#Neupogen
Explore tagged Tumblr posts
Link
Neulasta: Benefits, Side Effects, and Uses Neulasta is a medication that plays a crucial role in cancer treatment, particularly for individuals undergoing chemotherapy. It's essential for patients and caregivers to have a comprehensive understanding of Neulasta's significance in healthcare. In this article, we will delve into what Neulasta is, how it works, its indications, benefits, potential side effects, and much more. What Is Neulasta? Neulasta belongs to a class of drugs known as colony-stimulating factors. Its primary function is to stimulate the production of white blood cells, specifically neutrophils, which are vital for the body's immune system. Neulasta is often prescribed to cancer patients who are at risk of experiencing a significant drop in white blood cell counts due to chemotherapy. By boosting white blood cell production, Neulasta helps reduce the risk of infections and related complications during cancer treatment. [caption id="attachment_50673" align="aligncenter" width="598"] neulasta[/caption] Indications and Uses Chemotherapy-Induced Neutropenia: Neulasta is frequently prescribed to cancer patients undergoing chemotherapy. Chemotherapy can suppress the bone marrow's ability to produce white blood cells, leaving patients vulnerable to infections. Neulasta helps address this issue by stimulating the production of neutrophils, a type of white blood cell responsible for fighting off infections. Bone Marrow Disorders: In some cases, Neulasta may be used to treat bone marrow disorders that lead to low white blood cell counts. These conditions can occur independently of cancer and require specific medical management. Stem Cell Transplantation: Neulasta may also be part of the treatment plan for patients undergoing stem cell transplantation, as it aids in the recovery of white blood cell counts after the procedure. How Does Neulasta Work? Neulasta's mechanism of action revolves around stimulating the bone marrow to produce more neutrophils, a specific type of white blood cell essential for the body's immune response. Here's a simplified breakdown of how Neulasta works: Chemotherapy and Neutropenia: During chemotherapy, the bone marrow's ability to generate white blood cells, including neutrophils, can be severely compromised. Neutropenia, or a low neutrophil count, is a common side effect of chemotherapy. This condition weakens the patient's immune system, making them susceptible to infections. Stimulating Neutrophil Production: Neulasta contains a medication called pegfilgrastim, which is a synthetic version of a natural protein called granulocyte-colony stimulating factor (G-CSF). Pegfilgrastim works by binding to receptors on the surface of certain cells in the bone marrow, signaling them to increase the production and release of neutrophils into the bloodstream. Protection Against Infections: By boosting the production of neutrophils, Neulasta helps restore the patient's white blood cell count to more normal levels. This, in turn, enhances their ability to combat infections effectively. For cancer patients undergoing chemotherapy, Neulasta can be a critical component of their treatment plan, reducing the risk of serious infections and treatment interruptions. Benefits of Neulasta The use of Neulasta in cancer treatment offers several significant benefits: Reduced Infection Risk: Chemotherapy-induced neutropenia can leave patients highly vulnerable to infections. Neulasta mitigates this risk by elevating neutrophil counts, bolstering the body's defense against pathogens. Enhanced Treatment Continuation: Maintaining a proper white blood cell count allows patients to adhere to their chemotherapy schedule. Uninterrupted treatment is vital for the effectiveness of cancer therapy. Improved Quality of Life: With reduced infection-related complications, patients experience a better quality of life during their cancer treatment journey. They are less likely to face hospitalizations or treatment delays due to infections. Minimal Side Effects: Neulasta is generally well-tolerated, with few side effects. Common side effects, if any, are typically mild and temporary. Potential Side Effects While Neulasta is generally well-tolerated, it's crucial to be aware of potential side effects. These side effects can vary in severity, and not everyone will experience them. Common side effects may include: Bone Pain: Some patients may experience bone pain, typically in the lower back or pelvis. This pain is usually mild to moderate and can be managed with over-the-counter pain relievers as recommended by a healthcare provider. Muscle Aches: Muscle aches are another common side effect. Like bone pain, they are generally manageable and temporary. Headache: Headaches may occur but are usually mild and transient. Redness or Swelling at the Injection Site: If Neulasta is administered as an injection, redness or swelling at the injection site can occur. This is usually mild and short-lived. Nausea: Some patients may experience nausea, although this is less common. Neulasta Administration Neulasta is typically administered as a subcutaneous injection. The injection is usually given approximately 24 hours after chemotherapy to allow for optimal neutrophil production. The specific dosing and timing will be determined by your healthcare provider based on your treatment regimen. Safety Considerations When using Neulasta, safety considerations include: Allergic Reactions: While rare, allergic reactions to Neulasta can occur. Symptoms may include difficulty breathing, rash, itching, swelling, or dizziness. Seek immediate medical attention if you experience any signs of an allergic reaction. Monitoring: Your healthcare provider will monitor your white blood cell counts regularly to ensure that Neulasta is effectively increasing neutrophil production. Individualized Treatment: Neulasta dosing and administration are personalized to each patient's needs. It's crucial to strictly adhere to the prescribed treatment plan. Consultation: Always consult your healthcare provider if you have questions or concerns about Neulasta or experience any unusual symptoms. Patient Experiences Hearing about the experiences of others who have used Neulasta can provide valuable insights into its real-world impact. Here are a couple of patient stories: Sarah's Story Sarah, a breast cancer survivor, shared her experience with Neulasta. "During my chemotherapy, my immune system was weakened, and I was constantly worried about infections. Neulasta made a significant difference. I experienced some bone pain, but it was manageable with pain relievers. Thanks to Neulasta, I completed my treatment without any major setbacks." Mark's Journey Mark, a lymphoma patient, also benefited from Neulasta. "I was concerned about the risk of infections during my chemotherapy. My doctor recommended Neulasta, and it allowed me to stay on track with my treatment. The bone pain was there, but it was worth it to keep my white blood cell count up. I felt more confident throughout my treatment journey." These stories highlight how Neulasta can positively impact the lives of cancer patients, helping them maintain their treatment schedules and improve their overall well-being. Neulasta vs. Other Medications In some cases, Neulasta may not be the only option for managing neutropenia during cancer treatment. It's essential to consider how Neulasta compares to other medications in the same category. Here are some points of comparison: Neulasta vs. Neupogen Neulasta and Neupogen are both medications used to stimulate white blood cell production, but they differ in how they are administered. Neulasta is administered as a single, long-acting injection, usually 24 hours after chemotherapy. Neupogen, on the other hand, is given as a daily injection. While Neupogen may require more frequent dosing, it can be a suitable alternative for some patients. Neulasta vs. Zarxio Zarxio is another medication similar to Neulasta. It is a biosimilar to Neulasta, meaning it has a highly similar structure and function. Biosimilars like Zarxio can offer a more cost-effective option while providing similar benefits. Frequently Asked Questions (FAQs) 1. Is Neulasta the only medication for boosting white blood cell counts during chemotherapy? Neulasta is one of the medications used for this purpose, but there are alternatives like Neupogen and Zarxio. The choice depends on individual patient needs and preferences. 2. Are the side effects of Neulasta severe? Common side effects like bone pain and muscle aches are generally mild and manageable. However, it's essential to discuss any side effects with your healthcare provider. 3. Can Neulasta be self-administered at home? In some cases, patients may be taught to self-administer Neulasta injections at home. This allows for more flexibility in treatment. 4. How long does it take for Neulasta to increase white blood cell counts? Neulasta typically starts working within a day after administration. It boosts white blood cell counts, reducing the risk of infections. 5. Are there any dietary restrictions while using Neulasta? Neulasta doesn't usually require specific dietary restrictions. However, maintaining a balanced diet and staying hydrated is essential for overall health during cancer treatment. 6. Can Neulasta be used in pediatric cancer patients? Neulasta is generally indicated for adults. Pediatric patients may have different treatment options, and the decision is made based on their specific medical needs. 7. What should I do if I miss a Neulasta injection? If you miss a scheduled Neulasta injection, contact your healthcare provider for guidance. It's crucial to stay on track with your treatment plan. 8. How is Neulasta different from chemotherapy? Neulasta is not chemotherapy; it is a medication used in conjunction with chemotherapy. While chemotherapy directly targets cancer cells, Neulasta focuses on boosting the body's immune response to reduce infection risk. 9. Can I continue my regular activities while using Neulasta? Yes, you can generally continue with your daily activities while using Neulasta. However, it's essential to follow your healthcare provider's recommendations regarding rest and physical activity. 10. What should I do if I experience an allergic reaction to Neulasta? If you suspect an allergic reaction (e.g., difficulty breathing, rash, swelling), seek immediate medical attention. Allergic reactions to Neulasta are rare but should be addressed promptly. Conclusion: Neulasta plays a vital role in supporting cancer patients undergoing chemotherapy. By boosting white blood cell counts, it helps reduce the risk of infections and allows patients to stay on track with their treatment regimens. While it may have some side effects, the benefits of Neulasta in improving patients' quality of life during cancer treatment are significant. If you or a loved one is considering Neulasta as part of your treatment plan, consult with your healthcare provider to determine the most suitable approach tailored to your needs.
#Biosimilar#Chemotherapy_support#Neulasta_administration#Neulasta_benefits#Neulasta_drug#Neulasta_mechanism#Neulasta_medication#Neulasta_therapy#Neulasta_treatment#Neulasta_vs._Neupogen#Neupogen#Pegfilgrastim#Pegfilgrastim_injection#What_is_Neulasta#White_blood_cell_booster
0 notes
Text
Treating Bone Pain From Neulasta and Neupogen

Bone pain is a common side effect of Neulasta (pegfilgrastim) and Neupogen (filgrastim), which are medications given after chemotherapy to increase one's white blood cell count. While these medications are effective in reducing the risk of infection, they can also cause bone pain since they stimulate the bone marrow to produce more white blood cells. Pain can vary in intensity, from mild to severe. Commonly affected areas include the lower back, hips, thighs, and sternum. Here is a comprehensive approach to help manage and alleviate bone pain.
1. Dietary Adjustments
Anti-inflammatory Foods: Incorporate foods rich in omega-3 fatty acids (like salmon, flaxseeds, and walnuts), antioxidants (such as berries, leafy greens, and nuts), and spices like turmeric and ginger, which have natural anti-inflammatory properties.
Hydration: Staying well-hydrated helps maintain overall health and can alleviate some discomfort. Aim for at least 8-10 glasses of water a day.
Magnesium-Rich Foods: Magnesium can help relax muscles and ease pain. Include foods such as spinach, almonds, avocados, and bananas in your diet.
2. Natural Supplements
Omega-3 Fatty Acids: Omega-3 supplements can reduce inflammation and may help alleviate bone pain. Fish oil and kelp (which is vegan) are common sources. The recommended daily dose is 1,000 mg per day.
Vitamin D and Calcium: These nutrients are vital for bone health. Ensure adequate intake of both to support bone strength and potentially reduce pain. Most adults need 5,000 IU of vitamin D per day to maintain ideal levels, but ask your doctor based on your current vitamin D level. The recommended daily intake of calcium is 1,000 mg per day.
Magnesium: This mineral can help relax muscles and ease pain. Consider a magnesium supplement. The recommended dose is usually 300-400 mg per day.
3. Herbal Remedies
Turmeric and Ginger: Both herbs have potent anti-inflammatory effects. Turmeric can be taken as a supplement or added to meals, while ginger can be consumed as tea or in food.
Boswellia: Boswellia (frankincense) is an herbal extract that has anti-inflammatory properties and can be an effective natural pain reliever. The recommended dose is 500 mg, twice a day.
Willow Bark: Known as nature's aspirin, willow bark can help reduce inflammation and pain. The daily dose should be equal to 240 mg of salicin (active ingredient) per day.
4. Conventional Treatment
Tylenol: Ask your doctor before taking as tylenol should not be taken by those with liver disease. The recommended dose is 500 - 1,000 mg every 6 hours, as needed.
Loratadine: Loratadine (Claritin) is an antihistamine that can reduce bone pain due to reducing the amount of histamines in the body and reducing inflammation. The recommended dose for adults is 10 mg per day. Make sure to ask your doctor before taking this, especially if you have kidney or liver disease.
5. Physical Therapies
Acupuncture: Acupuncture has been proven to decrease pain by turning off pain signals in the brain, releasing endorphins in the bloodstream, reducing inflammation, and circulating Qi and Blood.
Massage Therapy: Professional massage can improve circulation, reduce muscle tension, and provide pain relief.
Gentle Exercise: Regular, low-impact exercises like walking, swimming, or yoga can help maintain mobility and reduce pain. Exercise also releases endorphins, which are natural painkillers.
6. Lifestyle Adjustments
Adequate Rest: Ensure you are getting enough sleep as it is essential for the body to heal and recover.
Stress Management: Chronic stress can exacerbate pain. Incorporate stress-reducing activities into your routine, such as hobbies, spending time in nature, or practicing meditation, and/or relaxation techniques.
Conclusion
Managing bone pain from Neulasta and Neupogen involves an integrative approach. Combining dietary adjustments, natural supplements, herbal remedies, conventional treatment, physical therapies, and lifestyle adjustments can offer significant relief. Always consult with healthcare providers before starting any new treatment to ensure it’s safe and appropriate for your specific condition.
#cancer#chemotherapy#side effects#bone pain#management#integrative oncology#eastwestintegrativeoncology#yourcancerguru
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
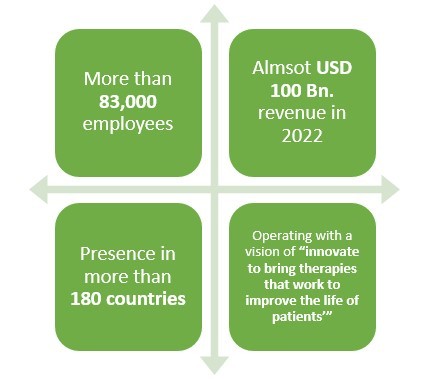
Click to read more about Pfizer
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
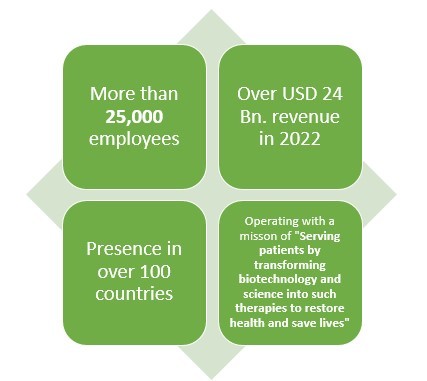
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
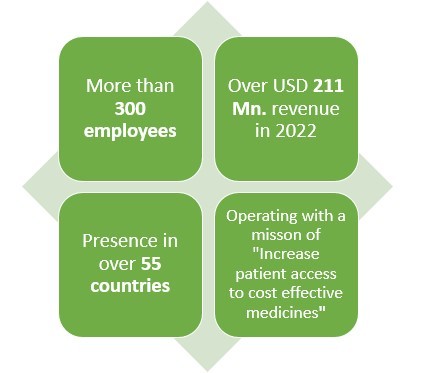
Click here to Ask for a Custom Report
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
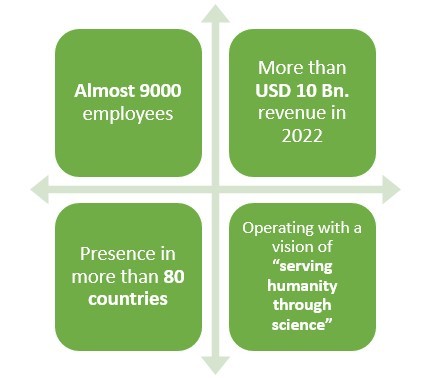
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
A Pot To Pee In
A Pot To Pee In
I started my second cycle of chemotherapy this morning with two hours of hydration, anti-nausea drugs, and gemcitabine. In order to qualify to receive the gemcitabine, I had to produce more urine. The nurses have been collecting my urine all day to insure I was fully hydrated. So don’t say to me, “You don’t have a pot to pee in!” Now rather cisplatin is dripping and I’ve been thinking about the…

View On WordPress
0 notes
Text
Filgrastim


Common Brand Names: Neupogen
Therapeutic Class: A hematopoietic growth factor which promotes proliferation and maturation of neutrophil granulocytes.
Common Injectable Dosage Forms:
Injection: 300 mcg/mL (1 mL, 1.6 mL), 600 mcg/mL (0.5 mL, 0.8 mL)
Dosage Ranges:
The recommended initial dose is 5 mcg/kg/day for up to 2 weeks or until the absolute neutrophil count reaches 10,000/mm3 for patients that are in post-antineoplastic therapy states.
In bone marrow transplantation, dosage is 10 mcg/kg/day and adjusted accordingly.
Administration and Stability: The commercially available solution (300 mcg/mL) may be given by direct rapid SQ injection. When given by IV infusion, the desired dose should be diluted with 50-100 mL of D5W to a concentration not less than 5 mcg/mL and may be given over 15-60 minutes or infused continuously over 24 hours. For continuous SQ infusion, the dose is diluted with 10-50 mL of D5W and administered at a rate not exceeding 10 mL/24 hours. When diluted to less than 15 mcg/mL, the solution should have 2 mcg/mL of albumin added to lessen the likelihood of adsorption. pH 4
Pharmacology/Pharmacokinetics: Filgrastim exerts its hematopoietic effects primarily by binding to neutrophil progenitor target cells that acts to stimulate proliferation of neutrophils within the bone marrow and possibly other sites. Following subcutaneous bolus administration, peak serum levels are observed in 2-6 hours. It is rapidly distributed with highest concentrations in the bone marrow, adrenal glands, kidney, and liver. The elimination half-life is 1.5-7 hours.
Drug and Lab Interactions: Filgrastim should not be administered within 24 hours before or after a dose of myelosuppressive antineoplastic agent. Because of transient decreases in platelet counts following filgrastim doses, caution should be used with patients on other platelet depressing agents. Lithium could theoretically potentiate the effects of filgrastim, but this has not been clinically established.
Contraindications/Precautions: Contraindicated in patients with known sensitivity to any proteins derived from E. coli. CBC and platelet counts should be performed prior to initiation and at least twice weekly during therapy to avoid the possibility of excessive leukocytosis or thrombocytopenia. Caution should be exercised in patients with suspected myeloid malignancies as tumor growth could be stimulated by filgrastim therapy. Pregnancy Category C.
Monitoring Parameters: CBC with diff., monitor for neutropenia.
Adverse Effects: The most common adverse effect is bone pain; other less common reactions include fever, elevations in serum uric acid, local cutaneous reactions, and occasionally, splenomegaly.
Common Clinical Applications: Effective in decreasing the incidence of febrile neutropenia associated with non-myeloid malignancies receiving myelosuppressive therapy, and in reducing duration of neutropenia when undergoing myeloablative chemotherapy.
#sigler injectable drug cards#7th edition#filgrastim#neupogen#hematopoietic growth factor#drug facts
0 notes
Text
dry eye disease market size
The global organic drugs industry could be largely classified as: vaccines, healing protein, and mAb. The part of healing proteins could be split into: Avonex, Enbrel, Neulasta, Lantus, NovoLog, Humalog, Aranesp, Rebif, Levemir, Epogen, Victoza, Neupogen, Betaseron, and Eylea. Likewise, the mAb part could be divided into: Remicade, Humira, Rituxan, Herceptin, Avastin, and Lucentis. And, the part for vaccines could be sub-segmented as: Cervarix, Gardasil, Varivax, Prevnar 13, and Fluzone.The industry for organic drugs could be classified and studied on the cornerstone of geographical regions. Thus, the marketplace could be split into: North America, Europe, Asia Pacific, and Remaining portion of the World. By industry share, North America emerges as the dominant region. It is dry eye market size in india
In North America, the global industry is fuelled by the rising utilization of such drugs for treating chronic diseases as well as diabetes and cancer. A case in point will be the National College of Rheumatology's advice of infection altering antirheumatic drugs as well as biologic brokers in treating rheumatoid arthritis in 2012. The National College of Rheumatology is definitely an business dedicated to developing treatment plans for rheumatic conditions via education and researchAs new breakthroughs are made in the biomedicine field, growth is also anticipated in the organic drugs market. According to U.S.-based industry study firm Persistence Market Research, this industry can record a 10% CAGR from 2014 through 2020, and will undoubtedly be dry eye disease market size
In different areas such as Europe, the growth of the marketplace could be ascribed to a spike in the geriatric population. A written report by the United Nations claims that at the time of 2000, seniors comprised 23.2% of the total citizenry of Germany. This percentage can properly scale to 33.2% by 2025. Glaucoma and macular degeneration are age-related problems that create possibilities for the development of treatment plans such as drugs.A healthy percentage of the total investments by organic medicine designers has become being noticed in Asian countries. The reduced production costs that win here, combined with rising move of pharmaceutical know-how from the West to the East, can support the growth of the marketplace here. A recent case would be that of Swiss biotech organization, Lonza, which invested a reported USD 350 million in two Asian places - Singapore and India - to improve organic drug-related activities. Dry Eye Syndrome Therapeutics Market size
1 note
·
View note
Text
Always Have Hope
New Story has been published on https://enzaime.com/always-have-hope/
Always Have Hope
A few months ago, I thought it was the beginning of the end; I thought I was dying. My cancer had been spreading like wild fire through my weak and tired body for eight months. My oncologist told me my treatment options were becoming limited. How could I not think I was dying?
I never give up hope and I believe in miracles. My family and friends give me much love and support, which helps get through the rough times and celebrate the good times. I was so happy this week to share some good news, finally. I am not dying, at least for the time being.
Last Tuesday I received scans. Thursday, I met with Dr. M to discuss the scans and treatment. Dr. M came in the room with a big smile on her face. She said hello, shook Mark and my hands, and sat down. The first sentence out of her mouth was, “I am thilled with your scans.” All tumors, lesions, and lymph nodes have shrunk. My tumor markers have come down 90%…. WOW! Dr. M and I hugged with big smiles on our faces. Mark had a big smile on his face too.
I love receiving good news about my condition, but I always remain cautious. Some treatments last for a long time, some do not. I have experienced both sides of treatments. However, I am thrilled, and rejoicing at this wonderful news.
Eribulin, my chemotherapy, will be given every other week instead of two weeks on and one week off. My white and blood red blood counts go way down after receiving Eribulin and take more than a week to come up in order to receive chemo. This is because my bone marrow is tired from receiving so much chemotherapy. I will also be taking neupogen injections to help keep the white counts from getting too low.
As you might know, I try to look for the silver lining in everything. Right now the silver lining is weight loss! Between my thyroid, and cancer treatments starting in 2010, my weight fluctuated quite a bit. In March of this year, I lost my appetite. This continued until June before starting the Eribulin. I am happy to say my appetite has returned! I am content with my weight now. I needed a new wardrobe, and Michaela and I had a lot of fun shopping for new clothes… And we still are!
Thanks for reading my blog, and supporting me through the ups and the downs. This photo was taken a couple of weeks ago by the talented Paul Vicario. He, and the talented Dee Lewis have captured the “real me” through their beautiful photos.
0 notes
Text
Swabbed my cheek to join the marrow registry today, It’s rare to be a match, but if I am- residency should give me a day off right?
I just hope I match for marrow rather than stem cells... I’ve had patients feel pretty crummy on Neupogen and I don't want to have to work feeling achy ann flu-like before the donation.
Also I encourage every healthy person to register!
7 notes
·
View notes
Text
It is so weird how my two worlds of music and my rare disease collide sometimes because I am sitting in the hospital right now while I am listening to my new single "Siren" which is my first release on a label!!
I'll guess I'll talk about what's going on with my SDS first. Over the past few weeks my blood counts have been gradually decreasing, something that was concerning my doctor and something that was very unexpected. Dr. Antin explained to me that in order to get my counts up and my transplant recovery progress back on track I would need to get more stem cells from Bradlee.
So we had Bradlee fly out for the week to receive his Neupogen shots (they allow your stem cells to rise to the surface of your blood for easier extraction) and yesterday he was hooked up to this crazy machine for a few hours while they collected and cleaned his blood! Luckily, no surgery this time for bone-marrow.
Today I am sitting in my little infusion room receiving his stem cells. To be honest, when I first got the news I was pretty frustrated that my body wasn't doing what I wanted it to, especially because there's nothing you yourself can do to improve your blood. When you get a cold, you can take care of yourself with cold medication and Vitamin C, when you want to lose weight you can start eating healthy and exercising...when you want your blood counts to do better there's literally nothing you can do except get infusions from a doctor. It's frustrating to not always be in control of your body and to feel like it is failing you.
But I have confidence that today's stem cells are going to do great and I want to thank my brother, Bradlee, for making the effort to come all the way from Detroit for the week and give me more of his blood!!
NOWWW onto the fun news!
In April I was contacted by a DJ named Kiba to topline one of their tracks. This was right around the time when my boyfriend, Louie, and I were gearing up to move from LA to Boston for the transplant. I got the track from Kiba and with my wonderful songwriting partner, Liza , I guess I just started writing about how I was feeling with everything going on. I sent them a rough draft of it and they instantly loved it! I finished the topline for them and they began shopping it around to different labels.
We finally landed upon Simplify Records for the release and now it is here today!!!!!
This song came from a very real place and it means a lot to me! I hope you can feel that when you listen to it =)
Please go stream!!
Thank you all for your continuous support as always!
With much love and gratittude,
Gracie

2 notes
·
View notes
Text
Biosimilar Litigation Trends and Best practices we have learnt in 2019
Almost 10 years since the U.S. Biosimilars Pathway the law which comprises of the Biologics Price Competition and Innovation was introduced. In the years 2015 the first of kind biosimilar product in U.S. history was introduced and launched in 2015. This consisted of long term court cases and later as the years passed Ten new biosimilars were approved and launched in 2019.

The Food and Drug Administration (FDA) till now has given green light to new 26 biosimilars in total, including biosimilars of nine of the world’s most important biologic medicines: Avastin, Rituxan, Enbrel, Herceptin, Humira, Neupogen, Neulasta, Remicade and Epogen. This has been possible due to the relentless efforts of the biosimilar litigations attorney.
The Supreme Court and Federal Circuit have given provisions of the enactment. 2019 did not offer any key insights into the enacment, but a number of trends emerged and lessons were learned.
How biosimilar litigation attorneys help you with Patent litigations between biosimilar competitors of the same biologic medicine
Biosimilar makers are filing for and obtaining manufacturing patent rights for their own as they do not have to use the manufacturing processes of the main manufacturers to obtain approval for their products. Then in 2019 we saw the first lawsuit between two biosimilar makers — both with biosimilars of AbbVie’s adalimumab (Humira). Then in January 2019, Coherus BioSciences, a biosimilar manufacturer, filed a complaint alleging that Amgen’s biosimilar of adalimumab (Amgevita) copied the patents for its own biosimilar version of adalimumab. From our resources we can say that Amgen launched Amgevita in Europe but officially it has manufactured Amgevita in the United States. Coherus claimed that production of Amgevita in the United States copied the main components of the patents. Now with the help of biosimilar litigation attorneys the parties settled the suit in November 2019.
According the experts Amgen holds the earliest launch date in the United States of any biosimilar adalimumab and definitely soon will launch in January 2023. Following that Coherus will launch in December 2023.
We are going to see patent copying litigation between biosimilar competitors in the years coming ahead as biosimilar makers continue to procure and enforce patents. So will the need for biosimilar litigations attorneys.
Expired patents may result in substantial damages imposed on the requester; the Safe Harbor does not save the companies from the pre-approved manufacturing.
Amgen’s lawsuit with Pfizer’s Hospira involving a biosimilar of erythropoietin (EPO) has given us quite lot of illustration to very important lessons: patents that are expired at the time of biosimilar approval may still find some value to innovators at the end of the day, and the Safe Harbor provision of 35 U.S.C. 271(e)(1) does not prevent innovators from all preapproval manufacture taken care by the biosimilar legislation attorney.
In December 2019, the Federal Circuit passed a new jury verdict that Amgen’s expired patent was copied and cannot be considered as originator drug. Then 14 batches of Hospira’s EPO biosimilar were not being protected by the Safe Harbor provision; and then the Amgen was entitled to $70 million in damages as a fine. The Federal Circuit then made it very clear that preapproval production of a biosimilar product is not necessarily protected by the Safe Harbor provision but they can be protected only if the biosimilar batches are made solely for regulatory purposes.
In another case that involved Amgen’s complaint that was filed in September 2015 alleged that Hospira infringed some of its U.S. Patents and related methods of developing EPO. When the complaint was filed, Hospira’s biosimilar was not approved and launched in the United States. Hospira claimed that before the ban its 21 batches of biosimilar EPO, which were prepared during the time period of 2013 and 2015, before the expiration of the patents, are protected within the Safe Harbor provision because the products were related to the development and approval of information.
During the trial, Amgen concluded that their 21 batches of Hospira’s EPO had the total estimated value of over $100 million. The jury found that the claims of the patent were surely infringed and not valid, and out which 14 of the 21 batches were not protected by the Safe Harbor provision. The jury awarded Amgen the case and was given $70 million in damages.
Settlements abound and the USPTO intervenes in biosimilar IPR appeals post-settlement
According to Biosimilar legislation attorney makers are taking advantage of the make use of inter partes review (IPR) proceedings to challenge against the verdict of the patents protecting biologic medicines and to finally get the approval for the drive settlements. When finally, the settlements occur after a final written verdict taken by the Patent Trial and Appeal Board (PTAB) which eventually leads to the biosimilar maker withdraws from the case, the U.S. Patent and Trademark Office (USPTO) is pulling up to protect the PTAB’s decision.
Another example is of AbbVie that appealed the PTAB’s Final Decision finding claims of U.S. Patent directed to the methods of using adalimumab which was not protected by the trademark. Although Coherus BioSciences and Boehringer Ingelheim settled with AbbVie and withdrew from the appeal, the USPTO had to come into the case to protect the PTAB’s decision.
In the same way, Biogen appealed against the PTAB’s Final Written Decision finding all claims of U.S. Patent No. 8,821,873. The case was related to the methods of treating diffuse large cell lymphoma with anti-CD20 antibodies. After the settlement between Pfizer and Biogen which was withdrawn from the court, eventually the United States government had to intervened in response to Biogen’s constitutional in order to challenge its pre-AIA patent to an IPR. Resulting in USPTO intervention to uphold the PTAB’s final verdict.
It would be interesting to see how biosimilar producers develop new scenarios for approval and launching the same in the market. But in the same way the experience required by biosimilar litigation attorneys is still very high. The biosimilar litigation attorneys would have to very minutely observe the present scenarios and the success failure rate connected to each case.
That way the past case experiences have been handled would eventually play a major role in creating future success rates.
0 notes
Text
PDF Science Lessons: What the Business of Biotech Taught Me About Management EBOOK BY Gordon Binder
Download Or Read PDF Science Lessons: What the Business of Biotech Taught Me About Management - Gordon Binder Free Full Pages Online With Audiobook.

[*] Download PDF Visit Here => https://best.kindledeals.club/1591398614
[*] Read PDF Visit Here => https://best.kindledeals.club/1591398614
Widely regarded as the most innovative, successful biotech firm ever, Amgen led its industry in revenue and sales growth in 2007. Top magazines including Fortune and Industry Week have repeatedly named it one of America's best companies to work for.In Science Lessons, Gordon Binder—CEO and chairman during 1988-2000—describes Amgen's climb to success. Revealing the highs and lows it experienced in the race to develop blockbuster drugs, he takes readers from the time Amgen had just three months of capital in the bank and no viable products in the pipeline to its spectacular success. The turning point? The 1989 launch of Epogen, which dramatically helped kidney dialysis patients suffering from debilitating anemia. Other landmark drugs, including Neupogen, would follow.Through engaging anecdotes and cogent insights, Binder weaves a fascinating tale while offering his unique brand of practical management advice. Using the principals of the scientific method, he shares his recommendations
0 notes
Link
0 notes
Link
#Amneal Pharmaceuticals#US Food and Drug Administration#top pharma companies news#latest pharma news#pharma industry updates
0 notes
Text
Day 18
Another day in the books, but this was probably the toughest one yet. My fever last night kept rising, so at around 1am (after consulting with the on-call night nurse at the oncology ward) I made my way into the hospital. They don't know for sure that I have an infection, but given how immunocompromised I am they need to treat any fever as such.
At around 1:30am I was hooked up to an IV and they started pumping antibiotics into my bloodstream. I had four bags to get through, so I was teetering on the edge of sleep until about 4am when I finally finished up for the night. I got a decent five hours of fitful rest--gotta love hospital beds--and then woke up to a chest x-ray and more IVs in the morning.
All day I have been feeling nauseous, and I can't tell if it's from the Neupogen shots, the antibiotics, or anticipatory nausea as a result of my surprisingly traumatic hospital stay in Days 1-3. I think a good chunk of it has to be the association, because I have been able to eat very little all day. I had a croissant courtesy of the hospital for breakfast, and ordered Nando's in for lunch. I could barely eat any of it, and a granola bar is pretty much all that supported me through the afternoon. For dinner my mom dropped off homemade chicken noodle soup in a thermos, which was absolutely delicious, and yet that too was a struggle to consume.
It's incredibly difficult to be locked up in one room for 24 hours a day. I left the room once today (for my x-ray). I wasn't even allowed to walk down to radiation; hospital policy dictates that I be wheeled down in a chair by a member of staff. Apart from that, I have been either on the bed or in the one provided chair. Nobody is allowed to visit, and I am not allowed to leave until I am discharged. I couldn't even break out if I wanted to--the elevators require an ID card in order to operate.
I'm really starting to see why people say that fighting cancer is as much mental as it is physical. I also don't think that there's a rigid separation between the two--the mental bleeds into the physical and vice versa. I'm sure that's not a hot take by any means, but I'm feeling the overlap very viscerally right now.
I start Cycle 2 on Friday, and I was desperately hoping to be recharging at home right now. Instead I'm sitting in a hospital room with no set end date, feeling perfectly fine but unable to leave. I'm really hoping my fever dissipates ASAP so I can get home for at least a day or two before the next cycle commences.
0 notes
Text
[jeonghan] sky full of stars
title: sky full of stars
premise: hospital au - you’re a nurse, and jeonghan is a patient on the twelfth floor.
pairing: jeonghan x reader
genre: angst, slow burn.
warnings: death, panic attacks (side effect of an illness), inaccurate medical terminology and how nurses work i’m sorry
word count: 5532
you make your way down the seventh floor, peering over unlit wards and drawn curtains. there’s 0710, neatly pressed and scrubbed down – just discharged this morning after her neupogen shot; 0714, who was kindly and always smiling, but left a terrible squash-shaped stain that had even kimmy squirming; or 0720 with her granddaughter and the cautious, messy cranes they folded every day. something stirs in you, lightly, and you find yourself bowing your head to keep from smiling widely at the thought.
the floor ends with a cluster from 0725 to 0730, and you brace yourself for the loud, unnatural snoring that 0728’s mr fujimoto never fails to emit. his wife had complained, too, her knitting sticks punctuating the air with her teasing jibes. he laughed, then, a hearty, full chortle, and held her hand to his ailing heart.
“but you love me for that,” he’d said. “always taking my breath away, this one.”
for a moment, it felt almost invasive to be standing and staring on, hiding your gasp behind the back of your hand; the quiet, glassy shine of her eyes – his tired, paling smile – a glass of water untouched, and a tray of capsules promising.
you stroll over, and the first chill that runs down your spine halts you. it’s silent. the lights are all out, 0730 has her curtains drawn, and the city twinkles spectacularly outside the window, forged together with the reflection of your hallway, lost in a space tipping between here and there.
something doesn’t sit right with you. you walk over to 0728, where mr fujimoto is resting, curled peacefully on his side. he’s always tucked politely into his blanket, mouth ajar and heaving.
he’s not moving.
your hand reaches for his shoulder – stiff, bony, cool. and he flops over onto his back. mr fujimoto doesn’t snore. doesn’t wince. doesn’t twist over. doesn’t.
“mr fujimoto?” you say, a shard of whisper clumsily ripping through the air. “mr fujimoto?”
shaking fingers dig into his papery neck, feeling for a pulse – and maybe you’re just bad at this, maybe he’s sleeping really deeply – but when your index finger hovers under his nose.
he’s not breathing.
fuck. fuck, fuck – chest compressions, first, fuck, a doctor – you fumble around in his bed, tugging for a wire. the remote is so small against the tremor of your palm, obedient to your jabs. the bell doesn’t ring loud enough, doesn’t – isn’t enough – you drop the remote, unlatch the grip bars and press your hands into his chest.
the rhythm’s supposed to be steady, but keeping count is hard now, and you’re yelling “code blue” too shrilly, the air burns up your nose, and everything’s blurring and you’re pumping hard, harder, fast, faster. something sickening crunches under your palms and that’s probably the ribs but he’s still not breathing and he has a wife –
– doctor hong has a tight grip on your shoulders, dragging you away.
“doctor hong,” you breathe, “he’s – code blue – he’s not breathing –”
“we can’t resuscitate him,” doctor hong levels you with a look, slow and firm. “mr fujimoto doesn’t have a code.”
he doesn’t – you were there, when he signed the order, his wife knitting away quietly, lips pulled taut into a line, dabbing at her tears subtly. he held her hand then, and she smiled back at him, gently, reassuringly, ready. and maybe you skipped lunch that day, resting your forehead against the chill of a bathroom sink, but you were okay with it, weren’t you? you were fine with it, had to be fine with it.
doctor hong lets you go.
“he shouldn’t,” you say, biting down the cracks in your voice.
doctor hong sighs, and places a palm on your shoulder. “please return to the break room. i’ll be taking care of things here.”
you nod, holding your breath as you march down the seventh floor. it isn’t when you get to the bed with the folded cranes that you break into a run.
the break room is the last place you want to be. so you slump down in an empty stairwell, pressing a fist into your mouth. you don’t want to cry. not like this. not for him.
it’s stupid, because it’s not even your loss, and even then mr fujimoto never saw it as a loss. he was ready to go, happy with his final days, grateful, relieved, resolved.
you think about his wife, think back to when she told you her name was “aiko”, when she held her knitting needles in a vice-like grip, fists going white and red and never letting go.
you don’t breathe, force your chest to harden, tighten into a ball and not feel. but it’s a lot. it’s a lot, and it’s so cold and you think back to the discharges in the morning, because they should have been mr fujimoto. he should have died in his home, not here.
a warm, woollen scarf drapes itself over your shoulders.
“you alright?” the voice is kind, gentle, and you shut your eyes.
he sits next to you, tweed coat brushing against your elbows. for a long time, you don’t speak. the man doesn’t seem bothered. he stares straight ahead, affording you the privacy you don’t deserve.
“i’m fine,” you force out, clenching your fingers and toes. “i’ll be – fine.”
he hums. you turn, and notice a slip of pink under his sleeve.
he doesn’t leave your side until dawn breaks.
-
the next day at work is brutal and unforgiving. there’s a procedural investigation into a violation of code, and you’re let off with a stern warning. aiko holds herself together better than you did, and thanks you for all the care you’ve offered her husband. you bow, not trusting yourself to speak.
you’re packing up his belongings for her, folding his dressing gown and slippers into a ziploc bag under her special request, when you notice his wrist tag. it’s pink.
-
you forget about the man from thursday night – after the violation, your review committee decided to ladle you with a couple more shifts to train your endurance and tenacity. in short, a punishment. you don’t mind, though, it gets you off other more distracting and despairing trains of thought.
it’s a relief to help people who come in with minor injuries or illnesses anyway. bandaging abrasive wounds and logging in prescriptions have become something like muscle memory, enough that you can make small talk easily and feel better about yourself.
even today, patching up kwon soonyoung – patient, 22, dancer and aspiring parkourist – next to seungkwan’s chatter feels normal.
“of course, otolaryngology takes in the best,” seungkwan says blithely, tapping on his keyboard. “but everyone knows that neurosurgery has the hottest shots.”
“woah, there’s such a thing?” soonyoung exclaims, sitting up and wincing immediately.
you tap his knee in chiding. “it’s all rumours.”
“and radiologists party the hardest,” seungkwan continues. “must be all the radiation from the x-rays.”
soonyoung frowns. “i didn’t know there were… otorary… or whatever. it’s all just medicine, isn’t it?”
“otolaryngology,” you correct.
seungkwan huffs. “please. don’t lump me in with the gynos.”
“gynos, that’s like, the pregnant thing right,” soonyoung says. “what’s wrong with pregnant people?”
“gynos are weird. who likes looking at babies before they’re born? and even then! all that blood,” seungkwan shivers.
you smile. “it must be nice, though, to be able to spread good news all the time.”
soonyoung’s deep in thought, doesn’t even notice when you move on to patch his right ankle. he chews on his lower lip.
“hey, you know that weird stickers patients have right,” he says, miming at his wrist. “the tags. are they because of the different departments of medicine that you have here?”
you shake your head. “nah, it’s just for us to know how to treat each patient – like, if they’re allowed to be out of bed themselves, if they’re staying for a long time…”
“what about pink?” soonyoung says hurriedly, flushing. “i saw this man earlier with a pink tag, and he was really good-looking!”
“ah, him,” seungkwan whispers, and then, “pink means they’re a long term patient. anyway, kwon soonyoung, no more parkour for the next two months! you need to let your joints rest, and remember to ice that sprain!”
soonyoung waves him away, turning to you instead. “thank you, miss!”
he hops off the bed and stumbles out of the room. you round up on seungkwan immediately.
“who’s he talking about?”
“who?”
“the patient with the pink tag.”
seungkwan presses the clipboard to his lips. “ah, right, you’re new; i can’t tell you much but – he’s the patient with ffi, he’s supposed to be with hospice care but they’ve been having him here for research and stuff.”
-
the last shift is a trip up to the seventh floor. you smooth a hand over your scrubs, straighten your shoulders, and plaster a smile up for this final round.
thankfully, unlike thursday, the wards are all awake, buzzing weakly, contained and conserved. visiting hours are almost over, but you know that most people don’t pay heed to the intercom announcements. you’ve learned to turn a blind eye to it.
instead, you walk straight to 0730, the bed opposite mr fujimoto’s, where the curtains are half-drawn. it’s occupied by an old lady who’s admitted for kidney stones, and has a nasty temper towards doctors advising her on her diet. a common case, really – elderly patients tend to distrust doctors, since, in spite of all those medical dramas and serials, nurses are really the ones who perform most of the rounds for food, medication and general welfare.
she’s a tough one, a little stubborn, but you think she might just be warming up to you.
“madam,” you say softly, peering over the curtains.
mdm lee turns away from her book, reading glasses perched on her nose bridge, and scoffs. you realise that there’s someone else with her, strange, because no one has visited her since her admission. the realisation spikes into something like embarrassment or surprise when you recognise the tweed coat around his frame.
“first him, now you,” she says without much bite. “sit down.”
you nod towards the patient, and take a seat next to mdm lee. “i didn’t know you had other visitors.”
“i don’t know him,” she sneers, returning to her book.
when your gaze shifts towards him, he places his hands up and laughs. “we’re not related, i just thought-”
“-badgering me about the bed across,” she mumbles.
you pale. “oh. you… heard about that.”
mdm lee places her books down and cleans her glasses gingerly. “everyone did, you were shouting so loudly that night.”
uncertain, you grimace.
mdm lee continues, “but i suppose it was reassuring. when we die, at least there’s one nurse in this place who cares.”
you shake your head at her generosity. “anyway, mdm lee, they said you were refusing the surgery.”
she huffs. “all those doctors – said it was a small thing, made me go through all those scans and medication, and now? now they want to stick more tubes in me.”
“i know,” you say, “but it’s a lot faster and certain if we can use a scope for that. then you wouldn’t need to come back except for further check-ups.”
“they said i’d be fine after a week of observation,” she insists, irritated. “and now, another operation?”
“medication is non-invasive, but because of that it’s also less direct and takes a longer time,” you say. “if you don’t want the operation, there’s nothing we can do.”
she hums, placing the glasses back on her nose. “if it’s an operation it must be bigger than we thought, yes?”
you nod. “i’ll do your follow ups when you’re discharged.”
mdm lee sighs heavily, blinking an eye open to assess you. “i’ll think about it. now get out so i can sleep.”
you do, making sure to close the curtains all the way. the man follows behind you, a tired smile on his face. it widens as he greets the other patients in the ward.
“which floor are you on?” you ask. the pink band is clearer around his wrist.
“twelfth tonight,” he says, and hesitates. “are you alright?”
“i’m better now, thank you,” you glance up at him, taking in his appearance – tired, but not sickly. “tonight?”
he shrugs, reaching up to comb his hair into place. “doctor hong sometimes has me on the fifteenth.”
the lights dim from bright fluorescent to a warmer orange. you continue walking briskly to the lobby, tapping your card once at the doors.
“the fifteenth…” you muse. twelve is a general ward, but fifteen is… “rare conditions?”
he smiles, peering down at you. “have you heard of fatal familial insomnia?”
you haven’t, but the name is simple enough to form a conclusion. you look at him again, then away, trying not to scrutinise or pin your own half-written diagnoses on him. he doesn’t look that sick, and though sleeping pills exist to sedate some part of the brain, it probably wouldn’t work on him.
“well,” he clears his throat. “the average age of onset is around fifty one, and it could take six years before the body wears out.”
“why are you here then?”
he shrugs. “research.”
-
the man’s name is yoon jeonghan. he’s twenty four with an incurable illness, and lazes around everywhere. you’re doing your rounds on the twelfth floor when you find him nestled among blankets and extra pillows (really, that many should count as a hazard). the evening light bathes him in orange and gold, and as he stares out the windows you wonder what he’s thinking.
you place his dinner down before him, and the light thud has him noticing you. he doesn’t bother looking over, though something in his shoulder relaxes against the pillows. you’re not sure if it’s distress at this point, or if he’s just insurmountably lazy.
when you return after half an hour, he hasn’t touched the food, much less shifted from his position. his eyes are wide open, however, gazing listlessly out at the city.
“you’re really good with patients,” he says softly when you sit down, ready to coax him into eating.
you shrug. “i listen.”
“that first time at the stairs,” he murmurs. “i heard you spent a lot of time with that patient. breaks, before shifts. talking to him.”
the memory dredges up something sour and acrid in you. you think you might be able to say his name without choking up. “yeah, mr fujimoto was very kind. we all knew he didn’t have much time left.”
“it must be hard being a nurse,” he says.
you turn to his meal, lifting the plastic lid and preparing the cutlery. “you could make it easier if you tried eating.”
he scowls, feigning hurt even as he shifts and sits up. “i knew it. you’re here to exploit my kind, unsuspecting nature.”
you grin, passing him the chopsticks. it’s bibimbap tonight, and you know for a fact that it’s the chef’s specialty. might even be better than most restaurants outside.
you tell him that, and he pouts. “i’m holding you to that.”
watching him, you cup your face in your hands and lean in. he takes a careful bite, and blinks.
“that’s really good!”
you chuckle, wagging your finger in his face. “told you.”
he grins, carrot slices stuck between his teeth.
-
[unknown]: hello! it’s me, jeonghan :)
[unknown]: i got your number from one of the nurses – please don’t blame her!
[unknown]: i have special permission to go out tonight!! from doctor hong ;)
[you]: oh hey
[unknown]: let’s get bibimbap!
[unknown]: :)
[you]: sure
-
jeonghan outside the hospital is not much different from jeonghan inside; he’s always wearing that heavy coat, even over his gown, and he smiles in the crinkly, sincere way that makes you want to smile back too. he waves you over, livelier than when he’d been in the wards. you skip ahead, too, relieved of your starched scrubs and monochrome uniform.
“why’d you ask me out for dinner?” you say.
“to prove a point,” he says. “because nothing beats eating out.”
you snort. “you bibimbap snob.”
jeonghan laughs, loudly. “food is the best thing there is! of course i’m picky!”
when you get to the restaurant, jeonghan removes his coat and reaches out for yours. you shrug out of your woolly cardigan and hand it to him. he’s wearing a black turtleneck and another thinner sweater. it’s kind of endearing, to see a man at least a head taller than you with sweater paws.
“so!” he says, clapping his hands and leaning forward. “i heard this place is really good.”
you hum noncommittally. it’s good, but it’s also on the pricier side – you’ll just have to live with cafeteria food for the next two days. being a student nurse has its perks, but the meagre allowance is not one of them.
he notices your dilemma. “it’ll be my treat! after all, i’m dragging you away from saving people’s lives.”
you narrow your eyes. “i can’t – you’re a patient.”
jeonghan scoffs. “i’m a research participant. i get money off that.”
you can’t argue with that. well, you try to, but he swats your concerns away, and instead points to the other people in the restaurant. his first pick is a waitress with dyed hair and large dangling earrings.
“she’s struggling to pay off her student loans, but she’s also a shopaholic and she’s gonna get a third piercing,” he says, leaning in conspiringly.
you snort. “you’re bad at this.”
he frowns. “i’m just trying to make conversation.”
sweeping your gaze across the restaurant subtly, you decide on a couple seated by the windows. jerking your head in the direction, you wait for jeonghan to catch on. he does, and gasps.
“he’s going to propose!” he squeaks.
“i thought they were going to break up,” you say dully. “isn’t he fidgeting and averting his eyes?”
jeonghan tsks, raising his brows at them. “there are extra flowers on their table, and the waitress keeps glancing over, and he’s been having his hand in his pocket for a while now.”
you turn to get a better look. indeed, he does. you swivel back to jeonghan, who now has a twinkle of interest in his eyes. he clasps his hands together, excitable and anticipating.
soon, the man does get up and down on one knee. everyone else in the restaurant’s staring at the scene, the lady pressing a manicured hand to her face. the man begins his speech nervously, and then starts to quote from a love poem. he’s stammering quite a bit, and though the lady seems touched, she’s also a little apprehensive at the attention.
“… when in eternal lines to time thou grow’st… so… so…”
you cringe. it’s sweet, but also a little embarrassing. the man continues to hem and haw.
jeonghan clears his throat, and says in a raised whisper, “it’s stuffy, i can’t really breathe. even the windows are too fogged up to see.”
you nudge him, heat flaring in your cheeks at possibly ruining the moment.
the man glances over at your table, then continues, “ah! so long as men can breathe or eyes can see, so long lives this, and this gives life to thee!”
it appears the be the right conclusion, because soon he’s sweeping the lady off her feet with a whoop, and applause is ringing around the entire restaurant. the waitress with the dyed hair from earlier brings a cake in and offers it to the newly-engaged couple as congratulations.
and when the man comes over to thank jeonghan for his intervention, you understand. lifting a hand you give him a high five. jeonghan grins, but the chill of his palm catches you completely off guard.
you don’t ask about it. “where did that come from?”
“the poem?” he asks. you nod. “it’s an old poem we learned in school.”
you shake your head. “i can barely remember anything from school.”
he pushes his food around with a fork, staring into the distance. you find that he has barely touched his food; he responds by teasing you about how maybe you were right, that bibimbap from the hospital was much better than that from outside.
when you take the bus back to the hospital, he dozes off intermittently, head bobbing curiously and landing on your shoulder. you let him, even if the sharpness of his jaw bites uncomfortably into your neck.
-
[jeonghan]: did you always want to be a nurse?
[you]: mm
[you]: i was very motherly all throughout high school
[jeonghan]: haha :)
[jeonghan]: i can see that.
[you]: i bet you were smart
[you]: top of the level
[jeonghan]: :o
[jeonghan]: i was! how did you know?
[jeonghan]: stalker ;)
[you]: …
[jeonghan]: i had a scholarship too, surprising huh!
[you]: why
[jeonghan]: everyone said i was like a sloth
[jeonghan]: always lying down
[jeonghan]: hey
[you]: whats up
[jeonghan]: can’t sleep ;)
-
jeonghan comes to help mdm lee with her discharge. he’s all smiles and wit despite her bluntness, pleasantly charming and sweet. you hide a snicker when mdm lee deadpans a bland, pointed remark about flattery.
“i’ll see you for my follow up,” mdm lee says to you, and hobbles out with her day bag. “i hope you have better taste in men.”
jeonghan raises a hand in salute, watching after her in admiration and envy. you roll your eyes.
“so?” jeonghan says. “what about your taste in men?”
“what about my taste in men?”
jeonghan licks his lips. “how terrible is it?”
you smack him on the forehead with a clipboard. “very.”
he winces. “why would you do that to a dying man?”
“if it’s you, it’s fine,” you quip.
he pouts, and trails after you anyway.
-
[jeonghan]: do you want to get bibimbap again?
[jeonghan]: :D
[you]: sure
[you]: sorry there’s a change of plans
[you]: i might be a bit busier the next few days
[jeonghan]: oh it’s okay then!
[jeonghan]: just text me whenever!
[jeonghan]: perks of being sick = always free :)
-
the next couple of weeks fly by, because there’s a sudden increase in influx of patients suffering from food poisoning. they’re all students from a foreign country on a school trip, and you’re swamped with the amount of paperwork and administrative details required – liaising with your own ministry of health, collecting allergies and dietary requirements.
most nights you end up in the break room, huddled up on the couch. it’s not a comfortable position to be sleeping in, but you really need the rest in order to catch up with your other duties.
one night, you’re just settling in on the couch, ready to shut your eyes and fade away into the depths of unconsciousness. your phone buzzes, once, twice, and you swat at it until it clatters to the floor. only with that crash do you sit up, cradling the poor, abused gadget.
[jeonghan]: gekkp
[jeonghan]: hdl0hekphdg3”p
it doesn’t make sense. you rub your eyes, half a mind to go back to sleep.
it hits you that you don’t exactly know what it is jeonghan’s suffering from. insomnia, yes, fatal, yes, but it’s never really struck you to search it up.
you kick the blankets off your body, launching yourself up to a dizzying blackout, before rushing out to the lift lobby. it’s either the twelfth floor or the fifteenth, but if he’s in trouble, it’s probably the twelfth, since most of the nurses are deployed to help with the food poisoning.
he’s not on the twelfth. you sprint back to the lift, jab it open, and go for the fifteenth.
[you]: where r u now
he’s not on the fifteenth either. there are rooms of empty beds and several strange, unnatural machines and labs. you bite down on your fist, steady yourself and the fluttering panic in your chest.
you climb down the stairs, shoes unkempt and noisy against concrete, when you catch the tail end of a whimper. it echoes around the stairwell, undecipherable.
“jeonghan?” you call.
there’s a muted wheeze this time. you scramble down, past thirteen and twelve and
at eleven, there’s a figure crouching, folded in on himself. you halt, and then tip toe down, quiet and unsteady. his shoulders are shaking, hard. you can barely make out the bruises on his bare feet. kneeling down to him, you find he’s covered in sweat, cold to the touch, and hardly breathing.
“jeonghan?” you say softly.
he doesn’t look up, flinching away from you and gasping. something cold balls up in your stomach.
“jeonghan, i need you to listen,” you try again, keeping your distance. “you’re having a panic attack, and i’m here to help you through it.”
“can’t,” he spits. “breathe.”
you nod, slipping into detached professionalism. “that’s alright. follow me – i want you to inhale on one, and then exhale on four. okay? one, yes, two, three, four – exhale, yes, five, six seven, eight, one – inhale, that’s right, three, four, exhale, yes, seven, eight, one-”
he struggles, fingers clenching involuntarily and chest shuddering all the way. you don’t let up, guiding him through his breathing.
the episode lasts half an hour. he’s exhausted by the end of it, limbs slack and neck drooping to the side. he leans in to your side, impossibly cold to the touch still, breath hitching at times.
“i’m proud of you,” you say. “i’ll get you some water.”
he wraps a hand around your wrist.
you still, and sit back down.
-
he doesn’t want anyone to see him for the next day. or week. the nurse who’s given authorisation to tend to him on the fifteenth floor tells you it’s creepy, how much he’s just lying there and staring out the window.
-
new note: untitled
last updated: 3.42pm
· age of onset: 18 – 72
· restless sleep > inability to fall asleep + weight loss, panic attacks, irregular body temperature > hyperventilation, hallucinations, delirium, myoclonus (muscle spasms) > dementia > inability to walk and talk
· death within 7 – 73 months
· immunotherapy??
· cardiac arrest
-
he’s a lot weaker this time. speaks slower, softer, and less. the sharp edges of his wrists and ankles are masked by his coat and the blankets, but the sallow complexion and deep eyebags are obvious. you listen to his voice, to the rumble of his throat, feel his exhaustion at not being quick or sharp as usual.
“you saved the man,” he says.
“who?”
jeonghan tilts his head. “the man. why?”
you set your book down. his meal’s untouched, even after you begged them for bibimbap.
“i couldn’t not save him,” you say. “you have to eat.”
he shuts his eyes, breathing too lightly to be asleep.
-
you bring him glow in the dark stars. he watches as you arrange them around the room.
“thanks,” he says. “they look nice.”
“do you know about constellations?” you ask.
“a little,” he says, wheezing into the pillow.
you hum, settling back in your seat. he drags his eyes up from the bed rails to the ceiling, face slack and white. he doesn’t look twenty four.
“i heard you,” he says, words jumbling. “back when – code blue – thursday – after lights, down the stairs. on the ninth.”
his brows furrow, trying to think.
“i heard you,” he says again. “i was…”
he trails off, uneasy.
you cut in, “thank you.”
jeonghan stops frowning. he blinks, once, and then again.
“alright.”
-
you’re laughing at something one of the students from the food poisoning said when jeonghan has another panic attack. doctor hong attends to it instead.
he sits in the chair to the side while you strip the sheets methodically, ignoring the wet outlines of his back. he doesn’t want to take off his coat, so you leave that on.
“sorry,” he says.
you shake your head. “it’s not your fault.”
jeonghan lifts a hand, pained. “i heard you, that night.”
he winces when you help him to the bed, a hand under his soaked armpit.
“i heard you, i shouldn’t,” he says, eyes unfocused. “my heart’s going really fast.”
you bite your lip. “i know, i’m such a charmer.”
he coughs a laugh weakly.
after you leave for your shift, doctor hong brings up the catheter.
-
one night, he rips all the glow in the dark stars out. you don’t stop him. don’t know how to. there’s a sudden burst of energy from him, hands clawing at the walls, heaving and panting furiously, scared. he winds up slumped against the wall, eyes shut and lungs too loud.
you help assess the damage late, bandaging his fingers (too thin) and taking down the rest of the stars. he makes you keep them in a side drawer.
-
he lies on his side, facing away. his pillow is wet, when you change it next morning.
“did you think?” he says softly.
“what?”
“i asked you,” he says.
you pause. “yeah, what did you say?”
he exhales, deliberate and long and winded and fragile enough to be human.
“did you want?” jeonghan says, eyes glued to the window.
you look away. hold your fist to your sides.
“yes.”
-
the thing about doctors is that they’re not allowed to give you a timeline. it’s impossible and irresponsible on all fronts to measure the amount of time you have left, which is possibly one of the biggest gripes you used to have with medical dramas overhyping the whole terminal illness thing.
a range is possible, however, and more legally rational; if you can’t make promises to a dying man on moral grounds, there’s much less reason to make them to his family from a legal perspective.
but when doctor hong lets up on the immunotherapy, lets up on the drugs and stops carting food at all from the hospital kitchen, you know. it doesn’t stop you from bringing store-bought lunch to share with jeonghan.
it doesn’t make him eat.
he’s silent a lot. you listen for the dips in his breathing.
“i won’t,” he says, a sigh barely caught.
“you won’t?”
he closes his eyes. “no code.”
-
he has a high fever through the night. for the first time, his neck is warm.
jeonghan doesn’t move around much. you press for a doctor, cupping his hand in your own, praying.
you’re not sure what you pray for.
-
it breaks. it breaks, and the next afternoon he looks slightly better, and even if all odds are screaming at you, as a medical professional, hope wins out. you smile at him, watch him smile back.
“need anything?” you ask.
he whispers, “no.”
“alright,” you say, getting up, stretching your stiff arms and thighs. “i gotta go to my next shift.”
he smiles, and coughs. “actually, maybe a tissue.”
-
0728 is now occupied by a hopeful girl with benign tumour in her chest. she laughs a lot, makes quick-witted jokes and clever judgements about the other occupants of the ward. she makes you tell her about yourself, insisting that otherwise she wouldn’t be able to sleep easy without knowing who’s really taking care of her.
you’d gone into nursing to treat patients as people. strange how that’s turned on you.
“do you like anyone?” she wheedles, wagging her eyebrows knowingly.
you smirk. “maybe.”
you leave the ward a little lighter, a little more relieved. for a moment, you wonder if you should head home first. take the first long shower in a week, and maybe sleep the first complete six hours in months.
0728 will go for a simple operation next monday, recover within half a day, and be discharged on tuesday. she’ll be laughing and pressing her face into her parents’ embrace.
that’s the part of the job you want.
-
the first thing you notice about jeonghan’s room is the peeling bunch of glow in the dark stars on his headboard. it’s all dark, so it’s more apparent. you smile to yourself, walking over to the side table and switching on the lamp.
he’s huddled up under a blanket, curled precociously. you wonder if he’s finally, miraculously, gotten some sleep.
and then it hits you how quiet it is.
you place a hand on his shoulder. he falls over.
-
you don’t yell code blue, don’t perform chest compressions, don’t wind up crying in a stairwell. instead, you call doctor hong in. he declares the time of death, cause of death, and signs it on your clipboard. the clipboard is passed to a medical staff at the counter, and by eleven, he’s carted off to the morgue.
you strip the mattress of its sheets, replace them with new ones, sanitize the bed frame and side tables as per protocol. when you peel off the stars you don’t know what to do with them. so they go in a huge white plastic bag.
someone tells you his parents are coming for his body. you stay back firmly, offering tissues and sweets. you can’t find the words to tell them how his final days were.
you go home, shower, and bury your head under a pillow.
you sleep.
#seventeen#seventeen fanfics#jeonghan imagines#jeonghan#angst#hospital au#very briefly researched#as u can tell because otherwise its a clear breach of#interests? like nurses n patients i think#also because nurses r underappreciated#and bc i have no medical background#save for trips to the hospital and prior experiences#so here#i just wanted sad
53 notes
·
View notes