#oral health in COVID-19
Explore tagged Tumblr posts
Text
Also preserved in our archive
#mask up#public health#wear a mask#wear a respirator#pandemic#covid#still coviding#covid 19#coronavirus#sars cov 2#mask bloc#history#oral history
26 notes
·
View notes
Text
Efficient Solutions for Tonsillitis White Patches: A Comprehensive Guide
Discover the underlying reasons behind white spots on tonsils, grasp their symptoms, and explore a spectrum of treatment options in this comprehensive guide that balances medical insights with patient-centered perspectives. Tonsils, those guardians of our throats, occasionally present an unsettling spectacle with the appearance of white spots. The interplay between the discomfort caused by these…

View On WordPress
#COVID-19 and Tonsil Symptoms#Infectious Mononucleosis#Mono Symptoms#Oral Thrush Candidiasis#Sore Throat Remedies#Strep Throat Causes#Throat Infections#Tonsil Care#Tonsil Discoloration#tonsil health#Tonsil Health Awareness#Tonsil Health Information#Tonsil Health Management#Tonsil Health Tips#Tonsil Infections#Tonsil Inflammation#tonsil stones#Tonsil Swelling#Tonsil Symptoms#Tonsil Treatment#Tonsiliths#tonsillectomy#Tonsillitis Infection#White Patches in Throat#white spots on tonsils
0 notes
Text

¡Aquí tenemos! Sobre el COVID y cómo puedes protegerte: edición de 2024
Traducción al castellano por Maru Florián Padrón (twitter: @unlocalizedling). ¡Todes pueden imprimir estas zines gratis!
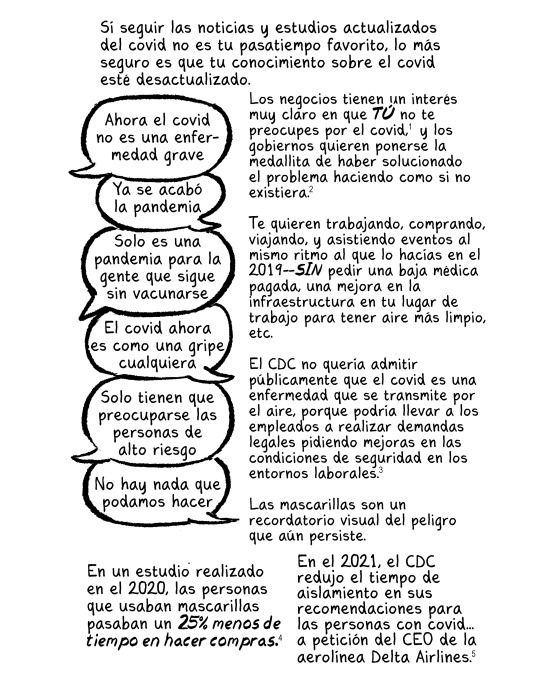

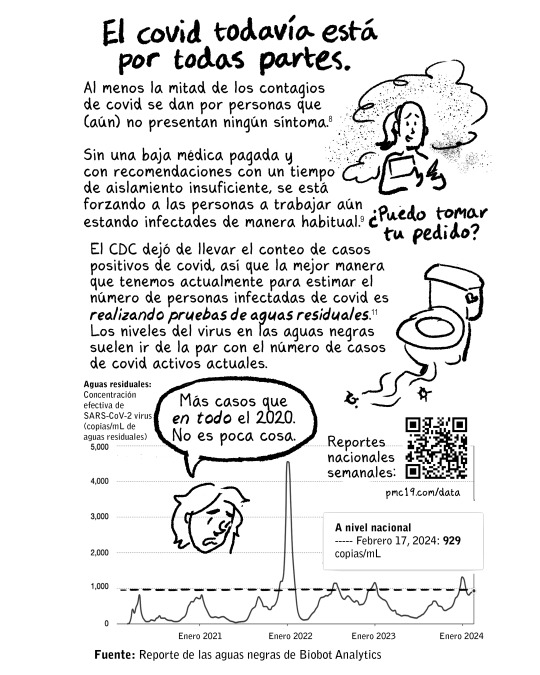
Reportes nacionales semanales sobre la concentración efectiva de SARS-CoV-2 virus en las aguas residuales: https://pmc19.com/data/
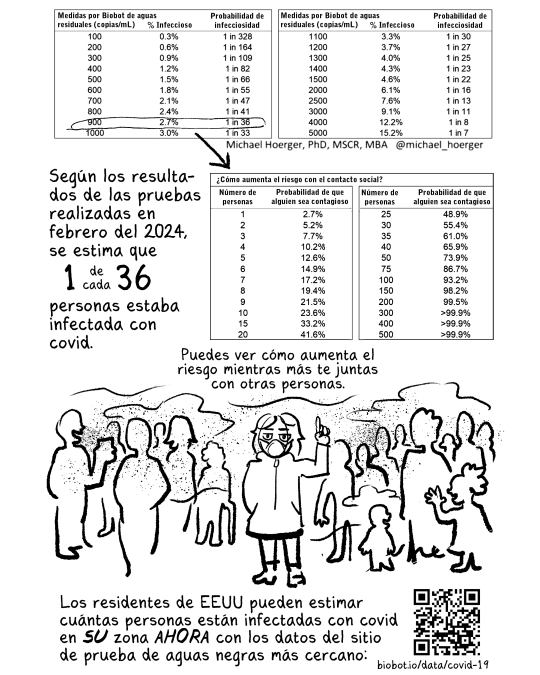
biobot.io/data/covid-19
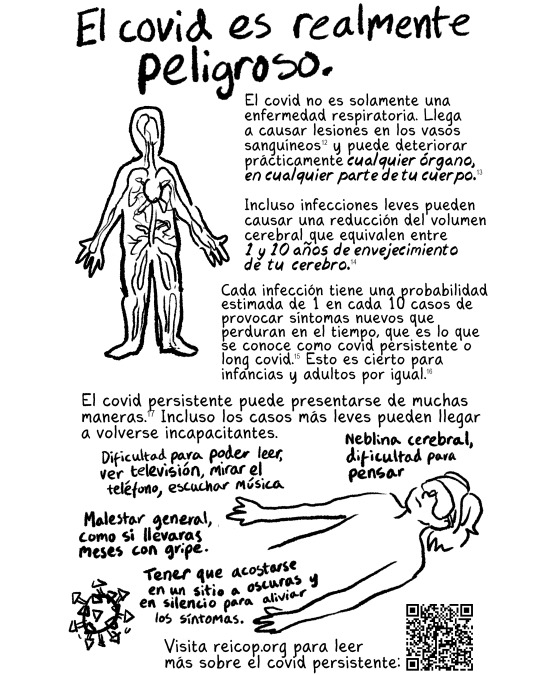
La Red Española de Investigación en COVID persistente: reicop.org
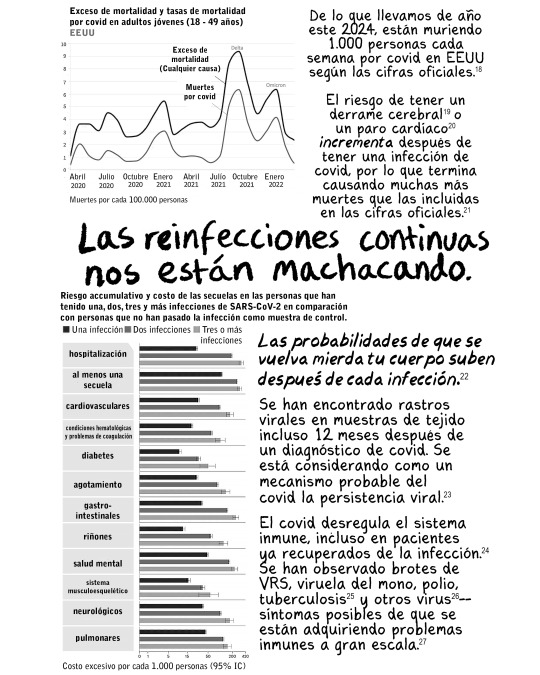
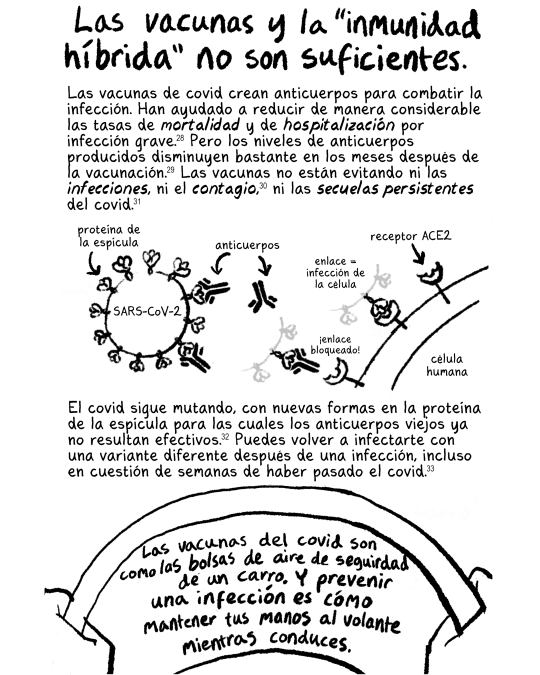
Las vacunas contra el covid actualizadas intentan coincidir con las variantes del covid que circulan actualmente, por lo que vale la pena adquirirlas. Simplemente no te hacen invencible.
Encuentre vacunas en los EEUU: https://vacunas.gov

Pide pruebas de covid gratuitas (si están incluidas en la cobertura de tu seguro médico): fastlabtech.com
Encuentra puntos gratuitos dónde realizarte una prueba: https://testinglocator.cdc.gov/homees
Cómo obtener una muestra: https://ontariohealth.ca/sites/ontariohealth/files/2022-05/RAT-Oral-nasal-collection-instructions-Spanish.pdf


Pruebas de ajuste facial y ajuste respiratorio por 3M: 3m.com.es
Compre un respirador 3M Aura: stauffersafety.com
Resultados de la prueba de talla: testtheplanet.org

Cómo hacer un filtro HEPA casero: https://renovablesverdes.com/filtro-hepa-casero/

Precaución: ¡el xilitol es venenoso para perros y gatos! Mantenga el aerosol nasal alejado de ellos.
Estudios sobre los sprays nasales (en inglés) aquí: https://newlevant.com/covidzine/es

Qué hacer si tiene COVID por el People's CDC: peoplescdc.org/es/2023/01/10/what-to-do-if-you-have-covid/…
Qué hacer cuando tengo COVID por Clean Air Club, con información relevante si vives en España: https://dropbox.com/scl/fi/999mutp

Encontrar un sitio de Pruebas para Tratar o un sitio del Programa de Asistencia a Pacientes de Paxlovid: https://treatments-es.hhs.gov
Virtual ExpressCare en NY: https://ondemand.expresscare.video/landing
Suplementos recomendados por RTHM Health (inglés): https://rthm.com/articles/youve-got-covid/

Gracias por leer. ¡Puedes imprimir y distribuir tus propias copias de este zine!
Referencias y más recursos aquí: http://newlevant.com/COVIDzine/es
Cualquier error en español es mío porque utilicé el traductor de Google. Maru tradujo muy bien el zine.
83 notes
·
View notes
Text
The tulsi gabbard Appointment and The 2024 U.S. Elections Aren't The Only Times donald j. trump and his Russian Asset Republicans Worked With Russians Against United States' Interests: COVID-19, USA Patriot Act/USA Freedom Act Sabotage, The Massive Russian SolarWinds Hack of 33,000 U.S. Government and Private Sector Computer Networks, and Russian 2024 Election Day Interference (compiled from Wikipedia):

In November 2019, a security researcher notified SolarWinds that credentials to a third party FTP server had a weak password of "solarwinds123", warning that "any hacker could upload malicious [code]" that would then be distributed to SolarWinds customers. The New York Times reported SolarWinds did not employ a chief information security officer and that employee passwords had been posted on GitHub in 2019.
December 1, 2019: COVID-19 pandemic: First known human case of Coronavirus disease 2019, in Wuhan, Hubei, China.
December 5, 2019: Speaker of the U.S. House of Representatives Nancy Pelosi asks the House Judiciary Committee to begin drafting the articles of impeachment against U.S. President Donald Trump.
December 9, 2019: The World Anti-Doping Agency votes unanimously to ban Russia from international sport for four years for doping offences, meaning it will be excluded from the 2020 Summer Olympics in Tokyo, the 2022 Winter Olympics in Beijing and the 2022 World Cup in Qatar.
December 10, 2019: Democrats in the United States House of Representatives announce formal charges against President Donald Trump, accusing him of abusing power and "obstructing Congress"; he becomes the third U.S. president in history to face impeachment.
December 18, 2019: The U.S. House of Representatives approves two articles of impeachment against President donald trump, making him the third president to be impeached in the nation's history.
December 29, 2019: The Taliban's ruling council agrees to a temporary cease-fire in Afghanistan, opening a door to a peace agreement with the United States.
In January and February 2020, U.S. intelligence agencies delivered over a dozen classified warnings in the President's Daily Brief about COVID-19, including its potential to inflict severe political and economic damage. President Donald Trump typically did not read daily briefs and often has "little patience" for oral summaries, The Washington Post reported. Each brief was also shared with other officials in the administration. The Office of the Director of National Intelligence, which produces the President's Daily Brief, denied that there were repeated mentions of COVID-19.
On January 8, 2020, the U.S. Centers for Disease Control and Prevention (CDC) released a health advisory regarding an outbreak of pneumonia in Wuhan, Hubei Province, China, which was being caused by a yet-unidentified virus.
January 16, 2020: The first impeachment trial of the President of the United States, Donald Trump, begins in the U.S. Senate.
On January 27, 2020, Anthony Fauci, head of the National Institute of Allergy and Infectious Diseases, predicted that "things are going to get worse before they get better". Three days later, Fauci stated that the COVID-19 outbreak "could turn into a global pandemic".
On February 10, 2020, Trump stated that "a lot of people think that [COVID-19] goes away in April with the heat … Typically, that will go away in April" (later, on April 3, he denied ever having given "a date" for the departure of the virus)
On February 13, 2020, CDC director Robert Redfield contradicted Trump, saying that the "virus is probably with us beyond this season, beyond this year". Redfield also predicted that it "will become a community virus at some point in time, this year or next year".
On February 16, 2020, Anthony Fauci warned that it was not necessarily true that COVID-19 would "disappear with the warm weather."
February 25, 2020, was the day that the CDC first warned the American public to prepare for a local outbreak. That day, Nancy Messonnier, head of the CDC's National Center for Immunization and Respiratory Diseases, said that "We are asking the American public to work with us to prepare for the expectation that this is going to be bad." Messonnier predicted that "we will see community spread in this country", and it was only a matter of time. As a result, "disruption to everyday life might be severe". Messonnier stated that the CDC is preparing, and "now is the time for hospitals, schools and everyday people to begin preparing as well."
On February 25, 2020, Anthony Fauci declared that given how COVID-19 was spreading in other nations, it was "inevitable that this will come to the United States" as well. On February 26, CDC Director Robert Redfield said it would be "prudent to assume this pathogen will be with us for some time to come".
On February 26, 2020, Trump contradicted Messonnier, stating: "I don't think it's inevitable" that a U.S. outbreak would occur, "It probably will, it possibly will … Whatever happens, we're totally prepared." Trump additionally declared that the number of infected was "going very substantially down, not up".
On February 27, 2020, The chairman of the Senate Intelligence Committee, Richard Burr, who helped to write the Pandemic and All-Hazards Preparedness Act (PAHPA), which forms the framework for the federal response, warned a private group of his constituents that COVID-19 is much more aggressive in its transmission than anything that we have seen in recent history, and is probably more akin to the 1918 Spanish Flu pandemic. "There will be, I'm sure, times that communities, probably some in North Carolina, have a transmission rate where they say, 'Let's close schools for two weeks. Everybody stay home,' We're going to send a military hospital there; it's going to be in tents and going to be set up on the ground somewhere, It's going to be a decision the president and DOD make."
On February 27, 2020, Trump said of the virus: "It's going to disappear. One day it's like a miracle, it will disappear. And from our shores, you know, it could get worse before it gets better. Could maybe go away. We'll see what happens. Nobody really knows." Also on February 27, Trump declared that the risk to the American public from COVID-19 "remains very low".
February 27, 2020 stock market crash: Triggered by fears of the spreading of COVID-19, the Dow Jones Industrial Average (DJIA) plunges by 1,190.95 points, or 4.4%, to close at 25,766.64, its largest one-day point decline at the time. This follows several days of large falls, marking the worst week for the index since the 2007–2008 financial crisis.
On February 29, 2020, Trump said that "additional cases in the United States are likely", but "there's no reason to panic at all." When a reporter asked Trump: "How should Americans prepare for this virus?" Trump answered: "I hope they don't change their routine".
February 29, 2020: A conditional peace agreement is signed between the United States and the Taliban. The U.S. begins gradually withdrawing combat troops from Afghanistan on March 10.
On March 4, 2020, donald trump appeared on Fox News's Hannity by phone, where he claimed a 3.4% mortality rate projected by the World Health Organization (WHO) was a "false number", and stated his "hunch" that the true figure would be "way under 1%". Trump also predicted that many people infected with COVID-19 would experience "very mild" symptoms, "get better very rapidly" and thus they "don't even call a doctor". Thus, there may be "hundreds of thousands of people that get better just by, you know, sitting around and even going to work—some of them go to work, but they get better."
From March 6 to March 12, 2020, donald trump stated on four occasions that the coronavirus would "go away". On March 10, Surgeon General Jerome Adams stated that "this is likely going to get worse before it gets better."
March 2020: The nightmare of empty grocery shelves begins. Stores capping/limiting purchases.
By March 11, 2020, the virus had spread to 110 countries, and the WHO officially declared a pandemic. The CDC had already warned that large numbers of people needing hospital care could overload the healthcare system, which would lead to otherwise preventable deaths. Director of the National Institute of Allergy and Infectious Diseases Anthony Fauci said the mortality from COVID-19 was ten times higher than the common flu. By March 12, diagnosed cases of COVID-19 in the U.S. exceeded a thousand. Trump declared a national emergency on March 13. On March 16, the White House advised against any gatherings of more than ten people. Three days later, the United States Department of State advised U.S. citizens to avoid all international travel.
The USA Freedom Act, which became law on June 2, 2015, reenacted the expired USA Patriot Act sections through 2019. However, Section 215 of the law was amended to disallow the National Security Agency (NSA) to continue its mass phone data collection program. Instead, phone companies will retain the data and the NSA can obtain information about targeted individuals with a federal search warrant.
On August 14, 2019, the outgoing Director of National Intelligence sent a letter to Congress stating the Trump Administration's intention to seek permanent extension of the provisions of FISA that under the terms of the USA FREEDOM Act are scheduled to expire on December 15, 2019, namely the "lone wolf" authority allowing surveillance of a suspected terrorist who is inspired by foreign ideology but is not acting at the direction of a foreign party, the roving wiretap authority regarding surveillance of a terrorist who enters the United States and the authority to allow the Federal Bureau of Investigation to obtain certain business records in a national security investigation, as well as the call detail records program undertaken by the NSA. In reference to the latter authority, the letter announced that "The National Security Agency has suspended the call detail records program that uses this authority and deleted the call detail records acquired under this authority."
Jurisdiction over the reauthorization of the expiring FISA provisions is shared by the Judiciary and Intelligence committees in the U.S. Senate and the U.S. House of Representatives; the House Committee on the Judiciary and the Senate Committee on the Judiciary held separate public hearings on the reauthorization in September 2019 and November 2019, respectively. Opposition to the call detail records program has led to some Congressional demands that the authority for the program not be renewed. Additional complications hindering reauthorization arose from a report of the US Department of Justice Inspector General finding fault with certain FISA applications in connection with the 2016 presidential campaign, which led some members of Congress to insist on reforms to FISA as a condition of reauthorizing the expiring USA FREEDOM Act provisions.With Congressional attention focused on dealing with the COVID-19 pandemic in the United States in 2020, the House of Representatives passed a long-term extension of the USA FREEDOM Act on March 11, 2020, just four days before the scheduled expiration of the Act on March 15, 2020, by a wide, bipartisan margin that kept the protections of the Act largely the same. Two months later, in May of 2020, the Senate passed an extension of the Act by an 80-16 vote that expanded some privacy protections, but the Senate version did not include protection of Americans’ internet browsing and search histories from warrantless surveillance, which was proposed by Sens. Ron Wyden (D-Ore.) and Steve Daines (R-Mont.) and failed by one vote.
According to former KGB major Yuri Shvets, donald trump became the target of a joint Czech intelligence services and KGB spying operation after he married Czech model Ivana Zelnickova and was cultivated as an "asset" by Russian intelligence since 1977: "Russian intelligence gained an interest in Trump as far back as 1977, viewing Trump as an exploitable target." Luke Harding writes that documents show Czechoslovakia spied on donald trump during the 1970s and 1980s, when he was married to Ivana Trump, his Czechoslovakia-born first wife. Harding writes that the Czechoslovakian government spied on donald trump because of his political ambitions and notability as a businessman. It is known that there were close ties between Czechoslovakia's StB and the USSR's KGB. Harding also describes how, already since 1987, the Soviet Union was interested in Trump. In his book Collusion, Harding asserts that the "top level of the Soviet diplomatic service arranged his 1987 Moscow visit. With assistance from the KGB." Then-KGB head Vladimir Kryuchkov "wanted KGB staff abroad to recruit more Americans". Harding proceeds to describe the KGB's cultivation process, and posits that they may have opened a file on Trump as early as 1977, when he married Ivana. "According to files in Prague, declassified in 2016, Czech spies kept a close eye on the couple in Manhattan, … [with] periodic surveillance of the trump family in the United States."
donald j. trump and His Republican Allies Allowed the USA PATRIOT ACT/FREEDOM ACT to Expire on March 15, 2020 and They Didn't Reinstate It until May of 2020.
March 2020: SolarWinds's Orion software hotfixes were released to 33,000 Orion customers.Hackers acquired superuser access to SAML token-signing certificates. This SAML certificate was then used to forge new tokens to allow hackers trusted and highly privileged access to networks. The attack used a backdoor in a SolarWinds library; when an update to SolarWinds occurred, the malicious attack would go unnoticed due to the trusted certificate. APT29, aka Cozy Bear, working for the Russian Foreign Intelligence Service (SVR), was reported to be behind the 2020 attack. Victims of this attack include the cybersecurity firm FireEye, the US Treasury Department, the US Department of Commerce's National Telecommunications and Information Administration, as well as the US Department of Homeland Security. Prominent international SolarWinds customers investigating whether they were impacted include the North Atlantic Treaty Organization (NATO), the European Parliament, UK Government Communications Headquarters, the UK Ministry of Defence, the UK National Health Service (NHS), the UK Home Office, and AstraZeneca.
December 13, 2020: SolarWinds begins notifying customers, including a post on its Twitter account, “SolarWinds asks all customers to upgrade immediately to Orion Platform version 2020.2.1 HF 1 to address a security vulnerability.”
December 15, 2020 SolarWinds Victims named and timeline moves back — Wall Street Journal reported that the U.S. Commerce and Treasury Departments, the Department of Homeland Security (DHS), the National Institutes of Health, and the State Department were all affected. Various security officials and vendors expressed serious dismay that the attack was more widespread and began much earlier than expected. The initial attack date was now pegged to sometime in March 2020, which meant the attack had been underway for months before its detection.
December 17, 2020: New SolarWinds victims revealed — The Energy Department (DOE) and National Nuclear Security Administration (NNSA), which maintains the U.S. nuclear weapons stockpile, were publicly named as victims of the attack.
On December 21, 2020, Attorney General William Barr, Secretary of State Mike Pompeo, the FBI, the Cybersecurity and Infrastructure Security Agency, or CISA, and the Office of the Director of National Intelligence all agreed that Russia was engaged in “a significant and ongoing cybersecurity campaign” against the United States. Russian asset, donald j. trump, immediately replied: “The Cyber Hack is far greater in the Fake News Media than in actuality,” Trump wrote. “I have been fully briefed and everything is well under control. Russia, Russia, Russia is the priority chant when anything happens because Lamestream is, for mostly financial reasons, petrified of discussing the possibility that it may be China (it may!).”
March 16, 2020: stock market crash: The the Dow Jones Industrial Average (DJIA) falls by 2,997.10, the single largest point drop in history and the second-largest percentage drop ever at 12.93%, an even greater crash than Black Monday (1929). This follows the U.S. Federal Reserve announcing that it will cut its target interest rate to 0–0.25%.
On March 19, 2020, donald trump told journalist Bob Woodward that he was deliberately downplaying the risk when communicating with the public. "I wanted to always play it down," Trump said. "I still like playing it down, because I don't want to create a panic."
On March 24, 2020, donald trump argued that: "We lose thousands and thousands of people a year to the flu … But we've never closed down the country for the flu." On March 27, he stated: "You can call it a flu. You can call it a virus. You know you can call it many different names. I'm not sure anybody even knows what it is."
On March 24, 2020, donald trump declared that "we begin to see the light at the end of the tunnel"; a day later the U.S. surpassed 1,000 COVID-19 deaths.
Throughout March and early April, several state, city, and county governments imposed "stay at home" quarantines on their populations to stem the spread of the virus. By March 26, The New York Times data showed the United States to have the highest number of known cases of any country. By March 27, the country had reported over 100,000 cases.
From March 30 to April 7, 2020, Trump stated on four occasions that COVID-19 would "go away".
On March 31, 2020, contradicting his many previous comparisons of COVID-19 to the flu, Trump said: "It's not the flu … It's vicious". When reporters asked him if his initial dismissive comments on the virus had misled Americans, he replied: "I want to give people a feeling of hope. I could be very negative … You know, I'm a cheerleader for the country." Asked further if he had known—despite his claims that the outbreak was under control—that the situation would turn out so severe, Trump replied: "I thought it could be. I knew everything. I knew it could be horrible, and I knew it could be maybe good."
On November 5, 2024, during the official Election Day, several non-credible bomb threats that originated from Russia briefly disrupted voting in two polling places in Fulton County, Georgia. Both re-opened after about 30 minutes. Republican Georgia Secretary of State Brad Raffensperger said Russian interference was behind the Election Day bomb hoaxes. In a statement, the FBI said it was aware of non-credible bomb threats to polling locations in several states, with many of them originating from Russian email domains. The bomb threats were solely made against Democratic-leaning areas. On the same day, U.S. federal officials again reported that Russian sources were actively engaged in "influence operations", citing disinformation in specific videos that falsely claimed Kamala Harris had taken a bribe and false news stories about the Democratic Party and election fraud in Georgia.
On November 8, 2024 it was reported that one of the Russian email addresses behind Election Day bomb threats was used in June 2024 bomb threats targeting LGBTQ+ events in Massachusetts, Minnesota and Texas.
If you're an American who wants this matter and the anti-American traitors responsible investigated by the U.S. Department of Justice, please contact Democratic Leaders Schumer and Jeffries, Marc Elias and Democracy Docket, and Citizens for Responsibility and Ethics in Washington regarding enforcing donald j. trump's insurrectionist disqualification and electing Kamala Harris as the 47th President of the United States. If you want this matter investigated and prosecuted, the best person to do it is an experienced criminal prosecutor sitting as the U.S. President.
#2024 presidential election#2024 election#election 2024#kamala harris#harris walz 2024#donald trump#trump vance 2024#trump 2024#president trump#trump#republicans#gop#evangelicals#democrats#us elections 2024#us elections#us election 2024#us politics#politics#american politics#uspol
23 notes
·
View notes
Text
On Tuesday, the Supreme Court of the United States will hear oral arguments in a challenge to abortion pill access across the country, including in states where abortion is legal. The stakes for abortion rights are sky-high, and the case is the most consequential battle over reproductive health care access since Roe v. Wade was overturned in 2022.
At the center of this fight is mifepristone, a pill that blocks a hormone needed for pregnancy. The drug has been approved by the US Food and Drug Administration for more than two decades, and it’s used to treat some patients with Cushing’s syndrome, as well as endometriosis and uterine fibroids. But its primary use is the one contested now—mifepristone is the first of two pills taken in the first 10 weeks of pregnancy for a standard medication abortion, along with the drug misoprostol.
If the justices side with the antiabortion activists seeking to limit access to mifepristone, it could upend nationwide access to the most common form of abortion care. A ruling that invalidates mifepristone’s approval would open the door for any judge to reverse the FDA approval of any drug, especially ones sometimes seen as controversial, such as HIV drugs and hormonal birth control. It could also have a chilling effect on the development of new drugs, making companies wary of investing research into medicines that could later be pulled from the market.
Pills are now the leading abortion method in the US, and their popularity has spiked in recent years. More than six in 10 abortions in 2023 were carried out via medication, according to new data from the Guttmacher Institute. Since rules around telehealth were relaxed during the Covid-19 pandemic, many patients seeking medication abortions have relied on virtual clinics, which send abortion pills by mail. And it keeps getting more popular: Hey Jane, a prominent telemedicine provider, saw demand increase 73 percent from 2022 to 2023. It recorded another 28 percent spike comparing data from January 2023 to January 2024.
“Telemedicine abortion is too effective to not be in the targets of antiabortion folks,” says Julie F. Kay, a longtime reproductive rights lawyer and director of the advocacy group Abortion Coalition for Telemedicine.
Tomorrow’s argument comes after a long, tangled series of legal disputes in lower courts. The Supreme Court will be hearing two cases consolidated together, including FDA v. Alliance for Hippocratic Medicine, in which a coalition of antiabortion activists filed a suit challenging the FDA’s approval of mifepristone, asking for it to be removed from the market. The Alliance for Hippocratic Medicine is represented by the Alliance Defending Freedom, a right-wing Christian law firm that often takes politically charged cases.
Despite decades of scientific consensus on the drug’s safety record, the Alliance for Hippocratic Medicine has alleged that mifepristone is dangerous to women and leads to emergency room visits. A 2021 study cited by the plaintiffs to back up their claims was retracted in February after an independent review found that its authors came to inaccurate conclusions.
In April 2023, the Trump-appointed judge Matthew Kacsmaryk of the Northern District of Texas issued a preliminary ruling on the FDA case invalidating the agency’s approval of mifepristone. The ruling sent shock waves far beyond the reproductive-rights world, as it had major implications for the entire pharmaceutical industry, as well as the FDA itself; the ruling suggested that the courts could revoke a drug’s approval even after decades on the market.
The US 5th Circuit Court of Appeals narrowed Kacsmaryk’s decision a week later, allowing the drug to remain on the market, but undid FDA decisions in recent years that made mifepristone easier to prescribe and obtain. That decision limited the time frame in which it can be taken to the first seven weeks of pregnancy and put telemedicine access, as well as access to the generic version of the drug in jeopardy.
Following the 5th Circuit ruling, the FDA and Danco Laboratories sought emergency relief from the Supreme Court, asking the justices to preserve access until it could hear the case. In its legal filing, Danco aptly described the situation as “regulatory chaos.”
SCOTUS issued a temporary stay, maintaining the status quo; the court ultimately decided to take up the case in December 2023.
As all this was unfolding, pro-abortion-rights states across the country were passing what are known as shield laws, which protect medical practitioners who offer abortion care to pregnant patients in states where abortion is banned. This has allowed some providers, including the longtime medication-abortion-advocacy group Aid Access, to mail abortion pills to people who requested them in states like Louisiana and Arkansas.
Though the oral arguments before the Supreme Court begin on Tuesday, it will likely be months before a ruling. Court watchers suspect a decision may be handed down in June. With the US presidential election in the fall, the ruling may become a major campaign issue, especially as abortion access helped galvanize voters in the 2022 midterms.
If the Supreme Court agrees with the plaintiffs that mifepristone should be taken off the market, some in the pharmaceutical industry worry that it will undermine the authority of the FDA, the agency tasked with reviewing and approving drugs based on their safety and efficacy.
“This case isn't about mifepristone,” says Elizabeth Jeffords, CEO of Iolyx Therapeutics, a company developing drugs for immune and eye diseases. Jeffords is a signatory on an amicus brief filed in April 2023 that brought together 350 pharmaceutical companies, executives, and investors to challenge the Texas district court’s ruling.
“This case could have easily been about minoxidil for hair loss. It could have been about Mylotarg for cancer. It could have been about measles vaccines,” Jeffords says. “This is about whether or not the FDA is allowed to be the scientific arbiter of what is good and safe for patients.”
Greer Donley, an associate professor of law at the University of Pittsburgh and an expert on abortion on the law, doesn’t think it’s likely that the court will revoke mifepristone’s approval entirely. Instead, she sees two possible outcomes. The Supreme Court could dismiss the case or could undo the FDA’s decision in 2023 to permanently remove the in-person dispensing requirement and allow abortion by telehealth. “This would be an even more narrow decision than what the 5th Circuit did, but it would still be pretty devastating to abortion access,” she says.
The Supreme Court could also decide that the plaintiffs lack a right to bring the case to court, says David Cohen, a professor of law at Drexel University whose expertise is in constitutional law and gender issues. “This case could get kicked out on standing, meaning that the plaintiffs aren't the right people to bring this case,” he says. “If most of the questions are about standing, that will give you a sense that that's what the justices are concerned about.”
As the current Supreme Court is considered virulently antiabortion, reproductive-health-care workers are already preparing for the worst. Some telehealth providers have already floated a backup plan: offering misoprostol-only medication abortions. This is less than ideal, as the combination of pills is the current standard of care and offers the best results; misoprostol on its own can cause additional cramping and nausea. For some providers who may have to choose between misoprostol-only or nothing, it’s better than nothing.
Abortion-rights activists have no plans to give up on telehealth abortions, regardless of the outcome of this particular case. “Let us be clear, Hey Jane will not stop delivering telemedicine abortion care, regardless of the outcome of this case,” says Hey Jane’s CEO and cofounder, Kiki Freedman.
“They’re not going to stuff the genie back in the bottle,” Kay says.
31 notes
·
View notes
Text
Make your voice heard and ask the CDC to:
Recommend updated 2024-2025 COVID vaccines for all ages AND
Strengthen our vaccine drive by recommending more frequent boosting (at least every six months) and more frequent updates to the vaccines, adjusted for the latest variants.
Submit a public comment using our sample language below.
You can also register to give Oral Public Comment at the upcoming June 26-28 online CDC ACIP Meeting at: https://www2.cdc.gov/vaccines/acip/acip_publiccomment.asp
Submit written comments and/or register to make oral comments at the meeting by Monday, June 17 at 11:59pm Eastern Standard Time.
It’s important to submit a personalized comment, which can be brief. Ideas for a personalized comment:
How you, your family, or your community would be impacted by fall vaccine eligibility being restricted to only high risk groups (such as older age or immunocompromised status)
Barriers to vaccination your have faced, particularly if your eligibility was questioned or misinterpreted by a vaccine provider
How out-of-pocket costs are a barrier to getting the latest vaccines
Also feel free to take inspiration from or borrow the language in our sample public comment below.
Docket No. CDC–2024–0043
Updated 2024-2025 COVID vaccines must be recommended for people of all ages, regardless of health status. A restrictive approach to eligibility would create undue barriers for vulnerable people and discourage high risk people from getting needed vaccine boosters.
The vaccine schedule should address waning efficacy in the months following vaccination [1-3] as well as emergence of new SARS-CoV-2 strains by recommending updated vaccination for all ages, at least every six months. Recent vaccination is also associated with a lower risk of developing Long COVID following a COVID infection [4] as well as a lower risk of Multisystem Inflammatory Syndrome in children (MIS-C) [5].
The CDC’s clear and unequivocal recommendation of updated COVID vaccination for all ages will influence what healthcare providers recommend, and what health insurances cover. Moreover, it will improve public awareness regarding the need for updated vaccination.
The CDC must ensure equitable and affordable access to updated vaccines and prevent limited access because of financial constraints or demographics. The CDC’s Bridge vaccine access program is slated to end August 2024 and must be extended to ensure uninsured and underinsured people have access to the updated vaccines this fall [6].
References:
1. Link-Gelles R. Effectiveness of COVID-19 (2023-2024 Formula) vaccines. Presented at: FDA VRBPAC Meeting; June 5, 2024. Accessed June 12, 2024. https://www.fda.gov/media/179140/download
2. Wu N, Joyal-Desmarais K, Vieira AM, et al. COVID-19 boosters versus primary series: update to a living review. The Lancet Respiratory Medicine. 2023;11(10):e87-e88. doi:10.1016/S2213-2600(23)00265-5
3. Menegale F, Manica M, Zardini A, et al. Evaluation of Waning of SARS-CoV-2 Vaccine–Induced Immunity: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023;6(5):e2310650. doi:10.1001/jamanetworkopen.2023.10650
4. Fang Z, Ahrnsbrak R, Rekito A. Evidence Mounts That About 7% of US Adults Have Had Long COVID. JAMA. Published online June 7, 2024. doi:10.1001/jama.2024.11370
5. Yousaf AR. Notes from the Field: Surveillance for Multisystem Inflammatory Syndrome in Children — United States, 2023. MMWR Morb Mortal Wkly Rep. 2024;73. doi:10.15585/mmwr.mm7310a2
6. https://www.cdc.gov/vaccines/programs/bridge/index.html
Full instructions for written and oral comment and meeting information can be found at: https://www.cdc.gov/vaccines/acip/meetings/index.html
You can also register to give Oral Public Comment at the upcoming June 26-28 online CDC ACIP Meeting at: https://www2.cdc.gov/vaccines/acip/acip_publiccomment.asp
You must register by June 17 at 11:59pm Eastern Standard Time
CDC’s ACIP meeting information on the Federal Register: https://www.federalregister.gov/documents/2024/05/24/2024-11439/meeting-of-the-advisory-committee-on-immunization-practices
Vaccination with the latest updated vaccines continues to be foundational to a multilayered approach to COVID, providing protection against both acute disease and Long COVID. Far too few Americans have received the latest vaccines. Only approximately 22.6% of adults and 14.8% of children have received the latest 2023-2024 vaccines (as of June 1, 2024), which have been available since Fall 2023. COVID vaccination rates in both groups lags far behind influenza vaccination rates. Only 7.1% of adults aged 65 and older received the recommended two doses of the 2023-2024 vaccine (as of April 27, 2024).
Vaccine efficacy wanes significantly four to six months following vaccination, making updated vaccination important for all people as COVID continues to spread in our communities. Vaccine approaches that restrict access based on age or risk status put all of us at risk and leave those at high risk of severe consequences of COVID infection��confused about whether they qualify to receive additional doses. A more frequent vaccination approach providing vaccination at least every six months as well as frequent updates to match current variants is needed to better protect all of us amid year-round COVID spread.
The CDC’s Bridge Access Program, which provides COVID vaccines to uninsured and underinsured adults free of charge, is due to end August 2024. The end of this program will unnecessarily put vulnerable people at risk, and public health officials must advocate for continuation and expansion of this program.
Submitted written comments or registration to make oral comments at the meeting must be received by the CDC no later than June 17 at 11:59pm Eastern Standard Time
28 notes
·
View notes
Text
Remember that the Supreme Court threw out Murthy v Biden. This past week Kennedy and CHD were at the 5th Circuit providing oral arguments before 3 judges about why they had standing for their First Amendment censorship case.
But the other side needs to kill free speech in order to take over, so the stakes are very high. Here is Tedros (today) who in his eloquence talks about trust, and how to get it back—by killing free speech, of course. See how he twists the free speech story.
Internet and social media platforms have given people unprecedented access to health information. But they have also turbo charged the spread of mis- and disinformation, which has contributed to mistrust in vaccines and other health interventions, fuelled stigma and discrimination, and even led to violence against health workers and marginalized groups. During the COVID-19 pandemic, falsehoods about masks, vaccines and “lockdowns” spread as fast as the virus itself, and were almost as deadly. Just as mis- and disinformation undermined the response to the pandemic itself, so it continues to undermine negotiations on the WHO Pandemic Agreement. Media, celebrities, social media influencers and politicians have spread false claims that the Agreement will cede national sovereignty to WHO and give it the power to impose “lockdowns” or vaccine mandates on countries. As you know, these claims are, of course, entirely false. Sovereign governments are negotiating the agreement; and sovereign governments will implement it, in accordance with their own national laws. It’s easy to blame, dismiss, ridicule or insult those who believe or spread mis- or disinformation. To be sure, governments and internet and social media companies have a responsibility to prevent the spread of harmful lies and promote access to accurate health information. WHO is working with a range of companies and researchers and partners to understand how misinformation and disinformation spreads, who is targeted, how they’re influenced, and what we can do to counter this problem. But we must also make sure that when we seek the trust of others, we are ourselves trustworthy. We cannot assume or expect trust; we must earn it. [Good luck with that—Nass]
2 notes
·
View notes
Text
Parents in Oregon are calling to replace a local school board following reports of a sexually explicit book in the curriculum and at least two instances where a teacher organized activities discussing sexual acts.
Fox News Digital previously reported how health class students who missed coursework at Churchill High School in Eugene, Oregon, were asked via Canvas, an online learning management system, to complete a 10-point assignment titled "Fantasy Story."
"For those students who were absent, you will write a short story of a paragraph or two. This story is a sexual fantasy that will have NO penetration of any kind or oral sex (no way of passing an STI)."
The assignment from teacher Kirk Miller also asked students to choose three items, such as candles, massage oil, feathers and flavored syrup, to use in the story.

But parent Justin McCall said his older daughter, who is in the 10th grade at Churchill High School, revealed the assignment had also been conducted in class and that the teacher had asked students to pick the sexual items written on a piece of paper out of a hat that he passed around.
Further scrutiny of the "Health 2 Human Sexuality" class found that students were also allegedly given an assignment called "With Whom Would You Do it." The project involved a virtual spinning wheel labeled with sexual categories. Students were allegedly instructed to respond when the wheel stopped and write the initials of the person they would engage in the sex act with.
"My daughter told me it was literally up on the board and it mentioned you know who are you going to have anal penetration with, oral sex, licking of the ear, kissing and vaginal sex," McCall said, calling the assignment "disgusting and wrong."
A Title VI and IX coordinator attempted to speak with McCall's daughter about the incidents but was directed to the family's legal counsel.
The Eugene 4J School District did not return Fox News Digital’s request for comment.
The above allegations, as well as a flurry of other complaints from parents, have prompted calls for new leadership on the Eugene School Board, which is holding elections in May. In the event leadership stays the same, a group of parents is in the process of knocking on doors and setting up tables outside the district schools to gather signatures for a recall.
Beth Ball, a 4J alumni who met her husband at Churchill High School in the 1990s, intentionally enrolled her children in the 4J school district following her positive educational experience. Her issues began when her child entered sixth grade at the Arts & Technology Academy in Eugene.
At home, Ball noticed a form that said she could opt her child out of health class if she disagreed with the contents of the syllabus or curriculum. However, after correspondence with the teacher, Ball was told the syllabus and curriculum had not yet been created and waited at the school for two days to get a meeting with the principal.
The principal informed her they could not access the syllabus and curriculum, but her child needed to be enrolled in health class to graduate. Given the lack of information, Ball instructed her child to wait in the office daily during health class. Her child received a "no pass" in the class, but because of COVID-19 school closures, the "no-pass" became a non-issue.
Once her child entered the ninth grade at Churchill, the same school Ball attended, he was assigned a novel called "I'll Give You the Sun."
"The first five pages of it the book talks about how [the main character] is getting beat up and he's turned on by it. He gets a boner," Ball said. "For me, that's completely unacceptable because it's saying that if you're getting abused or picked on, it's normal and in fact you should like it."
A review of the book by Fox News Digital found the scene described by Ball and takes place early in the book when the main character wrestles with another student.
After contacting the school to ask why the book was placed in the curriculum, she was simply told that it got great reviews and spurred conversation on complex topics. She then pulled her child out of Churchill and began homeschooling him.
"And then this sexual essay hit, and I wasn't surprised in the least," Ball said.
Following the backlash, school principal Missy Cole said, "the district has begun the process of reviewing and selecting a new health curriculum to replace the OWL content that will be completed by the end of the school year."
According to the board, the changes to the curriculum were not related to complaints from parents and had already been on the docket for the 2023 school year.
During a March 16 school board meeting, citing "failures in our practices," Eugene School District Superintendent Andy Dey said they had identified "shortcomings" in their curriculum and recommended that the sexual fantasy's lesson not be administered again.
Dey also said the rumors of "spinning wheels" with "salacious acts" had not been substantiated or reflected in the materials the teachers used. However, Dey did confirm an "online virtual randomizer wheel" that did not have sexual acts on it.
He also said that the sexual fantasy's assignment was given out due to "inadequate oversight" and that future lessons will follow the curriculum verbatim and refrain from using supplemental materials, as was done in this case.
In a statement to the New York Post, OWL program manager Melanie Davis said the district was following an "unauthorized" and "out-of-context" facilitated group activity currently out of print.
The United Church of Christ and the Unitarian Universalist Association claimed the material was not part of the comprehensive OWL curriculum it helped to develop.
Emails provided to Fox News Digital showed that McCornack Elementary asked McCall to sign paperwork agreeing to only correspond with the school over email and only through his wife and noted McCall was trespassed off the property "due to hostile behavior towards other McCornack parents, students, and staff that created fear."
McCall was allowed to speak at the March 16 board meeting despite his ban from the elementary school. Following his public comments at the meeting and his move to recall the board, McCall was banned from all 4J district property. The district said they contact McCall no later than April 20 about his formal complaints and trespass appeal.
At the meeting, McCall claimed the school was lying about the spinning wheel assignment and suggested the teacher had not yet been fired because he is also the football coach and the team was doing well.
"If you do not remove him, I'm giving you my word today that tomorrow morning I will go down to the County Clerk's office and I will file for the removal of every single one of you," he said as the room erupted in cheers.
Miller has been placed on administrative leave.
Dr. Michael Bratland, a local dentist and conservative running for school board in the May elections, said that he wants a comprehensive review of the situation but stopped short of supporting the termination of the teacher or board members. He also credited Superintendent Dey for his leadership and handling of the situation but was more critical of the current board.
"The school board's the top and they need to take responsibility and when I was at that school board meeting last week, I didn't really hear anybody take accountability," he said.
#nunyas news#if people that age want to write stuff like that for themselvess#that's on them#teachers should not have anything close to that
16 notes
·
View notes
Text
Also preserved in our archive (Daily updates!)
By Mary Van Beusekom, MS
New findings from two studies have tied use of the antiviral drug nirmatrelvir-ritonavir (Paxlovid) to a reduction in COVID-19 hospitalizations and death, as well as to faster resolution of symptoms and less use of healthcare resources.
Benefit seen only in older patients For the first study, published in Clinical Microbiology and Infection, a Medical University of Vienna–led research team compared the effectiveness of Paxlovid with that of the antiviral drug molnupiravir (Lagevrio)—and with that of not receiving an antiviral—against hospitalization and all-cause death from January 2022 to May 2023. Participants were adults with mild to moderate infections and one or more risk factors for severe illness caused by the SARS-CoV-2 Omicron variant.
"The oral antivirals nirmatrelvir-ritonavir and molnupiravir are the mainstay treatment for Covid-19 in non-hospitalised adults at increased risk of severe disease," the study authors wrote. "Both oral antivirals were approved at the time of the study period (2022/2023) for the treatment of non-hospitalised patients with mild-to-moderate Covid-19, but the current National Institute of Health guidelines favour nirmatrelvir-ritonavir over molnupiravir."
Of the 113,399 eligible COVID-19 patients in the retrospective cohort study, 10.7% received Paxlovid, 9.5% received molnupiravir, and 80.0% served as untreated controls. Over 96% of participants were previously infected with or vaccinated against COVID-19.
A total of 0.43% of Paxlovid recipients, 1.4% of molnupiravir users, and 1.13% of controls were hospitalized within 28 days (risk difference [RD], -0.7%; Paxlovid vs control RD, 0.26%). No Paxlovid recipients and 0.13% each of molnupiravir users and controls died.
The estimated risk of hospitalization was 0.57% in Paxlovid users and 1.09% in controls (adjusted RD [aRD], -0.53%). The estimated risk of death was 0.0% in the Paxlovid group and 0.13% in controls (aRD, -0.13%).
The number of patients needed to treat to prevent hospitalization and death was 190 in Paxlovid recipients and 792 in controls, respectively. These statistically significant aRDs were seen only among patients 60 years and older.
The estimated risk of hospitalization in the molnupiravir analysis was 1.36% in the molnupiravir group and 1.16% among controls (aRD, 0.2%). The estimated risk of death was 0.12% in molnupiravir recipients and 0.14% in controls (aRD, -0.01%).
"Among outpatients aged ≥60 years with Covid-19 in an Omicron-dominated era, treatment with nirmatrelvir-ritonavir was associated with a lower risk of hospitalisation and all-cause death within 28 days, albeit with wide confidence intervals and high numbers needed to treat," the study authors wrote.
"This finding was not observed in molnupiravir users and younger nirmatrelvir-ritonavir users. Future studies are needed to better define target populations that show greater benefit from treatment with nirmatrelvir-ritonavir," they concluded.
Proportion of patients seeking care slashed 73% The second study, a phase 2/3 randomized clinical trial published today in Clinical Infectious Diseases, also found protection against COVID-19 hospitalization and death in adults receiving Paxlovid and demonstrated a faster resolution of symptoms and lower use of healthcare resources compared with a placebo in high-risk patients.
The research was led by researchers from Pfizer, which developed Paxlovid. The drug was given to 977 symptomatic COVID-19 patients, while 989 were given a placebo, at 343 sites in 21 countries from July 2021 through December 2021, a Delta-predominant period.
Paxlovid significantly shortened the time to symptom relief (median, 13 vs 15 days; hazard ratio, 1.27) and resolution (16 vs 19 days; HR, 1.20) through 28 days and cut the number of COVID-related medical visits by 64.3% and the proportion of patients seeking care by 73.2%.
In total, 0.9% of Paxlovid recipients and 6.4% in the placebo group were hospitalized, for a relative risk reduction of 85.5%. Hospitalized Paxlovid recipients had briefer hospital stays, and none required intensive care or mechanical ventilation. Fewer patients in the Paxlovid group needed other COVID-19 treatments, and none died by 6 months, compared with 15 in the placebo group.
"The importance of having effective COVID-19 treatments such as NMV/r [Paxlovid] to reduce burden on healthcare systems, both ambulatory and hospital based, should not be underestimated," the authors wrote.
Study Links: www.clinicalmicrobiologyandinfection.com/article/S1198-743X(24)00508-1/fulltext
academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae551/7889107
#mask up#covid#pandemic#public health#wear a mask#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#paxlovid#covid treatments
56 notes
·
View notes
Link
2 notes
·
View notes
Text
Comment period Ends June 17th 2024 Copied directly from the People's CDC website https://peoplescdc.org/2024/06/13/acip/ Also feel free to take inspiration from or borrow the language in our sample public comment below. Docket No. CDC–2024–0043 Updated 2024-2025 COVID vaccines must be recommended for people of all ages, regardless of health status. A restrictive approach to eligibility would create undue barriers for vulnerable people and discourage high risk people from getting needed vaccine boosters. The vaccine schedule should address waning efficacy in the months following vaccination [1-3] as well as emergence of new SARS-CoV-2 strains by recommending updated vaccination for all ages, at least every six months. Recent vaccination is also associated with a lower risk of developing Long COVID following a COVID infection [4] as well as a lower risk of Multisystem Inflammatory Syndrome in children (MIS-C) [5]. The CDC’s clear and unequivocal recommendation of updated COVID vaccination for all ages will influence what healthcare providers recommend, and what health insurances cover. Moreover, it will improve public awareness regarding the need for updated vaccination. The CDC must ensure equitable and affordable access to updated vaccines and prevent limited access because of financial constraints or demographics. The CDC’s Bridge vaccine access program is slated to end August 2024 and must be extended to ensure uninsured and underinsured people have access to the updated vaccines this fall [6]. References: 1. Link-Gelles R. Effectiveness of COVID-19 (2023-2024 Formula) vaccines. Presented at: FDA VRBPAC Meeting; June 5, 2024. Accessed June 12, 2024. https://www.fda.gov/media/179140/download Wu N, Joyal-Desmarais K, Vieira AM, et al. COVID-19 boosters versus primary series: update to a living review. The Lancet Respiratory Medicine. 2023;11(10):e87-e88. doi:10.1016/S2213-2600(23)00265-5 Menegale F, Manica M, Zardini A, et al. Evaluation of Waning of SARS-CoV-2 Vaccine–Induced Immunity: A Systematic Review and Meta-analysis. JAMA Netw Open. 2023;6(5):e2310650. doi:10.1001/jamanetworkopen.2023.10650 Fang Z, Ahrnsbrak R, Rekito A. Evidence Mounts That About 7% of US Adults Have Had Long COVID. JAMA. Published online June 7, 2024. doi:10.1001/jama.2024.11370 Yousaf AR. Notes from the Field: Surveillance for Multisystem Inflammatory Syndrome in Children — United States, 2023. MMWR Morb Mortal Wkly Rep. 2024;73. doi:10.15585/mmwr.mm7310a2 https://www.cdc.gov/vaccines/programs/bridge/index.html Full instructions for written and oral comment and meeting information can be found at: https://www.cdc.gov/vaccines/acip/meetings/index.html You can also register to give Oral Public Comment at the upcoming June 26-28 online CDC ACIP Meeting at: https://www2.cdc.gov/vaccines/acip/acip_publiccomment.asp You must register by June 17 at 11:59pm Eastern Standard Time CDC’s ACIP meeting information on the Federal Register: https://www.federalregister.gov/documents/2024/05/24/2024-11439/meeting-of-the-advisory-committee-on-immunization-practices
Why? WHY??
U.S. CDC taking comments on potentially limiting COVID vaccine availbility by age or health status. Never mind that the vaccine is crucial to limiting long-term effects that could lead to immunocomprimisation. 🤬🤬🤬


You can comment here.
8K notes
·
View notes
Text
The 2024 U.S. Elections Weren't The Only Time donald j. trump Worked With Russians Against United States' Interests: COVID-19, USA Patriot Act Sabotage, The Massive Russian SolarWinds Hack of the U.S. Government and Private Sector, and Russian 2024 Election Day Interference (compiled from Wikipedia):

In November 2019, a security researcher notified SolarWinds that credentials to a third party FTP server had a weak password of "solarwinds123", warning that "any hacker could upload malicious [code]" that would then be distributed to SolarWinds customers. The New York Times reported SolarWinds did not employ a chief information security officer and that employee passwords had been posted on GitHub in 2019.
December 1, 2019: COVID-19 pandemic: First known human case of Coronavirus disease 2019, in Wuhan, Hubei, China.
December 5, 2019: Speaker of the U.S. House of Representatives Nancy Pelosi asks the House Judiciary Committee to begin drafting the articles of impeachment against U.S. President Donald Trump.
December 9, 2019: The World Anti-Doping Agency votes unanimously to ban Russia from international sport for four years for doping offences, meaning it will be excluded from the 2020 Summer Olympics in Tokyo, the 2022 Winter Olympics in Beijing and the 2022 World Cup in Qatar.
December 10, 2019: Democrats in the United States House of Representatives announce formal charges against President Donald Trump, accusing him of abusing power and "obstructing Congress"; he becomes the third U.S. president in history to face impeachment.
December 18, 2019: The U.S. House of Representatives approves two articles of impeachment against President donald trump, making him the third president to be impeached in the nation's history.
December 29, 2019: The Taliban's ruling council agrees to a temporary cease-fire in Afghanistan, opening a door to a peace agreement with the United States.
In January and February 2020, U.S. intelligence agencies delivered over a dozen classified warnings in the President's Daily Brief about COVID-19, including its potential to inflict severe political and economic damage. President Donald Trump typically did not read daily briefs and often has "little patience" for oral summaries, The Washington Post reported. Each brief was also shared with other officials in the administration. The Office of the Director of National Intelligence, which produces the President's Daily Brief, denied that there were repeated mentions of COVID-19.
On January 8, 2020, the U.S. Centers for Disease Control and Prevention (CDC) released a health advisory regarding an outbreak of pneumonia in Wuhan, Hubei Province, China, which was being caused by a yet-unidentified virus.
January 16, 2020: The first impeachment trial of the President of the United States, Donald Trump, begins in the U.S. Senate.
On January 27, 2020, Anthony Fauci, head of the National Institute of Allergy and Infectious Diseases, predicted that "things are going to get worse before they get better". Three days later, Fauci stated that the COVID-19 outbreak "could turn into a global pandemic".
On February 10, 2020, Trump stated that "a lot of people think that [COVID-19] goes away in April with the heat … Typically, that will go away in April" (later, on April 3, he denied ever having given "a date" for the departure of the virus)
On February 13, 2020, CDC director Robert Redfield contradicted Trump, saying that the "virus is probably with us beyond this season, beyond this year". Redfield also predicted that it "will become a community virus at some point in time, this year or next year".
On February 16, 2020, Anthony Fauci warned that it was not necessarily true that COVID-19 would "disappear with the warm weather."
February 25, 2020, was the day that the CDC first warned the American public to prepare for a local outbreak. That day, Nancy Messonnier, head of the CDC's National Center for Immunization and Respiratory Diseases, said that "We are asking the American public to work with us to prepare for the expectation that this is going to be bad." Messonnier predicted that "we will see community spread in this country", and it was only a matter of time. As a result, "disruption to everyday life might be severe". Messonnier stated that the CDC is preparing, and "now is the time for hospitals, schools and everyday people to begin preparing as well."
On February 25, 2020, Anthony Fauci declared that given how COVID-19 was spreading in other nations, it was "inevitable that this will come to the United States" as well. On February 26, CDC Director Robert Redfield said it would be "prudent to assume this pathogen will be with us for some time to come".
On February 26, 2020, Trump contradicted Messonnier, stating: "I don't think it's inevitable" that a U.S. outbreak would occur, "It probably will, it possibly will … Whatever happens, we're totally prepared." Trump additionally declared that the number of infected was "going very substantially down, not up".
On February 27, 2020, The chairman of the Senate Intelligence Committee, Richard Burr, who helped to write the Pandemic and All-Hazards Preparedness Act (PAHPA), which forms the framework for the federal response, warned a private group of his constituents that COVID-19 is much more aggressive in its transmission than anything that we have seen in recent history, and is probably more akin to the 1918 Spanish Flu pandemic. "There will be, I'm sure, times that communities, probably some in North Carolina, have a transmission rate where they say, 'Let's close schools for two weeks. Everybody stay home,' We're going to send a military hospital there; it's going to be in tents and going to be set up on the ground somewhere, It's going to be a decision the president and DOD make."
On February 27, 2020, Trump said of the virus: "It's going to disappear. One day it's like a miracle, it will disappear. And from our shores, you know, it could get worse before it gets better. Could maybe go away. We'll see what happens. Nobody really knows." Also on February 27, Trump declared that the risk to the American public from COVID-19 "remains very low".
February 27, 2020 stock market crash: Triggered by fears of the spreading of COVID-19, the Dow Jones Industrial Average (DJIA) plunges by 1,190.95 points, or 4.4%, to close at 25,766.64, its largest one-day point decline at the time. This follows several days of large falls, marking the worst week for the index since the 2007–2008 financial crisis.
On February 29, 2020, Trump said that "additional cases in the United States are likely", but "there's no reason to panic at all." When a reporter asked Trump: "How should Americans prepare for this virus?" Trump answered: "I hope they don't change their routine".
February 29, 2020: A conditional peace agreement is signed between the United States and the Taliban. The U.S. begins gradually withdrawing combat troops from Afghanistan on March 10.
On March 4, 2020, donald trump appeared on Fox News's Hannity by phone, where he claimed a 3.4% mortality rate projected by the World Health Organization (WHO) was a "false number", and stated his "hunch" that the true figure would be "way under 1%". Trump also predicted that many people infected with COVID-19 would experience "very mild" symptoms, "get better very rapidly" and thus they "don't even call a doctor". Thus, there may be "hundreds of thousands of people that get better just by, you know, sitting around and even going to work—some of them go to work, but they get better."
From March 6 to March 12, 2020, donald trump stated on four occasions that the coronavirus would "go away". On March 10, Surgeon General Jerome Adams stated that "this is likely going to get worse before it gets better."
March 2020: The nightmare of empty grocery shelves begins. Stores capping/limiting purchases.
By March 11, 2020, the virus had spread to 110 countries, and the WHO officially declared a pandemic. The CDC had already warned that large numbers of people needing hospital care could overload the healthcare system, which would lead to otherwise preventable deaths. Director of the National Institute of Allergy and Infectious Diseases Anthony Fauci said the mortality from COVID-19 was ten times higher than the common flu. By March 12, diagnosed cases of COVID-19 in the U.S. exceeded a thousand. Trump declared a national emergency on March 13. On March 16, the White House advised against any gatherings of more than ten people. Three days later, the United States Department of State advised U.S. citizens to avoid all international travel.
The USA Freedom Act, which became law on June 2, 2015, reenacted the expired USA Patriot Act sections through 2019. However, Section 215 of the law was amended to disallow the National Security Agency (NSA) to continue its mass phone data collection program. Instead, phone companies will retain the data and the NSA can obtain information about targeted individuals with a federal search warrant.
On August 14, 2019, the outgoing Director of National Intelligence sent a letter to Congress stating the Trump Administration's intention to seek permanent extension of the provisions of FISA that under the terms of the USA FREEDOM Act are scheduled to expire on December 15, 2019, namely the "lone wolf" authority allowing surveillance of a suspected terrorist who is inspired by foreign ideology but is not acting at the direction of a foreign party, the roving wiretap authority regarding surveillance of a terrorist who enters the United States and the authority to allow the Federal Bureau of Investigation to obtain certain business records in a national security investigation, as well as the call detail records program undertaken by the NSA. In reference to the latter authority, the letter announced that "The National Security Agency has suspended the call detail records program that uses this authority and deleted the call detail records acquired under this authority."
Jurisdiction over the reauthorization of the expiring FISA provisions is shared by the Judiciary and Intelligence committees in the U.S. Senate and the U.S. House of Representatives; the House Committee on the Judiciary and the Senate Committee on the Judiciary held separate public hearings on the reauthorization in September 2019 and November 2019, respectively. Opposition to the call detail records program has led to some Congressional demands that the authority for the program not be renewed. Additional complications hindering reauthorization arose from a report of the US Department of Justice Inspector General finding fault with certain FISA applications in connection with the 2016 presidential campaign, which led some members of Congress to insist on reforms to FISA as a condition of reauthorizing the expiring USA FREEDOM Act provisions.With Congressional attention focused on dealing with the COVID-19 pandemic in the United States in 2020, the House of Representatives passed a long-term extension of the USA FREEDOM Act on March 11, 2020, just four days before the scheduled expiration of the Act on March 15, 2020, by a wide, bipartisan margin that kept the protections of the Act largely the same. Two months later, in May of 2020, the Senate passed an extension of the Act by an 80-16 vote that expanded some privacy protections, but the Senate version did not include protection of Americans’ internet browsing and search histories from warrantless surveillance, which was proposed by Sens. Ron Wyden (D-Ore.) and Steve Daines (R-Mont.) and failed by one vote.
According to former KGB major Yuri Shvets, donald trump became the target of a joint Czech intelligence services and KGB spying operation after he married Czech model Ivana Zelnickova and was cultivated as an "asset" by Russian intelligence since 1977: "Russian intelligence gained an interest in Trump as far back as 1977, viewing Trump as an exploitable target." Luke Harding writes that documents show Czechoslovakia spied on donald trump during the 1970s and 1980s, when he was married to Ivana Trump, his Czechoslovakia-born first wife. Harding writes that the Czechoslovakian government spied on donald trump because of his political ambitions and notability as a businessman. It is known that there were close ties between Czechoslovakia's StB and the USSR's KGB. Harding also describes how, already since 1987, the Soviet Union was interested in Trump. In his book Collusion, Harding asserts that the "top level of the Soviet diplomatic service arranged his 1987 Moscow visit. With assistance from the KGB." Then-KGB head Vladimir Kryuchkov "wanted KGB staff abroad to recruit more Americans". Harding proceeds to describe the KGB's cultivation process, and posits that they may have opened a file on Trump as early as 1977, when he married Ivana. "According to files in Prague, declassified in 2016, Czech spies kept a close eye on the couple in Manhattan, … [with] periodic surveillance of the trump family in the United States."
donald j. trump and His Republican Allies Allowed the USA PATRIOT ACT/FREEDOM ACT to Expire on March 15, 2020 and They Didn't Reinstate It until May of 2020.
March 2020: SolarWinds's Orion software hotfixes were released to 33,000 Orion customers.Hackers acquired superuser access to SAML token-signing certificates. This SAML certificate was then used to forge new tokens to allow hackers trusted and highly privileged access to networks. The attack used a backdoor in a SolarWinds library; when an update to SolarWinds occurred, the malicious attack would go unnoticed due to the trusted certificate. APT29, aka Cozy Bear, working for the Russian Foreign Intelligence Service (SVR), was reported to be behind the 2020 attack. Victims of this attack include the cybersecurity firm FireEye, the US Treasury Department, the US Department of Commerce's National Telecommunications and Information Administration, as well as the US Department of Homeland Security. Prominent international SolarWinds customers investigating whether they were impacted include the North Atlantic Treaty Organization (NATO), the European Parliament, UK Government Communications Headquarters, the UK Ministry of Defence, the UK National Health Service (NHS), the UK Home Office, and AstraZeneca.
December 13, 2020: SolarWinds begins notifying customers, including a post on its Twitter account, “SolarWinds asks all customers to upgrade immediately to Orion Platform version 2020.2.1 HF 1 to address a security vulnerability.”
December 15, 2020 SolarWinds Victims named and timeline moves back — Wall Street Journal reported that the U.S. Commerce and Treasury Departments, the Department of Homeland Security (DHS), the National Institutes of Health, and the State Department were all affected. Various security officials and vendors expressed serious dismay that the attack was more widespread and began much earlier than expected. The initial attack date was now pegged to sometime in March 2020, which meant the attack had been underway for months before its detection.
December 17, 2020: New SolarWinds victims revealed — The Energy Department (DOE) and National Nuclear Security Administration (NNSA), which maintains the U.S. nuclear weapons stockpile, were publicly named as victims of the attack.
On December 21, 2020, Attorney General William Barr, Secretary of State Mike Pompeo, the FBI, the Cybersecurity and Infrastructure Security Agency, or CISA, and the Office of the Director of National Intelligence all agreed that Russia was engaged in “a significant and ongoing cybersecurity campaign” against the United States. Russian asset, donald j. trump, immediately replied: “The Cyber Hack is far greater in the Fake News Media than in actuality,” Trump wrote. “I have been fully briefed and everything is well under control. Russia, Russia, Russia is the priority chant when anything happens because Lamestream is, for mostly financial reasons, petrified of discussing the possibility that it may be China (it may!).”
March 16, 2020: stock market crash: The the Dow Jones Industrial Average (DJIA) falls by 2,997.10, the single largest point drop in history and the second-largest percentage drop ever at 12.93%, an even greater crash than Black Monday (1929). This follows the U.S. Federal Reserve announcing that it will cut its target interest rate to 0–0.25%.
On March 19, 2020, donald trump told journalist Bob Woodward that he was deliberately downplaying the risk when communicating with the public. "I wanted to always play it down," Trump said. "I still like playing it down, because I don't want to create a panic."
On March 24, 2020, donald trump argued that: "We lose thousands and thousands of people a year to the flu … But we've never closed down the country for the flu." On March 27, he stated: "You can call it a flu. You can call it a virus. You know you can call it many different names. I'm not sure anybody even knows what it is."
On March 24, 2020, donald trump declared that "we begin to see the light at the end of the tunnel"; a day later the U.S. surpassed 1,000 COVID-19 deaths.
Throughout March and early April, several state, city, and county governments imposed "stay at home" quarantines on their populations to stem the spread of the virus. By March 26, The New York Times data showed the United States to have the highest number of known cases of any country. By March 27, the country had reported over 100,000 cases.
From March 30 to April 7, 2020, Trump stated on four occasions that COVID-19 would "go away".
On March 31, 2020, contradicting his many previous comparisons of COVID-19 to the flu, Trump said: "It's not the flu … It's vicious". When reporters asked him if his initial dismissive comments on the virus had misled Americans, he replied: "I want to give people a feeling of hope. I could be very negative … You know, I'm a cheerleader for the country." Asked further if he had known—despite his claims that the outbreak was under control—that the situation would turn out so severe, Trump replied: "I thought it could be. I knew everything. I knew it could be horrible, and I knew it could be maybe good."
On November 5, 2024, during the official Election Day, several non-credible bomb threats that originated from Russia briefly disrupted voting in two polling places in Fulton County, Georgia. Both re-opened after about 30 minutes. Republican Georgia Secretary of State Brad Raffensperger said Russian interference was behind the Election Day bomb hoaxes. In a statement, the FBI said it was aware of non-credible bomb threats to polling locations in several states, with many of them originating from Russian email domains. The bomb threats were solely made against Democratic-leaning areas. On the same day, U.S. federal officials again reported that Russian sources were actively engaged in "influence operations", citing disinformation in specific videos that falsely claimed Kamala Harris had taken a bribe and false news stories about the Democratic Party and election fraud in Georgia.
On November 8, 2024 it was reported that one of the Russian email addresses behind Election Day bomb threats was used in June 2024 bomb threats targeting LGBTQ+ events in Massachusetts, Minnesota and Texas.
#2024 presidential election#2024 election#election 2024#kamala harris#harris walz 2024#donald trump#trump#trump 2024#president trump#trump vance 2024#republicans#gop#evangelicals#democrats#us elections#american politics#us politics#politics#uspol#us election 2024
21 notes
·
View notes
Text
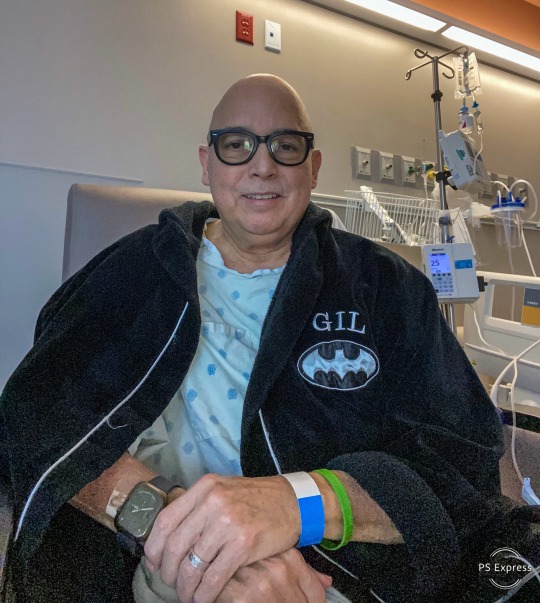
Just a matter of hours away from my 8th Lungaversary. This year, like the previous 7 years has not been without its health pitfalls. All of which are par for the course of a Bilateral Lung Transplant survivor. Covid 19 got me twice this year as well as a lung infection in between the two bouts of Covid. As I type this farewell to 2024 I am in-patient at University Hospital under treatment for Bacterial Pneumonia. The amazing news is I have just been discharged to go home and continue my treatment with oral antibiotics. I am ready to head home and be with my loving family for the new year!
Goodbye 2024!
0 notes
Text
just as a PSA, for anon and others, the reason (that the US government owns a patent for the medical benefits of cannabis, and that) cannabis has been kept illegal is because it would destroy the trillion dollar oncology industry that's existed for over a century.
If you could grow the cure for cancer easily in your backyard as a weed, and sell it / buy it at affordable prices with lots of trusted suppliers (hint- no monopolization of the market) then the precious pharmaceutical industry would shrink a lot and those people would have to go through the ever-so-tedious process of finding new jobs, which is the alternative to people continuing to die of cancer.
But not being able to call cannabis oil a cure for cancer is like not being able to call pizza delicious, because only the king is delicious. All hail the king, the monopolizer of medicine, the pharmaceutical industry and their lap dog Web MD.
Why hasn't the FDA ever done one study on orally ingested cannabis oil as a cure for cancer?
Why do they only test smoking flower, 19 times and they say smoking flower certainly doesn't cure cancer?... that's like having someone in cardiac arrest and using the defib paddles on their ass and concluding the paddles just don't work and should be discontinued, meanwhile patient dies.
If you want to sacrifice your life at the feet of the FDA, who for a time declared that masks don't stop covid so they could speculate on the price of those masks, then please, I can't stop you or anyone from fulfilling their life goal of being a sacrificial lamb for the feds. If having cancer and doing that whole dance is what makes someone happy, hey it's a free country, go right ahead. Just know that my generation, the young ones, we will be the first generation to no longer suffer from cardiac arrest or cancer. We refuse to eat the cow and pig, thus our arteries and cells don't get polluted so fast. Many of us are vegetarian, which further reduces toxin intake, many of us swear by using the sauna, which enables detoxification on a level unlike anything else.
It's a sad sight to see people still dying of cancer, open minded people like Bill Walton, a heroic basketball player and lovable weirdo famous for being open to cannabis. Somehow even this info didn't make it to Bill, which just highlights how suppressed it really is. Lifelong hippies are still dying of cancer unaware that cannabis can be refined into a high-potency oil that cures cancer when ingested inside a capsule. I'm begging the FDA to test this and publish the honest results, but they never will, because they're for profit, not science or health.
This is asking about any significantly life altering or life threatening physical disease, infection, or accident that a medical specialist would have needed to test/treat you for.
Anon had a cancer scare that thankfully turned out to be benign, but some bad info and a long waitlist made for a very stressful time. They're wondering how many others have been there!
–
We ask your questions so you don’t have to! Submit your questions to have them posted anonymously as polls.
4K notes
·
View notes
Text
Dental Scalers Market Set to Witness an Uptick during 2023-2033
The global dental scalers market was valued at USD 918 million in 2023 and it is anticipated to grow further to USD 1658 million by 2033, at a CAGR of 6.1% during the forecast period.
View The Full Report Here –https://www.globalinsightservices.com/reports/dental-scalers-market/
Dental Scalar is a specialized dental instrument used by oral health professionals to remove dental plaque, calculus (tartar), and other debris from the surfaces of teeth. It is an essential tool in dental cleanings and periodontal treatments, aiming to maintain optimal oral hygiene and prevent periodontal diseases. Dental scalers typically have sharp, curved tips designed to access hard-to-reach areas around the teeth and gums. During the scaling procedure, the dental scaler’s tip is gently applied to the tooth surface, effectively dislodging and scraping away the accumulated plaque and calculus. By removing these harmful deposits, dental scalers aid in reducing the risk of gum disease, tooth decay, and other oral health issues, promoting overall oral well-being for patients.
Market Trends and Drivers
Increasing prevalence of oral health issues and the growing awareness of the importance of oral hygiene. As dental conditions such as plaque buildup, tartar formation, and gum disease become more common, the demand for efficient and effective dental scalars rises. Additionally, advancements in technology and product innovation have led to the development of sophisticated dental scalar devices, offering better precision, ease of use, and patient comfort. Dental professionals are increasingly adopting these advanced tools to enhance their treatment capabilities, driving market growth. Furthermore, the rising geriatric population, with a higher propensity for dental problems, further contributes to the expanding market as dental scalars become a vital tool in geriatric oral care.
Market Restraints and Challenges
One significant restraint in the dental scaler market is the high cost associated with advanced dental scaling equipment and technologies. Dental scalers, which are essential tools for removing plaque and tartar from teeth, have seen considerable advancements in recent years, incorporating features like ultrasonic technology and ergonomic designs. However, the implementation of these innovations often results in higher production costs, which subsequently translate to elevated prices for dental professionals and consumers. The cost barrier may hinder smaller dental practices or those in economically constrained regions from adopting the latest scaler technologies, limiting their ability to provide the most cutting-edge dental care to their patients. Additionally, the high costs could also deter potential buyers from upgrading their existing equipment, thereby impacting the overall market growth for dental scalers. Manufacturers and stakeholders in the dental industry will need to find ways to balance technological advancements with affordability to address this restraint effectively.
Global Dental Scalers Market Segmentation
By Product
Powered Dental Scalers
Handheld Dental Scalers
Scaler Inserts
By Application
Periodontics
Endodontics
Others
By End User
Hospitals
Dental Clinics
Others
Unlock Growth Potential in Your Industry – Get Your Sample Report Now –https://www.globalinsightservices.com/request-sample/GIS10425/
Major Players in the Global Dental Scalers Market
The report analyses the major players such as Henry Schein, Inc., Dentsply Sirona, COLTENE Group, EMS Medical, Hu-Friedy Mfg. Co., LLC, Parkell, Inc., Nordent Manufacturing, Inc., and Micron Corporation among others.
COVID-19 Impact
The North American dental scalers market was significantly hit by the COVID-19 outbreak in 2020. The governments of several countries in the region were forced to impose restrictions on trade and transportation activities as a result of the exponential rise in COVID-19 cases. This forced the closure of manufacturing facilities and had an impact on a number of offline distributors of dental scalers. The frequency of dental appointments decreased by almost 27% in May 2020 compared to May 2019 due to the rising dangers of cross-infection. Sales of dental scaler goods decreased as a result in 2020.
Research Scope
Scope – Highlights, Trends, Insights. Attractiveness, Forecast
Market Sizing – Product Type, End User, Offering Type, Technology, Region, Country, Others
Market Dynamics – Market Segmentation, Demand and Supply, Bargaining Power of Buyers and Sellers, Drivers, Restraints, Opportunities, Threat Analysis, Impact Analysis, Porters 5 Forces, Ansoff Analysis, Supply Chain
Business Framework – Case Studies, Regulatory Landscape, Pricing, Policies and Regulations, New Product Launches. M&As, Recent Developments
Competitive Landscape – Market Share Analysis, Market Leaders, Emerging Players, Vendor Benchmarking, Developmental Strategy Benchmarking, PESTLE Analysis, Value Chain Analysis
Company Profiles – Overview, Business Segments, Business Performance, Product Offering, Key Developmental Strategies, SWOT Analysis.
With Global Insight Services, you receive:
10-year forecast to help you make strategic decisions
In-depth segmentation which can be customized as per your requirements
Free consultation with lead analyst of the report
Infographic excel data pack, easy to analyze big data
Robust and transparent research methodology
Unmatched data quality and after sales service
Contact Us:
Global Insight Services LLC 16192, Coastal Highway, Lewes DE 19958 E-mail: [email protected] Phone: +1-833-761-1700 Website: https://www.globalinsightservices.com/
About Global Insight Services:
Global Insight Services (GIS) is a leading multi-industry market research firm headquartered in Delaware, US. We are committed to providing our clients with highest quality data, analysis, and tools to meet all their market research needs. With GIS, you can be assured of the quality of the deliverables, robust & transparent research methodology, and superior service.
#Dental Scalers Market#Dental Scalers Market Analysis#Dental Scalers Market Demand#dental scalers Market Growth#dental scalers Market Size
0 notes