#CD4 cells (T cells)
Text
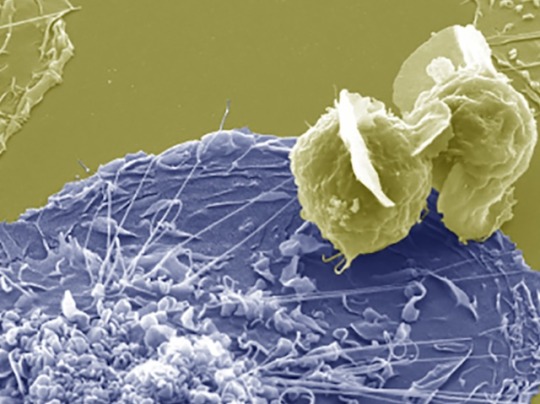
Infection by Fusion
Fusing HIV-infected CD4 T cells with macrophages – cells of the immune system that are a reservoir for HIV in tissue – infects them effectively, providing insight into the mechanisms of persistent infection
Read the published research paper here
Image from work by Rémi Mascarau and colleagues
Institut de Pharmacologie et Biologie Structurale (IPBS), Université de Toulouse, Centre National de la Recherche Scientifique, Université Toulouse III - Paul Sabatier (UPS), Toulouse, France
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Journal of Cell Biology, March 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#biology#hiv#immune system#macrophages#t cells#cd4#electron microscopy#scanning electron microscopy#viral infections
7 notes
·
View notes
Text

ideal threesome dynamics
#this is the thought that made me start this blog#CD4 T cells#CD8 T cells#dendritic cells#article is laidlaw et al 2016#redacted lab notes
4 notes
·
View notes
Text
Embracing Hope and Unity: A Reflection on World AIDS Day
As we come together on December 1st to observe World AIDS Day, it’s not just a day on the calendar; it’s a powerful reminder of solidarity, compassion, and the ongoing fight against HIV/AIDS. In this blog post, we’ll delve into the significance of World AIDS Day, the progress made, and the importance of continuing our collective efforts.
International Men’s Day: A Tribute to Men’s…

View On WordPress
#Acquired Immunodeficiency Syndrome#antiretroviral drugs#CD4 cells (T cells)#community engagement#compassion#December 1st#End inequalities. End AIDS. End pandemics#Ending the HIV/AIDS epidemic#fight against HIV/AIDS#HIV prevention#HIV treatment#pre-exposure prophylaxis (PrEP)#Putting communities at the center#significance of World AIDS Day#solidarity#Stigma and discrimination#WHO#World AIDS Day
0 notes
Text

COVID-19 and Its Impact on the Immune System: Increased Vulnerability to Future Pathogens
Introduction
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has had profound effects on global health. Beyond the immediate respiratory symptoms, emerging evidence suggests that COVID-19 can cause long-term alterations to the immune system, potentially making individuals more susceptible to future infections. This article explores how COVID-19 affects immune cells, particularly CD4+ and CD8+ T cells, and draws comparisons with the immunological impacts of HIV.
To listen click on View on Twitter.
42 notes
·
View notes
Text
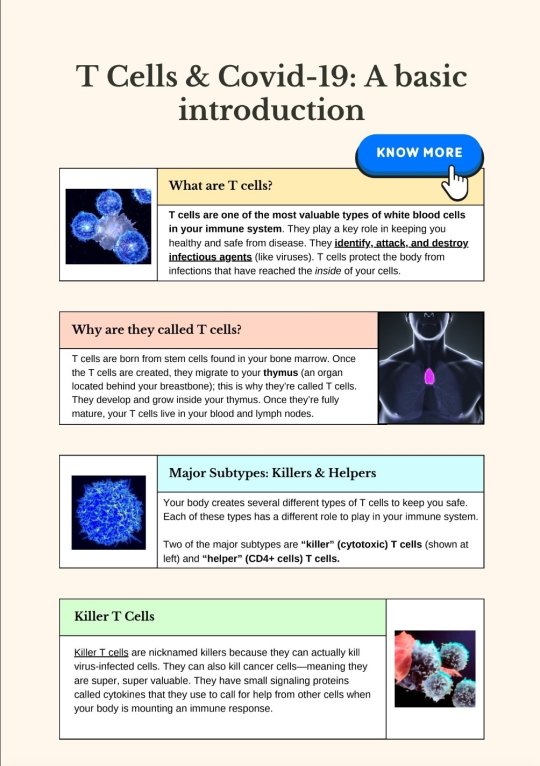
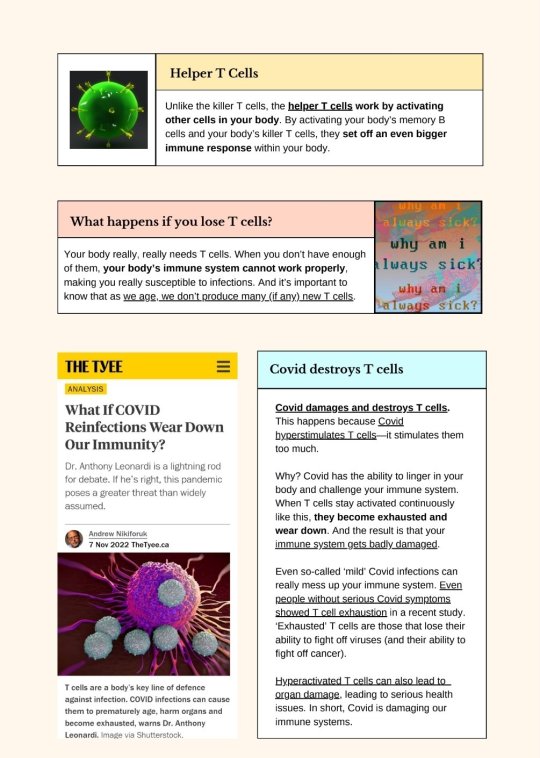
Source.
[ID: A two-page infographic titled "T Cells & Covid-19: A basic introduction."
A box labeled "What are T cells?" Text reads: "T cells are one of the most valuable types of white blood cells in your immune system. They play a key role in keeping you healthy and safe from disease. They identify, attack, and destroy infectious agents (like viruses). T cells protect the body from infections that have reached the inside of your cells." To the left of the box is an image of cells.
A box labeled "Why are they called T cells?" Text reads: "T cells are born from stem cells found in your bone marrow. Once the T cells are created, they migrate to your thymus (an organ located behind your breastbone); this is why they're called T cells. They develop and grow inside your thymus. Once they're fully mature, your T cells live in your blood and lymph nodes." To the right of the box is an image of a simplified human figure. The thymus' location is shown in bright pink in the center of the chest, roughly in the middle of the collarbone.
A box labeled "Major Subtypes: Killers & Helpers." Text reads: "Your body creates several different types of T cells to keep you safe. Each of these types has a different role to play in your immune system. Two of the major subtypes are 'killer' (cytotoxic) T cells (shown at left) and 'helper' (CD4+ cells) T cells." To the left of the box is a depiction of a blue cell.
A box labeled "Killer T Cells." Text reads: "Killer T cells are nicknamed killers because they can actually kill virus-infected cells. They can also kill cancer cells--meaning they are super, super valuable. They have small signaling proteins called cytokines that they use to call for help from other cells when your body is mounting an immune response." To the right of the box is an illustration of what appears to be killer T cells fighting infected or malign cells.
A box labeled "Helper T Cells." Text reads: "Unlike the killer T cells, the helper T cells work by activating other cells in your body. By activating your body's memory B cells and your body's killer T cells, they set off an even bigger immune response within your body." To the left of the box is a depiction of a green cell.
A box labeled "What happens if you lose T cells?" Text reads: "Your body really, really needs T cells. When you don't have enough of them, your body's immune system cannot work properly, making you really susceptible to infections. And it's important to know that as we age, we don't produce many (if any) new T cells." To the right of the box is a graphic where the words "why am i always sick?" appear multiple times in different colours.
A screenshot of an article from the Tyee, titled "What If COVID Reinfections Wear Down Our Immunity?" by Andrew Nikiforuk, dating from 7 November 2022. What text is visible reads: "Dr. Anthony Leonardi is a lightning rod for debate. If he’s right, this pandemic poses a greater threat than widely assumed", followed by an image of cells. Under the image, text reads: "T cells are a body’s key line of defence against infection. COVID infections can cause them to prematurely age, harm organs and become exhausted, warns Dr. Anthony Leonardi. Image via Shutterstock."
A box labeled "Covid destroys T cells." Text reads: "Covid damages and destroys T-cells. This happens because Covid hyperstimulates T cells--it stimulates them too much. Why? Covid has the ability to linger in your body and challenge your immune system. When T cells stay activated continuously like this, they become exhausted and wear down. And the result is that your immune system gets badly damaged. Even so-called 'mild' Covid infections can really mess up your immune system. Even people without serious Covid symptoms showed T cell exhaustion in a recent study. 'Exhausted' T cells are those that lose their ability to fight off viruses (and their ability to fight off cancer.) Hyperactivated T cells can also lead to organ damage, leading to serious health issues. In short, Covid is damaging our immune systems."
/end ID]
To read more on this topic:
How the Coronavirus Short-Circuits the Immune System (26 Jun, 2020)
Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection (21 Jul, 2021)
SARS-CoV-2 Actively Infects And Kills Lymphoid Cells (14 Apr, 2022)
In Cleveland and beyond researchers begin to unravel the mystery of long COVID-19 (22 Oct, 2022)
What if COVID Reinfections Wear Down Our Immunity? (7 Nov, 2022)
Single-cell multiomics revealed the dynamics of antigen presentation, immune response and T cell activation in the COVID-19 positive and recovered individuals (2 Dec, 2022)
SARS-CoV-2 infection weakens immune-cell response to vaccination: NIH-funded study suggests need to boost CD8+ T cell response after infection. (20 Mar, 2023)
Lymphocytopenia: Merck Manual (Revised Apr 2023)
Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2 (11 Jan, 2024)
91 notes
·
View notes
Text
COVID = AIDS?
@heresiae mi ha chiesto di commentare QUESTO POST (commentare non del tipo 'Maestro, illuminaci!' ma più tipo '???????') e mi tocca dire che nonostante alcuni punti siano meritevoli di approfondimento (perché, di massima, corretti) le informazioni vengono sparate in stecca con un tono allarmistico e, a mio avviso, esagerato.
In sintesi, nel post linkato si paragona il covid all'aids perché sono venute fuori evidenze (come leggerete, comuni ad altre infezioni virali) che la malattia da Sars-CoV2 abbia un effetto indebolente sul sistema immunitario... senza scendere in spiegazioni dettagliate su cosa siano i Linfociti T (T Cells nell'originale), in sostanza si correla l'infezione con un'aumentata APOPTOSI (morte cellulare programmata, utile al rinnovo) di queste cellule del nostro sistema immunitario, col risultato che dopo la malattia quest'ultimo diventerebbe più 'debole' e quindi più suscettibile ad altre infezioni nonché tumori.
Per amor di precisione, il paragone con l'aids è stato estrapolato da un'osservazione specifica che è stata posta fuori contesto, infatti HIV e il Sars-CoV2 sono due virus COMPLETAMENTE differenti e il Sars-CoV2 NON SI COMPORTA COME L'HIV che invece si aggancia e gemma all'interno dei linfociti CD4, inattivandone completamente la capacità immunitaria; inoltre sono stati riportati solo articoli scientifici che parlano dell'aumentata apoptosi cellulare senza specificare che:
SI TRATTA DI UN FENOMENO TEMPORANEO
E' PROPORZIONALE ALLA GRAVITA' DELL'INFEZIONE
Risultati: L’estesa linfopenia delle cellule T osservata in particolare nei pazienti con COVID-19 grave durante l’infezione acuta si era ripresa 6 mesi dopo l’infezione, accompagnata da una normalizzazione delle risposte funzionali delle cellule T agli antigeni virali comuni. Abbiamo rilevato un’attivazione persistente delle cellule T CD4+ e CD8+ fino a 12 mesi dopo l’infezione, in pazienti con COVID-19 lieve e grave, misurata dall’aumento dell’espressione di HLA-DR e CD38 su queste cellule. L’attivazione persistente delle cellule T dopo COVID-19 era indipendente dalla somministrazione di un vaccino COVID-19 post-infezione. Inoltre, abbiamo identificato un sottogruppo di pazienti con COVID-19 grave che presentava una conta di cellule T CD8+ persistentemente bassa al follow-up e mostrava un fenotipo distinto durante l’infezione acuta costituito da una risposta disfunzionale delle cellule T e segni di eccessivo processo pro-infiammatorio. produzione di citochine.
Conclusione: il nostro studio suggerisce che il numero e la funzione delle cellule T si riprendono nella maggior parte dei pazienti dopo COVID-19. Tuttavia, troviamo prove di attivazione persistente delle cellule T fino a 12 mesi dopo l’infezione e descriviamo un sottogruppo di pazienti affetti da COVID-19 grave con conteggi di cellule T CD8+ persistentemente bassi che mostrano una risposta immunitaria disregolata durante l’infezione acuta.
Fonte: [X]
Questi fenomeni non sono dicotomici e irreparabili come nell'infezione da HIV e nell'AIDS (che ricordo essere due cose diverse: si può essere positivi all'HIV e non sviluppare l'aids) e anche se nessuno (che abbia un QI perlomeno a due cifre) nega che ci possano essere queste complicazioni, esse NON SONO LA NORMA e condensare molteplici studi e osservazioni ancora in fieri in un unico post dandogli un taglio così netto e allarmistico a me pare controproducente ed esagerato.
Come avevo accennato all'inizio, sono parecchie le infezioni - spesso ritenute 'innocue' - che nel breve e nel lungo periodo possono potenzialmente dare GROSSI problemi al nostro sistema immunitario e all'organismo più in genere, però non ve le dico tanto non ci potete fare nulla e vivreste in un costante stato di paura che davvero non merita.
27 notes
·
View notes
Text
SARS-CoV-2-specific CD8+ T cells from people with long COVID establish and maintain effector phenotype and key TCR signatures over 2 years - Published Sept 16, 2024
Significance
Long COVID occurs in small but important minority of patients following COVID-19, reducing quality of life and contributing to healthcare burden. Although research into underlying mechanisms is evolving, immunity is understudied. As the recall of T cell memory promotes more rapid recovery and ameliorates disease outcomes, establishment of robust memory T cells is important for protection against subsequent infections, even when the virus mutates. We defined how SARS-CoV-2-specific T cell and B cell responses are established and maintained following infection and vaccination for 2 y in people with long COVID. We found robust and prototypical SARS-CoV-2-specific T cells with effector phenotype and key T cell receptor signatures in people with long COVID following SARS-CoV-2 infection and subsequent COVID-19 vaccination.
Abstract
Long COVID occurs in a small but important minority of patients following COVID-19, reducing quality of life and contributing to healthcare burden. Although research into underlying mechanisms is evolving, immunity is understudied. SARS-CoV-2-specific T cell responses are of key importance for viral clearance and COVID-19 recovery. However, in long COVID, the establishment and persistence of SARS-CoV-2-specific T cells are far from clear, especially beyond 12 mo postinfection and postvaccination. We defined ex vivo antigen-specific B cell and T cell responses and their T cell receptors (TCR) repertoires across 2 y postinfection in people with long COVID. Using 13 SARS-CoV-2 peptide–HLA tetramers, spanning 11 HLA allotypes, as well as spike and nucleocapsid probes, we tracked SARS-CoV-2-specific CD8+ and CD4+ T cells and B-cells in individuals from their first SARS-CoV-2 infection through primary vaccination over 24 mo. The frequencies of ORF1a- and nucleocapsid-specific T cells and B cells remained stable over 24 mo. Spike-specific CD8+ and CD4+ T cells and B cells were boosted by SARS-CoV-2 vaccination, indicating immunization, in fully recovered and people with long COVID, altered the immunodominance hierarchy of SARS-CoV-2 T cell epitopes. Meanwhile, influenza-specific CD8+ T cells were stable across 24 mo, suggesting no bystander-activation. Compared to total T cell populations, SARS-CoV-2-specific T cells were enriched for central memory phenotype, although the proportion of central memory T cells decreased following acute illness. Importantly, TCR repertoire composition was maintained throughout long COVID, including postvaccination, to 2 y postinfection. Overall, we defined ex vivo SARS-CoV-2-specific B cells and T cells to understand primary and recall responses, providing key insights into antigen-specific responses in people with long COVID.
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator#long covid
5 notes
·
View notes
Text
The T Cell Landscape
T cells, a critical component of the adaptive immune system, stand as the body's elite force in combatting infections and diseases. These specialized lymphocytes boast remarkable diversity, each type playing a distinct role in orchestrating a targeted and effective immune response.
T cells, like all blood cells, originate from hematopoietic stem cells residing in the bone marrow. However, their training ground lies within the thymus, a specialized organ located in the chest. Here, they undergo a rigorous selection process known as thymocyte education. During this process, immature T cells, called thymocytes, are presented with self-antigens (molecules unique to the body) by special cells. Thymocytes that bind too strongly to these self-antigens are eliminated, preventing them from attacking healthy tissues later. Only thymocytes that demonstrate the ability to recognize foreign invaders while exhibiting tolerance to self are released into the bloodstream as mature T cells.
Following this rigorous training, mature T cells exit the thymus and embark on their patrol, circulating throughout the bloodstream and lymphatic system. They remain vigilant, constantly scanning for their specific targets – antigens. Antigens are foreign molecules, such as fragments of viruses, bacteria, or even cancerous cells, that trigger the immune response.
The hallmark of a T cell is its T cell receptor (TCR), a highly specialized protein complex embedded on its surface. This receptor acts like a lock, uniquely shaped to fit a specific antigen, the "key." Each T cell develops a unique TCR capable of recognizing only a single antigen, enabling a highly specific immune response.
But how do T cells encounter these hidden antigens lurking within infected or cancerous cells? This critical role is played by antigen-presenting cells (APCs). APCs, such as macrophages and dendritic cells, engulf pathogens or abnormal cells, break them down into smaller fragments (peptides), and present them on their surface complexed with major histocompatibility complex (MHC) molecules. MHC molecules act as identification tags, allowing T cells to distinguish between "self" and "non-self." When a T cell's TCR encounters its specific antigen bound to an MHC molecule on an APC, a dance of activation begins. The T cell becomes stimulated, and a cascade of signaling events is triggered. This leads to the T cell's proliferation, producing an army of clones specifically tailored to combat the recognized threat.
T cells are not a single, monolithic entity. They comprise a diverse population, each type with a specialized function:
Helper T Cells (Th Cells):
Helper T cells, often abbreviated as Th cells, play a central role in coordinating immune responses. They express the CD4 surface marker and can recognize antigens presented by major histocompatibility complex class II (MHC-II) molecules. Subtypes of helper T cells include Th1, Th2, Th17, and regulatory T cells (Tregs), each with distinct functions and cytokine profiles.
Th1 cells mediate cellular immunity by activating macrophages and cytotoxic T cells, crucial for defense against intracellular pathogens.
Th2 cells are involved in humoral immunity, promoting B cell activation and antibody production, thus aiding in defense against extracellular parasites.
Th17 cells contribute to the immune response against extracellular bacteria and fungi, producing pro-inflammatory cytokines. Regulatory T cells (Tregs) maintain immune tolerance and prevent autoimmunity by suppressing excessive immune responses.
Cytotoxic T Cells (Tc Cells):
Cytotoxic T cells, also known as Tc cells or CD8+ T cells, are effector cells responsible for directly killing infected or aberrant cells. They recognize antigens presented by MHC class I molecules on the surface of target cells. Upon activation, cytotoxic T cells release perforin and granzymes, inducing apoptosis in target cells and eliminating the threat.
Memory T Cells:
Memory T cells are a long-lived subset of T cells that persist after the clearance of an infection. They provide rapid and enhanced immune responses upon re-exposure to the same antigen, conferring immunological memory. Memory T cells can be either central memory T cells (TCM), residing in lymphoid organs, or effector memory T cells (TEM), circulating in peripheral tissues.
γδ T Cells:
Unlike conventional αβ T cells, γδ T cells express a distinct T cell receptor (TCR) composed of γ and δ chains. They recognize non-peptide antigens, such as lipids and metabolites, and are involved in immune surveillance at epithelial barriers and responses to stress signals.
Beyond the Battlefield: The Expanding Roles of T Cells: The remarkable capabilities of T cells have opened doors for several groundbreaking applications in medicine:
Vaccines: By presenting weakened or inactivated forms of pathogens, vaccines "train" the immune system to generate memory T cells. This prepares the body to recognize and rapidly eliminate the real pathogen upon future exposure, preventing disease.
Cancer immunotherapy: CAR T-cell therapy, a revolutionary approach, genetically engineers a patient's own T cells to express chimeric antigen receptors (CARs) that recognize and target specific cancer cells. These "supercharged" T cells are then reintroduced into the patient, unleashing a potent attack against the tumor.
Autoimmune disease treatment: Researchers are exploring ways to manipulate T cells to suppress harmful immune responses that underlie autoimmune diseases like rheumatoid arthritis and multiple sclerosis.
The diverse array of T cells underscores the immune system's complexity and adaptability in mounting tailored responses against a myriad of threats. From orchestrating immune reactions to maintaining tolerance and establishing long-term immunity, T cells play multifaceted roles in safeguarding the body's health. Understanding the intricacies of T cell biology not only sheds light on immune-mediated diseases but also paves the way for developing novel therapeutic strategies harnessing the power of the immune system.
T cells represent a fascinating aspect of immunology, with their diversity and specificity driving the complexity of immune responses. As research advances, further insights into T cell biology promise to revolutionize immunotherapy and enhance our ability to combat diseases ranging from infections to cancer. By understanding and harnessing their power, we can unlock new avenues for protecting and improving human health.
#science sculpt#life science#science#molecular biology#biology#biotechnology#artists on tumblr#t cells#T helper cells#autoimmune#autoimmunity#helathcare#immunology#immunotherapy#medical care#cancer#human health#research#scientific research#the glass scientists#scientific illustration#research scientist
11 notes
·
View notes
Text
Immune Cells if they were Halo things
CD8+ T cells: Spartans
B cells: ODSTs
CD4+ T cells: Commanders and officers
Lymph nodes: UNSC ships
Organs: UNSC planets
Neutrophils: Marines
Macrophages: Planet side militias
Dendritic Cells: AI
Halo rings: Cytokine storm
The Flood: Roided out cancer or tuberculosis
14 notes
·
View notes
Text
19 notes
·
View notes
Note
alright but what cells do you think the other characters (Ada, Mendez, Salazar, Saddler, Krauser) are.
i personally think Salazar is a cancer cell specifically the ones that make up prostate cancer bc it's not that deadly if you have the right equipment but it can be annoying.
FINALLY GETTING TO THIS (I'll try to keep it more brief than the last one, we'll stick to human cells only)
I think you're spot-on with Salazar, especially because cancer cells aren't necessarily starting out that way, they can be normal cells that become cancerous (so like, getting a plaga infection for instance)
Ada: hmmmmmm she's kind of tricky but I'm gonna go with CD4 T-cells. They gather information and work to kill invading pathogens sort of behind the scenes, like a spy :)
Mendez: Monocyte, LARGE and aggressive white blood cells, for sure Mendez.
Saddler: dendritic cell on account of the big weird tendrils. Here's a picture... you get the idea.

Krauser: totally a neutrophil. Those cells are like if you walked into a bar and instead of figuring out who the bad guy is they just punch the first person they see. They literally explode themselves just on the off-chance they kill a pathogen too. Aggressive and ridiculous.
Thank you again for this it's so fun for me <3
5 notes
·
View notes
Note
Wait what, give us the cancer cell fun facts king
Sure thang 😎 Before I get into pancreatic cancer, let’s talk about lumps. One method of early tumor detection everyone should be periodically practicing is feeling yourself up for lumps. You should really get to know your testes if you got ‘em and it’s my scientific opinion that everyone should be feeling up their own chestes anyways, but it’s also good to do it on purpose to detect any abnormality as soon as possible.
What’s in a lump exactly? A healthy human body is a mix of many different cell types, and tumors are no different. I’m going to mostly correctly breakdown the various cell types as cells that do things/Epithelial cells, the in-betweeny/structurally important cells/Mesenchymal cells, and the blood cells where you got your cute lil red blood cells and immune cells. The interaction of all these cells forms something like an ecosystem, which we refer to as a microenvironment. Solid cancers arise from cells in tissues that lose the ability to die and/or gain the ability to keep dividing, so it makes sense that a lot of cell types present in the healthy microenvironment would be present in the tumor microenvironment/TME. However, the function of these cells have been found to be radically altered by the cancer cells in order to promote tumor growth and survival.
The pancreatic cancer lump is notable for two reasons: it is HARD and it is almost entirely made up of non-pancreatic cancer cells, with up to 90% of the tumor volume being non-cancer cells. A healthy pancreas is made up almost entirely of acinar cells which secrete digestive enzymes into the intestine, with some specialized regions for maintaining blood sugar levels. The pancreatic cancer TME on the other hand is an absolute circus. The lump is hard because the cancer has rewired certain mesenchymal cells to continuously churn out structural proteins, creating this densely fibrous netting which leads to drug permeability issues. There’s an influx of immune cells that should be performing anti-cancer duties but have instead been rewired to suppress immune responses. In order to prevent auto-immunity, the body’s own immune system targeting itself, there are various mechanisms by which the immune system will propagate a STOP response. The cancer cells adopt this process, secreting signals to override any productive immune response and push immune cells to suppress immune responses instead. As much as I’d love to get into the specifics and controversies of myCAFs, iCAFs, MDSCs, TAMs, Th1/2/17/regulatory CD4 family, and my poor downtrodden CD8 T cell babies, this post is already pretty long and I’m retired anyways 😮💨
in case anyone feels like reading the actual science version of this
#asked and answered babey!#it’s been like 2 years since I’ve thought about this stuff but I guess it’s like riding a bicycle#once you spend 5 years memorizing the intricacies of the pancreatic cancer TME you just never forget it 🤔
9 notes
·
View notes
Text
PNAS is one of those odd scientific journals that hasn't changed its girth breadth in 100 years, despite the field of science growing larger and more nuanced - most of the huge low hanging balls fruits of science have been picked. So you end up with highly specific artesticles articles about tissue resident CD4 inch T Cells long along side articles about the weather patterns of Neptune. This style of journal is very impotent important in the modern era to keep science interdickiplinary interdisciplinary. However, PNAS has significant issues with both its peer review and editorial processes, partially because of its wide shaft scope. PNAS articles also tend to be on the smaller side due to this scope, leaving some readers unsatisfied. Most scientists would agree there's no easy fix for these issues without irrevocably changing the journal - the problems are simply too deep and hard to penetrate.
10 notes
·
View notes
Text
Written by Don Ford based on the study listed below There's been a lot a talk comparing HIV and SARS2 but they also have some important differences...
And this affects how we interpret the chronic phase.
HIV affects a smaller portion of cells because it specifically binds to CD4 cells and makes syncytia from there but it gets bottlenecked into mostly just our immune system.
This will have very consistent deadly outcomes but it will need an opportunistic pathogen to finish the job. Fortunately we have developed treatments to manage HIV but we do need them to be more available...
Since most will ask, Syncytia is created when a cell is invaded and hijacked then it uses the cell's natural ability to fuse into neighboring cells in excessive amounts.
This is not unique to these pathogens, it's a thing cells can do but the pathogens do it to a destructive point.
With SARS2, this continues on in chains depending on each cell's access to other cells while still invading cells with ACE2 wherever this cell cluster finds it.
This happens because the Spike protein rotates to the outer surface of the infected cells. That's why we can detect spike protein but it's actually a sign of infected cells.
A secondary explanation is that when SARS2 invades and hijacks the nucleus of the cell it melts that cell into neighboring cells to create a multinucleated super cluster of YOUR cells and all of these cells are destroyed in the process but only after they are exhausted which includes releasing their cytokines and replicating A LOT of SARS2 virions.
This is what they mean when they say "COVID ages you."
Because it triggers a massive amount of cell death, which is described as physical damage in Dr. Iwaski's LC study, and cytokine inflammation which we call a cytokine storm, a process not unique to COVID.
SARS2 does not posses cytokines, those are released from a combination of the infected cells and the immune cells that are trying to destroy them to get at the virus inside.
When describing this process for SARS2 it starts with ACE2 and because this is so common, we use it to balance sugars among other things which is why this is all connected to diabetes, and each cell has additional access to other cells, remember it's invading and hijacking to replicate so it gains control of EVERY process, that gives it access to every type of cell eventually...
While HIV is limited by a less common cell entry access point which means everyone ultimately gets the same immune damage... That means SARS2 damage on the other hand is more widespread and while it can end up taking out your immune system it doesn't always.
But if it does, it will kill you.
This is why we have to change our conceptual understanding of disease in general because a SARS2 infection can affect so many things and extremely inconsistently muddying the water on traditional diagnostics...
We haven't really had something do such widespread damage in the modern era and leave people alive, but the big difference is that SARS2 is its own opportunistic pathogen while HIV is not... so when SARS2 wipes out your immune system...
You die, the end... And that's a terrible lottery to play over and over again but that also means there are limited patients with immune deficiency to monitor and track.
This is not a controversial statement, we've watched LC patients write their own obituaries and T cell apoptosis is already associated with severe COVID... That study is in my LC article from TWO YEARS ago.
So, if folks say "well... the immune deficiency isn't showing up in LC cohorts consistently enough"... well, I'd say...
Yeah, because they are dead.
So, when people ask me to say how close I think SARS2 is to HIV in risk...
It's airborne.
It's its own opportunistic pathogen.
Infection damage is cumulative.
Its Chronic phase is degenerative.
Treatment is extremely complicated.
It causes extreme cognitive dysfunction...
Or just ANY other type of dysfunction.
It almost certainly triggers cancer.
You can get reinfected constantly...
It creates new variants in every infection.
It is also recombinant and uses multiplicity reaction.
And you can die during the acute phase.
While the scale of infection tricks you into thinking it's mild and our leaders have all been infected multiple times.
...
It's probably worse.
5 notes
·
View notes
Link
Vinay Prasad, MD MPH; Physician & Professor Hematologist/ OncologistProfessor of Epidemiology, Biostatistics and MedicineAuthor of 450+ Peer Reviewed papers,...
3 notes
·
View notes
Text
Spatial immune landscapes of SARS-CoV-2 gastrointestinal infection: macrophages contribute to local tissue inflammation and gastrointestinal symptoms - Published July 16, 2024
Background: In some patients, persistent gastrointestinal symptoms like abdominal pain, nausea, and diarrhea occur as part of long COVID-19 syndrome following acute respiratory symptoms caused by SARS-CoV-2. However, the characteristics of immune cells in the gastrointestinal tract of COVID-19 patients and their association with these symptoms remain unclear.
Methodology: Data were collected from 95 COVID-19 patients. Among this cohort, 11 patients who exhibited gastrointestinal symptoms and underwent gastroscopy were selected. Using imaging mass cytometry, the gastrointestinal tissues of these patients were thoroughly analyzed to identify immune cell subgroups and investigate their spatial distribution.
Results: Significant acute inflammatory responses were found in the gastrointestinal tissues, particularly in the duodenum, of COVID-19 patients. These alterations included an increase in the levels of CD68+ macrophages and CD3+CD4+ T-cells, which was more pronounced in tissues with nucleocapsid protein (NP). The amount of CD68+ macrophages positively correlates with the number of CD3+CD4+ T-cells (R = 0.783, p < 0.001), additionally, spatial neighborhood analysis uncovered decreased interactions between CD68+ macrophages and multiple immune cells were noted in NP-positive tissues. Furthermore, weighted gene coexpression network analysis was employed to extract gene signatures related to clinical features and immune responses from the RNA-seq data derived from gastrointestinal tissues from COVID-19 patients, and we validated that the MEgreen module shown positive correlation with clinical parameter (i.e., Total bilirubin, ALT, AST) and macrophages (R = 0.84, p = 0.001), but negatively correlated with CD4+ T cells (R = −0.62, p = 0.004). By contrast, the MEblue module was inversely associated with macrophages and positively related with CD4+ T cells. Gene function enrichment analyses revealed that the MEgreen module is closely associated with biological processes such as immune response activation, signal transduction, and chemotaxis regulation, indicating its role in the gastrointestinal inflammatory response.
Conclusion: The findings of this study highlight the role of specific immune cell groups in the gastrointestinal inflammatory response in COVID-19 patients. Gene coexpression network analysis further emphasized the importance of the gene modules in gastrointestinal immune responses, providing potential molecular targets for the treatment of COVID-19-related gastrointestinal symptoms.
#covid#mask up#pandemic#covid 19#wear a mask#coronavirus#sars cov 2#still coviding#public health#wear a respirator
6 notes
·
View notes