#Coronavirus Vaccination
Explore tagged Tumblr posts
Text



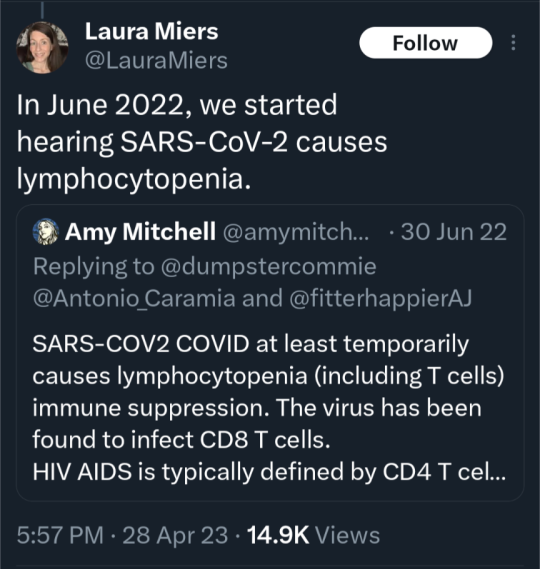
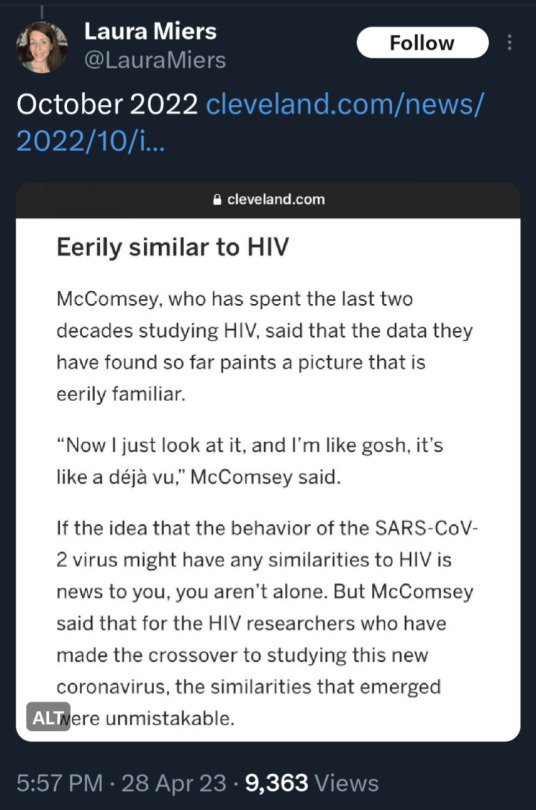
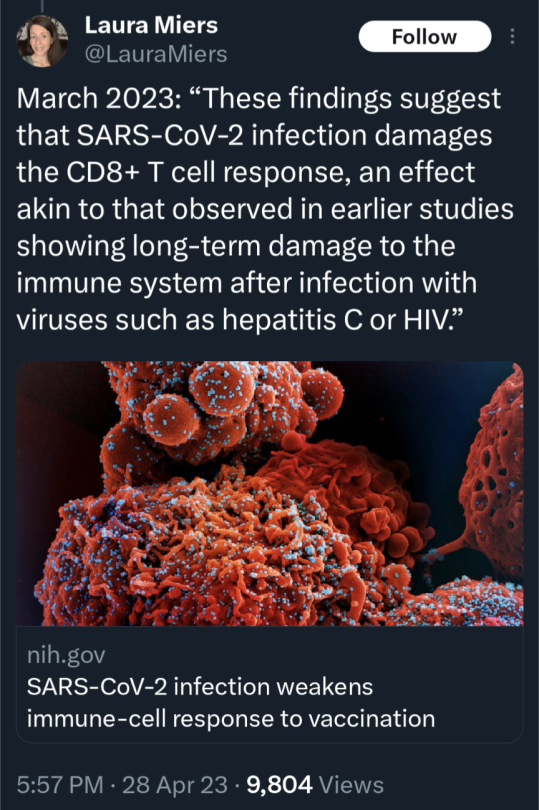

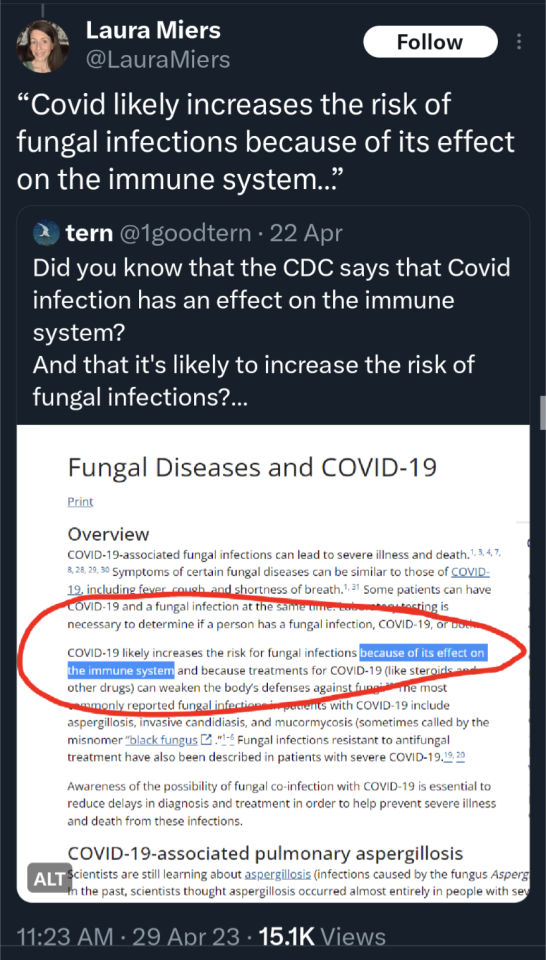
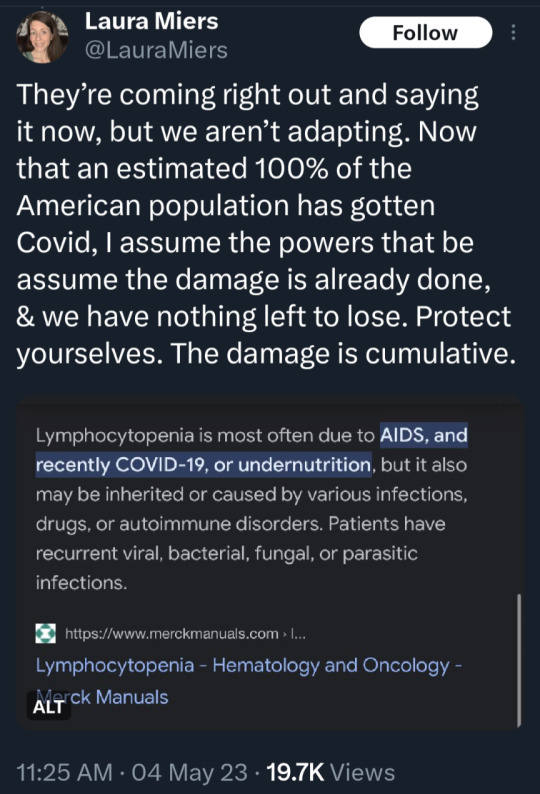

(source)
#covid 19#coronavirus#news#science#please get vaccinated#please get the booster#please stay safe#covid is not over#covid is not a hoax#please look out for each other#covid--19#long covid#yes the covid vaccine is safe
51K notes
·
View notes
Text
Great news for uninsured adults in the USA who want a COVID-19 booster! It now appears that ALL CVS locations are now active participants in the Bridge Access Program. The Bridge Access Program gives out free Covid-19 vaccinations to 18+ adults who otherwise can't afford one, so if you have a CVS near you, please go get one! For others who don't have a CVS near them, please go to vaccines.gov, click on "Find Covid-19 vaccines", fill out which vaccines you prefer (you can mix different vaccines if you have to so i reccomend just marking all of them for the age groups you need), and when the next page loads mark the "Bridge Access Program Participant" option to see only locations that are Bridge Access Program participants. Hopefully, other places that aren't CVS will start participating soon, so just check back every so often to see if there are any updates. The CDC Bridge Access Program website also has more details on what locations will be participating, but only CVS is appearing as an active participant on the vaccines.gov location finder at the moment.
#covid19#covid#coronavirus#vaccines#covid vaccine#bridge access program#CDC#signal boost#please share#coronavirus vaccine#covid19 vaccine#covid 19 vaccine#novavax#moderna#pfizer#also interesting side note but i havent been able to find any vaccine other than novavax near me#perhaps this is just a regional thing or maybe novavax is cheaper to make so those are the most common?#anyway thats why i made sure to tell people its okay to mix up because im going to have to bc i got moderna every other time lol#mayyybe other vaccines will become available in the future??? but ive had close family catch covid left and right so im not waiting#also does anyone know why the bridge program only bridges access to 18 or older individuals?#like i knew the gov didnt care about children but god damn lmao
16K notes
·
View notes
Text
I gotta say the Orwell quotes are getting way less snappy these days.
"the pandemic is over but covid is a leading cause of death" isnt as good of a soundbite as "war is peace freedom is slavery ignorance is strength"

#covid 19#coronavirus#anyways always remember MVV friends#mask ventilate and vaccinate#esp you northern hemisphere ppl going into winter
9K notes
·
View notes
Text
#covid 19#long covid#covid isn't over#still coviding#covid#coronavirus#corona virus#corona vaccine#mask up#wear a mask#mask#occupational health and safety#ausgov#politas#auspol#tasgov#taspol#australia#fuck neoliberals#neoliberal capitalism#anthony albanese#albanese government#health#mental health#healthcare#health & fitness#health and wellness#healthylifestyle#virus#germs
837 notes
·
View notes
Text
MRNA VACCINE MAY HAVE KILLED 17 MILLION?
European Parliment Member Christine Anderson has been a vocal opponent of the WEF and their plans. Here she explains the lies that were told to the public about this vaccine.
#the great awakening#wef#world economic forum#government corruption#fjb#democrats#joe biden#illegal immigration#bill gates#covid 19#covid vaccine#coronavirus#covid is airborne
2K notes
·
View notes
Text
Also preserved in our archive (Daily updates!)
At a Glance ~Researchers found that current COVID-19 vaccines fail to generate mature and durable antibody-producing cells in the bone marrow. ~The findings could help explain why protection tends to decline over time. ~Better understanding of long-term immune responses could lead to improved vaccines that provide enduring protection.
Some vaccines offer long-lasting protection. For instance, the tetanus vaccine provides protection for at least 10 years. With other vaccines, protection may begin to decline within a few months. To provide enduring immunity, a vaccine must elicit production of long-lived plasma cells, a type of immune cell that matures over time in the bone marrow and can rapidly trigger production of disease-fighting antibodies.
The mRNA vaccines developed for the SARS-CoV-2 virus have proven effective at preventing severe COVID-19 and reducing hospitalizations. These vaccines trigger production of antibodies that home in on the virus’s spike protein. But protective antibodies can begin to fade as soon as three months later and lead to breakthrough infections. Researchers have been puzzled by this waning protection, since SARS-CoV-2-specific immune cells can often be found in the bone marrow.
To better understand why protection against SARS-CoV-2 dwindles months after vaccination, a research team led by Dr. F. Eun-Hyung Lee of Emory University took a closer look at immune cells in the bone marrow of 19 healthy adults. Participants ranged in age from 20 to 65. All had previously received between two and five doses of mRNA COVID-19 vaccines. Samples of their bone marrow were evaluated within 33 months after receiving their initial COVID-19 vaccine shot.
The participants had also received an influenza vaccine within a year of giving their bone marrow samples. And all had previously received tetanus shots and boosters. Their responses to these previous vaccines were used for comparison.
The researchers used a cell-sorting technique called flow cytometry to separate each participant’s bone marrow immune cells into different groupings. These included short-lived antibody-secreting cells and long-lived plasma cells that confer lasting protection. Results appeared in Nature Medicine on September 27, 2024.
The scientists found that they could readily detect long-lived plasma cells that target tetanus and influenza. In contrast, while shorter-lived antibody-secreting cells specific to SARS-CoV-2 were abundant, long-lived ones were mostly absent. Even among five participants who had recent SARS-CoV-2 infections and vaccinations, long-lived plasma cells against the virus were scarce in the bone marrow samples.
The findings hint that newly created antibody-secreting cells against SARS-CoV-2 are unable to become fully mature and long-lasting once they reach and settle into the bone marrow. In contrast, vaccines against tetanus and influenza prompt antibody-producing cells to mature within bone marrow and become long-lived plasma cells. Future studies will need to investigate how to generate long-lived plasma cells against SARS-CoV-2.
“The holy grail of vaccine researchers is the generation of long-lived plasma cells,” Lee says. “Our findings demonstrate that current SARS-CoV-2 mRNA vaccines do not provide such long-lasting protection within bone marrow. Further research is needed to determine if updated vaccines, new delivery schedules, or other factors might provide such protection.”
—by Vicki Contie
#mask up#covid#pandemic#public health#wear a mask#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#covid vaccines#covid vaccine#vaccines
445 notes
·
View notes
Text
Rosemary Westwood at NPR:
A group of high-level managers at the Louisiana Department of Health walked into a Nov. 14 meeting in Baton Rouge expecting to talk about outreach and community events. Instead, they were told by an assistant secretary in the department and another official that department leadership had a new policy: Advertising or otherwise promoting the COVID, influenza or mpox vaccines, an established practice there — and at most other public health entities in the U.S. — must stop. NPR has confirmed the policy was discussed at this meeting, and at two other meetings held within the department's Office of Public Health, on Oct. 3 and Nov. 21, through interviews with four employees at the Department of Health, which employs more than 6,500 people and is the state's largest agency.
According to the employees, who spoke on the condition of anonymity because they fear losing their jobs or other forms of retaliation, the policy would be implemented quietly and would not be put in writing. Staffers were also told that it applies to every aspect of the health department's work: Employees could not send out press releases, give interviews, hold vaccine events, give presentations or create social media posts encouraging the public to get the vaccines. They also could not put up signs at the department's clinics that COVID, flu or mpox vaccines were available on site. The new policy in Louisiana was implemented as some politicians have promoted false information about vaccines and as President-elect Donald Trump seeks to have anti-vaccine activist Robert F. Kennedy Jr lead the U.S. Department of Health and Human Services. And some public health experts are concerned that if other states follow Louisiana, the U.S. could face rising levels of disease and further erosion of trust in the nation's public health infrastructure.
[...]
A blow to public health practice
Staff at Louisiana's health department fear the new policy undermines their efforts to protect the public, and violates the fundamental mission of public health: to prevent illness and disease by following the science.
[...]
Experts fear consequences of undermining trust in vaccine
Last year, 652 people in Louisiana died of COVID, including five children. Louisiana currently is tied with DC for the highest rate of flu in the U.S. In 2022 alone, flu killed 586 people in Louisiana. Every health department staff member, former staff member, public health official and vaccine expert contacted by NPR repeated the scientific consensus that vaccines are safe, effective, and essential for preventing illness, hospitalizations, and deaths.
[...]
Policy change follows new governor's election
Until becoming Louisiana governor in early 2024, Republican Jeff Landry served as the state's attorney general for eight years. During the pandemic, he criticized the state's COVID response and filed lawsuits over federal and state vaccine mandates. On Dec. 6, 2021, Attorney General Landry spoke at a state committee hearing against adding COVID to the childhood immunization schedule. At his side was Robert F. Kennedy, Jr., who presented false claims about COVID vaccines. This year the Republican-controlled legislature passed five bills — all signed by Gov. Landry — and two resolutions aimed at loosening vaccine requirements, limiting the power of public health authorities and sowing doubt about vaccine safety.
Gov. Landry also appointed Dr. Ralph Abraham, a family medicine doctor, to be the state's surgeon general. That position co-leads the Department of Health, and is tasked with crafting health policy that is then carried out by the departmental co-leader, the secretary. [...] Abraham said masking, lockdowns and vaccination requirements "were practically ineffective," that COVID vaccine adverse effects have been "suppressed," that "we don't know" whether blood from people who've been vaccinated is safe for donation and that "we hope and pray" COVID vaccines don't increase the risk miscarriages.
[...]
A slippery slope to future disease outbreaks
Experts told NPR they feared a policy that undermines COVID, flu and mpox vaccinations could have a spillover effect, reducing public trust in vaccinations overall, including those given to children to prevent a host of dangerous and deadly illnesses. "I believe that we will see measles cases. I believe we will see whooping cough cases. I believe we will likely see meningitis outbreaks," said Hood. In the Nov. 14 meeting, a staff member asked whether the ban on promoting vaccines applied to children's immunizations, but the answer was noncommittal, according to an employee with knowledge of the meeting's details. "My understanding was it's not clear to what extent we might be able to promote childhood vaccinations," the staff member said. (The Louisiana Department of Health's statement to NPR said the changes in policy and messaging do not apply to childhood immunizations.) Nationally, vaccination rates for serious childhood diseases have been falling in recent years, including in Louisiana.
[...]
The rise of public health officials promoting misinformation
Louisiana isn't the only state where public health officials have recently announced controversial decisions and repeated false or discredited health theories. Florida's surgeon general has made false claims about COVID vaccines, undermined school vaccine mandates for the measles and said local officials should stop adding fluoride to water supplies.
The consequences of anti-vaxxer extremism and anti-public health sentiments being normalized by Republicans: Louisiana bans the state's Department of Health from promoting COVID, flu, or mpox vaccines.
#Anti Vaxxer Extremism#Vaccines#Coronavirus Vaccines#mpox Vaccines#Flu Vaccines#Flu#mpox#Coronavirus#Louisiana#Public Health#Louisiana Department of Health#Jeff Landry#Ralph Abraham#Anti Vaxxers
128 notes
·
View notes
Text
#tiktok#drag queens#drag queen#drag#covid#long covid#covid isn't over#covid 19#coronavirus#corona vaccine#covid19#gay pride#pride month#lgbt pride#happy pride 🌈#lgbtq#lgbtqia#lgbtq community#lgbtqi community#lgbtq issues#lgbtqiia+#lgbtqiia#gaypride#gay
325 notes
·
View notes
Text

COVID SURGE! 🦠💉
Please get your Covid Vaccines (and boosters)!
There is a HUGE surge with a new variant — MASK UP AND GET YOUR SHOTS!
Help keep others and yourself safe! 🩹⭐️
430 notes
·
View notes
Text
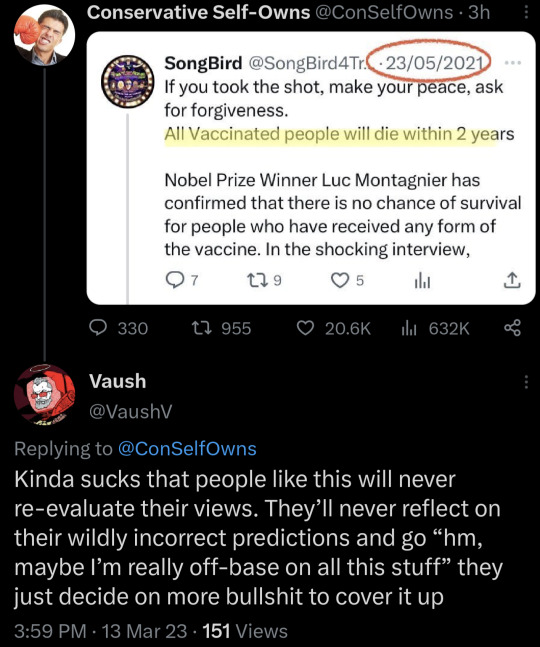
#us politics#twitter#tweet#republicans#conservatives#conservatives be like#republicans be like#2023#2021#covidー19#coronavirus vaccine#covid vaccine#coronavirus pandemic#conservative self owns#vaush#@vaushv#@conselfowns
2K notes
·
View notes
Text

Long Covid. In one meme
#chronic pain#chronically ill#covid#covid isn't over#covid19#long covid#chronic illness#long haul#create#art therapy#covid long hauler#post covid syndrome#post covid#covid is airborne#covid pandemic#covid vaccine#covid 19#still coviding#covid conscious#coronavirus#pandemic#still sick#i feel sick#sickness#chronic#chronic fatigue
123 notes
·
View notes
Text
the new covid vaccines are out and available and you can 100% get them for free if you're uninsured/insurance doesn't cover it through the CDC Bridge Program. PLEASE share and spread!!!
515 notes
·
View notes
Text
Covid19 was planned for a long time. Search for Dr. Judy Mikovits online.
#Judy Mikovits#government#united states#coronavirus#new world order#great reset#covid19#virus#vaccines#plandemic#pandemic#dr fauci#London real
435 notes
·
View notes
Text
An Edmonton constable who spoke at a "Freedom Convoy" rally, thanked protesters and posted a video suggesting vaccine mandates were "unlawful" and "unsafe" was sanctioned ten months of pay before being permitted to return to the job.
Edmonton Police Service Const. Elena Golysheva "acknowledged the inappropriateness of her actions" during a disciplinary hearing in June, according to documents CTV News Edmonton obtained through the Freedom of Information and Protection of Privacy Act.
Full article
Tagging: @politicsofcanada
#cdnpoli#canada#canadian politics#canadian news#canadian#edmonton#alberta#cops#police#freedom convoy#coronavirus#COVID-19#vaccine denial
352 notes
·
View notes
Text

This is another piece of art I made while processing my ongoing anger with how the US specifically, though also many other countries, have completely ignored managing the covid pandemic for the last several years.
This is your reminder: there was a new vaccine released in September of 2023. If you have not had a vaccine since then, you need one.
Threadless, Redbubble
#covid#covid 19#covid isn't over#long covid#covid19#coronavirus#pandemic#wear a mask#mask#mask up#get vaccinated#angry
162 notes
·
View notes
Photo
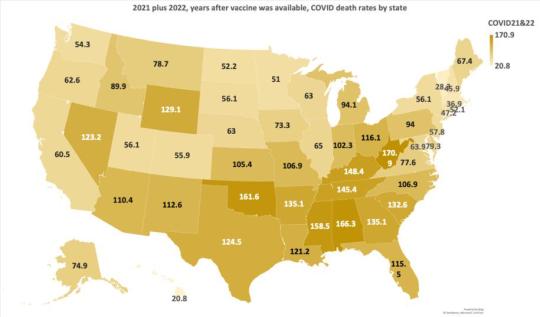
COVID Death Rate After Vaccines Became Available
146 notes
·
View notes