#Biosimilar Monoclonal
Explore tagged Tumblr posts
Text
#Biosimilar Monoclonal Antibody Market#Biosimilar Monoclonal Antibody#Biosimilar Monoclonal#Biosimilar#monoclo#monoclonal#antibody
0 notes
Text
Biosimilars Unleashed: The Future of Healthcare in the US
Buy Now
What is the Size of US Biosimilar Industry?
US Biosimilar Market is expected to grow at a CAGR of ~ % between 2017-2022 and is expected to reach ~USD Bn by 2028. Biosimilars enhance patient access to essential treatments, especially in therapies with high demand, like oncology, by providing more affordable options. Additionally, Growing evidence of biosimilars' comparable efficacy and safety fosters trust among healthcare professionals, driving adoption.

Click here to Download a sample Report
Biosimilars offer cost savings compared to originator biologics, addressing the need for affordable healthcare solutions in the face of rising medical costs. Favorable regulatory frameworks, like the BPCIA, streamline biosimilar approval processes, encouraging manufacturers to invest in development.
Furthermore, The expiration of patents for numerous reference biologics creates opportunities for biosimilar entry, leading to increased competition and market expansion. Pharmaceutical companies are investing in biosimilar R&D and production, expanding the pipeline and market availability. Supportive healthcare policies and reimbursement models incentivize biosimilar adoption, creating a favorable environment for market growth.
US Biosimilar Market by drug class
The US Biosimilar market is segmented by Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-inflammatory agents and Others. Based on drug class, Monoclonal Antibodies segment dominates the bio similar market in 2022.
Monoclonal antibodies have diverse applications across various therapeutic areas. From cancer treatment to autoimmune diseases, biosimilar Mabs addressed a wide range of medical needs, leading to a broad and growing market. Biosimilars, with their potential for cost savings while maintaining comparable efficacy and safety, gained significant attention as viable alternatives.
US Biosimilar Market by application
In US Biosimilar market, they are segmented by application into Oncology, Blood disorders, Chronic diseases and autoimmune conditions and Others. On the basis of application, Oncology segment was the dominant in 2022.
The increasing prevalence of cancer and the high cost of traditional biologics used in oncology treatment have created a strong incentive for the adoption of biosimilars. Biosimilars offer the potential to provide similar therapeutic outcomes at a lower cost, making them an attractive option for both healthcare providers and patients.
Additionally, the rigorous clinical trials and regulatory processes that biosimilars undergo to gain approval provide reassurance to healthcare professionals and patients regarding their safety and efficacy. This has led to increased acceptance and adoption of biosimilars in oncology.
US Biosimilar by Region
The US Biosimilar market is segmented by Region into North, East, West and South. In 2022, the dominance region is North region in US Biosimilar market.
The North region benefits from a concentration of healthcare providers and academic institutions that are at the forefront of adopting and integrating biosimilars into their treatment protocols. These institutions are more likely to have the expertise to evaluate and incorporate biosimilars effectively, driving their adoption among healthcare professionals and patients.
Click here to Download a Custom Report
Competition Scenario in US Biosimilar Market
The US biosimilar market has witnessed an evolving competitive landscape, with several key players competing for market share. Prominent pharmaceutical companies such as Amgen, Pfizer, Sandoz (Novartis), and Boehringer Ingelheim have been actively involved in developing and marketing biosimilar products. These established players have utilized their expertise in biologics and significant resources to navigate the regulatory landscape and compete effectively.
The competition in the US biosimilar market is characterized by a balance between established pharmaceutical giants and emerging biotech companies. While the major players possess the advantage of resources and experience, smaller biotech firms are also contributing to the market with innovative approaches and niche biosimilar offerings.
What is the Expected Future Outlook for the Overall US Biosimilar Market?
The US Biosimilar market was valued at USD ~Million in 2022 and is anticipated to reach USD ~ Billion by the end of 2028, witnessing a CAGR of ~% during the forecast period 2022- 2028. The US biosimilar market is likely to experience significant growth in the coming years, driven by several factors. Biosimilars are biologic drugs that are highly similar to already approved reference biologics. They offer potential cost savings, increased competition, and improved patient access to crucial treatments.
Firstly, the regulatory environment is becoming more favorable for biosimilars. The Biologics Price Competition and Innovation Act (BPCIA) established a pathway for biosimilar approval in the US, allowing for a smoother regulatory process. As more biosimilars receive approval, competition in the market is expected to intensify.
Secondly, patents for several blockbuster biologics are expiring or have already expired. This creates opportunities for biosimilar manufacturers to enter the market with more affordable alternatives, offering healthcare systems and patients a choice in treatment options.
Thirdly, as healthcare costs continue to rise, biosimilars present an attractive solution for reducing expenses. Their potential to offer cost savings without compromising therapeutic efficacy could lead to increased adoption by healthcare providers, insurers, and patients alike.
Physician and patient education are crucial, as misconceptions about biosimilars' safety and effectiveness might hinder their adoption. Additionally, legal and market access barriers, including patent litigation and complex distribution systems, could slow down the growth of the biosimilar market.
The biosimilar market witness consolidation as larger pharmaceutical companies acquire or partner with smaller biotech firms to bolster their biosimilar portfolios. This will lead to more resources being devoted to biosimilar development and marketing. Changes in healthcare policies, such as reimbursement models and value-based care initiatives, can influence the biosimilar market's growth. Favourable policies that incentivize biosimilar adoption drives their market growth.
#US Biosimilar Market#US Biosimilar Industry#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market#US homologous biologics market#US oncology biosimilar market#US immunology biosimilar sector#US insulin biosimilar industry#US Generics Biologics market challenges#US leading Biosimilar drug providers#US leading Biosimilar drug manufacturers
0 notes
Text
Immunology Market: Global Trends and Forecast 2024-2032

The Immunology Market size was valued at USD 107.14 Billion in 2023 and is expected to reach USD 165.68 Billion by 2031, growing at a CAGR of 5.6% over the forecast period 2024–2031. This growth is propelled by the increasing incidence of autoimmune diseases, advancements in immunotherapy, and the growing demand for biologics and targeted treatments across various chronic conditions.
Market Overview
Immunology is at the core of modern medicine, playing a vital role in managing diseases that involve immune system dysfunction, such as rheumatoid arthritis, lupus, multiple sclerosis, and inflammatory bowel disease. With rising healthcare awareness and the shift towards precision medicine, the immunology market is witnessing an upsurge in demand for novel therapies that offer higher efficacy and fewer side effects.
Key pharmaceutical companies are heavily investing in research and development to expand their immunology pipelines, particularly in biologics and monoclonal antibodies. The integration of artificial intelligence in clinical trials and drug discovery is also accelerating the pace of innovation within the sector.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/4188
Regional Analysis
North America dominates the global immunology market, driven by a high prevalence of autoimmune disorders, robust R&D infrastructure, and strong regulatory support.
Europe holds a significant share due to rising healthcare spending and early adoption of innovative therapies.
Asia-Pacific is expected to witness the fastest growth, with expanding healthcare access, increased government investments in biotechnology, and a growing patient pool.
Latin America, Middle East, and Africa are progressively evolving due to better diagnostic capabilities and expanding pharmaceutical distribution networks.
Market Segmentation
By Drug Class:
Monoclonal Antibodies
Immunosuppressants
Vaccines
Others
By Indication:
Rheumatoid Arthritis
Psoriasis
Inflammatory Bowel Disease (IBD)
Multiple Sclerosis
Systemic Lupus Erythematosus (SLE)
Others
By Distribution Channel:
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
KEY PLAYERS:
The key market players are Novartis AG, AbbVie, Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, Amgen Inc., F. Hoffmann-La Roche Ltd., Astellas Pharma Inc., Pfizer Inc., Janssen Global Services, LLC, Merck Sharp, Dohme Corp. & other players.
Key Highlights
Increasing global burden of chronic and autoimmune diseases.
Rising demand for targeted immunotherapies and biologics.
Accelerated R&D activities and clinical trials for novel immunomodulators.
Growth in biosimilar approvals fostering market competition.
Technological integration in drug development and disease monitoring.
Future Outlook
The immunology market is poised for transformative growth in the coming years, supported by scientific advancements and evolving treatment paradigms. The development of next-generation biologics, gene therapies, and personalized immunotherapies is expected to redefine disease management. Moreover, expanding patient access in emerging economies and collaborative efforts between academia and industry will further accelerate innovation. As precision medicine continues to gain ground, immunology is set to become one of the most dynamic and impactful areas in healthcare.
Conclusion
The immunology market stands at a pivotal juncture, driven by the urgent need for effective therapies and breakthroughs in biotechnology. With a strong development pipeline and increasing investment in immunological research, the industry is well-positioned to address some of the most challenging health conditions globally, delivering hope and improved outcomes for millions of patients.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Immunology Market#Immunology Market Share#Immunology Market Size#Immunology Market Trends#Immunology Market Growth
0 notes
Text
Biocon shares jump 5.5% as USFDA clears biosimilar Cancer drug JOBEVNE

Biocon’s share price surged 5.5 percent in intra-day trade on Friday, April 11, after the company’s subsidiary, Biocon Biologics, received approval from the US Food and Drug Administration (USFDA) for JOBEVNE, a biosimilar version of Bevacizumab for intravenous use. The development marks a key regulatory milestone for the company and reinforces investor confidence in Biocon’s growing biosimilar portfolio.
JOBEVNE: Biocon’s Seventh USFDA-Approved Biosimilar
The newly approved drug, JOBEVNE (bevacizumab-nwgd), is a biosimilar of the reference product Avastin (bevacizumab). It is a recombinant humanized monoclonal antibody designed to inhibit angiogenesis — the formation of new blood vessels — by blocking vascular endothelial growth factor (VEGF). This mechanism restricts the blood supply to tumors, thereby slowing or preventing their growth.
Shreehas Tambe, CEO and Managing Director of Biocon Biologics, described the USFDA approval as a major achievement for the company. “The U.S. FDA approval of JOBEVNE is a significant milestone — our seventh biosimilar approved in the U.S. and a strong addition to our robust oncology portfolio,” he said. Tambe added that the approval reflects Biocon’s scientific depth and ongoing commitment to making high-quality, affordable biologics accessible globally.
Strengthening Presence in Global Oncology Markets
With JOBEVNE, Biocon Biologics further cements its position in the U.S. oncology biosimilars market, where it already offers OGIVRI (Trastuzumab-dkst) and FULPHILA (Pegfilgrastim-jmdb). The company also sells its Bevacizumab biosimilar under the brand name ABEVMY in Europe (since February 2021) and Canada (since November 2021).
The U.S. market remains a significant opportunity, with sales of bevacizumab approximating USD 2 billion in 2023. Biocon’s expanded product pipeline is expected to enable the company to capture a larger share of this high-value market.
In addition to the USFDA nod for JOBEVNE, Biocon has been taking steps to strengthen its financial position. The company’s board recently approved the issuance of commercial papers worth up to ₹600 crore through private placement, aiming to improve liquidity and fund its growth initiatives.
Further, on March 24, Biocon’s subsidiary Biocon Pharma received USFDA approval for its ANDA (Abbreviated New Drug Application) for Norepinephrine Bitartrate Injection USP, used for managing acute hypotension in adult patients. This back-to-back regulatory clearance reinforces Biocon’s execution capabilities across therapeutic areas.
Stock Performance: Rebounding from Lows
The pharma stock rose to a day’s high of ₹322.25 on April 11, reflecting strong investor sentiment post-approval. While still trading over 20 percent below its 52-week high of ₹404.60 (hit in January 2025), the stock has shown resilience after touching a 52-week low of ₹260 in April 2024.
Over the past year, Biocon has gained around 10 percent. However, its monthly trajectory has been volatile: it declined 8 percent in April so far, after a 13 percent rally in March. This followed a steep 16.5 percent drop in February and a marginal 1 percent dip in January.
Intensify Research services is a Top SEBI registered Research analyst Indore committed to empowering investors with the most reliable stock market insights. Our team of expert analysts uses advanced tools and strategies to provide that enhance your chances of success. To visit- Intensifyresearch.com »
#sharemarketing#sharetrading#stockinvestment#stock market#investment#shareinvestor#sharetrader#share this post#stocks#sharemarket
0 notes
Text
Biologics and Biosimilars Market Consumer Behavior and Industry Shifts to 2033
Introduction
The biologics and biosimilars market is witnessing significant growth driven by advancements in biotechnology, increasing prevalence of chronic diseases, and rising demand for cost-effective biologic therapies. Biologics, which include complex molecules such as monoclonal antibodies and recombinant proteins, have revolutionized the treatment of various diseases. However, their high costs have paved the way for biosimilars—biological products that are highly similar to approved biologics—with comparable efficacy and safety at reduced prices.
As we look forward to 2032, the market dynamics are expected to shift further with policy reforms, technological innovations, and increasing competition shaping the future landscape.
Market Overview
In 2024, the global biologics and biosimilars market was valued at USD 400+ billion, and it is projected to surpass USD 750 billion by 2032, growing at a CAGR of over 8% during the forecast period. The market is segmented by product type, therapeutic area, manufacturing type, and region.
Download a Free Sample Report:-https://tinyurl.com/2k678792
Key Definitions:
Biologics: Medicinal products derived from living organisms, used to treat diseases such as cancer, autoimmune disorders, and infectious diseases.
Biosimilars: Biologic medical products that are almost identical copies of an original product but manufactured by a different company after the patent expiry.
Market Drivers
1. Patent Expirations of Biologic Blockbusters
The expiration of patents for blockbuster biologics such as Humira (adalimumab), Remicade (infliximab), and Herceptin (trastuzumab) has opened opportunities for biosimilars. These patent cliffs allow biosimilar manufacturers to enter the market with cost-effective alternatives, increasing competition and market penetration.
2. Rising Prevalence of Chronic Diseases
The growing global burden of chronic diseases like cancer, rheumatoid arthritis, diabetes, and cardiovascular disorders is fueling the demand for biologic therapies. Biologics offer high specificity and efficacy, making them the preferred choice for targeted treatment.
3. Cost-effectiveness of Biosimilars
Healthcare systems worldwide are increasingly adopting biosimilars to reduce the overall cost of treatment. Biosimilars typically cost 15-30% less than their reference biologics, which helps improve access, especially in emerging markets.
4. Regulatory Support and Guidelines
Regulatory bodies like the U.S. FDA, European Medicines Agency (EMA), and World Health Organization (WHO) have established stringent yet supportive pathways for biosimilar approval, ensuring their safety, efficacy, and quality.
Market Restraints
High Manufacturing Complexity: Biologics and biosimilars require sophisticated manufacturing processes, involving living cells and stringent quality controls.
Limited Patient and Physician Awareness: In certain regions, the lack of awareness and trust in biosimilars hinders adoption.
Patent Litigation and Exclusivity: Legal battles over patents and exclusivity rights can delay biosimilar entry and increase development costs.
Market Segmentation
By Product Type
Monoclonal Antibodies
Vaccines
Recombinant Hormones
Interferons
Insulin
Fusion Proteins
Monoclonal antibodies lead the biologics segment due to their application in cancer and autoimmune diseases.
By Therapeutic Application
Oncology
Autoimmune Diseases
Diabetes
Hematology
Infectious Diseases
Ophthalmology
The oncology segment is dominant, driven by the growing prevalence of cancer and the high efficacy of biologics and biosimilars in oncology treatment.
By Manufacturing Type
In-house Manufacturing
Contract Manufacturing Organizations (CMOs)
While large pharmaceutical firms often use in-house capabilities, small biotech firms increasingly rely on CMOs to reduce operational costs and enhance scalability.
Regional Insights
North America
North America dominates the market due to robust healthcare infrastructure, high R&D investment, and early adoption of biosimilars. The U.S. FDA’s Biosimilar Action Plan further encourages biosimilar development and competition.
Europe
Europe is a pioneer in biosimilar approvals and adoption. Countries like Germany, the UK, and France have implemented favorable reimbursement policies and strong physician awareness.
Asia Pacific
The Asia Pacific region is experiencing the fastest growth, driven by increasing healthcare spending, large patient populations, and growing investments by regional players in biosimilar development (e.g., India, South Korea, and China).
Competitive Landscape
The market is highly competitive, with both large pharmaceutical companies and specialized biotechnology firms operating globally.
Major Players:
Amgen Inc.
Roche Holding AG
Pfizer Inc.
Samsung Bioepis
Biocon Ltd.
Sandoz (a Novartis division)
Celltrion Healthcare
These companies focus on strategic alliances, product launches, biosimilar approvals, and manufacturing scale-up to gain competitive advantages.
Emerging Trends
1. Integration of AI and Digital Technologies
Artificial intelligence is being utilized for biologics discovery, molecular simulation, and process optimization. This enhances speed-to-market and reduces R&D costs.
2. Personalized Biologics
With advances in genomics, biologics are increasingly being tailored to individual patient profiles, leading to improved outcomes and reduced side effects.
3. Subcutaneous and Oral Biologics
There is a rising trend toward subcutaneous and oral biologics, offering patient convenience over traditional intravenous delivery methods.
4. Government Incentives and Reimbursement Policies
Governments in emerging economies are offering incentives and subsidies to promote biosimilar manufacturing and adoption, improving market access and affordability.
Future Outlook (2025–2032)
The biologics and biosimilars market is expected to evolve rapidly over the next decade. With major patents expiring and healthcare systems seeking cost-effective solutions, biosimilars will gain more ground. Meanwhile, continued innovation in biologics will lead to novel therapies for rare and complex diseases.
Key projections:
Biologics will continue to dominate in revenue, but biosimilars will grow at a faster rate due to increasing accessibility.
Asia Pacific will emerge as a biosimilar manufacturing hub due to lower production costs and favorable policies.
Strategic partnerships between pharma companies and CMOs or biotech startups will increase to meet global demand efficiently.
Biobetters—enhanced versions of original biologics—will gain popularity for offering improved efficacy and reduced dosing frequency.
Conclusion
The biologics and biosimilars market stands at a pivotal point. While biologics continue to drive innovation in treating life-threatening diseases, biosimilars are ensuring that these treatments become accessible and affordable for a broader population. As regulatory landscapes mature, technologies advance, and global healthcare priorities evolve, the market is poised for robust and inclusive growth through 2032.
Companies that invest in quality manufacturing, innovation, and regulatory navigation will be well-positioned to capture significant market share in this dynamic and transformative healthcare segment.
Read Full Report:-https://www.uniprismmarketresearch.com/verticals/healthcare/biologics-and-biosimilars.html
0 notes
Text
The Future of Pharmaceutical Contract Manufacturing Services: Trends & Innovations

Pharmaceutical Contract Manufacturing Services (PCMS) have become an essential component of the global healthcare ecosystem. As pharmaceutical companies seek to optimize costs, ensure compliance, and accelerate time-to-market, outsourcing manufacturing to specialized contract development and manufacturing organizations (CDMOs) has gained momentum. In this blog, we explore the latest trends shaping the future of pharmaceutical contract manufacturing services.
1. Growth of Biologics & Biosimilars
Biopharmaceuticals, including monoclonal antibodies, gene therapies, and biosimilars, are driving the demand for specialized contract manufacturing services. CDMOs are investing in advanced bioprocessing technologies and bioreactors to support the complex production of these therapies.
2. Adoption of Continuous Manufacturing
Traditional batch manufacturing is being replaced by continuous manufacturing (CM), offering increased efficiency, reduced waste, and enhanced scalability. CM enables real-time quality monitoring, improving overall product consistency and safety.
3. Emphasis on Regulatory Compliance & Quality Standards
With stringent regulations from the FDA, EMA, and other global authorities, CDMOs are focusing on compliance with Good Manufacturing Practices (GMP) and data integrity. Digitalized record-keeping, automation, and AI-powered analytics are enhancing regulatory adherence.
4. Integration of Digital & Smart Manufacturing
Industry 4.0 is transforming pharmaceutical contract manufacturing services with AI, IoT, and blockchain. Smart factories with predictive maintenance, automated workflows, and supply chain transparency are becoming the industry standard.
5. Growth of Specialized & Niche CDMOs
As personalized medicine and rare disease treatments gain prominence, niche CDMOs specializing in small-batch, high-potency, and orphan drug manufacturing are emerging. These companies offer tailored solutions to biotech and pharmaceutical firms.
6. Expansion of Global Manufacturing Networks
Pharmaceutical companies are diversifying their supply chains by partnering with CDMOs across different regions. This mitigates risks related to geopolitical instability, trade regulations, and supply chain disruptions.
7. Sustainability & Green Manufacturing
The industry is shifting towards eco-friendly manufacturing practices. Sustainable solvents, energy-efficient processes, and waste reduction strategies are being adopted to minimize the environmental footprint of pharmaceutical production.
Final Thoughts
Pharmaceutical contract manufacturing services are evolving rapidly, driven by technological advancements, regulatory requirements, and market dynamics. Companies that embrace these trends and invest in innovation will lead the next phase of growth in the pharmaceutical outsourcing sector.
0 notes
Text
Veterinary Services Strategies In The Global Market: Key Insights From The 2025 Report
The (CMO) / (CDMO) Market was valued at USD 23.32 billion in 2023 and is expected to experience significant growth, reaching an estimated USD 38.87 billion by 2032. With a compound annual growth rate (CAGR) of 5.86% from 2024 to 2032, the CMO/CDMO market is driven by the increasing demand for pharmaceutical outsourcing, advancements in drug development, and the growing focus on cost efficiency in the pharmaceutical industry.
Get Free Sample Report on CMO/CDMO Market
CMOs and CDMOs play a pivotal role in the global pharmaceutical and biotechnology sectors by providing outsourced manufacturing services for drug development, production, and supply chain management. These organizations offer a broad range of services, including research and development, manufacturing, packaging, and distribution, enabling pharmaceutical companies to streamline their operations, reduce costs, and bring new drugs to market more quickly.
Key Market Drivers
Several factors are driving the growth of the CMO/CDMO market:
Rising Demand for Outsourced Pharmaceutical Services: The global pharmaceutical industry continues to embrace outsourcing as a means to enhance efficiency, reduce operational costs, and focus on core competencies such as drug discovery and marketing. Contract manufacturers and developers provide pharmaceutical companies with the ability to scale production, access specialized expertise, and expedite time-to-market for new drugs. As the demand for high-quality, cost-effective, and timely drug production increases, more pharmaceutical companies are relying on CMOs and CDMOs for their manufacturing needs.
Growing Biopharmaceutical Sector: The increasing development and production of biologic drugs, such as monoclonal antibodies, gene therapies, and vaccines, has contributed significantly to the expansion of the CMO/CDMO market. Biopharmaceuticals require specialized manufacturing processes and facilities, which has led to a growing reliance on CDMOs that can offer state-of-the-art technology and expertise. Additionally, the global COVID-19 pandemic has further accelerated the demand for biopharmaceuticals and vaccines, highlighting the need for flexible, scalable manufacturing solutions.
Advancements in Manufacturing Technologies: The rise of new manufacturing technologies, such as continuous manufacturing, single-use technologies, and process optimization tools, is driving innovation in the CMO/CDMO sector. These technologies allow for greater production flexibility, improved efficiency, and reduced production costs. As pharmaceutical companies look to adopt these advanced technologies to stay competitive, they increasingly turn to CMOs and CDMOs with the expertise and infrastructure to support these innovations.
Cost Efficiency and Regulatory Expertise: Pharmaceutical companies are under pressure to reduce production costs while maintaining the highest quality standards. By outsourcing manufacturing to CMOs and CDMOs, companies can avoid the significant capital expenditure required to establish in-house manufacturing facilities and instead leverage the specialized expertise of third-party providers. Additionally, CDMOs often have extensive experience navigating the complex regulatory environment of the pharmaceutical industry, ensuring that products meet international regulatory requirements and avoiding costly delays in product approval.
Expansion of Generic and Biosimilar Markets: The increasing demand for generic drugs and biosimilars is another important factor driving the growth of the CMO/CDMO market. As patents for branded drugs expire, the market for generics and biosimilars has surged, creating new opportunities for contract manufacturers to produce high-quality, cost-effective alternatives. CDMOs are well-positioned to support the production of these drugs, as they have the infrastructure and regulatory knowledge required to produce large quantities of generics and biosimilars.
Market Segmentation
The CMO/CDMO market can be segmented based on service type, drug type, end user, and region.
By Service Type:
Drug Development Services: This segment includes services related to the early stages of drug development, such as preclinical studies, clinical trials, formulation development, and regulatory submissions. CDMOs providing these services are in high demand due to the increasing complexity of drug development processes and the growing need for specialized expertise.
Manufacturing Services: This segment covers the production of active pharmaceutical ingredients (APIs), biologics, and finished dosage forms. As the demand for both small-molecule drugs and biologics continues to rise, CMOs and CDMOs are expanding their capabilities to meet these needs, with a particular focus on biologics manufacturing.
Packaging and Distribution Services: Packaging and distribution are crucial aspects of the pharmaceutical supply chain. CMOs and CDMOs offering these services help ensure that drugs are packaged securely and delivered efficiently to healthcare providers and patients worldwide.
By Drug Type:
Small Molecule Drugs: This segment includes the production of traditional pharmaceutical drugs, which are typically produced using synthetic chemical processes. Despite the increasing prominence of biologics, small molecule drugs remain a dominant segment in the pharmaceutical industry, and CMOs continue to play a vital role in their production.
Biologics and Biosimilars: Biologics, including monoclonal antibodies, vaccines, and gene therapies, are rapidly growing in the pharmaceutical market. CDMOs specializing in biologics offer cutting-edge technology and facilities to support the complex manufacturing processes involved in biologic drug production.
Generics: As more branded drugs lose patent protection, the market for generic drugs is expanding rapidly. CMOs and CDMOs that specialize in generics are critical to meeting the global demand for affordable alternatives to branded pharmaceuticals.
By End User:
Pharmaceutical Companies: The largest end-user segment for CMOs and CDMOs is pharmaceutical companies, which outsource manufacturing and development services to reduce costs and improve efficiency.
Biotechnology Companies: Biotechnology companies, especially those focused on the development of novel biologic therapies, also rely on CDMOs for their manufacturing needs. These companies often do not have the resources or infrastructure to produce biologics in-house and turn to specialized contract providers.
Other End Users: Other end users of CMO/CDMO services include academic institutions, government organizations, and research laboratories involved in drug discovery and clinical trials.
Key Players and Products Related to CMO and CDMO
Swiss American CDMO
Pierre Fabre Group
Zymo Cosmetics
Make Enquiry about CMO/CDMO Market
Conclusion
The CMO/CDMO market is set for substantial growth, with a projected CAGR of 5.86% and an expected market value of USD 38.87 billion by 2032. Increasing demand for pharmaceutical outsourcing, advancements in manufacturing technologies, and the rise of biopharmaceuticals and generics are all contributing to the market’s expansion. As pharmaceutical and biotechnology companies continue to seek cost-effective and efficient manufacturing solutions, CMOs and CDMOs will play a critical role in supporting the development and production of life-saving drugs, ensuring that patients around the world have access to high-quality, affordable therapies. The future of the CMO/CDMO market looks promising, with ample opportunities for both established and emerging players to capitalize on the growing demand for outsourced pharmaceutical services.
About US:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
Contact Us:
Jagney Dave - Vice President of Client Engagement
Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
#CMO/CDMO Market#CMO/CDMO Market Size#CMO/CDMO Market Report#CMO/CDMO Market Trend#CMO/CDMO Market Share#CMO/CDMO Market Growth
0 notes
Text
The global Proteinase K market analysis covers its size, segmentation, regional insights, company share, key player profiles, and market forecast from 2025 to 2035.
Proteinase K Market Analysis, Trends, and Forecast (2025-2035)
Industry Outlook
The Proteinase K Market was valued at USD 4.88 Billion in 2024 and is projected to reach USD 12.15 Billion by 2035, growing at a CAGR of approximately 8.65% from 2025 to 2035. The market comprises global production volumes and demand for broad-spectrum serine protease, extensively used in biotechnology, diagnostics, and molecular biology.
Proteinase K plays a crucial role in DNA and RNA extraction, protein digestion, and clinical sample preparation. It is available in microbial-origin, animal-origin, and recombinant forms, catering to pharmaceutical companies, biotechnology firms, research institutes, diagnostic laboratories, and forensic investigation agencies. The rising adoption of Proteinase K in pharmaceutical manufacturing, food safety testing, and criminal forensics is driving market expansion, supported by advancements in recombinant enzyme technology and increasing R&D investments.
Get free sample Research Report - https://www.metatechinsights.com/request-sample/2160
Market Dynamics
Growing Demand for Molecular Diagnostics
Molecular diagnostics serve as a key driver of the Proteinase K market due to its indispensable role in DNA and RNA extraction. The surge in infectious diseases, genetic disorders, and cancer cases has heightened demand for rapid diagnostic techniques. PCR, RT-PCR, and next-generation sequencing (NGS) heavily rely on Proteinase K for pathogen detection, mutation analysis, and hereditary disease identification.
The COVID-19 pandemic significantly boosted the use of molecular diagnostics, increasing the application rates of Proteinase K. Modern point-of-care testing and liquid biopsy methods further expand its role in non-invasive diagnostics, enhancing precision medicine applications. Emerging technologies and government support for molecular research will continue to drive market growth.
Rising Biopharmaceutical Production
Biopharmaceutical production heavily relies on Proteinase K for impurity removal in monoclonal antibodies, recombinant proteins, and gene therapies. The increasing demand for biologic medicines and biosimilars, particularly for cancer and autoimmune treatments, is fueling the need for high-quality processing enzymes.
Proteinase K is widely used in cell culture processing, viral vector purification, and protein characterization. Regulatory authorities like the FDA and EMA enforce strict purification standards, pushing pharmaceutical companies toward Proteinase K adoption. The expansion of contract manufacturing organizations (CMOs) and bioprocessing facilities further drives market demand.
High Production and Purification Costs
One of the primary restraints in the Proteinase K market is the high cost of production and purification. Recombinant and purified Proteinase K variants require advanced fermentation technologies, stringent quality assurance processes, and costly culture mediums.
Liquid enzyme formulations necessitate controlled temperature storage, adding to transportation and logistical expenses. Small and medium-sized enterprises (SMEs) face significant challenges in market penetration due to financial constraints. Affordability issues in developing economies also limit widespread adoption, although technological advancements are gradually lowering costs.
Expansion of Personalized Medicine
The rise of personalized medicine is creating new growth opportunities for the Proteinase K market. Genetic profiling, NGS, and PCR-based diagnostics depend on Proteinase K for accurate DNA/RNA extraction, making it vital for precision medicine.
Companion diagnostics, which guide tailored treatments based on genetic markers, rely on pure nucleic acid material, increasing Proteinase K consumption. AI-driven genetic research and bioinformatics advancements further enhance its usage. Public and private funding in precision medicine is expected to propel market expansion in the coming years.
Increasing Demand for Recombinant Proteinase K
Recombinant Proteinase K is gaining traction due to its superior stability, high purity, and consistent enzymatic properties. Unlike microbial or animal-derived variants, recombinant Proteinase K offers contamination-free production, making it highly suitable for pharmaceuticals, diagnostics, and molecular biology applications.
This variant functions efficiently under extreme conditions, supporting DNA/RNA extraction, NGS, and PCR applications. Regulatory agencies mandate recombinant enzyme usage to eliminate contamination risks, further fueling market demand. With scalable and sustainable production techniques, recombinant Proteinase K is poised for substantial growth in industrial and research applications.
Read Full Research Report https://www.metatechinsights.com/industry-insights/proteinase-k-market-2160
Segment Analysis
By Source
Microbial-Derived Proteinase K
Animal-Derived Proteinase K
Recombinant Proteinase K (Dominant Segment)
Recombinant Proteinase K leads the market due to its high stability, purity, and regulatory approval for molecular diagnostics and biopharmaceutical applications.
By Form
Powder (Dominant Segment)
Liquid
Powdered Proteinase K dominates the market as it offers enhanced stability, longer shelf life, and convenient storage. It is extensively used in molecular biology applications, NGS, and biopharmaceutical production.
Regional Analysis
North America
North America holds a significant share in the Proteinase K market due to the widespread adoption of molecular diagnostics, forensic science, and biopharmaceutical production. The U.S. leads in market growth, driven by advanced research facilities, strong biopharma presence, and regulatory mandates favoring recombinant enzymes.
Asia-Pacific
The Asia-Pacific region is witnessing rapid market expansion, fueled by growing investments in biotechnology and pharmaceutical sectors. Countries like China, India, and Japan are heavily investing in life sciences research, precision medicine, and molecular diagnostics.
Increasing healthcare expenditures and the rising prevalence of genetic and infectious diseases are boosting market demand. The expansion of contract research organizations (CROs) and biomanufacturing facilities is also contributing to market growth.
Competitive Landscape
The Proteinase K market is highly competitive, with major players focusing on innovation, partnerships, and product line expansions. Key market participants include:
Thermo Fisher Scientific
Merck KGaA
QIAGEN
F. Hoffmann-La Roche Ltd.
New England Biolabs
Companies are investing in R&D to enhance Proteinase K applications in molecular diagnostics, biopharmaceutical manufacturing, and forensic science. Strategic collaborations with research institutions are also driving innovation in enzyme applications.
Buy Now https://www.metatechinsights.com/checkout/2160
Recent Developments
October 2023 – Sigma-Aldrich launched a new Proteinase K product to enhance nucleic acid extraction in research laboratories.
0 notes
Text

Learn cutting-edge Biopharmaceutical Technology with IGMPI's specialized program. This course covers biopharma manufacturing, biosimilars, monoclonal antibodies, and bioprocess engineering, equipping professionals with the expertise needed for the rapidly growing biopharmaceutical industry. Enroll now to advance your career in biotech and biopharma research!
#Biopharmaceutical Technology#Biopharma Course#Biopharmaceutical Industry#Biopharma Training#Biopharmaceutical ManufacturingDrug Development#Biotech Training
0 notes
Text
Global Chromatography Resin Market Analysis: Opportunities & Challenges
Rising Biopharmaceutical Research and Drug Development Drive Growth in the Chromatography Resin Market.

The Chromatography Resin Market Size was USD 2.6 billion in 2023 and is expected to reach USD 5.1 billion by 2032 and grow at a CAGR of 7.4% over the forecast period of 2024-2032.
The Chromatography Resin Market is experiencing significant growth due to its critical role in separation and purification processes across various industries, including pharmaceuticals, biotechnology, food & beverage, and environmental testing. The increasing adoption of monoclonal antibodies, vaccines, and biosimilars has fueled the demand for chromatography resins in biopharmaceutical manufacturing. Additionally, advancements in protein purification techniques and drug discovery research are further driving market expansion.
Key Players in the Chromatography Resin Market
Merck KGaA (Sepharose, Eshmuno)
Bio-Rad Laboratories, Inc. (Nuvia, Bio-Scale)
WIPRO GE HEALTHCARE PVT LTD (MabSelect, MabSelect SuRe)
Purolite (Chromalite, ProSep)
GRACE (Matrix, Discovery)
Mitsubishi Chemical Holdings Corporation (Kromasil, HICresin)
Danaher (Cytiva, ÄKTA)
Tosoh Corporation (Tosoh Biosep, TSKgel)
GE Healthcare (MabSelect, Sepharose)
Avantor Performance Materials Inc. (VWR, J.T. Baker)
Future Scope of the Market
The Chromatography Resin Market is expected to grow due to:
Increasing demand for biopharmaceuticals, including monoclonal antibodies and gene therapy products.
Rising investment in drug discovery, proteomics, and genomics research.
Expanding applications in food safety testing and environmental monitoring.
Technological advancements in resin manufacturing for enhanced separation efficiency.
Growth in personalized medicine and biotechnology-driven therapies.
Emerging Trends in the Chromatography Resin Market
The chromatography resin industry is undergoing rapid advancements driven by the need for high-purity separations and cost-effective purification solutions. The shift toward single-use chromatography systems is gaining traction in the biopharmaceutical sector due to its ability to enhance productivity and reduce contamination risks. Additionally, affinity chromatography resins are witnessing increased demand for their role in high-selectivity protein purification. The integration of nanotechnology and hybrid chromatography resins is further enhancing the efficiency of separation techniques. Sustainability efforts are also influencing the market, with manufacturers focusing on biodegradable and recyclable resins to reduce environmental impact.
Key Points:
Rising demand for chromatography resins in biopharmaceutical and life sciences research.
Increasing adoption of monoclonal antibody purification techniques.
Growth in single-use chromatography technologies for efficient drug manufacturing.
Advancements in hybrid and affinity chromatography resins for high-selectivity separation.
Sustainability initiatives promoting the development of eco-friendly resins.
Conclusion
The Chromatography Resin Market is set for substantial expansion, driven by biopharmaceutical innovations, technological advancements, and growing research activities. With increasing investments in drug discovery, precision medicine, and protein purification technologies, the demand for high-performance chromatography resins is expected to rise. As the industry moves toward sustainable and efficient purification solutions, manufacturers are focusing on innovation and product development to meet the evolving needs of pharmaceutical, food safety, and environment.
Read Full Report: https://www.snsinsider.com/reports/chromatography-resin-market-1693
Contact Us:
Jagney Dave — Vice President of Client Engagement
Phone: +1–315 636 4242 (US) | +44- 20 3290 5010 (UK)
#Chromatography Resin Market#Chromatography Resin Market Size#Chromatography Resin Market Share#Chromatography Resin Market Report#Chromatography Resin Market Forecast
0 notes
Text
Top 5 players in US Biosimilar Market
Buy Now
STORY OUTLINE
Pfizer: Excelling in the line of Biosimilar drugs with an experience of more than 10 years with presence in over 180 countries.
Amgen: Making pharmaceutical products with an experience of over 40 years and presence in over 100 countries.
Viartis: Presence in over 165 countries, and making Biosimilar drugs in over 75 markets, this pharmaceutical company is another leading contributor of US Biosimilar market.
Coherus Biosciences: Increasing patient access to cost effective medicines with a Biosimilar drugs experience of 13 years.
Biogen: serving humanity through science with a experiences of more than 40 years in the field of biologics.
According to Ken Research, the US Biosimilar market is anticipated to grow at a CAGR of ~40% in the next five years which currently has a market size of ~USD 9.4 Bn.
The US Biosimilar market is rapidly growing and will be witnessing a significant growth in the next five years.
There are various reasons behind the rapid growth of US Biosimilar market. Some of the major reasons behind the growth of US Biosimilar market include the cost effective nature of Biosimilar drugs, rising geriatric population, rising prevalence of chronic diseases, and growing partnerships between companies to develop Biosimilar drugs.
Various companies and players are contributing to their best efforts in the growth of the US Biosimilar market.
This article aims to put light on the contributions done by the major players towards the growth of the US Biosimilar market.
1.Pfizer
Click to read more about Pfizer
Pfizer is a leading American pharmaceutical company which is operating in the field of generics or original drugs for more than 30 years. But did you know that this pharma not only manufactures biologics but also biosimilar drugs?
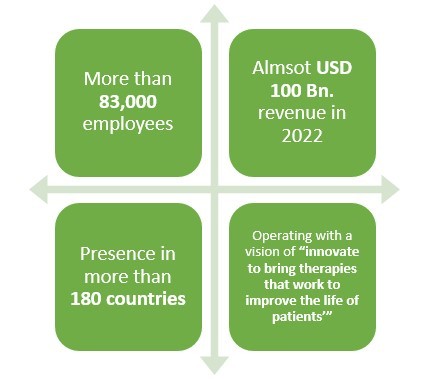
Pfizer has been in the business of biosimilar drugs for more than 10 years and have been quite successful as well. With more than 83,000 employees and presence in over 180 countries, this leading pharmaceutical company made almost USD 2 Bn. revenue only from its Biosimilar drugs sale in 2021.
Recently, this pharmaceutical company also collaborated with Samsung in two deals to produce various biosimilar drugs in South Korea. The deal size between these two companies happens to be approximately USD 900 Bn.
The major Biosimilar drugs of this pharmaceutical giant are primarily
ZIRABEV (a Biosimilar of Avastin)
TRAZIMERA (a Biosimilar of Herceptin)
RUXIENCE (a Biosimilar of Rituxan)
RITACRIT (a Biosimilar of Epogen)
NVYEPRIA (a Biosimilar of Neulasta)
NIVESTYM (a Biosimilar of Neupogen)
FILGRASTIM (a Biosimilar of Neupogen).
2.Amgen
Click here to Download a Sample Report
Amgen is another leading American pharmaceutical company which not only makes Biologics or generic drugs but also Biosimilar drugs. This pharmaceutical company has more than 40 years of experience when it comes to pharmaceutical line.
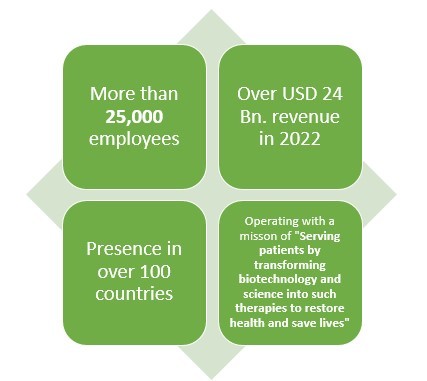
With over 25000 employees and presence in over 100 countries, this pharmaceutical company earned about USD 2 Bn. from their three biosimilar drugs which are reportedly MVASI, KANJITNTI, and AMJEVITA.
This pharma giant has also invested about USD 2 Bn. in the development of Biosimilar drugs.
This pharmaceutical company has made Biosimilar drugs primarily in 4 fields which are General Medicine, Oncology, and Hematology along with, Inflammation.
EPOTEIN ALFA
AMJEVITA
AVSOLA
KANJINTI
MVASI
RIABNI
are the various Biosimilar drugs of Amgen. And, STELARA, EYLEA, SOLIRIS are in their pipeline.
Recently Amgen revealed their Biosimilar report’s 8 version. It revealed a major information which said that the pharmaceutical company saved about USD 10 Bn. through their Biosimilar drugs in the past five years.
3.Viartis

Headquartered in Canonsburg, Pennsylvania, this American pharmaceutical company was founded only in 2020 yet they have achieved massive success in the pharmaceutical products with their revenue being USD 16 ~Bn. in 2022.
With presence in 165 countries and with over 45,000 employees worldwide, this pharmaceutical company makes pharmaceutical products in 10 areas which primarily are Cardiovascular, Dermatology, ophthalmology, Oncology, Gastroenterology, Women’s health, Infectious diseases, Diabetes & Metabolism, Immunology, CNS & Anesthesiology, Respiratory diseases and allergy.
Speaking of their first Biosimilar products, their first ever Biosimilar drug was launched in 2014. They have a variety of Biosimilar drugs which are primarily
TRASTUZUMAB
INSULIN ASPART
PEGFILGRASTIM
INSULIN GLARGINE-YFGN
ADALIMUMAB
BEVACIZUMAB
Their Biosimilar drug Insulin Glargine which is known as SEMGLEE was the first ever interchangeable Biosimilar drug in the United States which was FDA approved.
Their PEGFILGRASTIM also was the first ever FDA approved drug in the United States. They have launched their Biosimilar drugs in over 75 markets worldwide.
4.Coherus Biosciences:
Click here to Ask for a Custom Report
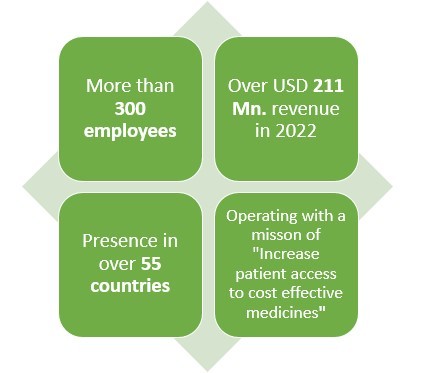
Headquartered in Redwood city, California this American pharmaceutical company earned a revenue of almost USD 211 Mn. In 2022.
With presence in over 55 countries and 300+ employees worldwide, this pharmaceutical company makes products in various areas such as solid tumors, non-small lung cancers, nasopharyngeal carcinoma, small cell lung cancer and hepatocellular carcinoma.
Speaking of their Biosimilar drugs, this pharma has been in the field of creating Biosimilar drugs since 2010 which has given them almost 13 years of experience.
This pharmaceutical company also disclosed that it plans to spend at least USD 1 Tn. on medicines worldwide, out of which at least 40% will be spent on Biosimilar drugs.
Their three major Biosimilar drugs which are also FDA approved include UDENCYA, YUSIMRY, and CIMERLI.
Udencya is a Biosimilar drug of Pegfilgrastim, Yusimry is a Biosimilar drug of Ranibizumab, and Cimerli is a Biosimilar drug of Adalimumab.
5.Biogen
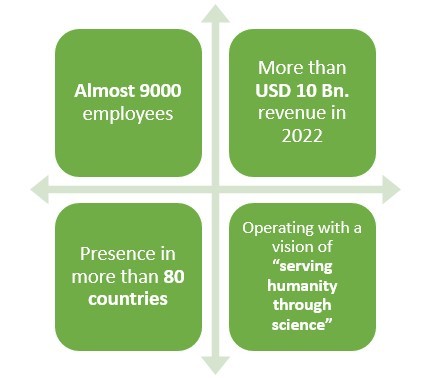
Headquartered in Cambridge, Massachusetts, this American pharmaceutical company earned a revenue of around USD 10 Bn. in 2022.
This company happens to have an experience of more than 40 years when it comes to making pharmaceutical products.
With presence in over 80 countries and more than 9000 employees worldwide, this pharmaceutical company primarily deals in Neurology, Specialized Immunology, Neuropsychiatry, Ophthalmology, and Rare Diseases.
ADUCANUMAB
LECANEMAB
TOFERSEN
ZURANOLONE
LITIFILIMAB
BENAPALI
FLIXABI
IMRALDI
are some of their Biosimilar drugs.
With their Biosimilar drugs, more than 250,000 people have gone on Anti-Tumor Necrosis Factor therapy.
Recently, this pharmaceutical company also made an agreement with Bio-Thera solutions to develop a Biosimilar drug for the treatment of Rheumatoid Arthritis.
#US Biosimilar Sector#United States Biosimilar Market#US Biosimilar Market forecast#US Biosimilar Market analysis#US Biosimilar Market trends#US Biosimilar Market share#US Biosimilar Market key players#US Biosimilar Market revenue#US Biosimilar Market growth#Monoclonal Antibodies in biosimilar market US#Recombinant Hormones in biosimilar industry US#Oncology in bio similar market US#Blood disorders in biosimilar market US#Research institutes in Biosimilar market US#US similar biotherapeutics products market#Hospitals in Biosimilar market US#Investors in Biosimilar market US#US comparable biologics products industry#US recombinant biosimilars industry#US replicate biologics sector#US analog biologics market
0 notes
Text
Biopharmaceutical Market: Global Trends and Forecast 2024-2032

The Biopharmaceutical Market size was valued at USD 370.13 Billion in 2023 and is expected to reach USD 871.92 Billion by 2031, growing at a CAGR of 11.3% over the forecast period 2024–2031. This rapid expansion is fueled by continuous innovations in biotechnology, the increasing prevalence of chronic conditions, and a growing demand for targeted therapies and personalized medicine.
Market Overview
Biopharmaceuticals, derived from biological sources, are revolutionizing the healthcare landscape by offering high specificity and fewer side effects compared to traditional drugs. These therapies, which include monoclonal antibodies, recombinant proteins, and gene therapies, are increasingly being adopted to treat cancer, autoimmune disorders, and infectious diseases.
The market is also benefiting from significant investments in research and development, alongside favorable regulatory policies that aim to accelerate the approval of novel therapies. Strategic partnerships between biotech firms and pharmaceutical giants are further accelerating innovation and global reach.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/4184
Regional Analysis
North America leads the global market due to its advanced healthcare infrastructure, strong presence of key market players, and high R&D expenditure.
Europe follows closely, supported by increasing regulatory support and rising geriatric population.
Asia-Pacific is emerging as the fastest-growing region, driven by government initiatives to boost biotech industries, rising healthcare awareness, and the expansion of clinical trial activities.
Latin America and the Middle East & Africa are gradually catching up due to improving healthcare access and increasing investments in biopharma infrastructure.
Market Segmentation
By Product Type:
Monoclonal Antibodies
Recombinant Proteins
Vaccines
Hormones
Gene Therapies
Others
By Application:
Oncology
Infectious Diseases
Autoimmune Disorders
Metabolic Disorders
Others
By End User:
Hospitals
Research Institutes
Biopharmaceutical Companies
Others
Key Biopharmaceutical Companies:
The Players operating in the biopharmaceutical market are:
1. Pfizer
2. F. Hoffmann-La Roche Ltd.
3. Johnson & Johnson
4. Novartis
5. Merck & Co.
6. AbbVie
7. Amgen
8. Biogen
9. Eli Lilly and Company
10. AstraZeneca
11. Bayer
12. Sanofi
13. Bristol Myers Squibb
14. Takeda Pharmaceutical Company Limited
15. Gilead Sciences
16. Vertex Pharmaceuticals
17. GlaxoSmithKline
18. AbbVie
19. Moderna
20. Regeneron Pharmaceuticals
21. Novo Nordisk
Key Highlights
Surge in demand for personalized medicine is a major growth driver.
Rapid progress in gene therapy and RNA-based treatments.
Growing use of AI and machine learning in drug development.
Expansion of biosimilar approvals is boosting market competition.
Strong pipeline of biologic drugs across various therapeutic areas.
Future Outlook
The future of the biopharmaceutical market looks highly promising. With advances in genomics, proteomics, and regenerative medicine, the industry is set to introduce more sophisticated therapies tailored to individual patient profiles. Furthermore, the integration of digital health technologies and real-time data analytics will significantly streamline drug discovery and development. Emerging markets are expected to play a key role in expanding global access, while sustainability and cost-efficiency in production will be critical focus areas.
Conclusion
The biopharmaceutical sector is undergoing a transformative phase, backed by scientific breakthroughs, growing healthcare demands, and favorable policy support. As the market continues to evolve, companies that prioritize innovation, patient-centric solutions, and strategic collaborations are poised to lead in this highly competitive landscape.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Biopharmaceutical Market#Biopharmaceutical Market Share#Biopharmaceutical Market Size#Biopharmaceutical Market Trends#Biopharmaceutical Market Growth
0 notes
Text
Oncology Market
The oncology market is expanding rapidly due to rising cancer prevalence, advancements in diagnostics, and innovative treatment options. Key drivers include targeted therapies, immunotherapy, personalized medicine, and AI-driven drug discovery. Pharmaceutical companies are investing heavily in research, with a focus on monoclonal antibodies, CAR-T cell therapy, and precision oncology. Governments and healthcare organizations are increasing funding for cancer research and early detection programs. The market is also shaped by regulatory approvals, biosimilars, and collaborations between biotech firms. Growth is strongest in developed regions, but emerging markets are expanding due to improved healthcare infrastructure and access to novel treatments.
For More : https://tinyurl.com/3d2scwe8

0 notes
Text
Biopharmaceuticals
1. Introduction to Biopharmaceuticals
Biopharmaceuticals, also known as biologics, are therapeutic drugs derived from living organisms such as bacteria, yeast, mammalian cells, or even transgenic animals and plants. Unlike traditional small-molecule drugs synthesized chemically, biopharmaceuticals involve complex biological production processes and offer targeted, highly effective treatments for various diseases.
2. Types of Biopharmaceuticals
Biopharmaceuticals encompass a broad range of biologically derived therapies, including:
Monoclonal Antibodies (mAbs) – Used in cancer immunotherapy, autoimmune diseases, and infectious diseases (e.g., Pembrolizumab, Trastuzumab).
Recombinant Proteins – Engineered proteins like human insulin, growth hormones, and clotting factors (e.g., Humulin, Erythropoietin).
Gene Therapy Products – Altering genetic material to treat inherited disorders and genetic diseases (e.g., Luxturna, Zolgensma).
Cell-Based Therapies – Including stem cell and CAR-T cell therapies (e.g., Kymriah, Yescarta).
Vaccines – Recombinant, viral vector, and mRNA vaccines (e.g., COVID-19 mRNA vaccines like Pfizer-BioNTech and Moderna).
Biosimilars – Biologic drugs designed to be highly similar to approved biologics, offering cost-effective alternatives.
3. Production & Manufacturing of Biopharmaceuticals
Biopharmaceutical production involves biotechnological processes such as: 🔹 Cell Culture & Fermentation – Using bacterial, yeast, or mammalian cells to produce biologic drugs. 🔹 Genetic Engineering – DNA recombination techniques to optimize drug production. 🔹 Purification & Downstream Processing – Ensuring high purity and potency of biologics. 🔹 Formulation & Delivery Systems – Development of injectable, oral, or nanoparticle-based drug delivery systems.
4. Applications of Biopharmaceuticals
✅ Oncology – Targeted cancer therapies using mAbs and CAR-T cells. ✅ Autoimmune Diseases – Biologics for rheumatoid arthritis, Crohn’s disease, and psoriasis. ✅ Infectious Diseases – Antibody-based therapies and novel vaccines. ✅ Neurological Disorders – Biologic treatments for Alzheimer’s, multiple sclerosis, and Parkinson’s. ✅ Regenerative Medicine – Stem cell-based therapies for tissue repair and organ regeneration.
5. Advantages of Biopharmaceuticals
✔️ Highly Specific & Targeted Therapy – Reduced side effects compared to conventional drugs. ✔️ Effective for Complex Diseases – Essential for treating conditions lacking small-molecule drug solutions. ✔️ Personalized Medicine Integration – Advances in genomics and precision medicine enhance effectiveness. ✔️ Long-Term Disease Management – Biologics improve quality of life in chronic diseases.
6. Challenges & Limitations
⚠️ High Production Costs – Biopharmaceuticals require sophisticated technology and facilities. ⚠️ Cold Chain Logistics – Many biologics need strict temperature-controlled storage. ⚠️ Regulatory Hurdles – Stringent FDA and EMA approval processes. ⚠️ Biosimilar Competition – Cost challenges due to patent expiration and biosimilar market entry.
7. Future Trends in Biopharmaceuticals
🚀 mRNA & RNA-Based Therapeutics – Expanding beyond COVID-19 to cancer, rare diseases, and gene editing. 🚀 Artificial Intelligence in Drug Discovery – AI-driven approaches for biologic design and optimization. 🚀 Synthetic Biology & Bioprocess Optimization – Enhancing biologic production efficiency. 🚀 3D Bioprinting & Regenerative Biologics – Innovating tissue engineering and personalized medicine.
Biotechnology Scientist Awards
Visit Our Website : http://biotechnologyscientist.com
Contact Us : [email protected]
Nomination Link : https://biotechnologyscientist.com/member-submission/?ecategory=Membership&rcategory=Member…
#sciencefather#researchawards#Scientist#Scholar#Researcher #Biopharmaceuticals #Biologics #MonoclonalAntibodies #GeneTherapy #CellTherapy #mRNATherapeutics #CancerImmunotherapy #Biosimilars #BiotechInnovation #RecombinantProteins #PersonalizedMedicine #PharmaceuticalBiotechnology #StemCellTherapy #CRISPRGeneEditing #RegenerativeMedicine #SyntheticBiology #Nanomedicine #BiologicDrugDevelopment #AntibodyTherapeutics #mRNAVaccines #TissueEngineering #Immunotherapy #Bioprocessing #PrecisionMedicine #AIinDrugDiscovery
👉Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Facebook : https://www.facebook.com/profile.php?id=61572562140976
Twitter : https://x.com/DiyaLyra34020
Tumblr : https://www.tumblr.com/blog/biotechscientist
Blogger: https://www.blogger.com/u/1/blog/posts/3420909576767698629
Linked in : https://www.linkedin.com/in/biotechnology-scientist-117866349/
Pinterest : https://in.pinterest.com/biotechnologyscientist/
0 notes
Text
Global Biopharmaceuticals Market: The Role of Emerging Technologies in 12% CAGR Growth by 2030
The global biopharmaceuticals market is set to witness a growth rate of 12% in the next 5 years. Rising prevalence of chronic diseases; advancements in biotechnology; growing geriatric population; expansion of biosimilars; and rising R&D investments are some of the key factors driving the biopharmaceuticals market.
Biopharmaceuticals are therapeutic products derived from biological sources, such as living cells or organisms, using advanced biotechnological processes. Unlike traditional chemical-based drugs, biopharmaceuticals are typically large, complex molecules, including proteins, nucleic acids, or living cells. Common examples include monoclonal antibodies, recombinant proteins, vaccines, and gene or cell therapies. These medicines are designed to target specific biological pathways, offering precision in treating diseases like cancer, autoimmune disorders, and genetic conditions. Biopharmaceuticals represent a cutting-edge approach to healthcare, providing innovative treatment options with improved efficacy and reduced side effects, often addressing previously unmet medical needs across a wide range of therapeutic areas. The biopharmaceutical industry remains optimistic about 2025 but less so than six months ago due to concerns over the US BIOSECURE Act, biotech funding recovery, and political changes. Geopolitical tensions and ongoing conflicts further add to industry uncertainty.
To request a free sample copy of this report, please visit below
Rising prevalence of chronic diseases to propel market demand
The rising prevalence of chronic diseases, such as cancer, diabetes, cardiovascular disorders, and autoimmune conditions, is a major driver of the biopharmaceuticals market. These diseases often require innovative and targeted treatments that biopharmaceuticals, including monoclonal antibodies, recombinant proteins, and gene therapies, can effectively address. Unlike traditional therapies, biopharmaceuticals provide improved specificity, efficacy, and reduced side effects. Additionally, the growing global burden of chronic conditions, fueled by aging populations and lifestyle factors, underscores the need for advanced therapeutic solutions. This demand incentivizes biopharmaceutical innovation, leading to increased research, development, and adoption, thereby fueling market growth.
Key trends and emerging technologies in the biopharmaceutical industry
Key trends shaping the biopharmaceutical industry include immuno-oncology (IO) drug development, anti-obesity medications, cell and gene therapies (CGTs), personalized medicine, real-world evidence (RWE), and rising clinical trial costs. IO drug development is the leading trend, with CGTs and precision medicine continuing to transform healthcare. AI, big data, and data integration are the most disruptive technologies, optimizing processes from drug discovery to patient outcomes. AI's ability to analyze vast datasets enhances efficiency, reduces R&D costs, and improves precision medicine. As AI advances, its integration with big data will further drive innovation in value-based healthcare.
Competitive Market Analysis
The global biopharmaceuticals market is marked by the presence of established and emerging market players such as AbbVie Inc., Amgen Inc., AstraZeneca, Eli Lilly and Co., Johnson & Johnson, Merck & Co., Novartis, Novo Nordisk, Pfizer Inc., and Roche Holding AG; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
🔗 Want deeper insights? Download the sample report here:
Market Segmentation
This report by Medi-Tech Insights provides the size of the global biopharmaceuticals market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product, type, therapy area, and distribution channel.
Market Size & Forecast (2023-2030), By Product, USD Million
Monoclonal Antibodies (mAbs)
Recombinant Proteins
Vaccines
Gene Therapies and Cell Therapies
Others
Market Size & Forecast (2023-2030), By Type, USD Million
Innovative
Biosimilars
Market Size & Forecast (2023-2030), By Therapy Area, USD Million
Autoimmune Diseases
Infectious Diseases
Cardiovascular Diseases
Neurological Disorders
Rare/Orphan Diseases
Others
Market Size & Forecast (2023-2030), By Distribution Channel, USD Million
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes