#Anodic Oxidation
Explore tagged Tumblr posts
Text
Comprehensive surface treatment process of materials
Surface treatment is a process that artificially forms a layer on the surface of a base material that has different mechanical, physical and chemical properties from base material.Purpose of surface treatment is to meet corrosion resistance, wear resistance, decoration or other special functional requirements of product. Our more commonly used surface treatment methods are mechanical grinding,…

View On WordPress
#Anodic Oxidation#electrolytic polishing#Electroplating#electroplating surface treatment#Electropolishing#Galvanizing#Hydro Transfer Printing#In-Mold Decoration#Metal Wired#metallurgical bonding#metallurgical bonding technology#Pad printing#Screen printing#Surface treatment#surface treatment process#Surface treatment technology#vacuum electroplating#vacuum electroplating process#Vacuum Metalizing#Vacuum plating
0 notes
Text
Figure 14.3 shows a schematic representation of an electrolysis cell for aluminium production.
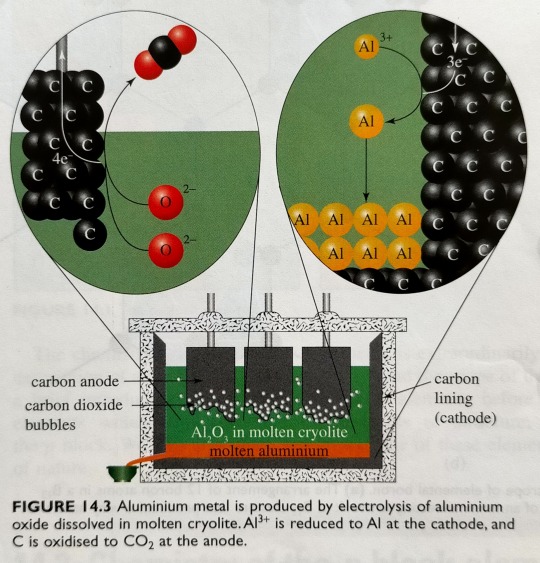
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#schematic#aluminum#electrolysis#carbon#carbon dioxide#cathode#anode#cryolite#oxidation
27 notes
·
View notes
Text
Anodic Aluminum Oxide Wafer Market Soars: Driven by Renewable Energy Sector and Expansion of the Biotechnology & Medical Devices
According to their "Anodic Aluminum Oxide Wafer Market" report, the global market was valued at USD 74.1 Million in 2023, with an expected compounded annual growth rate of 18.2% during the forecast period from 2024 - 2032 to reach USD XX Million by 2032.
AAO Wafer has a unique, nanoscale material architecture exploited in a variety of industries and scientific fields toward the development of material science and nanotechnology. These are finding increased demand due to the high surface area and tunability of pore sizes of AAO, from electronics and energy storage to biomedicine. It is mainly due to growing research and development activities for better options because of competition, the growing consumer electronics industry globally, and a surge in support provided by financial institutions for advancements in nanotechnology, resulting in high demand for miniature devices.

As a result, huge investments have been made in the market for improving production capacity and technology. Much prominence has been given to cost reduction and high-precision-manufacturing processes. Development in recent times has been related to the usage of AAO wafers in energy-efficient electronics and advanced sensor technologies. The expanding spectrum of application areas for AAO wafers has pushed businesses to explore their integration into new domains like healthcare and environmental monitoring, further extending the market's reach. AAO wafer market dominated by technologically advanced countries such as the US, China, and Japan, will further increase due to continuous R&D of advanced chipsets with high-quality transistors. Nanopore technology will play both a bad and a good role since it too will drive growth and sales. Still on the high would be renewable energy use, environmental technologies, and smart devices, with policies for the encouragement of innovation and sustainability such as investment by the US Department of Energy in nanotechnology for renewable clean energy solutions.
Growth in Renewable Energy Sector
This has been occasioned by the fact that the world is shifting towards the use of renewable sources of energy and therefore the need for materials that would improve efficiency of the energy conversion and storage systems. AAO wafers are also finding their applications in the renewable energy industry where there is high demand for surfaces with large surface area and controlled pore size for applications in solar cell and energy storage systems. In the case of solar cells, AAO wafers help as substrates for producing assorted nanostructures, which facilitate light trapping and enhance energy conversion efficacy hence boosting power conversion of photovoltaic systems. In the same way, the use of AAO as the anode or electrode for batteries and supercapacitors the porous structure wafers helps transfer ions more freely and store more energy density and enables a faster charging process. For instance: Renewable energy sector wafers in AAO include their use in fabrication of dye-sensitized solar cells (DSSCs). Scholars have employed the AAO wafers as molds to fabricate P25-TiO2 nanostructured photoanodes that enhance the light absorption capacity of DSSCs. This innovation has the potential of enhancing economies of scale in solar energy technologies hence encouraging the adoption of renewable energy.
Increased Research and Development Activities
The growing interest in material science and nano fabrication is considered as a major reason for growth of R&D activities associated with Anodic Aluminium Oxide (AAO) wafers. Hence, with the flexibility of employing AAO as a template material and the capability of forming organizable nanostructures, AAO becomes the research focus. This has made it possible to discover new uses of AAO wafers as well as improving the features of existing technologies hence increasing the utilization of AAO wafers. The research and development efforts are focused on the application of AAO wafers in optoelectronics, electronics, and biotechnology; to produce newer generation devices with improved performance and characteristics.
For example, Research & Development is encouraging the proposed application of AAO wafers in fabrication of superior performance nanostructured sensors. The possibility of applying templates synthesized in AAO structures for creating nanosensors with high sensitivity and selectivity to the environment, medical diagnostics, and technical process control have stimulated the interest of the research institutions and companies. These innovations are not only paving way for new use of AAO wafers but are also integrating technology into the society in other fields.
Request Free Sample Pages with Graphs and Figures Here https://univdatos.com/get-a-free-sample-form-php/?product_id=65174
Expansion of the Biotechnology and Medical Devices Industry
The biotechnology and the medical devices industry has been growing at a very fast pace and there is need to come up with new materials to support the development of better healthcare products. The most widely used substrates in this industry include Anodic Aluminum Oxide (AAO) wafers, which have biocompatible properties, high chemical stability, as well as well-defined nanostructures that are desirable for biosensor, drug delivery and tissue engineering applications. The reporters in biosensors benefit from AAO wafers since they allow distinction of biological molecules such as proteins by their uniform pore structure, while the drug delivery systems benefit from AAO wafers since they allow for the controlled release of therapeutic agents. Another factor that is contributing to increase in demand for AAO wafers in the medical industry is called up personalized medicine and diagnostics. For instance: Some of the uses of AAO wafers include in the industry of biotechnology as well as the medical devices industry, mainly in implantation of delivery systems of medicines. Such systems utilize the nature of porous AAO wafers to control the rate and duration of drug delivery to the disease site within the body, thus increasing therapeutic outcomes and at the same time minimizing side effects. As demonstrated in this application, AAO wafers may greatly transform the course of medical treatments and benefit the patients.
Conclusion
Overall, current trends point to the AAO Wafer's wide usage in a couple of high-impact industries like the renewable energy industry, increased research and development pursuits, and the biotechnology and medial devices industries. Their unique properties, including high surface area, controlled porosity, and biocompatibility, ensure progress in a variety of solar energy fields, nanostructured sensors, and novel treatments in health care. With AAO wafers playing a central role in technological development-from developing high-performance dye-sensitized solar cells and nanosensors to the fabrication of implantable drug delivery systems-continuously changing the face of such dynamic industries will only increase their importance and applicability, making AAO wafers a staple material in the progress and innovation of advanced technologies. The growth in the renewable energy sector and the expansion of the biotechnology & medical devices industry would drive the global scenario of the Anodic Aluminum Oxide Wafer market, according to analysis by Universal Data Solutions.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411Website -www.univdatos.com
0 notes
Text
Lithium-ion Battery Materials: Powering the Future of Energy Storage
Outline
Introduction to Lithium-ion Battery Materials
Components of Lithium-ion Batteries
Anode Materials
Cathode Materials
Electrolyte Materials
Importance of Battery Materials in Performance
Popular Lithium-ion Battery Materials
Graphite (Anode)
Lithium Cobalt Oxide (Cathode)
Lithium Iron Phosphate (Cathode)
Lithium Nickel Manganese Cobalt Oxide (Cathode)
Electrolyte Solutions
Advancements in Battery Materials Research
Environmental and Safety Considerations
Future Trends in Lithium-ion Battery Materials
Conclusion
FAQs
In the realm of modern energy storage, lithium-ion batteries have emerged as the frontrunner, powering everything from smartphones to electric vehicles. At the heart of these batteries lies a complex interplay of materials meticulously engineered to deliver optimal performance, efficiency, and safety. In this article, we delve into the world of lithium-ion battery materials, exploring their composition, significance, and future prospects.
Components of Lithium-ion Batteries
Anode Materials
The anode of a lithium-ion battery typically consists of graphite, which serves as a host material for lithium ions during charging and discharging cycles. Graphite's layered structure allows for the reversible intercalation and deintercalation of lithium ions, facilitating the battery's operation.
Cathode Materials
On the other side of the battery, the cathode houses materials like lithium cobalt oxide, lithium iron phosphate, or lithium nickel manganese cobalt oxide. These compounds play a crucial role in determining the battery's voltage, energy density, and cycle life.
Electrolyte Materials
The electrolyte, often a liquid or polymer solution containing lithium salts, enables the movement of lithium ions between the anode and cathode while preventing the direct contact of the two electrodes, thus ensuring the battery's stability and safety.
Importance of Battery Materials in Performance
The selection and optimization of battery materials significantly impact the overall performance and longevity of lithium-ion batteries. Factors such as energy density, charging rate, and cycle life are heavily influenced by the choice of anode, cathode, and electrolyte materials.
Popular Lithium-ion Battery Materials
Graphite (Anode)
Graphite remains the most common anode material due to its stability, conductivity, and low cost. Ongoing research aims to enhance graphite's performance through the development of advanced carbon structures and composites.
Lithium Cobalt Oxide (Cathode)
Despite safety concerns associated with cobalt, lithium cobalt oxide continues to be widely used in high-energy-density applications such as consumer electronics. Efforts are underway to reduce cobalt content or explore alternative cathode materials to address supply chain issues and environmental concerns.
Lithium Iron Phosphate (Cathode)
Lithium iron phosphate offers improved safety and thermal stability compared to lithium cobalt oxide, making it suitable for applications where safety is paramount, such as electric vehicles and grid storage systems.
Lithium Nickel Manganese Cobalt Oxide (Cathode)
This ternary cathode material combines the advantages of nickel, manganese, and cobalt to achieve a balance between energy density, power capability, and cost-effectiveness. It is commonly used in electric vehicles and stationary storage applications.
Electrolyte Solutions
Research into novel electrolyte formulations aims to enhance battery performance and safety by improving ion conductivity, stability, and compatibility with high-voltage cathode materials.
Advancements in Battery Materials Research
The field of battery materials research is dynamic and rapidly evolving, driven by the demand for higher energy density, faster charging, and longer-lasting batteries. Advanced characterization techniques, computational modeling, and material synthesis methods are enabling scientists to design next-generation battery materials with unprecedented performance attributes.
Environmental and Safety Considerations
While lithium-ion batteries offer numerous advantages, concerns persist regarding the environmental impact of raw material extraction, battery manufacturing processes, and end-of-life disposal. Efforts to develop sustainable and recyclable battery chemistries are underway to mitigate these challenges and promote the adoption of clean energy technologies.
Future Trends in Lithium-ion Battery Materials
Looking ahead, the future of lithium-ion battery materials is characterized by ongoing innovation and collaboration across academia, industry, and government sectors. Key areas of focus include the development of solid-state electrolytes, silicon-based anodes, and alternative cathode chemistries to further improve battery performance, safety, and sustainability.
Conclusion
In conclusion, lithium-ion battery materials play a pivotal role in shaping the performance, efficiency, and sustainability of modern energy storage systems. Continued research and development efforts aimed at advancing battery materials science are essential for realizing the full potential of lithium-ion technology and accelerating the transition to a clean energy future.
FAQs
Are lithium-ion batteries the best option for energy storage?
While lithium-ion batteries currently dominate the market, other technologies such as solid-state batteries and flow batteries are being actively researched as potential alternatives.
What are the main challenges facing lithium-ion battery materials research?
Key challenges include improving energy density, reducing costs, enhancing safety, and addressing environmental concerns associated with raw material sourcing and battery disposal.
How long do lithium-ion batteries typically last?
The lifespan of lithium-ion batteries varies depending on factors such as usage patterns, operating conditions, and battery chemistry. Generally, they can last several years with proper care and maintenance.
Are there any risks associated with lithium-ion batteries?
While lithium-ion batteries are generally safe when used as intended, there is a risk of thermal runaway and fire in cases of overcharging, physical damage, or manufacturing defects.
What role do government policies play in the advancement of battery materials research?
Government policies and incentives can significantly influence research funding, technology adoption, and market dynamics, thus playing a crucial role in shaping the trajectory of battery materials innovation.
#Lithiumion batteries#Battery materials#Anode materials#Cathode materials#Electrolyte solutions#Graphite#Lithium cobalt oxide#Lithium iron phosphate#Lithium nickel manganese cobalt oxide#Energy storage#Battery technology#Sustainable energy#Environmental impact#Battery research#Clean energy#Solidstate electrolytes#Siliconbased anodes#Energy density#Battery safety#Battery recycling.
0 notes
Text
The anode, which is also made of graphite, is oxidised during electrolysis.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Text
Elcoats is in the field of Anodising, Hard Anodising, Colour Anodizing, Chromatising and ElectroLess Nickel(ENP) for
#Anodising#Hard anodising#Colour anodising#Clear Chromatisation#Yellow chromatisation#Chromate conversion#Mil standard 5541F#Type 2 class 1#Clear Anodising#Harcoat Anodizing#Mil 8625 standards#Aluminium oxide blasting#Bead blasting
1 note
·
View note
Text

Team synthesizes a cost-effective, high-durability, non-noble metal alloy as alternative to iridium oxide anodes
Despite hydrogen's increasing prominence as a future energy source, the safe and large-scale transportation of gaseous hydrogen remains challenging. Researchers are exploring ways to transport hydrogen in the form of liquid methylcyclohexane (MCH) by reacting it with toluene. One such approach is organic hydride electrolytic synthesis. In this process, toluene is transferred from a cathode to an iridium oxide anode under highly acidic conditions. However, toluene oxidizes on the anode surface, forming a polymer coating that remarkably degrades the electrode's performance. This situation highlights the need for more durable and cost-effective anode materials. In response, researchers at the University of Tsukuba have developed a high-entropy alloy anode composed of nine non-precious metal elements using a conventional arc melting method. They also elucidated the mechanism underlying the catalytic poisoning by toluene, which considerably influences the anode durability in organic hydride electrolytic synthesis.
Read more.
#Materials Science#Science#Hydrogen#Liquids#Organic materials#Iridium#Oxides#Polymers#University of Tsukuba#High entropy materials#High entropy alloys#Catalysts
10 notes
·
View notes
Note
WiW:
What if you were the guest lecturer at a prestigious university. What would you lecture on?

"Blacksmithing, probably. More specifically how to change the color of your creation without using paint by means of heat oxidation, anodizing, chemical patinas, or quenching in oils and salts. With also touching on material type and durability in the results. It's a bit specific, but I think it's fascinating."
ty @cythion
7 notes
·
View notes
Text
Okay, so basically you have an anode and a cathode (your two ends of a battery).
They both contain an electrolyte and an electrode. The cathode has an electrode of a material that tends to be reduced, which means it wants to take in electrons. The anode has a material that tends to be oxidized, which means it wants to release electrons. Without a circuit these materials are not charged in the traditional sense (you don’t have a deficit of electrons or an excess, which would be positive or negative electric charges), but they are sort of ‘charged’ in the sense that one side wants to lose electrons and the other side wants to receive them. However, as you said, without a circuit, both reactions will only happen a little bit, since the electrolyte will quickly become saturated with ions.
When you plug in the circuit however, you are providing a path for the electrons to be passed from one side to the other, making it more favorable for the reactions to occur. When the anode is oxidized, it releases electrons into solution, giving the electrolyte a negative charge. The opposite happens at the cathode, which takes electrons from solution, leaving the electrolyte with a positive charge. The electrons will then pass from the electrolyte at the anode through the wire and into the electrolyte at the cathode.
However if both ends are completely isolated aside from the wire, then they will pretty quickly reach a new equilibrium. There won’t be enough electrons at the anode (and too many positively charged ions) and there will be too many at the cathode to support continued reactions. To fix this issue there’s actually a slight connection between the electrolyte at both ends, which allows a small ion current to pass positive ions that build up at the anode back to the cathode. However this connection can’t be very strong, because otherwise the charge would equalize entirely and there’s be no need to pass electrons through the wire at all.
okay so im trying to understand exactly how a battery works, given that the anode and cathode arent reacting with each other, but are instead reacting with the electrolyte. so the idea im forming is that the redox reactions (reduction on one side, oxidation on the other) would happen a little in the absence of the other electrode, but theyd quickly reach an equilibrium, because theyd make the electrolyte either positive or negative until the reaction was statistically unfavorable (because there arent reactants in the form of ions. or there are, but not a lot). and then the whole battery basically stops an accumulation of charge in the electrolyte, because the two effects balance out, so the reaction can happily proceed (as long as the charge on the electrodes themselves is being balanced by a circuit, otherwise THEY get charged which makes the reaction unfavorable, basically because there arent reactants in the form of ions)
#I hope this makes sense I need to go to bed so I can’t spend too much time#writing it up#also odds are good I swapped something somewhere#anode and cathode or positive and negative or oxidized and reduced#you get my point I switch words a lot it’s hard not to#essentially my point is you are pretty much right#but you don’t want to think of the electrolyte as connecting both ends of the battery until you do#like yes it does but also it doesn’t#think of the electrolyte as a carrier of ions and electrons and less of a reactant#and the wire is what allows electrons to pass from one side to the other#and there’s a way for ions to pass a little bit from one side to another
18 notes
·
View notes
Note
At work we have these metal inserts and some of them are the prettiest shade of dark pink (due to oxidation? Anodization? Idk) but everytime I see the metal I'm like "omg... Upgrade..."
Ohhhh that's so cool man , I love it when metal does funky things :] every time I see some kind of interesting looking metal I'm also instantly reminded of the bots lmao
Idk if you could do that but would you mind showing me ? I'm interested 👁️👁️
9 notes
·
View notes
Text
Guaranteed low price Dx51d Dx52d Dx66d galvanized steel coil
Galvanized steel coil is a specially treated steel product whose production process includes a number of fine steps. First, the raw material is all-hard board, which requires a pickling process to remove surface impurities and oxide layers, and then a rolling process to achieve the desired thickness and smoothness. The sheets are then fed into a zinc pan, where they are coated with a uniform and continuous zinc film. This layer of zinc film can not only effectively isolate the direct contact between the air and the substrate, thus greatly enhancing the corrosion resistance of the material; At the same time, because the zinc itself has good plasticity and ductility, the galvanized steel plate also has better processing and forming ability and good coating adhesion.

Hot-dip galvanizing technology is one of the most widely used methods for surface protection of steel products, which takes advantage of the unique electrochemical properties of zinc - when zinc is used as a sacrificial anode, it can be preferentially corroded by oxidation over iron, thereby protecting the internal base metal from damage. This method is especially suitable for those components or equipment that need to be exposed to the outside environment for a long time, such as building structures, Bridges, highway guardrails, etc.
According to the requirements of different application scenarios, the manufacturer can also adjust the specific thickness range of the galvanized layer, which can usually reach a maximum of about 120 grams per square meter. In addition, in cases where the appearance quality of the final product is required to be high, additional polishing treatment can also be selected to obtain a smoother and more delicate surface effect. According to whether such a follow-up processing process is carried out, the common galvanized steel coil on the market can be divided into two categories: zero spot type (that is, no additional treatment is done) and ultra-smooth type.
Our advantage
01. Advanced equipment: We have three production lines, all equipped with the most advanced production equipment in China. These devices not only improve production efficiency, but also ensure the quality and consistency of the product. Our factory covers an area of 30,000 square meters, and spacious production workshops provide ample space for large-scale production.
02. High-quality raw materials: We always adhere to the selection of high-quality raw materials, from the source to ensure the quality of products. We have established long-term and stable cooperation with many well-known suppliers to ensure that the quality of raw materials is reliable and meets international standards.
03. Strict quality inspection: We implement strict quality inspection standards, and strictly monitor and test every production link. Our products comply with ISO and SGS international standards, ensuring that every product can meet 100% of customer requirements.
04. Fast delivery: We use advanced production management processes, from order receipt to production arrangement to product delivery, the entire process is efficient and fast. Our goal is to complete customers' orders in the shortest possible time to ensure that customers receive satisfactory products in a timely manner.
05. Professional Sales services: We have a professional sales team who are fluent in Spanish, Portuguese, French, Arabic and Russian. This enables us to better communicate and cooperate with customers in different countries and regions, and provide customers with more intimate and professional services.
2 notes
·
View notes
Note
I need tips on how to study/revise for chemistry,especially physical chemistry.
Dear anon, I don't know whether you meant boards or competitive exams, so I covered for both since it is based on common ground anyway.
So👇
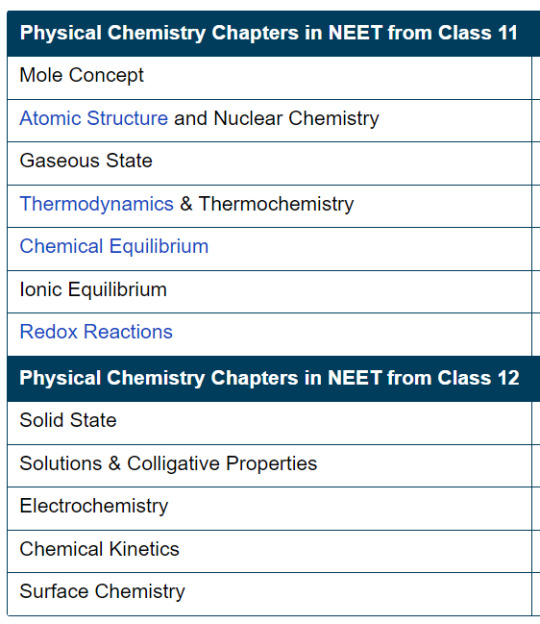
There is no other way that we could do well in physical chemistry except practice. A LOT.
What I understand about this dilemma (that I too have at this point, not fully solved) is that by the time you do at least 15 questions on a topic, you can get a grasp on the important formulae/which topics the questions hail from.
Mole concept : limiting reagent, M, m, w/w or w/v% (this particular option is not that frequent), reactions with stoichiometry coinciding with chemical kinetics, electrochemistry and metallurgy
Atomic Structure : which spectral series is in which region of the spectrum, sums with ratio of wavelengths (largest, smallest, comparison of different series), emission and absorption spectra, parts correlating to the physics part of energy levels and radius of hydrogen-like species
Gaseous state : GRAHAM'S LAW OF DIFFUSION (I cannot stress this enough, do it), the Cv and Cp values for Mono, Di and Polyatomic gases which connects thermo in Chem as well as Physics, mean free path proportionalities
Thermo (unit) : everything. All the laws, equations and graphs. adiabatic, isothermal, isochoric, my head and my tongue. Do every numerical in thermo. It's a weak point for a lot of us and we, right now, have the time to make it... Well, a not weak point.
Equilibrium : learn all the formulae and before you learn the formulae visualise/logically understand how something is happening. Log tables, roots, figure out some way to make decimal operations easier. A lot of sums from this one tpo because it isn't that connected to physics like thermo or electrochemistry
Redox : Make a trick for recognising which one is oxidation and which one is reduction. Balancing reactions must be practiced.
Solid state : Repetitive revision of the lattice examples is the only way we can remember them. Muscle memory can serve us well here. Make charts or stick it up on your wall to look at it every few days if that works for you. The rest are formulae and 4-5 numbers to be remembered. Density sums, chemical formula sums, voids sums <- practice
Solutions : formulae, how you get van't hoff factor for a compound, association and dissociation which is linked with electrochemistry molar conductivity part
Electrochemistry : formulae, graphs, molar conductivity sums, kohlrausch's law sums, electrolyte difference/spotting (will help in equilibrium), the cathode and anode of cells (this rarely comes)
Chemical Kinetics : some zero and first order reaction examples (will connect to radioactivity in nuclei chapter), half life formulae, and the 75% and 99% concentration formulae too (these 2 are not there in the tbk but it makes life easier in both phy and chem), all the graphs (should be able to read them even if they are messed around with or changed a bit)
Surface Chemistry : gold number sums, coagulation power and value orders in sols, recognising positive and negative sols, purification methods, electrophoresis definition (you'd be surprised how many times this came), helm holtz double layer theory, tindal's effect (connects a bit to optics, but vaguely), micelles (connects to bot biomolecules and cell unit). This chapter is very theoretical so keep revising stuff you don't get at first glance
Now briefly about Inorganic and Organic Chemistry:
Inorganic : write all the orders and the logics behind it. Some trends are weird so remember them with some trick (keep the tricks to the minimum in inorganic btw, it messes with your brain otherwise). That's as far as I've gotten with inorganic myself, but we can still work on it. If you have any advice regarding this, please do share.
Organic : understand the mechanism behind any reactions. Not just the way it's given in the textbook, but try to connect all organic chapters to each other. Practice a lot of questions, the direct ones as well as the weird ones. Organic does not have any tricks, it just requires practice and that can be done if we understand how each reaction goes about and why we do it.
Hope this helps you with Physical Chemistry and the like🤗 Thank you for approaching me with this so I could think out loud
Have a nice productive day!
#academiawho#physical chemistry#neet 2023#cbse boards#cbse 11#studyblr#chapter summary#cbse#ncert#cbse 2023#organic chemistry
55 notes
·
View notes
Text
Because F2 is an extremely powerful oxidising agent (E° = +2.87 V), the element cannot be prepared by chemical oxidation of F- and therefore obtained by electrolysis of KF·2HF, as depicted in figure 14.15; here, KF acts as an electrolyte.
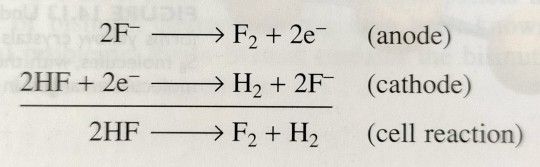
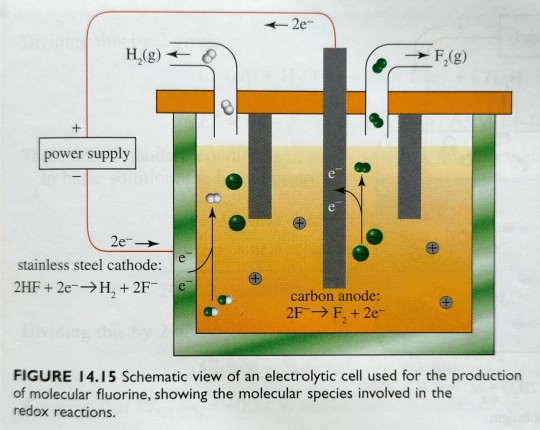
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#fluorine#oxidation#electrolysis#schematic#electrolytic cell#redox#reduction#cathode#anode#carbon#stainless steel
1 note
·
View note
Text
Aluminum Boat Maintenance Q&A: Expert Insights

As an aluminum boat owner, you know that proper maintenance is key to ensuring your vessel's longevity and performance on the water. However, with the unique properties of aluminum, there are specific considerations to keep in mind. In this comprehensive Q&A, we'll tap into expert insights to address some of the most common questions and concerns regarding aluminum boat maintenance.
Cleaning and Protecting the Hull
How should I clean the aluminum hull of my boat? According to experts, the best way to clean aluminum boat hull is to use a dedicated aluminum boat hull cleaner or a mild soap and water solution. Avoid harsh chemicals or abrasive cleaners, as they can damage the aluminum's protective oxide layer. Regular cleaning helps prevent the buildup of salt, dirt, and other contaminants that can lead to corrosion. Experts recommend using a soft brush or cloth to gently scrub the hull, paying extra attention to areas where dirt and grime tend to accumulate, such as around hardware and fittings. Rinse thoroughly with fresh water after cleaning to remove any residual soap or cleaner.What's the best way to protect the aluminum hull from corrosion? Applying a high-quality aluminum boat wax or sealant is crucial for protecting the hull from corrosion. These products create a barrier against moisture, salt, and other corrosive elements, while also enhancing the boat's appearance. Experts recommend waxing or sealing the hull at least once a year, or more frequently if you use your boat in harsh conditions. When selecting a wax or sealant, look for products specifically formulated for aluminum boats. These products often contain additives that help inhibit corrosion and provide additional protection against UV rays and other environmental factors.
Dealing with Dents and Scratches
How can I repair minor dents in my aluminum boat? For small dents, you can often use specialized aluminum dent repair tools or techniques, like suction cups, to gently massage the metal back into shape. However, for larger or more severe dents, you may need to seek professional assistance from a reputable boat repair shop or mobile fiberglass repair service near you. Aluminum Boat Hull Repair: Unraveling the CostsWhat's the best way to remove scratches from an aluminum boat? Depending on the depth and severity of the scratches, you can try using a fine-grit sandpaper or aluminum polish to buff them out. For deeper scratches, you may need to consider professional assistance, as they may require more extensive repair work, such as welding or patching. When addressing scratches yourself, start with the finest grit sandpaper or aluminum polish, and work your way up to coarser grits if necessary. Be sure to follow the manufacturer's instructions and take care not to over-sand or create additional scratches or swirl marks.
Corrosion and Electrolysis Prevention
How can I identify and address corrosion on my aluminum boat? Corrosion often manifests as pitting, discoloration, or flaking on the aluminum surface. To address it, you'll need to remove the affected areas, either by sanding or using a chemical treatment, and then apply a protective coating or paint. In severe cases, you may need to seek professional assistance for more extensive repairs or even partial replacement of corroded components. Identifying corrosion early is key to preventing it from spreading and causing more significant damage.What causes electrolysis in aluminum boats, and how can I prevent it? Electrolysis is a form of corrosion caused by electrical currents passing through the aluminum and the surrounding water. It can be prevented by ensuring proper bonding and grounding of your boat's electrical systems, as well as by using sacrificial anodes or zincs to protect the aluminum from galvanic corrosion. Restoring Aluminum Boats: Addressing Frequent Concerns
Leak and Hole Repairs
How can I repair a small hole or leak in my aluminum boat? For minor leaks or holes, you can often use a marine-grade epoxy or aluminum patching compound to seal the affected area. However, for larger holes or more extensive damage, you may need to seek professional assistance for welding or patching services.Can aluminum boats be repaired if they have significant hull damage? Yes, in many cases, even severe hull damage can be repaired on aluminum boats. Reputable boat repair shops and mobile fiberglass repair services can often perform welding, patching, or even partial hull replacement to restore the structural integrity of your vessel.By following these expert insights and addressing common concerns, you can keep your aluminum boat in top condition and enjoy many years of safe and enjoyable adventures on the water. Remember, proper maintenance is key to preserving the longevity and value of your aluminum vessel.
3 notes
·
View notes
Text
As a homecook of many years, I've experimented and did amateur research into the science of cooking and baking. Cooking/baking is full of chemical reactions that happen due to the order of ingredients and temperature of each step.
I feel like a mad scientist or a witch, but the important part is that I'm cackling wildly when a reaction goes as I expect, or even if it goes wrong!
A KEY component to this process that I had overlooked, I would even make a case that this is largely overlooked. It is under-advertised! It isn't hard to find, but you have to know what you're looking for.
Material of your cookware.
Why have I dedicated a lengthy post to this? It is because at my age of 29, I have found that not all non-stick is equal. My parents bought me a nice cookware set when I moved in with my then boyfriend (now husband🥰). It was Analon, so a brand that people recognize, known for quality, and marketed towards homecooks. It was a hard anodized nonstick set. Did I at 23 know what that meant? Absolutely not. Did I at 29 know what that meant? I KNOW NOW. If you do know, then you probably see where this is going.
If you're reading this and thinking "duh?? you deserve this??" then good for you! My post does not apply and I hope you educate the people in your life because after hours of forums on the internet, I feel sadly safe in the knowledge that I wasn't alone. So in case you're like me, let's go over what hard anodized is, what it does for your cooking, and how I fucked up.
"Anodizing is an electrochemical process that forms a layer of non-conductive anodic oxide on the surface of a non-ferrous (doesn't contain iron) metal especially aluminum. This process makes aluminum more durable, decorative, and corrosion resistant, informing its use for various finished parts across different manufacturing industries." -Wayken Manufacturing
What does this mean for cooking? From what my Google research tells me, this gives the aluminum a ceramic like finish to create the nonstick and nonscratch effect. Some will even go further and add a nonstick coating to the inside of the pan for extra durability (like mine did...) However, it also helps with the evenness of temperature and so that you're not using overly high heat when a bit of patience on medium heat will ensure less burned food and non-damaged cookware as you'll have more control over the temperature you're using. There's more to it and benefits as a cook, but I'm not a cooking blog and there are lots to read from if you're more interested.
Onto the point of this post: how I fucked up. Because I didn't know ANY of this, I followed rules for other nonstick that I've used. Which...were dishwasher safe. I'm horribly depressed. I need things to be an easy clean or I won't cook. I'll either starve or eat McDonald's dollar menu. As much as I love cooking/baking, finding joy in my hobbies is so hard on a good day. The thought of cleaning? Often enough to have me not bothering, but that's also a different post time. Because I'd say I could be a mental illness blog.
I digress.
To Analon's lasting credit, they held up in the dishwasher after years of abuse so so so well. Until the recent wash. When I finally noticed something was wrong. Now the nonstick coating on the inside? Perfectly 100% fine. The anodized coating on the outside? Is now a gray powder that comes off when touched. That I am HIGHLY allergic to, it seems. My poor hand still is recovering from the reaction. The burn was almost immediate and didn't spread beyond my fingers thank goodness. But apparently, the anodized coating is delicate. It does not like alkaline. What is mostly alkaline? Dishwashing detergents.
Parts of the aluminum and deep scratches are visible. I've ruined my set. "Not dishwasher safe" is on the website, down at the very very bottom. It was probably on the box, too. But I saw nonstick and that's what I was used to and knew so that's what I went with.
So now I warn you readers that have stuck with me this long, don't just trust keywords. Actively look up what your cookware is made out of. Understand the terms that you took for granted. Obsess over proper care and use of your sets. There are so many different materials to choose from and each brand has its own words and patents, it seems. This has been an expensive lesson in reading asterisks and fine prints and that cookware is not always an open the box and go deal.
Dishwashing also voids my warranty. Cooking at high heats would also void the warranty because the anodized coating is not built for high heats.
So yeah, recommend me some cookware sets😪
2 notes
·
View notes
Text
At the copper electrode, Cu²+ ions enter the solution when copper atoms are oxidised, leaving electrons behind on the electrode.

"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes