare solutions, rising prevalence of chronic diseases, and evolving healthcare infrastructure. Biologics, which are complex molecules produced using living cells, offer targeted treatments for various diseases such as cancer, diabetes, autoimmune disorders, and more. Biosimilars, on the other hand, are essentially copies of biologic drugs, similar in terms of safety, efficacy, and quality but at a reduced cost, making them an appealing option in markets with budget constraints.
Browse the full report https://www.credenceresearch.com/report/mena-biologics-and-biosimilars-market
Market Drivers
1. Rising Chronic Diseases: Chronic diseases such as diabetes, cardiovascular diseases, and cancers have seen a surge across the MENA region, largely due to lifestyle changes, urbanization, and aging populations. According to the World Health Organization (WHO), the burden of non-communicable diseases (NCDs) accounts for over 75% of all deaths in the region. This increase in chronic diseases is fueling demand for biologics, which offer cutting-edge, effective treatments, particularly for conditions like rheumatoid arthritis, cancer, and diabetes.
2. Government Initiatives and Healthcare Reforms: Several MENA countries are embarking on ambitious healthcare reforms to enhance access to quality care. Countries like the UAE and Saudi Arabia have made significant investments in modernizing their healthcare sectors as part of their respective Vision 2030 agendas. Saudi Arabia, for example, is heavily investing in biotechnology and life sciences, focusing on domestic production of biologics and biosimilars. Similarly, Egypt and Morocco are seeing increased attention from international pharmaceutical companies eager to establish a foothold in these emerging markets.
Regulatory bodies in the region, such as the Saudi Food and Drug Authority (SFDA) and the Egyptian Drug Authority (EDA), are also streamlining the approval process for biosimilars, further driving the market’s growth.
3. Cost-Efficiency of Biosimilars: The high cost of biologics has been a barrier for many patients and healthcare systems in the MENA region, especially where public healthcare budgets are constrained. Biosimilars, being more affordable alternatives, provide an opportunity for governments to offer cutting-edge treatments to larger populations at reduced costs. With increasing awareness about the safety and efficacy of biosimilars, their acceptance and adoption are steadily rising.
For instance, the launch of biosimilar versions of key biologics such as adalimumab (used to treat autoimmune diseases) and trastuzumab (used in cancer treatment) has made these therapies more accessible. The affordability of biosimilars is particularly crucial in the MENA region, where insurance coverage varies greatly, and out-of-pocket healthcare costs can be prohibitive for many patients.
Key Players in the MENA Market
Several international and regional pharmaceutical companies are playing pivotal roles in the growth of the biologics and biosimilars market in the MENA region. Major global players such as Roche, Pfizer, and Amgen have long dominated the biologics market, with blockbuster drugs in oncology, immunology, and other therapeutic areas. However, the introduction of biosimilars has opened up the market for new entrants, including companies from emerging markets.
Local manufacturers, particularly in countries like Saudi Arabia, Egypt, and Jordan, are increasingly focusing on producing biosimilars to meet local demand and reduce reliance on imports. For example, the Saudi pharmaceutical company Jamjoom Pharma has made strides in the biosimilars segment, contributing to the country’s self-sufficiency in advanced biologic treatments.
Challenges and Opportunities
Despite the promising growth prospects, the MENA biologics and biosimilars market faces several challenges:
1. Regulatory Barriers: The regulatory environment across the MENA region is diverse, with some countries having well-established frameworks for biologics and biosimilars approval, while others lag behind. Harmonizing these regulations and creating a more unified system could accelerate market growth.
2. Awareness and Education: While healthcare providers are becoming increasingly aware of biosimilars, there is still a need for greater education among physicians and patients to build confidence in their use. Misconceptions about the safety and efficacy of biosimilars remain a challenge.
3. Pricing and Reimbursement: Pricing remains a significant factor, particularly in markets where government support for healthcare expenditure is limited. Biosimilars, though more affordable than biologics, still need to be priced competitively to gain widespread adoption, particularly in countries with lower healthcare budgets.
Future Outlook
The future of the MENA biologics and biosimilars market is highly optimistic. Rising healthcare demand, coupled with increasing investments in biotechnology and life sciences, will continue to drive the market forward. The adoption of biosimilars is expected to accelerate as healthcare systems seek to balance the rising cost of care with the need for innovative treatments.
Key Player Analysis:
Pfizer Inc.
Hoffmann-La Roche AG
AbbVie Inc.
Novartis AG
Merck & Co., Inc.
Bristol Myers Squibb Co.
GSK plc
AstraZeneca
Eli Lilly & Co.
Bayer AG
Gilead Sciences
Amgen Inc.
Boehringer Ingelheim International GmbH
Novo Nordisk A/S
Viatris Inc.
Johnson & Johnson (Janssen Pharmaceuticals, Inc.)
Sanofi Winthrop Industries S.A
Serum Institute of India
Biocon Limited
Intas Pharmaceuticals Limited
Segmentation:
Based on Product Type:
Monoclonal Antibodies
Recombinant Proteins
Vaccines
Other Biologics
Based on Technology:
Mammalian Cell Culture
Microbial Fermentation
Plant-Based Systems
Other Production Technologies
Based on End-User:
Hospitals
Pharmaceutical Companies
Research Institutions
Biotechnology Firms
Based on Region:
Middle East (Saudi Arabia, UAE, Qatar, Kuwait, Oman, Bahrain)
North Africa (Egypt, Morocco, Algeria, Tunisia, Libya)
Gulf Cooperation Council (GCC) Countries (Saudi Arabia, UAE, Qatar, Kuwait, Oman, Bahrain)
Levant Region (Jordan, Lebanon, Syria, Palestine)
Browse the full report https://www.credenceresearch.com/report/mena-biologics-and-biosimilars-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Global CMO/CDMO Market Share: A Competitive Landscape Analysis
The global CMO/CDMO market revenue is experiencing significant growth, with the market size valued at USD 20.9 billion in 2023. Projections indicate the market will reach USD 51 billion by 2032, growing at a compound annual growth rate (CAGR) of 10.4% over the forecast period from 2024 to 2032.
The CMO/CDMO market plays a critical role in the pharmaceutical and biotechnology industries by offering outsourced services for the development and manufacturing of drugs, biologics, and other healthcare products. With increasing demand for pharmaceutical production efficiency and cost reduction, companies are increasingly turning to CMOs and CDMOs to support their drug development pipelines and manufacturing processes.
Key Market Drivers
Growing Biopharmaceutical and Pharmaceutical Demand: The rise of biopharmaceuticals, biologics, and personalized medicine has significantly increased the need for outsourced services in drug development and manufacturing. CMOs and CDMOs are essential in enabling biopharma companies to accelerate the commercialization of new drugs and biologics while reducing operational costs. The complexity of biologics, cell therapies, and gene therapies has further driven demand for CDMO expertise in these specialized areas.
Cost Efficiency and Focus on Core Competencies: The outsourcing of manufacturing and development services to CMOs and CDMOs allows pharmaceutical companies to focus on their core competencies, such as research and innovation. CMOs/CDMOs offer economies of scale, regulatory expertise, and advanced manufacturing facilities, helping companies reduce costs and time-to-market for new therapies. This trend is especially prevalent among small- and mid-sized biopharma companies that lack in-house capabilities for large-scale production.
Increasing Investment in Research and Development: Investment in research and development (R&D) is at an all-time high, particularly in the fields of oncology, immunology, and rare diseases. The surge in clinical trials and new drug approvals is driving demand for specialized CMO/CDMO services, from clinical-stage development to full-scale manufacturing. As pharmaceutical companies seek to streamline R&D processes, outsourcing to CDMOs has become an attractive solution to navigate complex production processes and regulatory requirements.
Rising Demand for Biologics and Biosimilars: The growing market for biologics and biosimilars is a key driver for CDMO services. The complexity and high manufacturing costs associated with biologics necessitate advanced production technologies, which CDMOs are equipped to provide. The growing acceptance and adoption of biosimilars, driven by cost savings and increasing healthcare needs, are further fueling the demand for contract manufacturing services.
Increasing Focus on Cell and Gene Therapy: The advancement of cell and gene therapies, including CAR-T therapies and gene editing technologies, is expanding the scope of CMO/CDMO services. These therapies require specialized production facilities and regulatory expertise, which CMOs and CDMOs are increasingly investing in to support the growing market for these innovative treatments.
Get Free Sample Report: https://www.snsinsider.com/sample-request/1289
Challenges and Opportunities
While the market presents substantial growth opportunities, challenges such as regulatory complexities, high manufacturing costs, and the need for skilled labor can present obstacles. Additionally, capacity constraints and lead times for large-scale biologics manufacturing may pose hurdles for CMOs and CDMOs in meeting the rising demand.
However, these challenges also present opportunities for investment in cutting-edge manufacturing technologies such as single-use bioreactors, continuous manufacturing, and automation. CMOs and CDMOs that invest in advanced capabilities will be well-positioned to capture market share, particularly in high-growth areas like biologics, cell therapies, and gene therapies.
Regional Insights
North America currently dominates the CMO/CDMO market, with significant investments in pharmaceutical research, strong healthcare infrastructure, and the presence of leading biopharmaceutical companies. Europe also holds a substantial market share, driven by its robust regulatory environment and focus on innovation in the life sciences sector.
The Asia-Pacific region is expected to experience the highest growth during the forecast period, bolstered by increasing pharmaceutical and biotech R&D activities, cost-effective manufacturing, and growing demand for innovative therapies in countries like China, India, and Japan. The region's favorable government policies and expanding healthcare infrastructure further support this growth.
Future Outlook
The global CMO/CDMO market is positioned for dynamic growth, driven by increasing demand for outsourced pharmaceutical services, the rise of biologics and biosimilars, and the rapid development of cell and gene therapies. As companies seek greater efficiency and specialization, outsourcing to CMOs and CDMOs will continue to be a strategic imperative in the pharmaceutical and biotech industries.
With a projected CAGR of 10.4% from 2024 to 2032, the CMO/CDMO market is set to expand significantly, from USD 20.9 billion in 2023 to an estimated USD 51 billion by 2032. The evolving landscape of drug development and manufacturing will further cement CMOs and CDMOs as key enablers of innovation and growth in the healthcare industry.
Other Trending Reports
Pancreatic Cancer Treatment Market Growth
gRNA Market Growth
Patient Portal Market Growth
Continuous Glucose Monitoring Market Growth
0 notes
Text
Clinical Trial Supplies Market Size, Trends, and Business Outlook 2024 - 2030
The global clinical trial supplies market size was estimated at USD 2.58 billion in 2023 and is anticipated to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030.
Globalization, and rise in the number of biologics & biosimilar drugs in clinical trials are among the major factors expected to drive the market growth. Rapid adoption of a supply chain management system to surmount R&D expenditure pressure and increase operational efficiency, as clinical trial supplies account for a large share of the total R&D expenditure of biopharmaceutical companies, is anticipated to propel market growth in near future. There has been a significant rise in biologics and temperature-sensitive drugs in clinical trials.
Most clinical trials are currently being conducted in developing economies. The increasing cost of clinical trials and complications in the recruitment of patients have encouraged biopharmaceutical companies to outsource clinical trials to regions such as Asia Pacific, Latin America, Central & Eastern Europe, and the Middle East. Disease variation in developing economies further aids biopharmaceutical companies in performing clinical trials on rare diseases. Some regions, such as Asia Pacific, also provide greater economic benefits to biopharmaceutical companies, as governments in Singapore and China allocate funds to promote biomedical research. In Latin America, patient recruitment is easy due to reduced language barriers, which can help obtain informed consent easily, resulting in a faster clinical trial process.
Gather more insights about the market drivers, restrains and growth of the Clinical Trial Supplies Market
Clinical Trial Supplies Market Report Highlights
• Based on the clinical phase, the market is anticipated to be dominated by the Phase III trial segment with a 52.7% revenue share in 2022. The presence of a large number of molecules currently under Phase III makes it the primary factor responsible for this deduction
• Among services, the storage, and distribution segment is anticipated to witness the fastest growth at a CAGR of 6.8% during the forecast period. The rise in global biologics pipeline and temperature-sensitive drugs is expected to increase the complexities related to the logistics of clinical trial supplies
• Biologics are expected to witness the fastest growth at 6.7% CAGR during the forecast period owing to the increasing research in the field of genetics and biotechnology such as the development of nanoparticle-based drug delivery systems
• In terms of therapeutic use, oncology dominated the market with a revenue share of 38.8% in 2022. According to the United Press International, hospitals in the U.S. are disposing of billions of cancer drug vials due to improper dosage, thereby indicating the need for appropriate supply management
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The 3D printed brain model market size was valued at USD 44.3 million in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 17.9% from 2024 to 2030.
• The global spinal fusion devices market size was valued at USD 7.03 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 4.9% from 2024 to 2030.
Clinical Trial Supplies Market Segmentation
Grand View Research has segmented the global clinical trial supplies market report based on clinical phase, product & services, therapeutic use, end-use, and region:
Clinical Phase Outlook (Revenue, USD Billion, 2018 - 2030)
• Phase I
• Phase II
• Phase III
• Other
Product & Services Outlook (Revenue, USD Billion, 2018 - 2030)
• Manufacturing
• Storage & Distribution
o Cold chain distribution
o Non-cold chain
• Supply chain management
End-use Outlook (Revenue, USD Billion, 2018 - 2030)
• Pharmaceutical
• Biologics
• Medical device
• Others
Therapeutic Use Outlook (Revenue, USD Billion, 2018 - 2030)
• Oncology
• CNS
• Cardiovascular
• Infectious disease
• Metabolic disorders
• Others
Regional Outlook (Revenue, USD Billion, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o India
o China
o Japan
o South Korea
o Australia
o Thailand
o Singapore
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa (MEA)
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the Clinical Trial Supplies Market Intelligence Study, published by Grand View Research.
#Clinical Trial Supplies Market#Clinical Trial Supplies Market size#Clinical Trial Supplies Market share#Clinical Trial Supplies Market analysis#Clinical Trial Supplies Industry
0 notes