#Implantable Biomaterials
Explore tagged Tumblr posts
Text
Increasing Construction Activities Boost the Glass Bonding Adhesives Market
The glass bonding adhesives market is valued at about USD 3,891.3 million in 2023, and will grow at a rate of 8.7% by the end of this decade, to touch USD 6,857.8 million by 2030. The growth has a lot to do with the use of these agents, the glass is smooth, as there will be no noticeable screws, bulging nuts and bolts. Furthermore, these materials offer protection against corrosion and possess…
View On WordPress
#Bioactive Materials#Biocompatible Materials#Biomaterial Innovations#Bone Grafts#Cartilage Repair#Healthcare Innovations#Implantable Biomaterials#Joint Health#Joint Replacement#market trends#Medical Implants#Musculoskeletal Health#Orthopedic Biomaterials#Orthopedic Devices#Orthopedic Prosthetics#Orthopedic Research#Orthopedic Surgery#Regenerative Medicine#Tissue Engineering
0 notes
Text

Biomaterials are at the forefront of regenerative medicine and implant technology! 🌱🔬 From creating sustainable solutions to advancing healthcare, these innovations are shaping the future of medical science. Discover how science and nature unite for better healing! 🌟
#Biomaterials in regenerative medicine#Innovative medical implants#Natural and synthetic biomaterials#Tissue engineering scaffolds#Advanced drug delivery systems#Biocompatibility challenges#3D printing in biomaterials
0 notes
Text
youtube
#Osteoclasts#osteoblasts#epigenetic regulation#long non-coding RNAs#lncRNAs#bone remodeling#titanium implants#nanotopography#bone regeneration#implant integration#bone healing#osteoimmunology#epigenetics#cellular interactions#biomaterials#tissue engineering#orthopedic research#regenerative medicine#gene expression#implant materials.#Youtube
0 notes
Text

I don't know much about 'skin things' but I do have a little bit of information on it for those who are interested! (I hope you don't mind me using your comment). I'll be supplementing my knowledge with some research under the read more.
Skin grafting is a dermatological procedure utilized to facilitate wound closure.
We'll talk about some commonly used techniques:
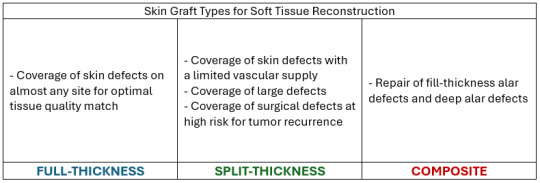
1. Split-Thickness Skin Grafts (STSG) are composed of the epidermic and a superficial part of the dermis. These grafts are commonly used to cover large wounds, burns, and areas of skin loss. They are thinner than full-thickness grafts, which allows them to cover larger areas.
2. Full-Thickness Skin Grafts (FTSG) contain both the full epidermis and the dermis. These grafts are typically used for smaller wounds in areas where aesthetics and durability are essential, such as the face, hands, or neck. Since FTSGs retain the full dermal layer, they offer better cosmetic outcomes, including improved texture, pigmentation, and reduced scarring compared to split-thickness grafts. They also tend to resist contracture better, making them ideal for regions requiring flexibility. - FTSGs are more complex because they require a well-vascularized wound bed to survive and heal. - FTSGs are the most commonly used graft.
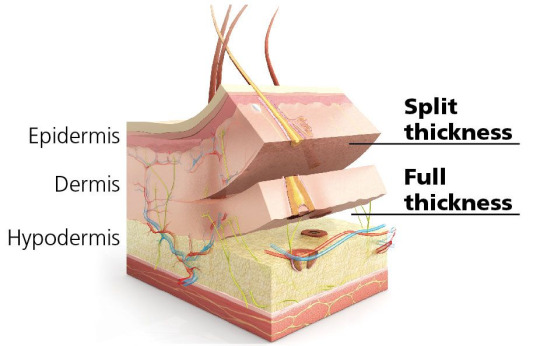
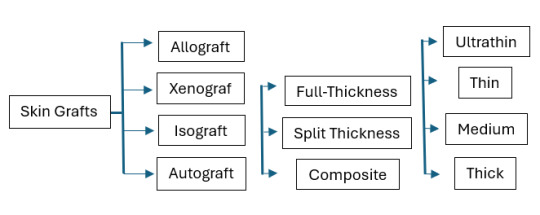
3. Composite Skin Grafts are usually small and include a combination of skin and underlying tissues, such as fat, cartilage, or muscle. These grafts are used to reconstruct areas where multiple tissue types are needed to restore both form and function, such as the nose, ears, or fingertips. - Composite skin grafts which combine allogeneic dermis and an expanded autologous epidermis can effect rapid wound closure.
It is further broken down by the following:
Permanent Skin Grafts
1. Autografts (autologous graft): skin collected from the patient 2. Isograft (syngeneic graft): skin collected from a genetically identical donor (twin)
Temporary Skin Grafts
1. Allocrafts (heterologous graft): skin from a cadaver (living donors are possible) 2. Xenograft (heterograft): skin from another species
Can be Temporary OR Permanent
1. Synthetic skin substitutes: use of manufactured skin - The only technique that is permanent is cultured epithelial autograft (CEA), which is essentially a skin graft grown from a patient's own skin cells.
NO NON-SELF TISSUE IS GUARANTEED TO COMPLETELY AVOID DEATH OR REJECTION.
Transplant Rejection: a patients immune system identifies the graft as a foreign body, which triggers an immune response to get "rid" of the tissue.
Skin implant compatibility is based on three highly polymorphic MHC genes (HLA-A, HLA-B, and HLA-C) that encode proteins and are a part of the Human Leukocyte Antigen (HLA) system. This system identifies foreign bodies.
Knowing this, the use of modified donor animals, such as pigs, to provide transplantable organs, is gaining some renewed research. It involves excising the genes in the pig that are most responsible for the rejection reaction after transplantation. However, finding these genes and effectively removing them is a challenge.
The use of autologous skin grafts is the most common approach in the treatment of chronic wounds. However, in the case of deep and/or large wounds or with extensive severe burns, the use of autografts is limited, and either allogeneic (from cadaver) or xenogeneic skin grafts are used for transplantation.
The use of allogenic/xenogenic tissue carries a high risk of graft rejection, limiting their clinical applications.
Tissue Engineering
Advanced therapies for chronic wounds involve application of bioengineered artificial skin substitutes to overcome graft rejection as well as topical delivery of mesenchymal stem cells to reduce inflammation and accelerate the healing process.
Photo shows potentially ideal artificial skin graft:
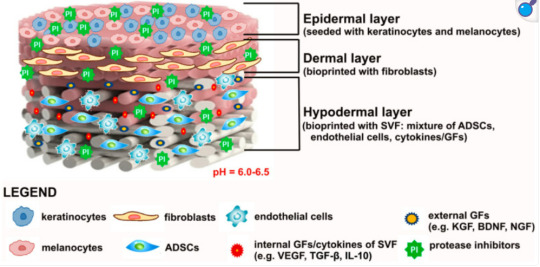
Modern treatment includes skin tissue engineering aiming to produce bioengineered biomaterial-based artificial skin grafts. Therefore, the main roles of bioengineered skin grafts is to supply oxygen (by being oxygen permeable), keep the wound from dehydration, promote healing, and prevent infections. - Depending on the type of biomaterial used for the production of artificial skin grafts, they may function as skin equivalents providing temporary wound covers or permanent skin substitutes. - When the biomaterials are pre-seeded or have cells incorporated within their matrix, they are classified as cellular artificial skin grafts, whereas biomaterials without or deprived of cells are defined as acellular artificial skin grafts.
Here are some current commercially available synthetic skin grafts I found applicable to Curly's injuries:
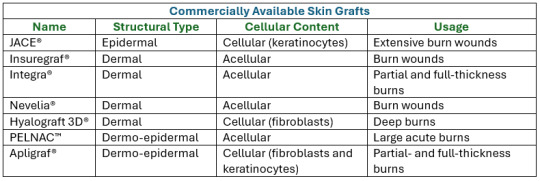
Definitions for Clarity: 1. Epidermal: Pertaining to the outermost layer of the skin. 2. Cellular Content: the complex structures and biomolecules that make up cells, the smallest units of life. 3. Acellular: not consisting of, divided into, or containing cells. 4. Fibroblasts: a cell in connective tissue which produces collagen and other fibers. 5. Keratinocytes: an epidermal cell which produces keratin (a fibrous protein forming the main structural constituent of hair).
Articles to Reference
Organ Transplantation and Rejection by Libretexts biology. LINK
(CW: Images) Skin Grafting by Joseph Prohaska and Christopher Cook. LINK
A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? by Agata Przekora LINK
Composite skin graft: frozen dermal allografts support the engraftment and expansion of autologous epidermis by E L Heck, P R Bergstresser, C R Baxter LINK
#curly mouthwashing#mouthwashing#mouthwashing theories#leo vomits medical jargon#i didnt proof read this if there are errors you dont see them
20 notes
·
View notes
Text

One-step, high-speed, thermal-electric aerosol printing of piezoelectric bio-organic films
Amidst the ongoing surge in demand for bio-MEMS, wearable/implantable electronics and bio-tissue therapeutics, the pursuit of piezoelectric biomaterials has become a priority, thanks to their remarkable electromechanical properties, biocompatibility, and bioresorbability. However, their technological potential is restrained by the challenges of precise manipulation of nano-biomolecules, controlling their growth across the nano-to-macro hierarchy, and tuning desirable mechanical properties. Since the discovery of biological piezoelectricity in wool and hair in 1941, attempts to activate piezoelectricity in biomaterials through external electrical poling have proven largely unsuccessful. For 80 years, the challenge has remained unaddressed, forming a huge gap between laboratory piezoelectric biomaterials and practical bio-devices.
Read more.
#Materials Science#Science#Piezoelectric#Aerosol#Thin films#Biomaterials#Organic materials#Materials Synthesis#Additive manufacturing
9 notes
·
View notes
Text
Pristyn Care: Innovations in ACL Surgery: The Development and Use of Synthetic Ligaments

Anterior cruciate injuries are one of the most common but most crippling sports-related injuries caused to athletes from amateur to professional levels. It limits the knee joint from unstable activities for rotating and forward movements. The injury of the ACL can reduce the mobility of joints, causing chronic pain, and deteriorating the quality of life drastically.
In most of the cases, it necessitates surgery. New developments, techniques of production, and ways to use synthetic ligaments nowadays represent a true revolution in orthopedic medicine. All the novelties in treatment were taken into consideration by Pristyn Care in its work to innovate the Pristyn healthcare services, and as soon as the patients began to take the benefit of the most advanced options for ACL repair through minimally invasive interventions, the expanded benefits of minimally invasive techniques began to be evident clearer.
Importance of ACL Reconstruction
The ACL plays a very important part in the stability and movement of the knee joint; basically, activities such as walking, running, and jumping are regulated. These micro-injuries, if not halted in the growth phase, can grow into serious knee instability. The individual is put at risk of further injuries and a high probability of degenerative, destructive joint conditions of the osteoarthritic type. It is estimated that about 200,000 reconstructions of the ACL are carried out annually across the globe, and the question of finding and applying an integral treatment suddenly becomes urgent.
Most notably, the introduction of artificial ligaments entirely changed the idea of ACL reconstruction; it was the arrival on the market of a durable commercial analog of traditional grafts. In most cases, the period of recovery was elongated by the occurrence of pain in the donor area and the need to recover two traumatized areas and to use transplant tissue taken from the body of the patient. Contemporary solutions, backed by state-of-the-art equipment and the teams of surgeons in the Pristin Care facilities, have brought initiation of repair of an ACL tear to a new level—the maximum quality of life that individuals devastated by such an injury can hope for.
Synthetic ligaments. What exactly are synthetic
Some of the commonly used and developed synthetic implants are the synthetic ligaments used in orthopedic surgery. More precisely, they are artificially created biomaterials used in cases of a technique for replacing damaged natural ligaments. The synthetic ligaments are not autografts, allografts, or autografts harvested from the body of the patient but, on the contrary, they are biocompatible polymers in origin.
The various available options of synthetic ligaments and benefits brought by it include good quality, which is consistent; unlimited supply; and no morbidity at the donor site. The extraction of autografts can be a bit frightening to the patient, and most of the time, more time for recovery is needed. Other than that, synthetic ligaments minimize the time used during the surgery because grafting is not necessary.
That is why advanced synthetic ligaments are increasingly being used as a substitute for grafting, since, in some places, the availability of appropriate and right donor grafts is very limited. This, therefore, enhances the access and timeliness of the surgeries for anybody in need of ACL.
Where Technological Innovations Impact Development on Synthetic Ligaments
The development of an artificial ligament, and more so that of an artificial fiber, is an extremely interdisciplinary field of materials science and engineering, aiming to produce fibers with properties analogous to natural ligaments. If performed on a human knee, that material should be strong, flexible, and durable enough to bear the dynamic stresses. Modern ones are made using advanced polymers, usually polyethylene terephthalate, and polyurethanes for their qualities of strength and elasticity.
This is particularly the case since such developments are specifically targeted at the improvement in the integration of such material with human tissue, the further reduction of risks for tissue rejection, as well as the enhancement of the healing process itself. Particularly, "Pristyn Care" is engaged in doing the given research process further enhancing the boundary of what is possible to be done with synthetic ligament technology. Focusing on knee biomechanics allows developers to adapt how a ligament's structure is formed to recreate natural motion while at the same time reducing complications and inducing new methods of natural and efficient ways of healing.
The newest technologies will save artificial knee ligaments from being inferior or just equal to the traditional knee-ligament grafts and most of the time, they will be far above benchmark levels by such a huge amount, marking a huge leap in ACL reconstruction technology.
How Pristyn Care is Changing the Way ACLs are Reconstructed
Pristyn Care leads from the front in the adaptation and innovation of methods for synthetic ligaments in the reconstruction of ACLs. There is a deep commitment to the assimilation of the latest in medical innovations with the aid of advanced technology that supports conducting such complex procedures in ultra-modern surgical facilities. With patient safety and surgical efficacy set to be a priority, the surgical centers of Pristyn Care can perform every ACL surgery under the best of conditions. Moreover, Pristyn Care is actively involved in partnering with advanced medical researchers and bioengineers to further enhance effectiveness and safety in the use of synthetic ligaments.
Reviews of ACL surgery operations under the aegis of Pristyn Care state that the medical staff were professional, the facilities were modern, and generally, surgeries were performed with good outcomes. All these statements refer not to new medical technologies but to the high rates of patient satisfaction and trust.
Advantages of Synthetic Ligaments in ACL Reconstruction
Using synthetic ligaments in ACL reconstruction has one major benefit which is the elimination of donor site morbidity. Unlike conventional techniques that include removing body tissue from a patient, these issues can be avoided by using synthetic ligaments. Therefore, it is widely recognized as an effective approach that lessens the chances of postoperative infection and pain reduction along with a quicker & easier recovery process. Pristyn Care reviews indicate that patients have highly ranked this method because they recover within a short time and experience minimal postoperative discomfort.
Pristyn healthcare reviews indicate that Pristyn Care’s use of synthetic ligament in ACL surgery has yielded outstanding results, which have enabled patients to make fast comebacks for sports and their normal duties.
In Pristyn Care reviews, people talk about how much they are satisfied with the minimal interruption to their lives, and the quickness with which they recovered. Good testimonials through Pristyn Healthcare reviews emphasize how advanced surgical practices and individualized treatments offered by Pristyn Care are geared towards providing high-quality health services resulting in the best outcomes.
Identify the Challenging Issues and Consider
The utilization of synthetic materials in surgical procedures presents both opportunities and challenges. Although these materials can enhance the recovery process due to their design to integrate with tissue without triggering an immune response, their long-term durability remains a concern. Pristyn Care reviews often highlight the rigorous monitoring of synthetic-tendon integration, particularly under the continuous stress imposed by daily activities. This is an area of active research within Pristyn healthcare reviews, focusing on enhancing the material's ability to withstand long-term use without compromising safety.
Additionally, regulatory and ethical factors about these techniques are vital. In addition, all synthetic materials used are strictly regulated by Pristyn Care for maximum safety. This commitment to safety standards is reflected in Pristyn healthcare reviews where the rigorous clinical trials and medical & ethical constraints are highly acknowledged. These materials undergo stringent safety checks before their approval for use so that they conform with the highest possible safety standards hence maintaining the reputation of Pristyn Care as a provider of safe, innovative, and effective Pristyn health care solutions.
Patient Success Stories and Case Studies
Against this backdrop, many life-changing benefits make patients at Pristyn feel that their ACL is rebuilt with synthetic ligaments. This is not on paper, but one watches how a patient describes getting back to sports activity after months of surgery and says great recovery is credited to great care. Another case in point reflects a patient who almost felt no pain with rapid rehabilitation due to advanced surgical techniques at Pristyn Care. These stories strengthen the good reviews about high satisfaction rates and successful surgical outcomes on Pristyn healthcare platforms.
Conclusion
ACL injuries are mostly known as anterior cruciate ligament injuries and they are common and severe, especially in high-impact sports or activities that involve the knee joint heavily. These injuries negatively affect performance, so it requires a well-planned recovery plan to safely go back to what one was doing before. One main surgical procedure through which the normal functions of the knee can be restored is known as ACL reconstruction. The Return to Play (RTP) protocols post-surgery should be focused on total recuperation, prevention of future traumas, and restoration of peak levels of performance. For Pristyn Care, emphasis has been put on RTP post-ACL reconstruction to achieve full patient recovery and enable them to regain their best level of performance. In Pristyn care reviews, there is usually mention of how effective its customized RTP protocols are because these protocols help in preventing reinjuries. Further, Pristyn Healthcare reviews also hail the individualistic approach taken during the rehabilitation period which has significantly improved the success rates for ACL surgeries conducted at Pristyn Care.
#pristyn care#pristyn care reviews#pristyn healthcare#pristyn healthcare reviews#pristyn care lybrate#pristyn care company
3 notes
·
View notes
Text
Prosecutors in northern China have charged dozens of suspects in a decade-long scheme in which corpses marked for cremation were instead sold to one of the country's largest biomaterial firms, a company with ties to the state.
Owners and operators of Shanxi Osteorad Biomaterial Co. and Sichuan Hengpu Technology Co. are accused of receiving more than 4,000 cadavers via illegal means, allowing the former to turn over nearly $53 million in revenue from 2015-2023.
The alleged corpse trafficking spanned at least seven provinces and nearly a dozen localities, where dead bodies were either transferred, dissected, stored or turned into material devices for bone-grafting procedures, according to a leaked May indictment by the Taiyuan People's Procuratorate in Shanxi province.
The case involves at least 75 suspects, including funeral home managers, doctors and company shareholders. The wide investigation was still ongoing as of Thursday, prosecutors told the Shanghai news site The Paper.
The report was carried briefly by Chinese state broadcaster CCTV on Thursday, but both were removed just hours later. Conversations about the topic were also being restricted on Weibo, China's largest social media website, at the time of writing.
Newsweek reached out to China's National Health Commission for comment.
Shanxi Osteorad was founded in 1999 as a subsidiary of Taiyuan's China Institute for Radiation Protection, part of the state-owned China National Nuclear Corp. headquartered in Beijing.
It is among the country's largest bone-graft and substitutes providers in a market to be worth a half-billion dollars by the end of the decade. Its biggest shareholder also owns Sichuan Hengpu, a medical devices manufacturer.
The medical industry uses bone grafting to repair injuries such as severe fractures. Bone tissue can be obtained from the patient—an autograft—but possible complications mean it is commonly harvested from cadavers—an allograft—with donor consent and strict regulatory oversight.
Shanxi Osteorad was accused of forging donor agreements to create allogeneic bone implant material used to treat Chinese patients. Its staff also mistreated cadavers, prosecutors alleged.
In China, the biomedical devices market is overseen by the National Medical Products Administration. The funeral industry falls under the Civil Affairs Ministry. Newsweek reached out to both for comment.
Chinese authorities have for years encouraged small burials, sea burials or cremations to save land. The pandemic also saw a surge in cremations as families were left without access to traditional funeral rites.
But four funeral homes in China's southwestern provinces of Yunnan, Guizhou and Sichuan, as well as in the Chongqing municipality, collected cremation fees from family members before selling the corpses to two hospitals for dissection and storage, according to the indictment.
Prosecutors said some family members paid for cremation services but did not wish to bring home the ashes. In other cases, unclaimed cadavers were selected instead.
A staff member at Guilin Medical University's anatomy department was alleged to have knowingly purchased at least 450 corpses from three of the funeral homes named in the complaint. The employee bought the bodies for $125 each and later sold more than 300 to Shanxi Osteorad for $1,400 each.
A transplant surgeon at the Affiliated Hospital of Qingdao University, in China's eastern Shandong province, was accused of processing and selling at least 10 corpses to Shanxi Osteorad for $1,400-$3,000 each. Newsweek reached out to both hospitals for comment.
Authorities were alerted to the scandal in September 2023, when China's National Audit Office probed the irregular earnings of the Guilin teaching hospital's faculty member, according to The Paper.
Chinese authorities seized more than 34,000 biomaterial products and over 18 tons of semi-finished goods, as well as 16 properties and 29 vehicles, it said. All suspects have reportedly admitted to their roles.
#nunyas news#that's the governments job you ninny#only they get to do things like that#as well as killing inmates to harvest organs
5 notes
·
View notes
Text
3D Printing Medical Devices Market: Industry-Specific Segmentation and Applications
The global 3D printing medical devices market is experiencing significant expansion, driven by technological advancements and the increasing demand for personalized healthcare solutions. Valued at USD 2.69 billion in 2023, the market is projected to reach USD 11.46 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 17.49% over the forecast period 2024-2032.
Market Segmentation:
The 3D printing medical devices market is segmented based on component, type, technology, and region:
By Component:
Software and Services
Equipment
3D Printers
3D Bioprinters
Biomaterials
By Type:
Prosthetics and Implants
Surgical Instruments
Tissue Engineering Products
By Technology:
Laser Beam Melting
Electron Beam Melting
Photopolymerization
Droplet Deposition
Get Free Sample Report @ https://www.snsinsider.com/sample-request/1185
Regional Analysis:
North America: Leading the market due to early adoption of advanced technologies and supportive regulatory frameworks.
Europe: Experiencing substantial growth with significant contributions from countries like Germany and the UK.
Asia-Pacific: Anticipated to witness the fastest growth, driven by increasing healthcare investments and technological advancements in countries such as China and India.
Key Players
1. Stratasys Ltd.
J750 Digital Anatomy Printer
F370 CR
2. EnvisionTEC
Perfactory P4K
3D-Bioplotter
3. 3D Systems, Inc.
ProX DMP 320
Figure 4
4. EOS GmbH
EOSINT M 280
EOS P 396
5. Renishaw plc
RenAM 500Q
Additive Manufacturing System
6. GE Additive
Arcam EBM
Concept Laser M2 Cusing
7. Desktop Metal, Inc.
Studio System
8. CELLINK
BIO X
INKREDIBLE
9. Materialise
Materialise Magics
Materialise Mimics
10. 3T Additive Manufacturing Ltd.
3T Metal 3D Printing
11. General Electric Company
Arcam Q10
12. Carbon, Inc.
Carbon M1
13. Prodways Group
ProMaker L6000
14. SLM Solutions
SLM 280
15. Organovo Holdings Inc.
NovoGen MMX Bioprinter
16. FIT AG
FIT Additive Manufacturing
17. Wacker Chemie AG
Silicone-based 3D printing materials
18. Dentsply Sirona
SIRONA CEREC
19. DWS Systems SRL
XFAB 2000
20. Roland DG
DWX-52DC
21. HP, Inc.
HP Jet Fusion 3D
22. regenHU
3D-Bioprinting Platform
23. Fluicell
Bioprinting System
24. Proto Labs
Protolabs 3D Printing
25. GESIM
Bio 3D Printer
26. Triastek
3D Printed Drug Delivery Systems
27. Inventia
Rastrum
28. FabRx
FDM Printers
29. Apprecia Pharmaceuticals
3D-Printed Tablets
Key Highlights:
The FDA approved over 100,000 3D-printed medical devices in 2023, indicating growing acceptance in healthcare.
Advancements in bioprinting are paving the way for the development of functional human tissues for therapeutic purposes.
Healthcare institutions are increasingly utilizing 3D-printed anatomical models for pre-surgical planning, enhancing surgical precision.
Future Outlook:
The 3D printing medical devices market is poised for substantial growth, driven by continuous technological innovations and the rising demand for customized medical solutions. The ability to produce patient-specific devices efficiently positions 3D printing as a transformative force in healthcare. As research and development efforts intensify, and as more healthcare providers recognize the benefits of 3D-printed medical devices, the market is expected to witness robust expansion in the coming years.
Conclusion:
The global 3D printing medical devices market is on a promising trajectory, with significant growth anticipated across various segments and regions. Stakeholders, including manufacturers, healthcare providers, and investors, are well-positioned to benefit from the evolving landscape of 3D printing technology in healthcare.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Medical Display Market Size
Medical Waste Containers Market Size
IoT Medical Devices Market Size
eClinical Solutions Market
#3D Printing Medical Devices Market#3D Printing Medical Devices Market Share#3D Printing Medical Devices Market Size#3D Printing Medical Devices Market Trends#3D Printing Medical Devices Market Growth
0 notes
Text
3D Bioprinting in Musculoskeletal Tissue Engineering

Introduction: In the future, we will be able to print muscles, cartilage, and bones in the same way that we print papers! Doesn't that seem like science fiction? But 3D bioprinting is making this a reality. In musculoskeletal tissue engineering, one of the most exciting uses of 3D bioprinting is the creation of live tissues to replace or repair injured muscles, cartilage, and bones. Millions of people with bone-related illnesses, arthritis, and injuries are finding hope because to this technology, which is transforming regenerative medicine. We will discuss the definition, operation, and ways that 3D bioprinting is changing musculoskeletal tissue engineering in this blog. We will also examine its advantages, difficulties, and potential. READ ALSO: WHY IS TALKING ABOUT INFERTILITY IS NECESSARY?
3D bioprinting:
A cutting-edge technique called 3D bioprinting employs specialized printers to produce three-dimensional constructs composed of growth factors, biomaterials, and live cells. Bioprinting use bio-inks, which contain live cells and biomaterials that replicate the natural structure of tissues, as opposed to conventional 3D printing, which utilizes plastic or metal. How Does Bioprinting in 3D Operate? Several steps are involved in the 3D bioprinting process:
Imaging and Design: First, physicians and scientists do medical scans of the injured tissue, such as CT or MRI scans. They make a three-dimensional model of the necessary tissue using this data. Bio-ink Production: A blend of hydrogels, biomaterials, and live cells is used to create bio-inks. For the cells, these bio-inks offer nutrition and structural support. Printing: The required tissue structure is created by the 3D bioprinter layering on the bio-ink. Maturation: The printed tissue is grown and developed into a functioning tissue while being housed in a bioreactor. Implantation: The tissue is placed into the patient's body to replace or repair damaged tissue once it is prepared.
Use in Tissue Engineering for the Musculoskeletal System 1. Bone renewal In bone tissue engineering, 3D bioprinting is one of the most promising applications. Traditional bone transplants frequently have problems such limited supply, infection, and rejection. Rejection risk can be decreased by using a patient's own cells to manufacture personalized bone transplants using 3D bioprinting. 2. Cartilage Repair Athletes and older people frequently suffer cartilage degradation as a result of injuries and arthritis. 3D bioprinting presents a viable answer by producing customized cartilage implants, as cartilage has a limited capacity for self-healing.
3. Engineering of Muscle Tissue Accidental or surgical muscle injuries can result in significant functional loss. By creating muscle tissues that blend in perfectly with the body, bioprinting helps to restore strength and mobility. Advantages of Musculoskeletal Tissue Engineering using 3D Bioprinting
Tailored Care
Decreased Rejection Risk
Faster Recuperation
Addresses Organ Scarcity
Cost-Effective
Difficulties and Restrictions
Although 3D bioprinting has great potential, there are still a number of obstacles to overcome in musculoskeletal tissue engineering:
Cell viability
The intricacy of tissues
Regulatory Approval
Cost and accessibility
How 3D Bioprinting Will Advance Musculoskeletal Engineering With ongoing developments in cell engineering, printing methods, and biomaterials, 3D bioprinting has a promising future. Now, researchers are investigating:
Printing whole, functional organs, such as kidneys, livers, or hearts, is the ultimate objective. Smart bio-inks: creating bio-inks with the ability to release growth factors to promote recovery. Hospital on-demand printing: Imagine a medical facility where surgeons could print muscle or bone tissue for last-minute operations in a matter of hours! In conclusion: For those who suffer from damage to their bones, cartilage, and muscles, 3D bioprinting is transforming musculoskeletal tissue engineering and providing fresh hope. We are getting closer to a time when organ and tissue replacements can be manufactured on demand thanks to continued research and technical developments. The promise of 3D bioprinting is boundless, opening the door to individualized and efficient medical treatments, even though there are still obstacles to overcome.
0 notes
Link
0 notes
Text
Tooth Regeneration Market Insights Regional Market Growth and Expansion
The tooth regeneration market is rapidly evolving, driven by groundbreaking advancements in regenerative medicine, stem cell therapy, and biotechnology. Unlike traditional dental implants, bridges, or dentures, tooth regeneration focuses on biological restoration, stimulating the body’s natural ability to regrow lost or damaged teeth. This revolutionary approach is transforming dental care by providing long-lasting and more natural solutions to address tooth loss caused by aging, trauma, or disease.

With an increasing global demand for innovative dental treatments, the tooth regeneration market is attracting substantial investments from biotech firms, research institutions, and healthcare companies. This article explores key market insights, growth drivers, technological innovations, challenges, and future market projections.
Market Growth Drivers and Opportunities
The growth of the tooth regeneration market is fueled by several critical factors, including:
Rising Prevalence of Dental Disorders – Conditions like periodontitis, tooth decay, and oral trauma are increasing globally, creating a strong demand for regenerative dental solutions.
Aging Population – As life expectancy rises, more individuals suffer from tooth loss due to aging, boosting demand for long-term and biological tooth replacement options.
Technological Advancements – Innovations in stem cell research, tissue engineering, and biomaterials are accelerating the development of effective tooth regeneration therapies.
Consumer Awareness and Demand – Patients are increasingly seeking natural, long-lasting alternatives to traditional prosthetic dental solutions, driving market expansion.
Investment in Regenerative Medicine – Governments, private investors, and research institutions are funding new studies and clinical trials, supporting the growth of regenerative dentistry.
Role of Stem Cells and Biotechnology in Tooth Regeneration
Stem cell therapy is at the core of tooth regeneration, offering immense potential for regrowing dental tissues such as dentin, enamel, and pulp. Scientists are leveraging various types of stem cells, including:
Dental Pulp Stem Cells (DPSCs) – Found in tooth pulp, these cells show strong regenerative potential for dental tissue repair.
Mesenchymal Stem Cells (MSCs) – Derived from bone marrow or adipose tissue, MSCs play a crucial role in stimulating tooth regeneration.
Induced Pluripotent Stem Cells (iPSCs) – These reprogrammed adult cells mimic embryonic stem cells and hold promise for customized regenerative treatments.
Biotechnology companies are also exploring genetic engineering, bioactive molecules, and scaffolding technologies to enhance tooth regrowth. The integration of bioprinting and tissue engineering is expected to revolutionize the market in the coming years.
Emerging Technologies and Innovations in the Market
The field of tooth regeneration is witnessing rapid technological advancements, including:
Biodegradable Scaffolds – These structures act as a framework for tooth regrowth, supporting cell attachment and tissue formation before dissolving naturally.
3D Bioprinting – Researchers are using 3D printing to create complex, patient-specific tooth structures from living cells and biomaterials.
Growth Factor-Based Therapies – Bioactive proteins and molecules stimulate stem cells to regenerate tooth tissues effectively.
Gene Therapy – Scientists are exploring genetic modification techniques to activate tooth regrowth mechanisms in patients.
Nanotechnology in Regenerative Dentistry – Nanomaterials are being developed to enhance the efficiency of stem cell therapy and improve dental tissue regeneration.
Competitive Landscape and Key Players
The tooth regeneration market is highly competitive, with biotech firms, pharmaceutical companies, and research institutions leading the race to develop commercialized treatments. Some of the key players involved in this sector include:
Straumann Group – A global leader in dental solutions, investing heavily in regenerative dentistry technologies.
BioEden – A pioneer in dental stem cell storage and regenerative applications.
Riken Center for Developmental Biology – Conducting cutting-edge research on tooth regeneration using stem cells.
Unilever and Other Consumer Goods Companies – Exploring innovative oral health products with regenerative properties.
These companies are actively collaborating with academic institutions and government agencies to accelerate research and clinical trials.
Regulatory and Ethical Challenges in the Market
Despite its promising potential, the tooth regeneration market faces several regulatory and ethical hurdles, including:
Stringent Approval Processes – Regulatory bodies such as the FDA and EMA impose strict guidelines on regenerative therapies, delaying market entry.
High Costs of Research and Treatment – The complexity of developing safe and effective regenerative treatments results in high costs, limiting accessibility.
Ethical Concerns Over Stem Cell Use – The sourcing and manipulation of stem cells, especially embryonic stem cells, raise bioethical issues.
Long Clinical Trial Durations – Tooth regeneration therapies require extensive testing and validation, prolonging commercialization timelines.
Limited Public Awareness – Many patients remain unaware of regenerative dental treatments, affecting early market adoption.
Regional Market Growth and Expansion
The tooth regeneration market is expanding globally, with significant growth observed in:
North America – The U.S. leads in research funding, clinical trials, and technological innovation.
Europe – Countries like Germany, the UK, and Switzerland are investing in regenerative dental research.
Asia-Pacific – Japan and China are emerging as key players due to advancements in biotechnology and government support for regenerative medicine.
Latin America & Middle East – These regions are gradually adopting advanced dental solutions, but market penetration remains low.
Future Outlook and Industry Forecast
The future of the tooth regeneration market looks promising, with several key trends expected to shape the industry:
Increased Commercialization of Regenerative Dental Products – More biotech companies are expected to launch clinically approved products in the coming years.
Integration of AI and Digital Health in Regenerative Dentistry – AI-driven diagnostics and treatment planning will enhance precision in regenerative dental care.
More Clinical Trials and FDA Approvals – As research progresses, more regenerative therapies will receive regulatory approval, increasing market accessibility.
Affordability and Accessibility Improvements – With further advancements, costs are expected to decrease, making tooth regeneration treatments more affordable.
Consumer Acceptance and Market Expansion – Growing awareness and education about regenerative dentistry will drive adoption among patients and dental professionals.
Conclusion
The tooth regeneration market represents a transformative shift in dentistry, offering natural, long-term solutions for tooth loss. While technological advancements, stem cell research, and biotech innovations continue to drive the market, challenges such as regulatory approvals, high costs, and ethical considerations need to be addressed. As clinical trials progress and commercialization accelerates, tooth regeneration has the potential to become a mainstream dental treatment, significantly improving oral health worldwide.
#Tooth Regeneration Market#Tooth Regeneration Market trends#Tooth Regeneration#Tooth Regeneration treatement#Tooth Regeneration medications
0 notes
Text
Spinal Implants and Surgical Devices Market Trends, Challenges, and Forecast 2031
Spinal Implants and Surgical Devices Market Growth, Demand and Forecast 2031
The Spinal Implants and Surgical Devices Market sector is undergoing rapid transformation, with significant growth and innovations expected by 2031. In-depth market research offers a thorough analysis of market size, share, and emerging trends, providing essential insights into its expansion potential. The report explores market segmentation and definitions, emphasizing key components and growth drivers. Through the use of SWOT and PESTEL analyses, it evaluates the sector’s strengths, weaknesses, opportunities, and threats, while considering political, economic, social, technological, environmental, and legal influences. Expert evaluations of competitor strategies and recent developments shed light on geographical trends and forecast the market’s future direction, creating a solid framework for strategic planning and investment decisions.
Brief Overview of the Spinal Implants and Surgical Devices Market:
The global Spinal Implants and Surgical Devices Market is expected to experience substantial growth between 2024 and 2031. Starting from a steady growth rate in 2023, the market is anticipated to accelerate due to increasing strategic initiatives by key market players throughout the forecast period.
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-spinal-implants-surgical-devices-market
Which are the top companies operating in the Spinal Implants and Surgical Devices Market?
The report profiles noticeable organizations working in the water purifier showcase and the triumphant methodologies received by them. It likewise reveals insights about the share held by each organization and their contribution to the market's extension. This Global Spinal Implants and Surgical Devices Market report provides the information of the Top Companies in Spinal Implants and Surgical Devices Market in the market their business strategy, financial situation etc.
Medtronic (US),Stryker Corporation (US), Zimmer Biomet (US), Globus Medical (US), Exactech, Inc. (US), Orthofix Medical Inc. (US), ATEC Spine, Inc. (US), Integra LifeSciences (US),NORMAN NOBLE, INC (US), XTANT MEDICAL (US), Spine Wave, Inc. (US),Captiva Spine, Inc. (US), Wenzel Spine. (US), Jayon Implants. (India), Tecomet, Inc. (US), SINTX Technologies, Inc. (US), Abbott (US), Meditech Spine, LLC (US), Boston Scientific Corporation (US) and GPC Medical Ltd. (India).
Report Scope and Market Segmentation
Which are the driving factors of the Spinal Implants and Surgical Devices Market?
The driving factors of the Spinal Implants and Surgical Devices Market are multifaceted and crucial for its growth and development. Technological advancements play a significant role by enhancing product efficiency, reducing costs, and introducing innovative features that cater to evolving consumer demands. Rising consumer interest and demand for keyword-related products and services further fuel market expansion. Favorable economic conditions, including increased disposable incomes, enable higher consumer spending, which benefits the market. Supportive regulatory environments, with policies that provide incentives and subsidies, also encourage growth, while globalization opens new opportunities by expanding market reach and international trade.
Spinal Implants and Surgical Devices Market - Competitive and Segmentation Analysis:
**Segments**
- By Product (Thoracic Fusion and Lumbar Fusion Devices, Cervical Fusion Devices, Spinal Biologics, Vertebral Compression Fracture Treatment Devices, Spinal Decompression Devices, Non-Fusion Devices, Spinal Bone Growth Stimulators, Spinal Cord Stimulators) - By Technology (Spinal Fusion and Fixation Technologies, Spinal Non-Fusion Technologies) - By Type (Implantable Spinal Devices, Instrumentation and Spine Devices, Biomaterials) - By Surgery (Open Surgery, Minimally Invasive Surgery)
The global spinal implants and surgical devices market is expected to witness significant growth and evolution by the year 2031. The market is segmented based on product, technology, type, and surgery type. The products segment includes thoracic fusion and lumbar fusion devices, cervical fusion devices, spinal biologics, vertebral compression fracture treatment devices, spinal decompression devices, non-fusion devices, spinal bone growth stimulators, and spinal cord stimulators. The technology segment is divided into spinal fusion and fixation technologies and spinal non-fusion technologies. Furthermore, the market is categorized by type into implantable spinal devices, instrumentation and spine devices, and biomaterials. Lastly, the segmentation based on surgery includes open surgery and minimally invasive surgery, reflecting the diverse landscape of the market.
**Market Players**
- Medtronic - DePuy Synthes (Johnson & Johnson Services, Inc.) - Stryker - NuVasive, Inc. - Zimmer Biomet - Globus Medical Inc. - Alphatec Holdings, Inc. - B. Braun Melsungen AG - Orthofix Holdings, Inc. - RTI Surgical Holdings, Inc.
Key market players in the global spinal implants and surgical devices market play a crucial role in driving innovation, research, and development in the industry. Companies such as Medtronic, DePuy Synthes, Stryker, NuVThe global spinal implants and surgical devices market is anticipated to witness substantial growth over the forecast period, with key market players such as Medtronic, DePuy Synthes (Johnson & Johnson Services, Inc.), Stryker, NuVasive, Inc., and Zimmer Biomet leading the way in driving innovation and research within the sector. Medtronic, a renowned name in the medical technology industry, offers a wide range of spinal implant products and surgical devices that cater to various spinal conditions. DePuy Synthes, a part of Johnson & Johnson Services, focuses on advancing healthcare solutions through cutting-edge technologies in spinal surgery. Stryker is another prominent player known for its innovative spinal fusion and fixation technologies, contributing to the advancement of minimally invasive surgical procedures. NuVasive, with its focus on transforming spine surgery through disruptive technology, has established itself as a key player in the market. Zimmer Biomet, a leading musculoskeletal healthcare company, also offers a diverse portfolio of spinal implants and surgical devices, enhancing patient outcomes and surgical experiences.
Moreover, other major market players such as Globus Medical Inc., Alphatec Holdings, Inc., B. Braun Melsungen AG, Orthofix Holdings, Inc., and RTI Surgical Holdings, Inc. also contribute significantly to the global spinal implants and surgical devices market. Globus Medical specializes in innovative spine solutions, including implants, instruments, and biologics, aimed at improving patient care and surgical outcomes. Alphatec Holdings focuses on developing novel technologies for spinal disorders, catering to both fusion and non-fusion surgical approaches. B. Braun Melsungen AG offers a wide range of spinal implants and instrumentation, emphasizing quality and precision in spinal surgeries. Orthofix Holdings is known for its comprehensive spine care solutions, including spinal implantable devices and biologics, enhancing the overall treatment of spinal conditions. RTI Surgical Holdings specializes in providing biologic, metal, and synthetic implants for spine surgeries, addressing a wide range**Market Players**: - Medtronic (US) - Stryker Corporation (US) - Zimmer Biomet (US) - Globus Medical (US) - Exactech, Inc. (US) - Orthofix Medical Inc. (US) - ATEC Spine, Inc. (US) - Integra LifeSciences (US) - NORMAN NOBLE, INC (US) - XTANT MEDICAL (US) - Spine Wave, Inc. (US) - Captiva Spine, Inc. (US) - Wenzel Spine (US) - Jayon Implants (India) - Tecomet, Inc. (US) - SINTX Technologies, Inc. (US) - Abbott (US) - Meditech Spine, LLC (US) - Boston Scientific Corporation (US) - GPC Medical Ltd. (India)
Market analysis: The global spinal implants and surgical devices market is poised for substantial growth over the forecast period. Key market players, such as Medtronic, DePuy Synthes, Stryker, NuVasive, Inc., and Zimmer Biomet, are expected to lead the way in driving innovation and research within the industry. These companies offer a diverse range of spinal implant products and surgical devices that cater to various spinal conditions, contributing to enhanced patient outcomes and surgical experiences. Additionally, other major players like Globus Medical Inc., Alphatec Holdings, Inc., B. Braun
North America, particularly the United States, will continue to exert significant influence that cannot be overlooked. Any shifts in the United States could impact the development trajectory of the Spinal Implants and Surgical Devices Market. The North American market is poised for substantial growth over the forecast period. The region benefits from widespread adoption of advanced technologies and the presence of major industry players, creating abundant growth opportunities.
Similarly, Europe plays a crucial role in the global Spinal Implants and Surgical Devices Market, expected to exhibit impressive growth in CAGR from 2024 to 2031.
Explore Further Details about This Research Spinal Implants and Surgical Devices Market Report https://www.databridgemarketresearch.com/reports/global-spinal-implants-surgical-devices-market
Key Benefits for Industry Participants and Stakeholders: –
Industry drivers, trends, restraints, and opportunities are covered in the study.
Neutral perspective on the Spinal Implants and Surgical Devices Market scenario
Recent industry growth and new developments
Competitive landscape and strategies of key companies
The Historical, current, and estimated Spinal Implants and Surgical Devices Market size in terms of value and size
In-depth, comprehensive analysis and forecasting of the Spinal Implants and Surgical Devices Market
Geographically, the detailed analysis of consumption, revenue, market share and growth rate, historical data and forecast (2024-2031) of the following regions are covered in Chapters
The countries covered in the Spinal Implants and Surgical Devices Market report are U.S., Canada, Mexico, Brazil, Argentina, Rest of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E, South Africa, Egypt, Israel, and Rest of the Middle East and Africa
Detailed TOC of Spinal Implants and Surgical Devices Market Insights and Forecast to 2031
Part 01: Executive Summary
Part 02: Scope Of The Report
Part 03: Research Methodology
Part 04: Spinal Implants and Surgical Devices Market Landscape
Part 05: Pipeline Analysis
Part 06: Spinal Implants and Surgical Devices Market Sizing
Part 07: Five Forces Analysis
Part 08: Spinal Implants and Surgical Devices Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers And Challenges
Part 13: Spinal Implants and Surgical Devices Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Browse More Reports:
https://www.databridgemarketresearch.com/jp/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/zh/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/ar/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/pt/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/de/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/fr/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/es/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/ko/reports/global-spinal-implants-surgical-devices-market
https://www.databridgemarketresearch.com/ru/reports/global-spinal-implants-surgical-devices-market
Data Bridge Market Research:
Today's trends are a great way to predict future events!
Data Bridge Market Research is a market research and consulting company that stands out for its innovative and distinctive approach, as well as its unmatched resilience and integrated methods. We are dedicated to identifying the best market opportunities, and providing insightful information that will help your business thrive in the marketplace. Data Bridge offers tailored solutions to complex business challenges. This facilitates a smooth decision-making process. Data Bridge was founded in Pune in 2015. It is the product of deep wisdom and experience.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1676
Email:- [email protected]
#Spinal Implants and Surgical Devices Market#Spinal Implants and Surgical Devices Market size#Spinal Implants and Surgical Devices Market scope
0 notes
Text
Global flexible spinal implants market Size, Growth Outlook 2035
Flexible Spinal Implants Market Size was valued at USD 3.9 Billion in 2023. The Global Flexible Spinal Implants industry is projected to grow from USD 4.5 Billion in 2024 to USD 9.2 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 14.5% during the forecast period (2024 - 2032).
Executive Summary
The global flexible spinal implants market is witnessing significant growth due to rising incidences of spinal disorders, increasing demand for minimally invasive spinal surgeries, and advancements in biomaterials and implant technology. Flexible spinal implants offer greater mobility, reduced recovery time, and improved spinal stabilization compared to traditional rigid implants. The market is benefiting from the increasing geriatric population, a growing preference for motion-preserving spinal implants, and the rising number of spinal surgeries worldwide. However, high costs, stringent regulatory requirements, and limited reimbursement policies remain key challenges.
Market Overview
Flexible spinal implants are designed to provide stability and controlled motion while preserving the natural biomechanics of the spine. Unlike rigid fusion implants, flexible implants such as dynamic stabilization devices, motion-preserving disc replacements, and expandable rods help reduce complications associated with spinal fusion, including adjacent segment degeneration. The rising adoption of minimally invasive spine procedures (MISS) and advancements in 3D-printed implants and bioresorbable materials are further fueling market growth.
Market Size and Growth Analysis
Flexible Spinal Implants Market Size was valued at USD 3.9 Billion in 2023. The Global Flexible Spinal Implants industry is projected to grow from USD 4.5 Billion in 2024 to USD 9.2 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 14.5% during the forecast period (2024 - 2032). Increasing spinal deformities, technological advancements in implant materials, and growing awareness about non-fusion spine surgery alternatives are driving market expansion.
Market Dynamics
Growth Drivers
Rising Prevalence of Spinal Disorders: Increasing cases of degenerative disc diseases, scoliosis, and herniated discs are driving demand for motion-preserving spinal implants.
Growing Adoption of Minimally Invasive Spine Surgery (MISS): Patients prefer less invasive procedures with shorter recovery times and minimal post-surgical complications.
Technological Advancements in Implant Materials: The development of titanium-based, bioresorbable, and 3D-printed spinal implants is enhancing treatment efficacy.
Aging Population and Higher Incidence of Spinal Conditions: The elderly population is more prone to spinal disorders, increasing demand for flexible spinal implants.
Challenges and Restraints
High Cost of Advanced Spinal Implants and Procedures: The expensive nature of flexible spinal implants limits affordability, especially in developing economies.
Stringent Regulatory Approvals: The complex approval process for implantable medical devices slows down product launches.
Limited Reimbursement Policies for Spinal Implants: Many insurance plans provide partial or no coverage for motion-preserving implants, affecting patient adoption.
Regional Analysis
North America
Largest market, driven by high healthcare spending, strong presence of key market players, and a rising number of spinal procedures.
The United States leads the market due to favorable reimbursement policies and technological advancements.
Europe
Second-largest market, benefiting from advancements in spinal surgery techniques, aging demographics, and increasing R&D investments.
Germany, the UK, and France are the key contributors.
Asia-Pacific
Fastest-growing region, driven by an increasing patient population, expanding healthcare infrastructure, and rising medical tourism.
China, India, and Japan are major contributors due to rising healthcare investments and improving access to spinal care.
Latin America, Middle East & Africa
Emerging market with gradual adoption of flexible spinal implants and increasing government focus on orthopedic healthcare services.
Market Segmentation
By Product Type:
Dynamic Stabilization Devices
Artificial Disc Replacements
Interspinous Process Devices
Expandable Rods & Screws
By Material Type:
Titanium-based Implants
Bioresorbable Implants
Polyetheretherketone (PEEK) Implants
By End-User:
Hospitals
Specialty Clinics
Ambulatory Surgical Centers
Key Market Players
Key company profiled in the flexible spinal implants market report are
Paradigm Spine LLC
Medtronic plc
Abbott Spine, Inc.
Raymedica
K2M Group Holdings
Recent Developments
Launch of Next-Generation Artificial Discs: Companies are developing advanced disc replacement technologies with improved mobility and longevity.
Increasing FDA Approvals for Motion-Preserving Implants: Regulatory agencies are approving more non-fusion spinal devices, boosting adoption.
Growing Investments in 3D Printing for Spinal Implants: Custom-designed spinal implants are improving patient outcomes and surgical success rates.
Future Outlook and Opportunities
The global flexible spinal implants market is expected to continue its strong growth trajectory, driven by increasing demand for motion-preserving treatments, advancements in implant materials, and a rising number of minimally invasive spinal surgeries. Expanding reimbursement policies and increasing surgeon awareness of non-fusion alternatives will further support market penetration.
For more information please visit @marketresearchfuture
#Global flexible spinal implants market Size#Global flexible spinal implants market Share#Global flexible spinal implants market Growth#Global flexible spinal implants market Analysis#Global flexible spinal implants market Trends#Global flexible spinal implants market Forecast#Global flexible spinal implants market Segments
0 notes
Text
0 notes
Text
Two years ago, a medical professional approached scientists at the University of Tabriz in Iran with an interesting problem: Patients were having headaches after pacemaker implants. Working together to investigate, they began to wonder if the underlying issue is the materials used in the pacemakers. "Managing external noise that affects patients is crucial," author Baraa Chasib Mezher said. "For example, a person with a brain pacemaker may experience interference from external electrical fields from phones or the sounds of cars, as well as various electromagnetic forces present in daily life. It is essential to develop novel biomaterials for the outlet gate of brain pacemakers that can effectively handle electrical signals."
Read more.
#Materials Science#Science#Medical technology#Biomaterials#Electronics#Composites#Nanotechnology#Polymers#Clay#Graphene#Polypropylene
5 notes
·
View notes
Text
Collagen Membrane Market Analysis 2025-2033: Emerging Trends, Growth Opportunities, and Forecast
Collagen Membrane Market : Comprehensive Analysis and Growth Forecast (2025-2033)
Global Collagen Membrane Market Size will approximately grow at a CAGR of 10.1% and North America is the dominant region of this market.

Market Overview
The Collagen Membrane Market Report delivers a detailed exploration of a dynamic market cutting across multiple industries. Forecasts, trend analyses, and practical insights covering the years 2024–2033 are all included in this thorough study. The research explores important aspects such product innovation, adoption patterns, pricing tactics, and regional penetration by fusing quantitative data with professional comments.
According to Market Strides the Collagen Membrane Market Size will approximately grow at a CAGR of 10.1% during the forecast period.
Collagen membranes have been widely employed in the field of tissue regeneration due to their well-established biocompatibility and their ability to facilitate wound healing processes. Collagen membranes, when combined with grafting materials, have demonstrated efficacy in facilitating tissue regeneration processes. Collagen membranes are commonly employed in the field of regenerative dentistry for the purpose of restoring dental tissues and facilitating bone grafting procedures. The global market for dental procedures, specifically minimally invasive surgery and bone grafting, is experiencing significant growth due to the escalating prevalence of dental issues worldwide.
Collagen membranes commonly employed in dental medicine are derived from diverse sources, including tendons, dermis, skin, and the pericardium. The membranes under consideration are commonly sourced from either bovine or porcine species. Collagen membranes have gained significant traction in various health procedures, including bone grafting, dental interventions, plastic surgeries, and more. The escalating demand for these procedures has consequently fueled the rapid Collagen Membrane Market Growth. Additionally, it considers macroeconomic indicators like GDP growth and socio-economic trends to contextualize market dynamics effectively, enabling stakeholders to make informed decisions.
Get Sample Research Report: https://marketstrides.com/request-sample/collagen-membrane-market
Key Focus Areas
Sectors Utilizing Collagen Membrane Market Products/Services: A detailed examination of industries leveraging the offerings
Market Leaders and Consumer Preferences: Insights into leading participants and evolving trends in customer behavior.
Competitive Landscape: An analysis of competitive positioning, regulatory influences, and emerging technologies shaping the market.
Organized into well-structured segments, the Collagen Membrane Market report fosters a multi-dimensional understanding of the industry, ensuring actionable insights across economic, political, and cultural contexts.
Growth and Emerging Trends
The Collagen Membrane Market industry is undergoing transformative changes driven by pivotal trends, which include:
Digital transformation: is the use of cutting-edge technologies to improve customer engagement and expedite processes through data-driven solutions.
Consumer-Centric Innovation: Using customized products to meet the increasing need for ease and personalization.
Regulatory Evolution: To stay competitive, quickly adjust to new regulations and more stringent compliance standards.
Top Key Players in Collagen Membrane Market
Geistlich Pharma
Zimmer Dental
Cook Biotech
Genoss
MegaGen Implant
Sunstar Americas
Integra LifeSciences
BioHorizons
Osteogenics
Nobel Biocare
Keystone Dental
Biotech Dental
Implant Direct
Collagen Matrix
Straumann
Dentsply Sirona
Maxigen Biotech
Datum Dental
Yantai Zhenghai Bio-Tech
Qingdao Jieshengbo Biomaterials
This section provides a SWOT analysis of the top players, focusing on their strategies, opportunities, and challenges. Highlights include:
Top 3-5 Companies: Comprehensive profiles and analysis of key strengths, weaknesses, and growth strategies.
Competitive Landscape: Insights into recent developments, such as partnerships, mergers, acquisitions, and product launches.
Regional Influence: Assessment of regional presence and contributions using the Ace matrix criteria to evaluate market share and growth potential.
Collagen Membrane Market Segmentation
Segmentation by Type
Cowhide Collagen
Pigskin Collagen
Segmentation by Application
Hospitals
Clinics
allowing stakeholders to identify specific opportunities and tailor strategies effectively.
Browse Details of Collagen Membrane Market with TOC: https://marketstrides.com/report/collagen-membrane-market
Research Methodology
The report is backed by a meticulous research approach:
Primary Research: In-depth interviews, surveys, and consultations with industry experts, supported by corporate press releases, annual reports, and government publications.
Secondary Research: Extensive analysis of market drivers using industry reports, trade publications, and academic research.
Data Validation: Rigorous cross-verification with expert input to ensure accuracy and credibility.This methodology guarantees a reliable and actionable market perspective, empowering stakeholders with informed decision-making tools.
Regional Analysis Collagen Membrane Market
The Collagen Membrane Market report provides an in-depth regional breakdown, offering insights into unique opportunities and characteristics across
North America
Europe
Asia-Pacific
Latin America
The Middle East and Africa
Our Reports Empower Clients Through:
Market sizing and competitive analysis
Strategic guidance for due diligence
product expansion
plant setup
acquisition intelligence
Buy Now:https://marketstrides.com/buyNow/collagen-membrane-market
About Us:
Market Strides is a Global aggregator and publisher of Market intelligence research reports, equity reports, database directories, and economic reports. Our repository is diverse, spanning virtually every industrial sector and even more every category and sub-category within the industry. Our market research reports provide market sizing analysis, insights on promising industry segments, competition, future outlook and growth drivers in the space. The company is engaged in data analytics and aids clients in due-diligence, product expansion, plant setup, acquisition intelligence to all the other gamut of objectives through our research focus.
Contact Us📧: [email protected]
#Collagen Membrane Market Size#Collagen Membrane Market Share#Collagen Membrane Market Growth#Collagen Membrane Market Trends#Collagen Membrane Market Players
0 notes