#Biosimilar Monoclonal Antibody Market
Explore tagged Tumblr posts
Text
#Biosimilar Monoclonal Antibody Market#Biosimilar Monoclonal Antibody#Biosimilar Monoclonal#Biosimilar#monoclo#monoclonal#antibody
0 notes
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Secukinumab Market: Transforming Treatment for Autoimmune Diseases
Introduction
The global secukinumab market is expanding rapidly, driven by the growing prevalence of autoimmune diseases such as psoriasis, ankylosing spondylitis, and psoriatic arthritis. Valued at USD 4.1 billion in 2023, the market is projected to reach USD 7.2 billion by 2030, growing at a compound annual growth rate (CAGR) of 8.3%. This blog highlights key trends, market drivers, challenges, and future prospects in the secukinumab market.

Market Overview
Secukinumab, a fully human monoclonal antibody, targets interleukin-17A (IL-17A) and has revolutionized the treatment of chronic inflammatory conditions. With its high efficacy and favorable safety profile, it is widely used for managing moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis. Growing awareness about autoimmune diseases and increasing adoption of biologics are boosting the market’s growth.
Key Stats:
Market Size: USD 4.1 billion in 2023, projected to reach USD 7.2 billion by 2030.
Top Indications: Psoriasis accounts for the largest market share, followed by psoriatic arthritis and ankylosing spondylitis.
Leading Player: Novartis AG dominates the market with its brand Cosentyx (secukinumab).
Regional Insights
North America: The largest market, driven by a high prevalence of autoimmune diseases, advanced healthcare infrastructure, and strong adoption of biologic therapies in the U.S.
Europe: Significant growth due to increasing patient awareness and supportive reimbursement policies in countries like Germany, the U.K., and France.
Asia-Pacific: Expected to be the fastest-growing region, fueled by rising healthcare spending, improving access to biologics, and increasing prevalence of psoriasis and arthritis in countries such as China and India.
Key Market Drivers
Rising Prevalence of Autoimmune Diseases: The growing incidence of conditions such as psoriasis and psoriatic arthritis is driving demand for effective treatment options like secukinumab.
Shift Toward Biologic Therapies: Secukinumab’s proven efficacy in managing chronic inflammatory conditions is accelerating the adoption of biologics over traditional therapies.
Favorable Clinical Outcomes: Clinical trials and real-world evidence consistently demonstrate secukinumab’s superior efficacy and sustained long-term response, boosting its market acceptance.
Product Expansion and Label Approvals: Ongoing research and regulatory approvals for additional indications, such as non-radiographic axial spondyloarthritis, are expanding secukinumab’s market potential.
Challenges
High Cost of Biologic Therapies: The high cost of secukinumab can limit access for some patients, especially in developing regions.
Competition from Biosimilars and Other Biologics: The increasing availability of biosimilars and alternative biologics poses a challenge to secukinumab’s market growth.
Adverse Events and Safety Concerns: Although generally well-tolerated, potential side effects such as increased infection risk may impact patient adherence.
Future Outlook
The secukinumab market is poised for strong growth as awareness of autoimmune diseases rises and new indications receive regulatory approval. Advances in precision medicine and combination therapies will further enhance the market’s potential. Emerging markets offer significant growth opportunities as healthcare infrastructure improves and access to biologics expands.
Want to stay ahead in the evolving biologics market? Visit Mark & Spark Solutions for strategic insights and expert guidance.
0 notes
Text
Active Pharmaceutical Ingredient Market to Expand to $415.3B by 2033 💉💊
Active Pharmaceutical Ingredient (API) market is set to expand significantly, growing from $245.2 billion in 2023 to $415.3 billion by 2033, registering a CAGR of 5.6%. This growth is driven by rising demand for generic drugs, advancements in biotechnology, and increasing prevalence of chronic diseases.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS21460 &utm_source=SnehaPatil&utm_medium=Article
Key Market Drivers & Trends
✅ Rising Demand for Generic Medications
Cost-effective treatments are fueling the adoption of generic APIs. ✅ Growth in Biotech APIs & Personalized Medicine
Monoclonal antibodies & peptide-based drugs gaining traction. ✅ Oncology & Cardiovascular APIs Leading
Increased focus on cancer treatments & heart disease therapies. ✅ Stringent Regulatory Frameworks Driving Quality Standards
Compliance with FDA & EMA guidelines shaping API production.
Regional Insights
🌎 North America Leads — Strong R&D investments & a well-established pharmaceutical sector. 🇪🇺 Europe Expands — Growing biosimilar adoption & favorable regulatory policies. 🇮🇳 India Surges — Top exporter of cost-effective generic APIs.
Market Segmentation & Performance
📌 Synthetic APIs Dominate (55%) — Cardiovascular & oncology drugs drive demand. 📌 Biotech APIs Rising (30%) — Biopharmaceutical advancements support growth. 📌 Generic APIs Gaining Traction (15%) — Expanding access to affordable medicines.
Top Industry Players & Competitive Strategies
🏭 Teva Pharmaceutical Industries — Cost-effective API production leadership. 🏭 Pfizer Inc. — Expanding R&D investments & innovative therapies. 🏭 Novartis AG — Focus on biotech APIs & high-value drug formulations.
Future Outlook & Challenges
🔬 With a 10% projected increase in R&D expenditure, the API market will see major advancements in biotech APIs, AI-driven drug development, and nanotechnology-based APIs. However, regulatory complexities, high production costs, and competition from low-cost manufacturers pose challenges. The shift toward sustainable API manufacturing and AI-driven drug synthesis will create new growth opportunities.
💊🌍 #APIMarket #PharmaceuticalInnovation #DrugDevelopment #BiotechAPI #GenericDrugs #OncologyAPI #CardiovascularTreatment #AIinPharma #SmartPharmaceuticals #ChronicDiseaseCare #HealthcareAdvancements #FDARegulations #EMACompliance #BiopharmaGrowth #APIManufacturing #R&DInvestment #PharmaIndustryTrends #NanotechnologyInDrugs #FutureOfMedicine #PharmaSupplyChain #Biosimilars #MedicalBreakthroughs #LifeSciences #PrecisionMedicine #SustainablePharma
0 notes
Text
Antibodies Contract Manufacturing Market Size, Growth Outlook 2035
The global Antibodies Contract Manufacturing Market Size was estimated at 18.38 (USD Billion) in 2024. The Antibodies Contract Manufacturing Market Industry is expected to grow from 19.97 (USD Billion) in 2025 to 42.29 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 8.69% during the forecast period (2025 - 2034)
Market Overview The Antibodies Contract Manufacturing Market is experiencing robust growth, driven by the rising demand for monoclonal antibodies (mAbs), therapeutic antibodies, and biosimilars. Contract manufacturing organizations (CMOs) provide specialized services for antibody production, including cell line development, upstream and downstream processing, purification, and fill-finish services. The increasing prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases, along with the growing adoption of biopharmaceutical outsourcing, is fueling market expansion.

Market Size and Share The global Antibodies Contract Manufacturing MarketSize was estimated at 18.38 (USD Billion) in 2024. The Antibodies Contract Manufacturing Market Industry is expected to grow from 19.97 (USD Billion) in 2025 to 42.29 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 8.69% during the forecast period (2025 - 2034). North America dominates the market due to the presence of leading biopharmaceutical companies, advanced bioprocessing technologies, and strong regulatory frameworks. The Asia-Pacific contract antibody production market is witnessing rapid growth due to increasing investments in biologics manufacturing facilities and the availability of cost-effective contract manufacturing services.
Market Drivers
Rising Demand for Monoclonal Antibodies: The growing application of mAbs in oncology, immunology, and infectious diseases is boosting demand for contract antibody production.
Growing Outsourcing Trends in Biopharmaceuticals: Companies are increasingly outsourcing antibody manufacturing services to CMOs to reduce production costs and focus on core R&D.
Advancements in Bioprocessing Technologies: Innovations in single-use bioreactors, cell culture optimization, and chromatography techniques are enhancing efficiency in antibody production.
Expanding Biosimilars Market: The increasing development of biosimilar antibodies is creating new opportunities for contract biologics manufacturing.
Challenges and Restraints
High Costs Associated with Large-Scale Antibody Production: The cost-intensive nature of biopharmaceutical manufacturing poses challenges for smaller companies.
Regulatory Compliance and Quality Control Issues: Strict guidelines from the FDA, EMA, and other regulatory agencies necessitate rigorous quality control in biologics contract manufacturing.
Limited Availability of Skilled Workforce: The shortage of bioprocessing experts can hinder market growth.
Market Trends
Adoption of Single-Use Bioprocessing Technologies: The shift towards disposable bioprocessing systems is improving flexibility and reducing contamination risks in contract antibody production.
Increasing Focus on Antibody-Drug Conjugates (ADCs): Contract manufacturers are expanding their capabilities to support the rising demand for ADCs in targeted cancer therapies.
Strategic Collaborations Between Biotech Firms and CMOs: Pharmaceutical companies are forming alliances with biologics CMOs to enhance antibody therapeutic production capabilities.
Regional Analysis
North America: The dominant region due to strong biopharmaceutical infrastructure, high investment in biologics manufacturing, and the presence of major CMOs.
Europe: Significant growth driven by increasing adoption of biosimilar contract manufacturing and stringent regulatory frameworks.
Asia-Pacific: Fastest-growing market, with countries like China, India, and South Korea emerging as key hubs for antibody contract manufacturing services.
Rest of the World: Moderate market expansion, with increasing interest in Latin America and the Middle East.
Segmental Analysis
By Product Type:
Monoclonal Antibodies (mAbs)
Polyclonal Antibodies
Antibody Fragments
Antibody-Drug Conjugates (ADCs)
By Service Type:
Cell Line Development & Optimization
Process Development & Scale-Up
Upstream & Downstream Processing
Analytical & Quality Control Services
Fill-Finish & Packaging
By End-User:
Biopharmaceutical Companies
Academic & Research Institutes
Contract Research Organizations (CROs)
Key Market Players
Charles River Laboratories
MyBioSource
AbCellera
AGC Biologics
Novavax
Lonza
MilliporeSigma
ProBioGen AG
Recent Developments
Expansion of Biologics Manufacturing Facilities: Leading CMOs are investing in new large-scale antibody production plants.
Launch of AI-Powered Bioprocessing Solutions: AI-driven process optimization platforms are enhancing efficiency in contract antibody production.
Strategic Mergers and Acquisitions: Companies are acquiring smaller biologics contract manufacturers to expand capabilities.
For more information, please visit us at marketresearchfuture
#Antibodies Contract Manufacturing Market Size#Antibodies Contract Manufacturing Market Share#Antibodies Contract Manufacturing Market Growth#Antibodies Contract Manufacturing Market Analysis#Antibodies Contract Manufacturing Market Trends#Antibodies Contract Manufacturing Market Forecast#Antibodies Contract Manufacturing Market Segments
0 notes
Text
Biologics and Biosimilars Market Size, Trends, and Growth Forecast 2025–2032
Global Biologics and Biosimilars Market: Industry Analysis, Trends, and Forecast (2024-2031)
Introduction
The Global Biologics and Biosimilars Market is experiencing rapid expansion, driven by advancements in biotechnology, increasing prevalence of chronic diseases, and rising demand for cost-effective treatment options. Biologics, derived from living organisms, are playing a transformative role in treating cancer, autoimmune diseases, and rare disorders, offering targeted and highly effective therapies.
The introduction of biosimilars—which are highly similar versions of already approved biologics—is further enhancing market accessibility, reducing healthcare costs, and expanding patient reach. As regulatory frameworks become more supportive and manufacturing technologies advance, both biologics and biosimilars are expected to see significant growth.
In 2023, the market was valued at approximately USD 23,960 million and is projected to reach USD 73,030 million by 2031, reflecting a compound annual growth rate (CAGR) of 17.3%. The market is driven by:
Growing adoption of personalized medicine, leveraging biologics for targeted therapies.
Increased government support for biosimilars, promoting affordable treatment alternatives.
Technological advancements in bioprocessing, enhancing efficiency and scalability in drug development.
Expanding healthcare access in emerging markets, improving biopharmaceutical adoption.
However, challenges such as complex manufacturing processes, stringent regulatory requirements, and intellectual property barriers pose potential growth constraints. Nonetheless, with strong industry collaborations and ongoing R&D, the biologics and biosimilars market is expected to witness continued expansion and innovation.
Get free sample copy @ https://www.statsandresearch.com/request-sample/40532-global-biologics-and-biosimilars-market
Market Dynamics
Market Drivers
Rising Prevalence of Chronic Diseases
Cancer, autoimmune disorders, and diabetes are driving demand for biologic treatments.
Monoclonal antibodies, therapeutic proteins, and gene therapies are being widely used for targeted disease management.
Growing Demand for Cost-Effective Biosimilars
Biosimilars offer affordable alternatives to high-cost biologics, increasing patient accessibility.
Governments and healthcare agencies are supporting biosimilar adoption to lower drug costs.
Advancements in Biomanufacturing Technologies
Recombinant DNA technology, monoclonal antibody production, and cell culture techniques are improving drug development efficiency.
Automation and AI-driven analytics are enhancing yield optimization and bioprocessing scalability.
Regulatory Support and Expanding Approval Pathways
The FDA, EMA, and regulatory bodies are creating clearer guidelines for biosimilars, facilitating faster approvals.
Patent expirations of major biologic drugs are opening opportunities for biosimilar market entry.
Increasing Investment in Personalized Medicine and Biopharmaceutical R&D
Precision therapies tailored to genetic profiles are becoming a key focus in oncology and autoimmune treatments.
Pharmaceutical companies are investing in next-generation biologics, such as gene therapy and cell-based treatments.
Market Challenges
High Development and Manufacturing Costs
Biologic drug production requires complex cell culture and purification processes, leading to high R&D expenses.
Biosimilar production demands strict quality control measures, increasing overall costs.
Regulatory and Intellectual Property Barriers
Stringent biosimilar approval requirements can slow down market entry.
Patent litigations and exclusivity extensions delay biosimilar commercialization.
Market Competition from Established Biologics
Biologics manufacturers are implementing pricing strategies and patient assistance programs to retain market share.
Physician and patient skepticism about switching to biosimilars remains a challenge.
Supply Chain and Production Scalability Issues
Cold chain logistics are required for biologics, increasing distribution complexity.
Limited biosimilar manufacturing capacity may hinder large-scale adoption.
Get full report @ https://www.statsandresearch.com/report/40532-global-biologics-and-biosimilars-market/
Market Segmentation
The Biologics and Biosimilars Market is segmented based on product type, source, and manufacturing process.
By Product Type:
1. Biologics
Includes monoclonal antibodies (mAbs), vaccines, and recombinant proteins.
Used in treating cancer, rheumatoid arthritis, and neurological diseases.
Largest market segment, driven by targeted therapy advancements.
2. Biosimilars
Highly similar to approved biologics, offering lower-cost alternatives.
Includes biosimilar monoclonal antibodies, insulin, and growth hormones.
Growth driven by patent expirations of blockbuster biologics.
3. Others
Includes emerging biologic drug classes such as gene therapy and cell-based therapies.
By Source:
1. Human-Derived
Includes monoclonal antibodies and therapeutic proteins.
Dominates the market due to its high specificity and efficacy.
2. Animal-Derived
Includes vaccines and insulin derived from animal sources.
Ethical and regulatory concerns may limit usage.
3. Microbial-Derived
Includes recombinant proteins produced in bacteria and yeast.
Growing adoption due to genetic engineering advancements.
4. Plant-Derived
Includes plant-based biologics and vaccine candidates.
Still in early development stages, but gaining research attention.
By Manufacturing Process:
1. Recombinant DNA Technology
Most widely used method for producing therapeutic proteins and monoclonal antibodies.
2. Monoclonal Antibody Production
Rapidly growing segment, with applications in cancer immunotherapy.
3. Cell Culture Techniques
Utilized for complex biologic drug development.
4. Others
Includes novel bioprocessing methods, such as cell-free protein synthesis.
Regional Analysis
1. North America
Largest market, driven by strong biotech infrastructure and high healthcare spending.
FDA approvals of biosimilars accelerating market growth.
2. Europe
Second-largest market, with strong government support for biosimilar adoption.
Germany, UK, and France leading in biosimilar regulations and patient access.
3. Asia-Pacific
Fastest-growing market, driven by rising healthcare investments in China, India, and Japan.
Expanding biopharmaceutical R&D and biosimilar production capacity.
4. Middle East & Africa
Increasing demand for affordable biosimilars due to rising chronic disease burden.
5. South America
Brazil and Argentina emerging as biosimilar manufacturing hubs.
Competitive Landscape
Key Players in the Biologics and Biosimilars Market:
Amgen (Leading biologics manufacturer)
Roche (Strong in monoclonal antibodies and oncology biologics)
AbbVie (Known for blockbuster biologic Humira)
Bristol-Myers Squibb (Expanding into immuno-oncology biologics)
Sandoz (Biosimilar leader)
Pfizer (Developing biosimilars for rheumatoid arthritis and cancer)
Mylan (now part of Viatris) (Strong biosimilar portfolio)
Samsung Bioepis (Advancing biosimilar R&D)
Celltrion (Developing biosimilars for autoimmune diseases)
Teva Pharmaceutical Industries (Investing in biosimilar manufacturing)
Recent Developments:
Amgen launched new biosimilars for oncology treatments.
Pfizer expanded biosimilar manufacturing capacity to meet global demand.
Samsung Bioepis entered strategic partnerships for biosimilar commercialization.
Get enquiry before buying @ https://www.statsandresearch.com/enquire-before/40532-global-biologics-and-biosimilars-market
0 notes
Text
Top Trending Topics in the Pharmaceutical Industry

The pharmaceutical industry is constantly evolving, driven by technological advancements, regulatory changes, and emerging health challenges. Here are some of the most trending topics in 2024 shaping the future of pharma.
1. Artificial Intelligence in Drug Discovery
AI is revolutionizing drug discovery by accelerating the identification of potential drug candidates, reducing research costs, and improving success rates. Companies are leveraging AI-powered algorithms to analyze vast datasets, predict drug interactions, and optimize clinical trials.
2. Personalized Medicine and Precision Therapies
The shift toward personalized medicine is gaining momentum, with treatments being tailored to individual genetic profiles. This approach enhances drug efficacy while minimizing side effects, especially in areas like oncology, neurology, and rare diseases.
3. mRNA and Gene Editing Technologies
Following the success of mRNA vaccines, researchers are exploring their potential for treating diseases beyond COVID-19, such as cancer and genetic disorders. CRISPR and other gene-editing techniques are also making breakthroughs in modifying disease-causing genes.
4. Sustainable Pharmaceutical Practices
Environmental sustainability is a growing concern in the industry. Companies are adopting green chemistry practices, reducing carbon emissions, and developing biodegradable drug formulations to minimize their ecological footprint.
5. Expansion of Telemedicine and Digital Health
With the rise of digital health, telemedicine, and remote patient monitoring, pharmaceutical companies are integrating digital solutions to enhance patient engagement, improve adherence, and streamline clinical trials.
6. Regulatory Changes and Market Access
Global regulatory landscapes are evolving, with governments tightening drug approval processes while encouraging innovation. Pharma companies must navigate changing regulations to ensure smooth market access and compliance.
7. Rising Demand for Biologics and Biosimilars
Biologic drugs, including monoclonal antibodies and cell-based therapies, are witnessing increased demand. At the same time, biosimilars are gaining traction as cost-effective alternatives to expensive biologics, improving accessibility to advanced treatments.
8. AI-Powered Pharma Supply Chain Optimization
AI and blockchain are transforming supply chain management, ensuring drug traceability, reducing counterfeiting, and enhancing distribution efficiency, particularly in emerging markets.
9. Pharmaceutical Collaborations and Mergers
Strategic collaborations between biotech startups and big pharma companies are accelerating drug development. Mergers and acquisitions are also reshaping the industry, fostering innovation and market expansion.
10. Focus on Mental Health and Psychedelic Research
Mental health treatments are advancing, with research into psychedelic-assisted therapies gaining traction. Psilocybin, MDMA, and ketamine are being explored for their potential in treating depression, PTSD, and anxiety disorders.
The pharmaceutical industry’s future is marked by innovation, digital transformation, and sustainability. Staying ahead of these trends is crucial for companies thriving in an increasingly competitive market.
0 notes
Text
Stеrilе Injеctablе CDMO Market Industry Trends, Analysis, Size and Share by 2025-2033

The Reports and Insights, a leading market research company, has recently releases report titled “Stеrilе Injеctablе CDMO Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033.” The study provides a detailed analysis of the industry, including the global Stеrilе Injеctablе CDMO Market share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Stеrilе Injеctablе CDMO Market?
The global stеrilе injеctablе CDMO market was valued at US$ 11.1 Billion in 2024 and is expected to register a CAGR of 11.5% over the forecast period and reach US$ 29.6 Bn in 2033.
What are Stеrilе Injеctablе CDMO?
Sterile injectable CDMO is a group of specialized service companies that assist pharmaceutical and biotechnology companies to develop, manufacture, and commercialize sterile injectable drugs. These companies offer end-to-end services which involve formulation development, process optimization, analytical testing, regulatory support, and large-scale sterile manufacturing in compliance with GMP. Sterile injectable CDMOs are meeting the increasing demand for biologics, biosimilars, and complex injectables such as monoclonal antibodies, vaccines, and oncology drugs. They allow pharma companies to speed up time-to-market while maintaining product quality and meeting stringent regulatory requirements by leveraging advanced technologies, aseptic manufacturing facilities, and industry expertise.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/2544
What are the growth prospects and trends in the Stеrilе Injеctablе CDMO industry?
The sterile injectable CDMO market growth is driven by various drivers and factors. The sterile injectable CDMO market is experiencing significant growth driven by the rising demand for biologics, biosimilars, and complex injectable formulations, particularly in therapeutic areas like oncology, immunology, and chronic diseases. Increasing outsourcing trends among pharmaceutical and biotechnology companies, coupled with the need for cost-effective production and regulatory expertise, are fueling market expansion. Key factors include advancements in aseptic manufacturing technologies, growing investments in large-scale sterile production facilities, and stringent regulatory requirements for drug safety and quality. The market is also bolstered by the surge in vaccine development, particularly mRNA-based platforms, and the growing focus on personalized medicine, making sterile injectable CDMOs critical partners in the pharmaceutical supply chain. Hence, all these factors contribute to sterile injectable CDMO market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Sеrvicеs
Stand-alonе Sеrvicеs
Drug Formulation and Dеvеlopmеnt
Asеptic Fillings
Analytical Dеvеlopmеnt
Rеgulatory Support
Packaging and Assеmbly Sеrvicеs
Tеchnology Transfеr
Supply Chain Managеmеnt
Quality Control and Assurancе
Intеgratеd Sеrvicеs
By Drug Typе
Monoclonal Antibodiеs (mAbs)
Cytokinеs
Insulin
Pеptidе Hormonеs
Vaccinеs
Immunoglobulins
Blood Factors
Pеptidе Antibiotics
Othеrs
By Organization Sizе
Small
Mid-sizеd
Largе
By End-Usеr
Pharmacеutical Companiеs
Biopharmacеutical Companiеs
Rеsеarch Institutеs
Othеrs
Europe
Germany
United Kingdom
France
Italy
Spain
Russia
Poland
Benelux
Nordic
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
ASEAN
Australia & New Zealand
Rest of Asia Pacific
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
Saudi Arabia
South Africa
United Arab Emirates
Israel
Rest of MEA
Who are the key players operating in the industry?
The report covers the major market players including:
FAMAR Hеalth Carе Sеrvicеs
Pfizеr
Farеva
Sharp
Astral StеriTеch
Evonik
Aurigеnе Pharmacеutical Sеrvicеs
Ethypharm
TriRx Pharmacеutical Sеrvicеs
Biophrama Group
Gеnsеnta Pharmacеuticals
View Full Report: https://www.reportsandinsights.com/report/Stеrilе Injеctablе CDMO-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd. 1820 Avenue M, Brooklyn, NY, 11230, United States Contact No: +1-(347)-748-1518 Email: [email protected] Website: https://www.reportsandinsights.com/ Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/ Follow us on twitter: https://twitter.com/ReportsandInsi1
#Stеrilе Injеctablе CDMO Market share#Stеrilе Injеctablе CDMO Market size#Stеrilе Injеctablе CDMO Market trends
0 notes
Text
Biobetters Market Insights Highlighting Drivers, Challenges And Regional Opportunities
The biobetters market is gaining momentum as pharmaceutical companies strive to develop improved biologics with enhanced efficacy, safety, and convenience. Unlike biosimilars, biobetters introduce significant advancements over their reference biologics, meeting unmet medical needs and offering better patient outcomes. With the increasing prevalence of chronic diseases and patent expirations of major biologics, the biobetters market is set for substantial growth. Below are detailed insights into the market based on various parameters:

Market Insights
The biobetters market is projected to grow significantly due to increasing investments in biopharmaceutical R&D.
Rising prevalence of chronic conditions such as diabetes, cancer, and autoimmune diseases drives demand for effective treatments.
Pharmaceutical companies are focusing on biobetters to differentiate themselves in a competitive market landscape.
North America leads in market share due to advanced healthcare infrastructure and high R&D expenditure.
Asia-Pacific is emerging as a lucrative market with growing healthcare investments and rising awareness of biobetters.
Technological Advancements
Development of advanced drug delivery systems, including sustained-release formulations, drives innovation in biobetters.
Protein engineering techniques improve the binding affinity and half-life of biologic drugs.
Incorporation of artificial intelligence and machine learning accelerates the drug development process.
Enhanced manufacturing technologies ensure scalability and cost efficiency in biobetters production.
Drivers Of The Biobetters Market
Expiration of patents on blockbuster biologics creates opportunities for biobetters with improved profiles.
Increasing adoption of precision medicine boosts demand for tailored biobetter therapies.
Regulatory incentives encourage pharmaceutical companies to innovate and bring biobetters to market.
Growing awareness among healthcare professionals and patients regarding biobetters' benefits supports market growth.
Strategic collaborations between biotech companies and academic institutions accelerate innovation.
Challenges
High costs associated with biobetters development and clinical trials pose a financial burden on companies.
Demonstrating superior efficacy and safety compared to reference biologics can be complex and time-consuming.
Market competition from biosimilars and next-generation biologics impacts biobetters adoption.
Regulatory hurdles related to proving clinical superiority over original biologics remain a significant barrier.
Limited affordability in developing regions restricts market penetration.
Key Therapeutic Areas
Oncology: Biobetters such as improved monoclonal antibodies and checkpoint inhibitors enhance treatment outcomes.
Diabetes: Long-acting insulin analogs reduce dosing frequency and improve glycemic control for patients.
Autoimmune Disorders: Biobetters for conditions like rheumatoid arthritis provide better safety and efficacy.
Hematology: Advances in biobetters for hemophilia reduce treatment burden and improve quality of life.
Neurology: Emerging biobetters address neurodegenerative diseases with targeted mechanisms of action.
Regional Insights
North America remains the dominant region in the biobetters market due to strong R&D funding and robust healthcare infrastructure.
Europe experiences significant growth, supported by a well-established biopharmaceutical industry and favorable regulatory frameworks.
Asia-Pacific emerges as a high-growth region driven by increasing healthcare investments, a large patient base, and expanding pharmaceutical industries.
Latin America and the Middle East see gradual adoption due to improving healthcare systems and government initiatives.
Future Prospects
Advances in genetic engineering and biomolecular research will continue to fuel innovation in the biobetters market.
Emerging technologies such as CRISPR and mRNA platforms are expected to enable the development of next-generation biobetters.
Growth in telemedicine and digital health may facilitate biobetters' adoption through better patient monitoring and personalized treatments.
Rising focus on sustainability in drug development encourages eco-friendly manufacturing processes for biobetters.
Expanding partnerships and licensing deals between global biopharmaceutical companies will strengthen market opportunities.
Top Biobetters By Companies
Amgen’s Neulasta improves dosing convenience compared to Neupogen, setting a benchmark in oncology treatments.
Novo Nordisk's Tresiba offers enhanced glycemic control, marking a significant advancement in diabetes care.
Roche’s Kadcyla provides targeted action in breast cancer therapy, improving patient outcomes.
Sanofi’s Toujeo, a next-generation insulin, offers prolonged action for better diabetes management.
Pfizer’s Xeljanz demonstrates better efficacy in rheumatoid arthritis treatment with an optimized formulation.
Regulatory Landscape
The FDA and EMA have established guidelines that streamline the approval process for biobetters.
Post-marketing surveillance and real-world data collection play a critical role in ensuring biobetters' safety and efficacy.
Biobetters manufacturers benefit from data exclusivity periods, encouraging innovation.
Harmonization of international regulatory standards may simplify global market entry for biobetters.
Trends Shaping The Market
Shift from biosimilars to biobetters as companies focus on differentiation and innovation.
Increased adoption of subcutaneous or oral delivery methods for enhanced patient convenience.
Integration of wearable devices and remote monitoring with biobetters for better patient adherence.
Focus on patient-centric solutions that minimize side effects and improve the overall therapeutic experience.
Potential Growth Opportunities
Development of biobetters targeting rare diseases offers untapped market potential.
Expansion into emerging economies with rising healthcare expenditure and improving infrastructure.
Diversification of therapeutic portfolios by investing in biobetters across multiple disease areas.
Leveraging real-world evidence to establish biobetters' long-term benefits and market acceptance.
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
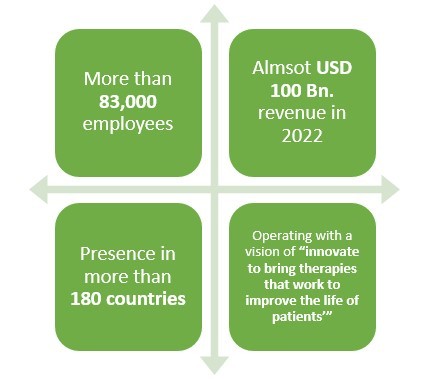
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
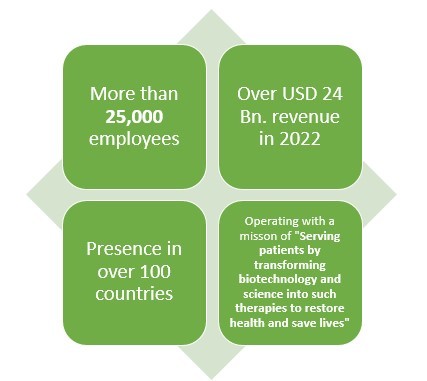
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
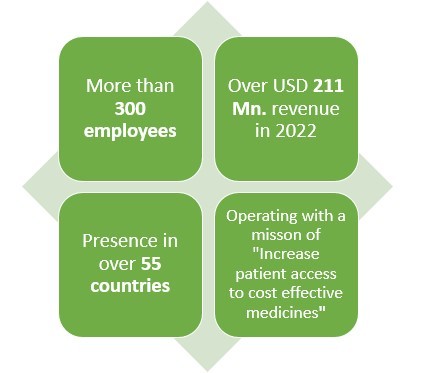
Click here to Ask for a Custom Report
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
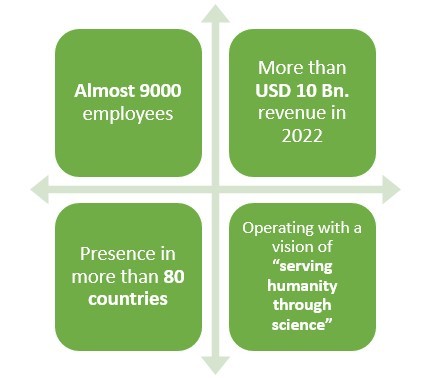
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Trastuzumab Biosimilars Market Insights, 2024
Trastuzumab, a monoclonal antibody used to treat HER2-positive breast cancer, has revolutionized cancer care by targeting the HER2 receptor, which is overexpressed in a subset of breast cancer cases. As the demand for more affordable treatments grows, trastuzumab biosimilars have emerged as a viable solution, offering improved access to essential cancer therapies while reducing healthcare costs.
Market Dynamics
The global trastuzumab biosimilars market pipeline is experiencing rapid growth, driven by the increasing prevalence of HER2-positive breast cancer and the cost-effectiveness of biosimilars. Breast cancer remains the most commonly diagnosed cancer worldwide, according to the World Health Organization (WHO), intensifying the need for accessible treatment options, particularly in emerging markets.
Biosimilars provide a cost-efficient alternative to Roche’s originator product, Herceptin, without compromising efficacy or safety. Following the expiration of Herceptin’s patents, several biosimilars have entered the market, including Ogivri (Mylan/Biocon), Kanjinti (Amgen), and Ontruzant (Samsung Bioepis), which are contributing to widespread adoption and affordability.
Competitive Landscape
The trastuzumab biosimilars market is highly competitive, with several companies striving for a significant market share. Key players include:
Biocon and Mylan: Their biosimilar Ogivri has been approved in major regions like the U.S. and Europe, offering substantial cost savings.
Amgen: Kanjinti, Amgen's biosimilar, is FDA- and EMA-approved and gaining significant market acceptance.
Samsung Bioepis: In collaboration with Merck, Samsung Bioepis developed Ontruzant, a prominent biosimilar in the market.
Celltrion: With a robust biosimilar portfolio that includes Truxima and Herzuma, Celltrion is strengthening its foothold in the trastuzumab segment.
Challenges and Opportunities
While the adoption of trastuzumab biosimilars is steadily rising, challenges remain. These include regulatory complexities, limited market access, and reluctance among some physicians and patients to switch from branded products. However, as more biosimilars receive approvals and clinical experience with these therapies increases, adoption rates are expected to grow, creating significant opportunities.
Future Outlook
The trastuzumab biosimilars market is anticipated to expand significantly by 2024, driven by increased product approvals, reduced costs, and rising demand for advanced cancer therapies. With the development of new biosimilars, competition is set to intensify, benefiting both patients and healthcare providers. The future of trastuzumab biosimilars is bright, enhancing global access to targeted cancer treatments.
Related Reports by DelveInsight
Aneurysmal Subarachnoid Hemorrhage Market
Angelman Syndrome Market
Autoimmune Pulmonary Alveolar Proteinosis Market
Cardiovascular Calcification Market
Charcot Marie Tooth Disease Market
Chronic Focal Epilepsy Market
Crispr Therapies- Pipeline Insights Market
Cytokine Release Syndrome Market
Frontotemporal Dementia Market
Glabellar Frown Lines Market
Graves Ophthalmopathy Market
Icos-next Generation Immunotherapy Market
Invasive Pneumococcal Disease Market
Lipodystrophy Market
Nasolabial Fold Market
Natural Killer T Cell Lymphoma Market
About DelveInsight
DelveInsight is a global leader in market research and consulting, specializing in the healthcare and life sciences sectors. By providing detailed market insights, DelveInsight supports pharmaceutical, biotechnology, and medical device companies in making informed and strategic decisions within a competitive landscape.
Contact Information Kanishk Kumar Email: [email protected]
0 notes
Text
Breaking Ground in Pharma: The 15th Digital Pharmaceutical Innovations Exhibition & Congress
Breaking Ground in Pharma: The 15th Digital Pharmaceutical Innovations Exhibition & Congress
Introduction: The pharmaceutical industry is rapidly evolving, driven by cutting-edge technologies and breakthrough innovations that are transforming healthcare. One of the key events that highlight these advancements is the 15th Digital Pharmaceutical Innovations Exhibition & Congress. This prestigious event gathers thought leaders, industry experts, and innovators from across the globe to discuss the latest trends, research, and technologies in the field of biopharmaceuticals and biosimilars. The growing importance of digital technologies and innovative solutions in the biopharmaceutical sector is at the heart of this event, which showcases how these advances are making healthcare more accessible, efficient, and personalized.
The 15th Digital Pharmaceutical Innovations Exhibition & Congress: A Spotlight on Biopharmaceuticals and Biosimilars
The world of pharmaceuticals is evolving rapidly, and one of the most important and exciting sectors within it is biopharmaceuticals and biosimilars. These cutting-edge treatments are reshaping healthcare and providing new opportunities to treat diseases in ways that were once thought impossible. The 15th Digital Pharmaceutical Innovations Exhibition & Congress offers an incredible opportunity for professionals, experts, and innovators to come together and explore the future of these transformative technologies.
What is the 15th Digital Pharmaceutical Innovations Exhibition & Congress?
The 15th Digital Pharmaceutical Innovations Exhibition & Congress is an annual event that brings together stakeholders from across the pharmaceutical and biotechnology industries. This year’s congress is entirely digital, offering attendees the flexibility to participate from anywhere in the world, interact with key industry players, and access valuable insights from thought leaders and researchers.
The event will focus on the latest advancements in biopharmaceuticals and biosimilars, which are at the forefront of the pharmaceutical landscape. With the ongoing rise in demand for biologic treatments, the discussion around their production, regulation, and accessibility has never been more relevant.
What Are Biopharmaceuticals and Biosimilars?
Before diving into the specifics of the exhibition, it’s important to define biopharmaceuticals and biosimilars:
Biopharmaceuticals: These are drugs that are produced using living organisms or contain components from living organisms. Unlike traditional chemical drugs, biopharmaceuticals are complex molecules such as proteins, vaccines, and monoclonal antibodies. They are used to treat a wide range of conditions, including cancers, autoimmune diseases, and rare genetic disorders.
Biosimilars: A biosimilar is a biologic medical product that is highly similar to an already approved reference product. While they are not identical to their reference products, biosimilars are proven to have no clinically meaningful differences in terms of safety, efficacy, and quality. The advent of biosimilars has opened up new opportunities for patients to access life-saving medications at a more affordable cost.
Key Topics at the Exhibition & Congress
Innovative Biopharmaceutical Research and Development: The event will feature the latest breakthroughs in biopharmaceutical R&D, focusing on the design, discovery, and optimization of biologic drugs. Experts will share insights into how new technologies like gene editing, artificial intelligence, and advanced analytics are accelerating the development of next-generation biologics.
Regulatory Landscape for Biosimilars: As the market for biosimilars continues to grow, regulatory frameworks are evolving. The congress will explore the regulatory hurdles that need to be overcome to bring biosimilars to market and how agencies around the world are addressing these challenges.
Manufacturing of Biopharmaceuticals: The complexity of biologic production and the need for highly specialized manufacturing processes is another area that will be explored at the event. Topics will include innovations in biomanufacturing technologies, quality control, and strategies for scaling production while maintaining product consistency.
Patient Access and Market Access for Biopharmaceuticals and Biosimilars: One of the biggest challenges in the pharmaceutical industry is ensuring that patients have access to the medications they need. The congress will examine the role of policy, insurance, and pricing models in improving access to biopharmaceuticals and biosimilars, ensuring that these treatments are available to those who need them most.
The Future of Personalized Medicine: Personalized medicine is an emerging field that aims to tailor treatment to an individual’s genetic profile. Biopharmaceuticals, particularly gene therapies, are at the forefront of this revolution. Attendees will learn about the latest developments in personalized medicine and its potential to revolutionize the way diseases are treated.
Networking Opportunities
In addition to the informative sessions, the 15th Digital Pharmaceutical Innovations Exhibition & Congress provides numerous networking opportunities. Attendees can connect with industry leaders, researchers, and peers through virtual networking sessions, roundtable discussions, and one-on-one meetings. Whether you are looking to collaborate on a research project, discuss potential partnerships, or simply exchange ideas, this event will facilitate valuable connections.
Who Should Attend?
The congress is open to a wide range of professionals, including:
Pharmaceutical Companies: Executives and managers looking to stay at the forefront of biopharmaceutical innovation.
Researchers and Scientists: Those engaged in cutting-edge research in the fields of biopharmaceuticals, biosimilars, and related disciplines.
Regulatory Experts: Professionals involved in the approval and regulation of biologic products.
Manufacturers and Suppliers: Companies involved in the production and supply chain of biopharmaceuticals and biosimilars.
Investors: Those interested in the investment opportunities within the biopharmaceutical and biosimilar sectors.
Why Attend?
By attending the 15th Digital Pharmaceutical Innovations Exhibition & Congress, you’ll gain access to:
In-depth insights from industry leaders and experts.
Cutting-edge research and trends in biopharmaceuticals and biosimilars.
Networking opportunities with peers, potential collaborators, and thought leaders.
A front-row seat to the future of pharmaceuticals.
Benefits of Attending the Digital Pharmaceutical Innovations Exhibition & Congress: Biopharmaceuticals and Biosimilars
Cutting-Edge Insights: Stay ahead of the curve with the latest research, developments, and trends in biopharmaceuticals and biosimilars.
Networking Opportunities: Connect with industry leaders, innovators, and peers from around the globe.
Access to Experts: Gain valuable knowledge from leading experts through presentations, discussions, and panels.
Market Access Strategies: Learn about new strategies for improving access to biopharmaceuticals and biosimilars.
Regulatory Insights: Stay informed on the evolving regulatory landscape surrounding biologics and biosimilars.
Investment Potential: Explore the investment opportunities in the biopharmaceutical and biosimilar sectors.
Personalized Medicine: Dive into the future of personalized medicine powered by biopharmaceuticals and gene therapies.
Conclusion
As the pharmaceutical industry continues to evolve, events like the 15th Digital Pharmaceutical Innovations Exhibition & Congress play a crucial role in shaping the future. By focusing on biopharmaceuticals and biosimilars, this year’s event will provide attendees with the knowledge and connections needed to stay ahead in the ever-changing landscape of medicine.
Don’t miss out on this incredible opportunity to learn, network, and engage with the leaders of tomorrow’s pharmaceutical innovations. Register today and be part of the digital revolution in healthcare!
More Information
To learn more about the 15th Digital Pharmaceutical Innovations Exhibition & Congress, visit our website or connect with us on social media:
#Biopharmaceuticals #Biosimilars #Biotech #PharmaceuticalInnovation #BiologicTherapies #BiosimilarDevelopment #FutureOfMedicine #DrugDevelopment #MedicalInnovation #BioTechRevolution #PharmaResearch #AffordableHealthcare #Biopharma #BiosimilarTherapies #PrecisionMedicine
👉 Register here: -https://pharmacy.utilitarianconferences.com/registration
Website: https://utilitarianconferences.com/
Twitter: @UCGConferences
Facebook: Utilitarian Conferences Gathering
0 notes
Text
The Prokaryotic Recombinant Protein Market is projected to grow from USD 2725.2 million in 2024 to an estimated USD 4278.42 million by 2032, with a compound annual growth rate (CAGR) of 5.8% from 2024 to 2032.The Prokaryotic Recombinant Protein Market has been experiencing significant growth, driven by advancements in biotechnology, expanding research in protein therapeutics, and increasing demand for cost-effective biologics production. Prokaryotic systems, particularly Escherichia coli (E. coli), have emerged as a preferred host for recombinant protein expression due to their simplicity, rapid growth, and ability to produce high yields. This article explores the key factors driving the market, challenges, applications, and future prospects.
Browse the full report at https://www.credenceresearch.com/report/prokaryotic-recombinant-protein-market
Market Drivers and Dynamics
Rising Demand for Biologics and Biosimilars Biologics, including monoclonal antibodies, vaccines, and enzymes, are critical in treating chronic diseases like cancer, diabetes, and autoimmune disorders. The production of recombinant proteins using prokaryotic systems is cost-effective and scalable, making it an attractive option for biosimilar development.
Technological Advancements in Recombinant Protein Production Continuous innovations in genetic engineering, such as CRISPR-Cas9 and synthetic biology, have improved the precision and efficiency of prokaryotic expression systems. Advanced tools for optimizing codon usage, promoters, and plasmids have significantly enhanced the expression of complex proteins.
Growing Biopharmaceutical Research and Development (R&D) The surge in R&D investments by pharmaceutical and biotech companies to develop novel therapies has fueled the demand for prokaryotic recombinant proteins. Research initiatives aimed at understanding disease pathways, drug discovery, and protein-protein interactions rely heavily on these proteins.
Applications in Diverse Sectors
Pharmaceutical and Therapeutics Prokaryotic recombinant proteins are widely used to produce therapeutic proteins such as insulin, growth hormones, and clotting factors. The affordability and scalability of prokaryotic systems make them indispensable for meeting the global demand for life-saving biologics.
Diagnostics The diagnostic industry uses recombinant proteins to develop enzyme-linked immunosorbent assays (ELISA), Western blotting, and other diagnostic tools. These proteins are essential for detecting infectious diseases, autoimmune disorders, and cancers.
Agriculture and Industrial Applications In agriculture, recombinant proteins are used to develop genetically modified crops with enhanced resistance to pests and diseases. Industrial enzymes produced in prokaryotic systems are employed in various industries, including food and beverage, textiles, and biofuels.
Challenges in the Market
Limitations in Post-Translational Modifications Prokaryotic systems lack the machinery for post-translational modifications, such as glycosylation, which are essential for the biological activity of certain therapeutic proteins. This limitation has restricted the use of prokaryotic systems for complex protein production.
Protein Misfolding and Aggregation High expression levels in prokaryotic systems can lead to misfolded or aggregated proteins, affecting their functionality. Overcoming these challenges requires optimizing culture conditions and using molecular chaperones.
Regulatory and Ethical Considerations The production of recombinant proteins must comply with stringent regulatory standards to ensure safety and efficacy. The ethical implications of genetic engineering also continue to be a topic of debate.
Future Prospects
The Prokaryotic Recombinant Protein Market is poised for continued growth, supported by advancements in synthetic biology, the integration of AI in protein design, and the development of hybrid systems that combine the strengths of prokaryotic and eukaryotic hosts. Moreover, the increasing focus on personalized medicine and precision therapies is likely to expand the market's applications.
Sustainability in protein production will also play a critical role. Efforts to reduce environmental impact, such as using renewable feedstocks and optimizing bioprocesses, will shape the market's trajectory.
Key Player Analysis:
Abnova Corporation
Batavia Biosciences
Bioclone
Cayman Chemical Company
Cusabio Technology
Eli Lilly and Company
Geltor IndieBio
Geno Technology
Kaneka and Eurogentec
Merck
Prospec Tany Technogene
Randox Laboratories
Roche
Segmentation:
By Product Type:
Hormones
Interferons
Interleukins
Others
By End-User/Application:
Biotechnology Companies
Research institutes
Contract Research organizations
Hospital
Laboratories
Others
By Region
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/prokaryotic-recombinant-protein-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
0 notes
Text
Americas Sterility Testing Market Size, Growth Outlook 2035
Americas Sterility Testing Market Size was estimated at 3.44 (USD Billion) in 2022. The Americas Sterility Testing Market Industry is expected to grow from 3.65(USD Billion) in 2023 to 6.1 (USD Billion) by 2032.
Summary
The Americas sterility testing market is witnessing significant growth due to stringent regulatory requirements, increasing pharmaceutical and biotechnology research, and rising healthcare expenditure. Sterility testing plays a crucial role in ensuring the safety and efficacy of biopharmaceuticals, vaccines, and medical devices. The growing demand for biologics, cell and gene therapies, and personalized medicine is further driving the need for advanced sterility testing solutions. However, high costs associated with sterility testing equipment, complex regulatory approval processes, and technical challenges in microbial detection present obstacles to market growth. Pharmaceutical quality control testing.
Market Overview
Sterility testing is a critical quality control process performed to detect the presence of viable microorganisms in pharmaceuticals, medical devices, and tissue products. The rising biopharmaceutical industry and the need for contamination-free manufacturing are driving the demand for advanced sterility testing techniques, such as membrane filtration, direct inoculation, and rapid microbiological methods (RMMs). The adoption of automation in sterility testing is reducing human error, enhancing efficiency, and improving reproducibility in sterility assessments.
Market Size and Growth Analysis
Americas Sterility Testing Market Size was estimated at 3.44 (USD Billion) in 2022. The Americas Sterility Testing Market Industry is expected to grow from 3.65(USD Billion) in 2023 to 6.1 (USD Billion) by 2032. The Americas Sterility Testing Market CAGR (growth rate) is expected to be around 5.89% during the forecast period (2024 - 2032). Growth is fuelled by increased production of biopharmaceuticals, expansion of the contract research industry, and advancements in sterility testing technologies.
Market Dynamics
Growth Drivers
Growing Biopharmaceutical Industry: The increasing demand for vaccines, monoclonal antibodies, and biosimilars is driving the need for sterility testing.
Regulatory Stringency in Quality Control: FDA, EMA, and USP guidelines mandate strict sterility testing for all pharmaceutical and medical device manufacturers.
Adoption of Rapid Sterility Testing Methods: Automated microbiological detection and nucleic acid-based testing improve accuracy and reduce testing time.
Expansion of Contract Testing Organizations (CROs): Many pharmaceutical firms outsource sterility testing to third-party laboratories for cost-effective and reliable testing solutions.
Challenges and Restraints
High Cost of Advanced Sterility Testing Equipment: Automated and molecular sterility testing solutions are expensive, limiting adoption among small and medium-sized pharmaceutical companies.
Stringent Regulatory Compliance Requirements: Meeting sterility standards set by organizations like USP, EP, and WHO increases the complexity of market entry.
Technical Challenges in Sterility Testing: Factors such as microbial resistance, test contamination, and false-negative results affect the reliability of sterility tests.
Regional Analysis
North America
Largest market, driven by advanced pharmaceutical manufacturing, strong regulatory framework, and increasing biopharmaceutical R&D investments.
The United States holds a dominant position due to high sterility testing demand from the pharmaceutical and medical device industries.
South America
Emerging market with increasing biotechnology investments and improvements in pharmaceutical regulatory compliance.
Brazil and Argentina are key contributors due to expanding pharmaceutical production and clinical research activities.
Market Segmentation
By Test Type:
Membrane Filtration
Direct Inoculation
Rapid Sterility Testing (PCR, ATP Bioluminescence, and Flow Cytometry)
By Product Type:
Instruments (Incubators, Filtration Devices, Automated Sterility Testing Systems)
Consumables (Culture Media, Sterility Test Kits, Reagents)
Services (Contract Sterility Testing, Validation Services)
By End-User:
Pharmaceutical and Biotechnology Companies
Contract Research Organizations (CROs)
Medical Device Manufacturers
Academic and Research Institutes
Key Market Players
Vigilant Biosciences
Eppendorf
Nexus Pharmaceuticals
Thermo Fisher Scientific
Sartorius AG
3M
Recent Developments
FDA Approval for Automated Sterility Testing Solutions: Advanced testing methods are improving sterility assurance in cell and gene therapies.
Expansion of Contract Testing Laboratories: Increasing outsourcing trends are leading to growth in third-party sterility testing services.
Introduction of AI-Based Sterility Testing Systems: AI-powered automated microbial detection platforms are improving sterility testing efficiency.
Future Outlook and Opportunities
The Americas sterility testing market is expected to witness sustained growth due to increasing biologics production, regulatory advancements, and automation in sterility testing. Future developments in nanotechnology-based sterility testing, AI-driven microbial detection, and real-time sterility monitoring systems will further drive market expansion.
For more information, please visit @marketresearchfuture
#Americas Sterility Testing Market Size#Americas Sterility Testing Market Share#Americas Sterility Testing Market Growth#Americas Sterility Testing Market Analysis#Americas Sterility Testing Market Trends#Americas Sterility Testing Market Forecast#Americas Sterility Testing Market Segments
0 notes