#moles per litre
Explore tagged Tumblr posts
Text
Physics "Friday" #11 [Opinion]: Okay so I have a few more problems with SI Units
Preamble: Now what Missy?
Education Level: Primary School (Y5/6)
Topic: Measuring Systems (Metrology)
Ok so this was going to be my necessary follow up to my first opinion post, Is Fahrenheit the better temperature scale?
I was meant to save it for the next time I had too much stuff to deal with on Friday and needed to write something easier. In fact, that's going to be how I do opinion posts - when I don't need to do as much a-thunkin'.
Regardless, there are of course other problems with the SI units system that many people point out. This is basically me doing it myself.
What are the SI Units?
During the french revolution, there were several attempts to metricise the existing unit system. Generating standardised units on length, volume, weight, temperature ... they even tried to decimalise time.
These units were based off physical constants:
The metre was one 10 millionth the distance from the north pole to the equator
The litre was the volume taken up by one kilogram of water
Celsius was based on the boiling/melting points of water
A kilogram was based on the weight of a specific Platinum-Iridium Alloy called the IPK (International Prototype Kilogram)
Now obviously, these weren't perfect constants. The earth's radius changed, the boiling point of water depends on the pressure of the environment, and the IPK can vary based on surrounding conditions.
This was fixed in 2019, when every SI measurement unit was defined using a universal constant rather than physical objects like a mass or the earth:
Hyperfine transition frequency of Caesium
Speed of light
Planck Constant
Charge of the Electron
Boltzmann Constant
Avogadro's Constant
Luminous Efficacy of 540 THz radiation
This gives our seven SI units:
Time (Second)
Distance (Meter)
Mass (Kilogram)
Charge (Columb)
Temperature (Kelvin)
Amount (Mole)
Luminous Intensity (Candela)
Problem #1: Unit-less units
There are several units that come from mathematics that appear to be unitless, like:
Angle (Radians)
Solid Angle (Steradians)
Amount (Number count)
We use this all the time like in lumens (candela steradians), angular velocity (radians per second), and number density (particles per cubic metre).
Wait ... amount was already listed as an SI quanity - it was a mole wasn't it?
That's exactly it. A mole is just defined as 6.022×10²³ objects of something. This is where we experience our first problem.
Why is radian and steradian not considered a unit in it's own right but a mole is?
Radians are defined as being one metre per metre. The angle required to create an arc with a length of 1 metre given a radius of one metre.

Image Credit: BYJU's
This comes from the formula S=Rθ where circular arc length (S) is equal to the radius (R) multiplied by angle in radians (θ).
The problem with this, is that the radian is still an arbitrary dimension. We could've chosen our standard angle unit to be a degree, and all we need to do is change the definition of the sine function and the above arc length definition:
S=kθR were kθ is dimensionless and k = 1 rad⁻¹
It's almost as if, while the radian is dimensionless, it still behaves and acts like a unit. Much like how we can redefine everything and make the unit for length be a foot instead, we can do the same with angle.
You may object, as S=Rθ looks a lot more fundamental than adding an extra unneeded constant. But it's like if we were to use natural units, where the speed of light is equal to 1, making E = mc² actually E = m.
This doesn't mean that m is somehow 'fundamental' when we define it in terms of energy. All we've done is redefine our unit system in a way that makes a few constants equal to one.
The same thing goes for radians.
A little side-note on Intensity
You could argue that this also applies to relative intensity units like Magnitude or Bels. However, I'd argue that they don't count as their unit systems are defined as being relative. The strength of a signal or luminosity is just choosing a unit system of power.
For example, relative magnitude is based on the luminosity of the sun. Sound/Signal amplitude is based on watts or volts.
Problem #2: Redundant Units
Many of the quantities in the SI unit system are technically redundant. Temperature can just be redefined as energy density, mass can be redefined as energy, etc.
What I'm concerned with is one unit in particular: the Candela - what exactly is it?
I often find that a lot of people attempting to explain the SI unit system often brush over this unit, even though it feels rather important given it's called 'Luminous Intensity'.
But hold on, isn't luminosity just about energy production? After all, radiation is just energy, and it's emitted as a form of energy.
A candela is equal to one lumen per steradian, where a lumen is the total luminous intensity of an object that emits light everywhere. Candelas concern luminous intensity from a specific solid angle view of the object.
Watts, the measurement of power and bolometric luminosity, measure the total (or bolometric) luminosity of an object at all wavelengths of light.
Lumens, on the other hand, represent the power produced by an object after each wavelength is passed through some filtering function that accounts for how the human eye sees light. This weighting function is determined by the ISO.
Of course, our eyes only see light within a particular range, and inside of that range, different wavelengths appear more intense to us because we pick it up more.
But this fails to recognise that this is still just a glorified unit of power. Just because it's weighted based on some function, shouldn't change what unit is necessarily uses. Hence it is technically redundant as it can be defined as a combination of other SI units - similar to radians.
Problem #3: When the unit system isn't used
I mentioned it in the last post, but there are a lot of occasions where the standard SI units aren't used. The worst culprit I feel is Astronomy, with Physics only having issues in lieu of SI vs. Natural units vs. Electron/Atomic Units. Again here's the list:
My beloved SI units
CGS Units
Whatever the fuck a Jansky is
Don't even start with natural units I can't live without big G
"Ampere in CGS units is g1/2 cm3/2 s−2"
Solar Luminosity/Mass of Sun
Angstroms (like please can we just use nanometers?)
How many Jupiters or Earths fit into this cloud of gas?
The vomit of parallax units i.e. AU, pc, Mpc, arcseconds, radians
Steradians (Solid angles can be finicky)
Logarithms, logarithms everywhere!
Hubble's constant being in km/s/Mpc but then having to turn that into Hz or per year - like can someone please acknowledged how cursed this is?
When you do Kepler's 3rd law on Mercury and realise it doesn't work (because you forgot Einstein existed) ... so no units end up working
ADUs and/or whatever you get when you deal with telescope outputs
Sidereal time, J2000, etc.
Sky Coordinates (it always depends on the telescope mount)
(the last two are new entries into the list I forgot!)
A lot of these units are very, very, annoying to handle. Often at times because they are just so unnecessary. We have scientific notation for a reason - so why are we using Jansky? Why ever use the mass of planets unless if we are talking about specifically exoplanets?
It can especially be annoying when in astronomy course subjects, unit conversions make up like 50% of the work and 50% of the error in calculations.
And the biggest problem, of course, is the CGS units system. I hate it. So much. From what I know CGS is used simply because it appears as more correctly "scaled" for a lot of astronomical processes.
However, the problem is that it just adds extra conversion factors into every equation. Now I have to remember big G in both CGS units as well as in SI or solar units etc. And it doesn't jive with a lot of other astronomical units.
J2000 is also rather annoying - why are we using the Julian calendar at all? Shouldn't we try and strive for using a more accurate year instead? Because what happens is that every four years the calendar shifts by a day.
The "Ampere in CGS units is g1/2 cm3/2 s-2" is a direct quote from one of my professors. And it also makes very little sense - because it says that the coulomb and ampere units are actually redundant.
However, CGS units aren't usually the only thing we use. Sometimes we put things in Gaussian CGS units, where we define the coulomb's constant (kₑ) as being 1. Thus, similar to natural units, we can define a statCoulomb in terms of our three base units:
1 statC = 1 g1/2⋅cm3/2⋅s−1.
Now this is where we find our ampere definition! But wait ... this is materially different to the real ampere. In fact, we can determine the difference between coulombs and statcoulombs:
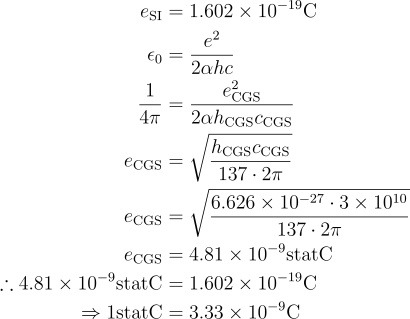
So they are different! And thus, after several paragraphs, my lecturer is wrong and they shouldn't've used the Ampere but a different stat ampere.
So what would I do?
Well, the big problem with all unit systems is that they are just equivalent to eachother dimensionally.
Because of all the seperate equations surrounding each of the SI units, we can define any SI unit in terms of any other SI unit combination just by setting certain constants to one.
So there's quite a lot of units that we could me missing.
But here's how I would do it ...
There are five unique SI units:
Distance - Metre
Time - Second
Mass - Kilogram
Charge - Coulomb
Temperature - Kelvin
There are also three dimensionless/special units:
Amount - Mole
Angle - Radian
Luminosity - Lumen
These three units are special as they can be expressed as dimensionless constants or in terms of other units, however we want them to be expressed in our own specific units.
Solid Angle is just determined from square radians where 1 sr = π/4 rad² (ratio of square and circle of same diameter).
The constants which define these unique units are:
Hyperfine transition frequency of Caesium
Speed of light
Planck Constant
Charge of the Electron
Boltzmann Constant
For the non-unique units we have:
Avogadro's Constant
Pi
Luminous Efficacy of 540 THz radiation
Conclusion
This post did end up taking longer than I expected ... much longer. Nevertheless it was fun to do!
As stated in the start, opinion posts are for those Fridays where things aren't as easy as spending 2-3 hrs writing a post. Because when you want to argue a point you have a little more passion behind it.
Anywho. Did you know that only 5% of people who read this post actually follow this channel?. SMASH THAT FUCKING LIKE AND SUBSCRIBE. Brought to you by Raid Skillspace VPN™.
Feedback's always welcome. Come and debate me you cowards. Follow if you like it ... or don't.
#physics friday#stem#academics#stemblr#science#astronomy#si units#Finding hashtags for metrology is a bit difficult
10 notes
·
View notes
Text
Different methods of expressing the concentration of solution

In Chemistry, “Concentration” expresses the amount of solute present in the solution. Suppose you have been given two jars containing lemon juice and added different quantities of sugar. How will you express its concentration? You will say lemon juice in one jar is “too sweet” and “less sweet” in another. But Chemistry doesn’t use the word “too” to express concentrations. We have to define it.
A solution in which a relative amount of solute is less is known as a dilute solution, and one with a large amount of solute is known as a concentrated solution. But these are the relative terms and do not give quantitative information about the solution. Thus, in this article, we will look at the different methods of expressing the concentration of solutions.
So, let’s start!
1) Solution concentration or strength:
It describes the amount of solute present in one litre of solution. It is denoted by C or S, and its unit is g/L. It is also known as the strength of the solution.

For example: If 5 grams of salt is added to 0.5 L water, then the concentration of the solution will be 5/0.5 = 10 g/L.
2) Concentration in parts per million (ppm):
It describes the part of a particular component per million (106) parts of the solution. It is expressed as milligrams of solute present in one litre of solution, i.e., mg/L. Another unit for ppm is μg/mL.

There are some more units used to express concentration in parts: parts per billion (ppb, parts per 109) and parts per trillion (ppt, parts per 1012) and parts per quadrillion (ppq, parts per 1015).
3) Mass/weight percentage or Weight/mass percentage (w/w):
The mass percentage of a solution is the concentration of the solution expressed as the per cent of one component in the solution by mass. Suppose a solution consists of two components: solute A and solvent B, then the mass percentage of A will be

For example, 30% (w/w) glucose means 30 grams of glucose (solute) is present in 100 grams of the solution, i.e., 30 grams of glucose is dissolved in 70 grams of water.
4) Volume percentage (v/v):
The volume percentage of a solution is the concentration of the solution expressed as the per cent of one component in the solution by volume. Suppose a solution consists of two components: solute A and solvent B, then the volume percentage of A will be

For example, 20% (v/v) ethanol means 20 mL of ethanol is present in 100 mL of the solution, i.e., 20 grams of ethanol is mixed with 80 mL of water.
5) Mass by Volume percentage (w/v):
It means the mass of a solute dissolved per 100 mL of the solution. This phenomenon is majorly used in the pharmaceutical industry.

6) Molarity (M):
Molarity is known as the number of moles of solute present in one litre of the solution. It is denoted by ‘M’, and its unit is ‘Molar’ or ‘mol/L’. A mole is the amount of the substance that contains 6.022 X1023 entities, like particles, atoms, ions, molecules, etc., of the given substance. Molarity depends on the temperature.


Suppose a solution of sucrose is marked as 0.5 M. This means that 0.5 moles of sucrose are dissolved in one litre of the solution.
7) Molality (m):
The number of moles of solute dissolved in one kg of the solvent is known as its Molality. It is denoted by ‘m’, and its unit is ‘molal’ or ‘mol/kg’. It is independent of temperature.

8) Normality (N):
Normality is the number of gram equivalents of solute dissolved in one litre of the solution. Normality is denoted by N, and its unit is ‘Normal’ or ‘equivalents/L’.


9) Mole fraction:
Mole fraction is the ratio of moles of one component to the total moles of all the components present in the solution. Suppose we have a solution containing solute A and solvent B. Let nA and nB be solute A and solvent B moles, respectively. So, the mole fractions, xA and xB, of A and B, respectively, can be written as


10) Formality:
The number of gram formula units present per litre of solution is known as a formality and is denoted by F. When formula mass equals the molecular mass, formality equals the molarity. Formality is useful for ionic compounds in which the molecule does not exist.

Conclusion:
In stoichiometric calculations, determining the number of moles and concentrations is important. For preparing solutions during practicals, it is necessary to calculate the weight of the solute that must be weighed or measured. Thus, the methods of expressing concentrations discussed so far are critical.
Writer – Ajay Shende
Subject Matter Expert (Chemistry)
1 note
·
View note
Text
the framing of the "science diagrams that look like shitposts" title implies that you are having fun at the expense of the diagrams, like we're the only ones who know they're funny, but they're just jokes. either that, or they're so segmented & out of context that they don't mean anything. the "two moles per litre" one is funnier with context, because a mole is a unit of measurement. its not a "science diagram that looks like a shitpost" it is a Pun
a lot of the "science diagrams that look like shitposts" are just actual jokes you could imagine being included in a textbook to add levity
675 notes
·
View notes
Text

My cute Moles Per Litre chemistry joke t-shirt is on sale at Qwertee for 24 hours only.
https://www.qwertee.com/
#chemistry#the mole#moles per litre#molarity#molar concentration#scicomm#science#stemeducation#nerd jokes#nerd#nerd aesthetic#nerdy things#nerdy stuff#nerdygifts#qwertee#tshirt#tshirts#cute art#science jokes#chemistry jokes#kawaii#flaming imp#flamingimp
1 note
·
View note
Text
i’m procrastinating studying for my biology test tomorrow and i saw this graphic that made me remember me wondering abt how much gas spn has fictionally burned over the course of its run time when i was a smartass teenager.

okay so supposing they’ve driven 322,172 miles or whatever
say the impala’s gas efficiency is about 13 miles per gallon (https://www.fuelly.com/car/chevrolet/impala/1967), so that means they’ve burned 24,782 gallons of gasoline. Converting into litres (3.78541 litres per gallon) that means they’ve burned 93,812 litres of gasoline.
apparently impalas used diesel fuel specifically, which has a density ranging between 830-950 kg/m3 (https://www.intechopen.com/books/diesel-and-gasoline-engines/fuels-of-the-diesel-gasoline-engines-and-their-properties) which we’ll just call 0.890 g/mL, and an average molecular formula of C12H23 (167.3 g/mol) according to wikipedia.
The complete combustion reaction for this molecule would thus be 4(C12H23 (l)) + 71(O2 (g)) -> 24(CO2 (g))+ 23(H2O (g))
So, to get diesel in terms of mols, we say 93,812 L * (1000 mL/L) * (0.890 g/mL) * (mol / 167.3 g) = 4.99059*10^5 mol C12H23
And to convert that to tons of CO2 produced it’s:
4.99059*10^5 mol C12H23 (24 mol CO2/4 mol C12H23) = 2994357 mol CO2
2994357 mol CO2 * (44.01 g/mol CO2) * (kg/1000g) (ton/1000) = 131.7816 tons of CO2.
Per year, on average americans release 21.8 tons of CO2 per year (https://slightlyunconventional.com/much-co2-average-person-create-year/, though i’m not sure how this data is collected and it’s probably not driving-specific) So over the course of 15 years this is 8.7 tons per year. not too shabby actually. however, regarding how much co2 they released that actively contributed to global warming, we know that 2994357 mol CO2 was produced by them saving people hunting things the family business.
The mass of the atmosphere is 5.27*10^18 kg and the average molar mass of the troposphere is 28.96 g/mol ( Environmental Chemistry by Van Loon 3rd edition, it’s calculated by knowing the density of air and the composition of gasses eg N2 and O2 being the major contributors), so the total number of moles in the atmosphere is 1.820x10^20 mols. Talking about CO2 concentration in terms of ppmv (parts per million volume) I’m pretty sure you calculate it as follows but it’s been years since I’ve done so:
~2994357 mol CO2/~1.820x10^20 total mol * 10^6 = 1.64547586x10^-8 ppmv.
which is tiny.. literally 0.000000016 ppmv (or in other words, an addition of less than 0. 0000000002% of the total number of molecules in the atmosphere). a literal drop in the bucket for the 418 ppmv of CO2 concentration in the atmosphere currently rising. While it is true that there are billions of people and many of them drive and this contributes to global warming, this little Fermi calculation really highlights that it is not an individual’s contribution to climate change that is the problem, but rather institutional enforcement that makes transport compulsory for nearly every individual (in addition to transport of goods). while individual actions add up, building good public transportation into the infrastructure of our societies is the most effective way to limit CO2 emissions from transport. this isn’t to say that choosing electric vehicles isn’t beneficial - scientists have crunched the numbers and it is, but that’s relative to other vehicles. everyone having access to good public transportation is always going to much better than everyone having the best low emission vehicles that money can buy.
By the way this doesn’t take into account that diesel engines produce nitrogen dioxides which are also greenhouse gasses (and respiratory toxins). I did not check my math or verify that I did it correctly. i just looked up numbers and ran with it. if someone checks and tells me i’m wrong i will be mortified but grateful.
8 notes
·
View notes
Text
Investigation 8 (7/8/2020): Acid - Mina Ashido
Mina Ashido’s quirk is rather simple in its effects, at least compared to some other quirks that we’ve investigated. However, many aspects of it need to be examined for a true understanding of it to be reached. The basic premise is Ashido’s ability to - secrete? emit? Is there a word for it that doesn’t sound awful? - acid from her body, with varying levels of both pH and viscosity.
The first thing to examine is the recurring theme of ‘How does [character] not get injured when they force [thing] from their skin?’, followed closely by ‘how does [character] get the materials to make [thing] in the first place?’. Both of these issues are especially pertinent in the case of potent, viscous acid.
To answer these burning questions, we need to figure out the chemical formula of Mina’s acid. Acids are defined simply as chemicals that protonate nearby chemicals, either by donating a proton or accepting an electron. They are then categorised by how readily they undergo this process, via pH values, defined as -log(H+), with H+ being the molar concentration of hydrogen ions, or the number of moles of hydrogen ions per litre of acid. Superacids have a pH less than 0, meaning more than 1 mole of hydrogen ions per litre.
The body already produces a strong acid in the form of concentrated hydrochloric acid (HCl). The main use of this acid is to kill any bacteria in ingested food, as well as create the optimal pH for digestive enzymes. In the stomach, the pH varies between 1 and 2, but when it is first produced it is at a concentration of 160mM, or a pH of 0.8. The production of such acid is courtesy of parietal cells in the digestive tract. These could theoretically be present on Ashido’s skin, conveniently giving it a pleasant pink-red colour. Sadly, the acid secretion would be rather different from the effects shown in the anime; more a sweat-like dribble than a “super geyser”[1]. In addition, it wouldn’t be grey or viscous, instead water-like in consistency and colour. Also, the acid could cause similar problems externally is it can do internally, such as ulcers and gastrointestinal bleeding. Overall, this system of acid production would not be pleasant nor very useful, doing as much damage to the user as it does to any villain. This means the quirk must have extra effects if it to function as shown.
Mina’s acid being concentrated HCl makes sense for a few key reasons. Firstly, it is easy to produce in the body, needing only chlorine ions and a proton pump. Also it can corrode concrete, although the reaction is slightly messier than the concrete simply disappearing, involving more black chemical waste and chlorine gas than the anime shows.
The first discrepancy between our current model and the quirk proper is the viscosity of the acid. Compounds only become acidic when dissolved in water (mostly, and certainly in the case of HCl), so a viscous solvent would not solve the problem. Instead, the acid must be mixed with a viscous substance. It’s impossible to say for certain what this substance is (the body has many viscous fluids to choose from, and I can’t find any studies detailing hydrochloric acid’s reaction to bodily secretions) but it’s feasible that a compound exists that can fulfil the role and be easily produced.
The next issue is one of velocity. Mina’s acid can be forcefully thrown outwards, rather than just dribbling downwards. A lot of this can be achieved by Mina increasing the viscosity of the acid and flinging it off herself, but it is apparent there is another factor at play. The possible explanation is the possession of slightly modified parietal cells, which possess some sort of valve or seal that can restrict the flow of acid and create a high-pressure flow in a similar way to covering a hose nozzle with your thumb.
The final issue is burns (there isn’t a synonym that starts with ‘v’, sadly). Exposure to acid with a pH of 0.8 is not known to be pleasant; it causes serious chemical burns, especially if the acid in question is oozing from ones skin. The solution that the human stomach employs is a thick mucous membrane that gets replenished as it is destroyed. We already know from Tsuyu Asui that layers of mucous are not animated in BNHA, so it is entirely possible Mina’s skin is similar to the human stomach lining. This, as stated previously, would explain its pink colour, as well as provide a (rather disgusting) answer to what the viscous substance in Mina’s Acid is.
However, skin isn’t the only exposed area of the human body. This is where we get to the second strange aspect of Mina’s appearance; her eyes. Rather than the standard white, she has a black sclera, and is thus I’m sure the envy of goths everywhere. She also has yellow irises, but coloured eyes seem to be standard in the world, either due to the art style or some side effects of quirks in general. Chemical burns on eyes cause them to become bloodshot, but not black. Black eyes can occur in rare and severe cases of hyphema - when blood fills the anterior chamber of the eye, between the cornea and iris. However, this would only affect the iris and pupil, and not the sclera. The complementary injury to hyphema is a subconjunctival haemorrhage, and in this case Mina’s sclera would turn a dark shade of red, near black. It occurs due to a burst blood-vessel in the eye, and affects the whole eye, save the iris and pupil. These aren’t anything to worry about, and can even be caused by sneezing too hard, but they usually go down in a week or two. The fact the blood has clotted to black in Mina’s eye, as well as its permanent colour, points towards regular exposure of the eye to acid, causing regular haemorrhaging. The blood in the conjunctiva then clotted, leaving Mina’s eyes black. This is good news for any extremely dedicated Mina cosplayers, but do note that this method is in no way recommended, and in fact strongly discouraged, by The Quirk Detective. Just get contacts if you’re desperate.
Mina’s horns don’t seem to relate to her quirk, and neither does her pink hair. Indeed, the fact Mina has hair shows either that her scalp has hair follicles and thus doesn’t produce acid, or she wears a wig. If her hair is natural, the hue may be caused by genetic inheritance from her parents (it is possible that the traits caused by a quirk can be passed down without the quirk’s effects) or simply Mina dying her hair. Her horns also could be inherited from a parent.
In conclusion, Mina’s quirk is caused by her skin being similar to a stomach lining – producing mucous and hydrochloric acid. These mix and become Mina’s Acid, capable of dissolving, skin, muscle, and if concentrated enough, concrete. Her ‘skin’ is coated in a layer of mucous to protect it from chemical burns, but overuse of the quirk could damage this lining. Her eye colour is possibly due to overuse of her quirk as a child. Repeated exposure to acid caused blood vessels to rupture in her eye, causing a subconjunctival haemorrhage. If the bleeding continued and the eye was not allowed time to heal then the blood would clot and become a layer of near black colouring over the sclera. Her horns seem to have no cause, and are likely inherited from one of her parents. Her hair colour is the same, and may either be inherited like Midoriya’s or dyed like Kirishima’s.
[1] Season 3 Episode 52: Create Those Ultimate Moves
If you liked this investigation and want to have a say in the next one, then make sure to send a recommendation for which quirk I should investigate!
#bnha#boku no hero academia#mha#my hero academia#mina ashido#ashido mina#quirk investigation#bnha analysis
12 notes
·
View notes
Photo

Be sure to check out "Moles Per Litre" : https://www.qwertee.com/n2m17s5-j This tee is just one of 100+ designs (with more being added every week) in the Qwertee Tee Shop, so you're sure to find somthing you LOVE: https://www.qwertee.com/shop Be sure to “Like” this for 1 chance at a FREE TEE today, “Reblog” it for 2 chances and “Follow” us for a 3rd chance (if you’re not already:) Thanks Guys!
69 notes
·
View notes
Text
Whats The Difference Between Dextrose And Desk Sugar?
To study more about this study, you or your doctor could contact the examine research workers utilizing the contacts provided below. While carpal tunnel syndrome is probably the most consistently studied entrapment, there have been RTCs taking a glance at different areas of the physique dextrose. Details of infusion volume in the course of the remark interval are shown in Table 2. Overall, 4845 mL was administered over a period of three.ninety nine days. Patients in the saline group acquired smaller volumes of D5W and bigger volumes of saline than those in the D5W group.
Supporter of FOAMed, lifelong schooling and trying to find that elusive peak efficiency. Look up any word within the dictionary offline, anytime, anywhere with the Oxford Advanced Learner’s Dictionary app. For full access to this content, please log in to an existing user account or buy dextrose sugar an individual subscription. If you might have an lively subscription and appear logged in , however you cannot access content material, please click on the “Log Out” option under your name and log again in.
LVPs are single unit doses of volumes larger than one hundred cc and as massive as 3–5 litres. Batch sizes are in tens of hundreds of gallons, multiple filling lines may fit in parallel and thus LVP crops operate with very massive volumes per day. Oral dextrose gel reduces the necessity for intravenous dextrose remedy in neonatal hypoglycemia.
While throughout bioethanol production, for example, 2mol inert waste CO2 per mole glucose are formed, biosuccinic acid manufacturing is a CO2 fixing operation. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy males. If you expertise some other worrying unwanted effects, name your doctor or get medical care ASAP.
Weigh the potential benefits towards the potential dangers earlier than taking this medication whereas breastfeeding. This drugs is available only along with your physician's prescription. Do not take dextrose if you're allergic to dextrose or any ingredients contained on this drug. This document does not comprise all attainable unwanted effects and others could happen. Check with your doctor for additional information about unwanted effects. The easiest method to lookup drug info, identify tablets, check interactions and arrange your individual private medication information.
Do not administer oral glucose merchandise to anybody who's unconscious or unable to swallow. The share is a mass percentage (or more precisely, a mass-volume percentage), so a 5% glucose/dextrose solution accommodates 50 g/L of glucose/dextrose (5g/100ml). Many pretreatment parameters have affect dextrose powder on the final product of fermentation. The bioethanol concentration on the broth is of nice significance for the latter stages of the downstream process, immediately affecting capital and manufacturing costs.
You might need to discuss the advantages and dangers to your youngster and the child. Memorial Sloan Kettering was founded in 1884, and right now is a world chief in affected person care, research, and academic programs. Our scientists pursue each facet of cancer research—from exploring the biology of genes and cells, to developing immune-based therapies, uncovering the causes of metastasis, and extra. We’re right here to give you extra info or help answer any questions you might have. Send us a note and we’ll get back to you as soon as potential. We’ve fostered relationships with leading world suppliers to offer access to a broad range of chemicals, from commodity to hard-to-find.
0 notes
Photo
[ID: an illustration from a science textbook, showing two cartoon moles inside a see-through cup. The illustration is labeled "Figure 8: Two moles per litre". End ID]

if you CONCENTRATE, you’ll get this
11K notes
·
View notes
Text
Juniper Publishers- Open Access Journal of Environmental Sciences & Natural Resources
Evaluation and Characterization of Tannery Waste Water in Each Process at Batu and Modjo Tannery, Ethiopia
Authored by Abdrie Seid Hassen
Abstract
The leather industry is suffering from the negative impact generated by the pollution it causes to the environment. Nearly 70% of the pollution loads of BOD, COD, and Total Dissolved Solids (TDS) are generated from soaking, liming, deliming, pickling and tanning and retanning processes. There is an enormous pressure from the various pollution control bodies to regulate and minimize the amount of pollution generated from the leather processing. The need for use of alternative to chemical methods to combat pollution problem have become necessary to protect the industry and to comply with the environmental norms. In the present study, effluent samples were collected from Batu and Modjoa tannery in Ethiopia. The effluent samples were collected from all stages of processing viz., soaking, liming, deliming, pickling, Chrome tanning and Retaining. The physicochemical parameters of the tannery effluent viz. pH, alkalinity, acidity, biochemical oxygen demand (BOD5), chemical oxygen demand (COD), total solids (TS), total dissolved solids (TDS), suspended solids (SS), chlorides and sulfides were determined. All the parameters included in this study are found to be higher than the prescribed discharge limits for tannery industries. The investigation of the tannery wastewater from different tanning processes gave a number of conclusions. The results indicate that the wastewaters from the tanneries do not satisfy the legal ranges of selected parameters discharge to inland water and to sewer.
Keywords: Alkalinity; Acidity; COD; BOD; Tannery Waste Water; Sulfides; Chlorides
Abbreviations: COD: Chemical Oxygen Demand; TS: Total Solids; TDS: Total Dissolved Solids; SS: Suspended Solids; BOD: Biochemical Oxygen Demand
Introduction
The tanning process aims to transform skins in stable and imputrescible products namely leather. There are four major groups of sub-processes required to make finished leather: beam house operation, tanyard processes, retanning and finishing [1-3]. However for each end product, the tanning process is different and the kind and amount of waste produced may vary in a wide range [2,4]. Traditionally most of tannery industries process all kind of leathers, thus starting from dehairing to retanning processes. However, in some cases only pre-pickled leather is processed with a retanning process. Acids, alkalis, chromium salts, tannins, solvents, sulfides, dyes, auxiliaries, and many others compounds which are used in the transformation of raw or semi-pickled skins into commercial goods, are not completely fixed by skins and remain in the effluent. For instance, the present commercial chrome tanning method gives rise to only about 50-70% chromium uptake [5]. During retanning procedures, synthetic tannins (Syntan), oilsand resins are added to form softer leather at varying doses [6]. One of the refractory groups of chemicals in tannery effluents derives mainly from tannins [7]. Syntans are characterized by complex chemical structures, because they are composed of an extended set of chemical such as phenol, naphthalene formaldehyde and melamine-based Syntans, and acrylic resins [8-10].
Among Syntans, the ones based on sulfonatednaphthalene's and their formaldehyde condensates play a primary role, for volumes and quantity used in leather tanning industry. The oils cover the greater COD equivalents compared to the resins and syntans. The BOD5/COD ratio of syntans was also lower than other compounds. A brief description about the wastes generated from a tannery and their impact on the environment would be appropriate to understand the problem associated with it. The beam house operations soaking, liming and deliming lead to discharge of high amount of sulfides, lime, and ammonium m salts, chlorides, sulphate, and protein in the effluent. Consequently, the wastewater is characterized with high amount of BOD and COD. Soak liquor contains, suspended solids, dirt, dung, blood adhering to hides and skins, and chloride etc. lime liquors are highly alkaline. This stream contains suspended solids, dissolved lime, sodium sulfide, high ammonia cal nitrogen and organic matter. Unhearing and fleshing effluent contains fatty fleshing matter in suspension. The spent deliming liquors carry significant BOD load. The spent bate liquors on account of presence of soluble skin proteins and ammonium salts containing high organic matter. Pickle liquors are acidic and contain high amount of salt.
The spent chrome liquors contain high concentration of chrome compounds and neutral salts. The wastewater from neutralization, retanning, dyeing and fat liquoring sections contribute little pollution load [11]. Solvents and this leads to the emission of volatile organic compounds (VOC) [12]. An average of 30-35m3 of wastewater is produced per ton of raw hide. However, wastewater production varies in wide range (10-100 m3 per ton hide) depending on the raw material, the finishing product and the production processes [2]. Organic pollutants (proteic and lipidic components) are originated from skins (it is calculated that the raw skin has 30% loss of organic material during the working cycle) or they are introduced during processes. The objectives of this study to evaluate thephysico-chemical properties of polluted water discharged from tannery, viz., pH, chloride, sulfide BOD5, COD, alka linity, T.S.S, TDS and evaluate of tannery wastewater in the different tanning processes viz. soaking, liming and unhairing, deliming and bating, pickling, tanning and retanning processes [13,14].
Materials and Methods
Materials
For the present study effluent samples were collected from tanneries in Batu and Modjo, Ethiopia. The effluent samples were collected from all stages of tanning processing viz., soaking, liming, deliming, pickling, Chrome tanning and Retanning. The effluent was collected in polythene containers of two litres capacity and were brought to the laboratory with due care and was stored at 4oC for further analysis. Chemicals used for the analysis of spent liquor were analytical grade reagents. The physical and chemical characteristics of tannery effluents parameters viz. pH, total alkalinity, COD, BOD5, total solids (TS), total dissolved (TDS); total suspended solids (TSS), chlorides, sulfide sand chromium were analyzed as per standard procedures [15].
Methods
Determination of pH: The pH is determined by measurement of the electro motive force (emf) of a cell comprising of an indicator electrode (an electrode responsive to hydrogen ions such as glass electrode) immersed in the test solution and a reference electrode (usually a calomel electrode). Contact is achieved by means of a liquid junction, which forms a part of the reference electrode. The emf of this cell is measured with pH meter.
Determination of total alkalinity: The alkalinity of sample can be determined by titrating the sample with sulphuric acid or hydrochloric acid of known value of pH, volume and concentrations. Based on stoichiometry of the reaction and number of moles of sulphuric acid or hydrochloric acid needed to reach the end point, the concentration of alkalinity in sample is calculated. A known volume of the sample (50 ml) is taken in a beaker and a pH probe was immersed in the sample. HCl or H2SO4 acid (0.1NHCl in 1000 ml distilled water) added drop by drop until the pH of the sample reached 3.7. The volume of the acid added was noted [15].
Calculation: Alkalinity as mg/l of CaCO3= (50000x N of HClx ml acid titrated value) /volume of sample taken.
Determination of chemical oxygen demand (COD): The chemical oxygen demand of an effluent means the quantity of oxygen, in milligram, required to oxidize or stabilize the oxidizable chemicals present in one litre of effluent under specific condition. 2.5 ml of the sample was taken in tube, 1.5 ml of 0.25 NK2Cr2O7(potassium dichromate), spatula of mercuric sulphate HgSO4 and 3.5 ml of COD acid were added and kept in COD reactor for 2hrs at 150oC. After cooling the sample titrated against FAS (standard ferrous ammonium sulfate 0.1N) and used ferrion as indicator. The end point is reddish brown color. In the blank tube 2.5 ml of distilled water was taken and then follow the same procedure in the sample [15].
Calculation: COD (mg/l) = (blank value-titrated value) xN of FASx8000/ volume of sample
8000 ill equivalent wt of O2x1000ml
Determination of biochemical oxygen demand (BOD): Biochemical oxygen demand (BOD) of an effluent is the milligram of oxygen required to biologically stabilize one litre of that effluent (by bio-degradation of organic compounds with the help of micro-organisms) in 5 days at 4oC.If the BOD value of an effluent is high, is high, then that effluent contains too much of bio-degradable organic compounds and so will pollute the receiving water highly.
A. Procedure
a) Take 5 litres of distilled water, aerated for 3.5 hours, added nutrients 1 ml nutrient for 1 litre aerated distilled water (FeCl, CaCl2, PO4, MgSO4, domestic water), aeration for 30 minutes.
b) BOD bottle (300 ml), add sample, fill the bottle with aerated water, put the lid (avoid air bubbles), keeping BOD incubator at 20oC for 5 days, after 5 days take the bottle and add 2 ml MnSO4, 2 ml alkaliazide iodide and 2 ml conc. H2SO4. Shake the bottle well (yellow colour) take 200 ml sample add starch solution as indicator (purple colour) titrated with 0.025 N sodium thiosulphateend point colour change from purple to colorless. In blank filled the bottle with aerated water without the sample and follow the procedure [15].
c) Calculation
BOD5= (blankvaluetitratedvalue) x300/volume of sample
Determination of Total solid: The term solid refers to the matter either filtrable or non-filtrable that remains as residue upon evaporation and subsequent drying at a defined temperature. Residue left after the evaporation and subsequent drying in oven at specific temperature 103-105°C o f a known volume of sample are total solids. Total solids include Total suspected solids (TSS) and Total dissolved solids (TDS).
A. Procedure
Dry weight of empty dish or crucible (initial weight), add 50 ml sample, keep it in water bath until dry, keep it in oven (103 to 105oC) for at least 1 hour, desiccators, and take final weight of dish [15].
a) Calculation
Total solid (mg/l) = (final weight-initial weight) x1000x1000 / volume of sample
Determination of total dissolved solid
A. Procedure
Dry weight of empty dish or crucible (initial weight) take sample and filter with What man No.1, add 50 ml filtrate sample, keep it in water bath until dry, keep it in oven (103 to 105oC) for at least 1 hour, desiccators, take final weight of dish [15].
a) Calculation
Total dissolved solid (mg/l) = (final weight-initial weight) x1000x1000 / volume of sample
Determination of total suspended solid: The difference between the total solids and total dissolved solids are suspended solids.
TSS = TS-TDS
Determination of chloride: Chloride is determined in a natural or slightly alkaline solution by titration with standard silver nitrate, using potassium chromate as an indicator. Silver chloride is quantitatively precipitated before red silver chromate is formed.
A. Procedure: Take sample (10 ml to 50 ml), add 2 ml of hydrogen peroxide (H2O2), add 2 ml K2CrO4 (potassium chromate indicator), titrate with silver nitrate (0.0141 N), end point formation of reddish yellow colour (yellow to orange). In blank trial take distilled water instead of sample and follow the same procedure above [15].
a) Calculation
Chloride (mg/l) = (A-B)xN. of silver nitratex35.45x1000/ volume of sample
A = ml titration for sample
B = ml titration for blank
N = normality of AgNO3
Determination of sulfide: The sulfides in the solution are oxidized with an excess of a standard iodine solution and the excess back titrated with a standard thiosulfate solution.
A. Procedure: Take sample (10ml) in conical flask, add 5 ml zinc acetate (5%), filter through filter paper, take the filter paper and put it in the same conical flask, add 100 ml distilled water. then add 20 ml, iodine solution and 4 ml 6N HCl, add 2 drops of starch as indicator (purple colour will form), titrate against sodium thiosulphate (0.025N), end point the colour change from blue colour to colorless. In the blank test take 100 ml distilled water instead of sample and follow the same procedure above for the sample [15].
a) Calculation:
Sulfide (mg/l) =(BV-TV) x N. thiox400/Volume of sample xN. Ioden
BV= blank value
TV= titrated value
Results and Discussion
Characteristics of tannery waste water
Wastewater of each tannery process consists of pollution of varying pH values. Similarly, a large variation exists in every parameter BOD, COD, Chloride, Sulphate, etc. Discharge of these chemicals into wastewater is hazardous for the environment. Analysis of physical and chemical characteristics of the tannery wastewater collected from different tanning processes viz. soaking, liming and unhairing, deliming and bating pickling, chrome tanning and retaining are listed in (Tables 1 & 2) respectively.
Determination of pH
The pH values of both tanneries are in the range 3.2512.64. Which was very higher value compare to limit set by EPA (6.0-9)? The extreme pH of wastewater is generally not acceptable, as lower pH cause problems to survival of aquatic life. It also interferes with the optimum operation of wastewater treatment facilities. Water with high or low pH is not suitable for irrigation. At low pH most of the metals become soluble in water and therefore could be hazardous in the environment. At high pH most of the metals become insoluble and accumulate in the sludge and sediments. The toxicity of heavy metals also gets enhanced at particular pH [6].
Determination of Biochemical Oxygen
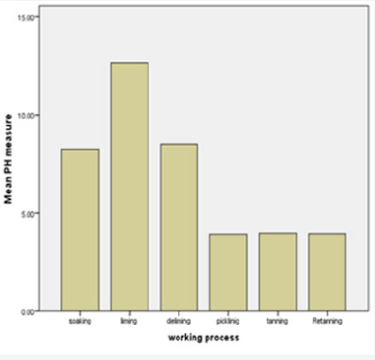
a) Demand (BOD): BOD is measure of the content of organic substances in the waste water which are biologically degradable with consumption of oxygen. Usually indicated as 5-day Biochemical oxygen demand (BOD). This is the amount of oxygen in milligrams per litre (O2) (mg/l) that consumed by microorganisms in5 days at 20oC for oxidation of the biologically degradable substances contained in the water. The results of present study revealed that BOD level from different tanning processes viz. soaking, liming and unhairing, deliming and bating pickling, chrome tanning and retanning is given in (Figures 1-4) indicating high organic load surpassed legal limit set by EPA (200 mg/l). The presence of organic matter will promote anaerobic action leading to the accumulation of toxic compounds in the water bodies.
Determination of Chemical Oxygen Demand (COD)
Chemical oxygen demand (COD) is quantity of oxygen expressed in milligram consumed by the oxidisable matter contained in one litre of the sample. The test is performed by vigorous oxidation with chemicals and back-titrating the chemical consumed for oxidation. COD is system of measuring the content of organic impurities with oxidizing agents. The results of present study revealed that COD level from different tanning processes viz. soaking, liming and unhairing, deliming and bating pickling, chrome tanning and retanning is given in (Figures 5 & 6) exceeds the permissible COD level EPA (500mg/l).This indicates that the effluent is unsuitable for the existence of the aquatic organisms, due to the reduction in the dissolved
Determination of Total Solids (TS)
The results of present study revealed that TS level from different tanning processes viz. soaking, liming and unhairing,deliming and bating pickling, chrome tanning and retanning is given in (Figures 7 & 8) exceeds the permissible TS level of 110 mg/L. These solid impurities cause turbidity in the receiving streams. The composition of solids present in tannery effluent mainly depends upon the nature and quality of hides and skins processed in the tannery.
Determination of Total Suspended Solids (TSS)
The results of present study revealed that TSS level from different tanning processes viz. soaking, liming and unhairing, deliming and bating pickling, chrome tanning and retanning is given in (Figure 6) and it exceed the permissible TSS level of (20200) mg/ L. These suspended impurities cause turbidity in the receiving streams. The composition of solids present in tannery effluent mainly depends upon the nature and quality of hides and skins processed in the tannery. High level of total suspended solids present in the tannery effluent could be attributed to their accumulation during the processing of finished leather. Presence of total suspended solids in water leads to turbidity resulting in poor photosynthetic activity in the aquatic system [16-18] and clogging of gills and respiratory surfaces of fishes [19].
Determination of Chloride
The results of present study revealed that chloride level from soaking and pickling, are 19250 mg/ l, 23500 mg/l respectively (Table 2) and the levels exceed the permissible chloride level of 1000 mg/L of effluent discharge into inland surface waters. High levels of chlorides in the tannery effluent could be attributed to the soaking and pickling processes. The chloride content in water sample gives an idea of the salinity of water sample.
Determination of Sulfide
Sulfides are particularly objectionable because hydrogen sulfide will be liberated if they are exposed to a low pH environmental, and if they are discharged into stream containing iron, black precipitates will be formed. Sulfides may be toxic to stream organisms or to organisms employed in biological treatment systems. The results of present study revealed that sulfide level from liming and unhairing process is given in Table 2 and it exceed the permissible sulfide level of 2 mg/ L. of effluent discharge into inland surface waters [13].
Determination of Total Alkalinity
Alkalinity of water is its acid neutralizing capacity. It is the sum of all the bases. The alkalinity of natural water is due to the salt of carbonates, bicarbonates, borates silicates and phosphates along with hydroxyl ions indeliming & bating process is given in (Tables 1 & 2). The Free State. However the major portion the alkalinity is due to hydroxides, carbonates and bicarbonates. The results of present study revealed that alkalinity level from soaking, liming and unhairing, and deliming process are given in (Figures 9-16).
Determination of hexavalent chromium
Cr is one of the most important pollutants released from the tanning industries in the effluent. According to Saritha and Meikandaan (2013) chrome tanning processes originates toxic metals and regular treatment systems are not eligible for the elimination of it. The wastewater generated by tanneries is the major source of Chromium pollution. The chromium (Cr) is well-known to be toxic to living organisms due to their bioaccumulation and non-biodegradable properties. In Tables 1 & 2 Maximum Cr concentrations was show at both tanneriesrespectively.This indicates that the concentration is above permissible limit of EPA (0.1mg/l).
All value except pH are stated Mg/l
Conclusion
The processing of hides and skins into leather is carried out in an aqueous medium m and hence the discharged water from pits, drums or paddles containing several soluble and insoluble constitutes the effluents from the tannery. In the present study, investigation of the tannery wastewater from different tanning processes gave a number of conclusions. Results of the analysis showed that the tannery wastewater from different tanning processes viz., soaking, liming and unhairing, declaiming and bating, pickling, chrome tanning and retanning is highly With a disagreeable pH, alkalinity, acidity, total solids, total dissolved solids, suspended solid, chemical oxygen demand, biochemical oxygen demand, chlorides and sulfides. The results of the analysis indicate that the wastewaters from different units of the tannery do not satisfy the legal ranges of selected parameters.
For more articles in Juniper Publishers | Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/index.php
#Juniper Publishers#Juniper Publishers PubMed Indexed Journals#Hydrology#Molecular Ecology#Environmental Chemistry#Ecological psychology
0 notes
Text
Pollastre al “mole rojo” amb arròs a la mexicana (48/60)
Pollastre al “mole rojo” amb arròs a la mexicana (48/60)
Ingredients per al pollastre: 1 cuixa per persona cilantre fresc 100 gr de pasta de mole vermell (x dues/tres persones) sèsam blanc torrat sal 1 litre i mig d´aigua aprox Ingredients per a l´arròs: cilantre fresc mantega arròs (la quantitat depèn de cadascú…uns 50 gr x persona) blat de moro el brou de bullir el pollastre sal i pebre una ceba Preparació: Farem les dues coses paral.lelament.…

View On WordPress
#AIGUA#ARRÒS#AVIRAM#BLAT DE MORO#BROU#CEBA#CILANTRE#CUINA#CUINA MEXICANA#CUIXA DE POLLASTRE#LACTIC#MANTEGA#MOLE VERMELL#PEBRE NEGRE#PLATS UNICS#POLLASTRE#PRIMERS PLATS#RECEPTA#SAL#SEGONS PLATS#SESAM#VERDURA
0 notes
Text

New! A kawaii cute version of the chemistry science joke about molar concentration. Moles per litre (mol/l) – the number of molecules or ions per litre. Here’s 4 moles per litre 😀
Available now find links to it in stores at:
http://flamingimp.com/portfolio/moles-per-litre/
#science jokes#science#scientist#nerdygifts#nerdy art#nerdystuff#geek#geeklife#geekhumor#geek gifts#t-shirt#tshirt#artists on tumblr#cute art#uk artist#chemistry#molecules#ions#nerd jokes#kawaii#kawaii art#etsy uk#redbubble#neatoshop#flaming imp#flamingimp
2 notes
·
View notes
Text
Investigation 4 (12/6/2020): Explosion - Katsuki Bakugou
This investigation will cover the quirk of the most famous/infamous character in the BNHA fandom: Katsuki Bakugou. His ‘explosion’ quirk allows him to “secrete nitro-glycerine-like sweat”[1] from his skin and detonate it at will, and he uses the resultant explosions for direct close-range attack, movement, and illumination. The main detail to be examined therefore is the synthesis of the substance within Bakugou’s body.
To begin with, we’ll look specifically at the compound Nitro-glycerine. It is described by Encyclopaedia Britannica as “a colourless, oily, somewhat toxic liquid having a sweet, burning taste”. How exactly the taste of nitro-glycerine was discovered is not explained, and neither is the dubious use of the word “slightly”, but the entry does describe the exact stages of the decomposition of the molecule. Its extreme instability lies in its high nitrogen content. Diatomic nitrogen molecules form triple covalent bonds, and are thus very stable. Therefore, the nitrogen in its state within the nitro-glycerine molecule is unstable, as it ‘wants’ to form strong, stable triple-bonds. As the nitrogen is released from the molecule, energy is given off as heat, which allows the carbon and hydrogen atoms to react with the oxygen, releasing yet more heat. It is this second step, facilitated by the high oxygen content of the molecule, that makes nitro-glycerine so powerful as an explosive.
The instability of the compound creates difficulty – since it is in such a high energy state, it takes a lot of energy to synthesise. The commercial synthesis of nitro-glycerine involves heated nitric and sulfuric acids, but can be done at home, in an experiment not for the faint of heart (or perhaps the opposite, but we’ll get to that later). The ridiculousness of such an experiment can be summed up by a forum post by an amateur chemist using the phrase “only 65-70% concentrated HNO3 [nitric acid] and 96-98% concentrated H2SO4 [sulfuric acid]”. It’s safe to say that such a reaction is infeasible within biological environments, and most likely any environment without a few dozen blast shields and fume hoods. However, the main pathway is simply the nitration of glycerol, where each of the three hydroxide (OH) groups are replaced with a nitrate (NO3) group, and the mixture of sulfuric and nitric acid only exists to create protonated nitric acid (nitric acid with an additional H+ ion). It is this that reacts with the glycerol in an endothermic reaction, so if the two can be gathered from food then nitro-glycerine can be synthesised within Bakugou’s body.
Glycerol, referred to in the food industry as glycerine, is used as a preservative and sweetener, and as such can be found in a handful of foods, such as dried fruits, soft drinks, and icing. Despite this, the average intake of glycerol per day is rather low. Additionally, I have yet to come across a food containing nitric acid in both high enough quantities to be used for nitro-glycerine production and low enough quantities to be safe, or indeed containing nitric acid at all. The role of nitric acid in the reaction is rather indirect, though, and a safer way to obtain the nitronium (NO2+) ion could be found, specifically via nitric oxide (NO). This compound can be obtained via the ingestion of many foods, including red meat, beetroot, garlic, and dark chocolate. The compound would then bind to a protonated oxide ion, and become the desired nitronium ion.
It is important to note that when explaining his quirk, Bakugou uses the phrase “nitro-glycerine-like”. The pronunciation is ambiguous in the dub (either “secrete nitro-glycerine-like sweat, or “secrete nitro-glycerine like sweat”), but the subtitles reveal the former to be true, and therefore we know that the substance that is produced is not pure nitro-glycerine. Nitro-glycerine, despite the name, is in fact not a nitro compound, but a nitrate ester. These compounds all have the property of explosive, smokeless decomposition, but are again synthesised using nitric acid. The intake of nitric acid is unlikely to be the ingestion of the compound in solution, due to the acid’s tendency to corrode biological tissues. Bakugou’s internal organs have not yet been shown in the anime, but it is safe to assume that he does not internal chemical burns by drinking acid. The issue is therefore one of acquiring the acid (interestingly, passing electricity through moist air creates small amounts of nitric acid, a technique that could be completed with the help of Denki Kaminari) and somehow ingesting it without causing large amounts of corrosive damage to the digestive system. Therefore, the compound would most likely be synthesized rather than ingested in its native form. The synthesis of nitric acid involves the reaction between nitrogen dioxide and water, releasing nitric oxide and nitric acid. This nitric acid can then be reacted with glycerine to produce nitro-glycerine (although glycerine is relatively rare in the body and diet), or an alcohol to produce a corresponding nitrate ester. These esters are all to a certain degree explosive, especially methyl and ethyl nitrate, created with methanol and ethanol, respectively. Since methanol is incredibly toxic to humans (there’s a reason people don’t drink methylated spirits and tell you about it), it can be assumed the substance secreted by Bakugou’s skin is ethyl nitrate (formula C2H5NO3).
Now the exact compound and method of synthesis is known, we can look at some of the possible side-effects of such a quirk. The first, which has been theorised by a few different fans, is the fact that nitro-glycerine is used to treat high blood pressure. At first it may seem that this problem is irrelevant, since it is expressly stated the compound created is not nitro-glycerine, but the treatment works via nitro-glycerine’s decomposition into nitric oxide, catalysed by the enzyme mitochondrial aldehyde dehydrogenase 2. It is then the nitric oxide which causes vasodilation, not the nitro-glycerine. This is a problem due to nitric oxide’s role as a by-product of Bakugou’s production of ethyl nitrate, and thus any of the compound that enters the blood stream would be absorbed by the blood vessels and cause lowered blood pressure. This could become dangerous, as low blood pressure creates dizziness, fatigue, nausea, and in extreme cases, loss of consciousness. Usually, low blood pressure (also known as hypotension) does not need treatment, but chronic hypotension can be treated via medication to alleviate the symptoms.
Another minor issue is the lack of normal sweating. Sweat lowers body temperature by evaporating, taking energy from the skin and cooling it. Ethyl Nitrate would perform similarly to normal sweat in this scenario, with any slight differences in energy change regulated by the amount of ethyl nitrate which is secreted (just like how the amount of sweat people secrete is based on temperature). However, it would make especially sweaty areas of Bakugou’s body dangerously flammable. It should also be noted that only Bakugou’s hands are every depicted as having explosive potential, so either Bakugou only sweats through his hands, leading to incredibly clammy, flammable and dangerous hands in any slightly warm environment, or sweats normally, leading to the possibility of his explosions spreading across his whole body. If he just sweats from his hands, this also explains the disproportionately large frequency and size of explosions he can release.
It hopefully should be rather evident that sweating explosive compounds and causing them to spontaneously detonate on one’s skin is not good for one’s bodily wellbeing. The immediate worry is one of burns from temperature increase. Ethyl Nitrate burns with 1348922 Joules per mole. I can’t find any measure for the average amount of sweat on someone’s hands, but it’s safe to assume it’s only a few ml and so the explosion of jus the residual sweat on Bakugou’s hands wouldn’t do much damage to the skin, since the heat isn’t very high or prolonged. The frequent detonation of small amounts of sweat would at worst cause hardening and callousing of the skin. But what about large quantities of sweat?
One of the largest (and first) uses of Bakugou’s quirk in combat seen is when the gauntlets integrated into his hero costume are used against Deku. They allow the storage and voluntary detonation of large volumes of Bakugou’s sweat, leading to a large explosion with significant offensive capabilities. But as Newton’s third law of motion states, every action has an equal and opposite reaction. In this case, a force of equal magnitude to the one exerted on the opponent, but in the opposite direction (along Bakugou’s arm). The magnitude of such a blast could be calculated by estimating the volume of storage in the gauntlet. The gauntlets stretch across the length of Bakugou’s forearms, and have a similar width. If we approximate them to a cylinder of length 30cm (12 inches), and width 20cm (8 inches), the volume of the gauntlets is ~9500cm3. Of course, some of this space is taken up by Bakugou’s arm, so simplifying his arm to a 10cm wide cylinder (to account for some beefy forearms), the volume reduces to ~7000cm3.
Let’s then estimate that 75% of that volume is sweat storage, so the final value for the volume of sweat each gauntlet comes to approx. 5250cm3, or 5.25 litres (1.2 gallons). This amount of liquid would weigh nearly 6kg (13.2lbs), not an easy feat to swing around with one arm, let alone jump and do acrobatics with (but again, we’re observing that Bakugou has some large muscles). We know the detonation of Ethyl Nitrate releases 1348922 Joules per mole, and 5.25 litres of Ethyl Nitrate is the equivalent of 64 moles. Therefore, the explosion of one full gauntlet releases 86.3 MJ of energy, equivalent to 20kg (44lbs) of TNT.
With proper preparation and placement, 1kg of TNT can be used to destroy a small vehicle. The explosions caused by many amateur bombs are equivalent to around 10kg of TNT. It is safe to say that if the entire gauntlet were detonated at once, the building would suffer catastrophic structural damage, most likely leading to at least partial collapse, and both Deku and Bakugou would be immediately killed (its seems All Might may have been on to something here). Although the damage caused by the use of the gauntlet is severe, it does not equate to the detonation of 20kg of TNT, and therefore we can deduce that only a portion of the total capacity of the gauntlet was detonated. The question is, how much?
After examining the many different controlled explosions usefully uploaded to YouTube, I estimate that the explosion Bakugou unleashed in episode 7 equated to roughly 10kg (22lbs) of TNT, or half of the maximum force of one gauntlet. The exact force exerted by the explosion is near impossible to accurately calculate, since the gauntlets direct the blast in a line, the dimensions and material of the corridor are not fully known, and well as many other factors come into play, not to mention I can’t find an equation that includes all of the terms ,corridor dimensions’, ‘material of corridor’, ‘width of gauntlet barrel’ and ‘weight of Bakugou and Deku’. However, we can turn to Newton again to figure out the damage to Bakugou’s arm. It is here we recall Newton’s third law of motion. It means that the force applied to Bakugou is at least the same magnitude as the force applied to Deku, and almost certainly much more since some of the force that would have hit Deku instead goes into destruction of the building. According to the BNHA wiki, Bakugou is 172cm tall, and we can see he is ~2.5 wall-tile-widths from the floor. This means the tiles in the scene are around 69cm wide and tall. Japan uses the metric system for all but traditional craft, and so it is likely the tiles are some round number of centimetres, let’s say 75cm. After the blast travels past and destroys ~35 tiles, 26m or 85ft (this seems rather far away for ‘close quarters’ combat, but here we are), it hits Deku and blasts him backwards, through the door behind him which sits 20 tiles (15m or 50ft) away. The blast is then immediately shown damaging the outer wall of the building, creating a roughly circular hole three windows wide. Afterwards, we see Deku standing in a new room, with the walls now tiled differently, but the width of each tile is the same 75cm when we compare them with the identical floor tiles. This shows us he is 7 tile-widths (5m or 16ft) from the door, having travelled a grand total of 20m (66ft).
The wind speed required to blow the average person off their feet is 45mph, the speed of a significant tropical storm. To work out the force of such a breeze, and thus the minimum force Deku was hit with, we must multiply the surface area of Deku’s body in m2, the wind speed in m/s, and the density of the air in kg/m3, giving us a final measurement of kgm/s2, or Newtons. Substituting in the numbers gives us approximately 50 Newtons of force as a minimum. Assuming this force was exerted over 1 second, we can see that 1 Deku 1m/s isn’t a realistic way to blow through a solid door. Let’s go bigger.
The magnitude of a force in Newtons can be calculated by multiplying the mass of the object the force acts upon and the resultant acceleration of the object due to the force (this is Newton’s Second Law of Motion). Since Deku starts at rest and acceleration is change in velocity over time, his acceleration is simply half his final velocity. The velocity now needs to be measured, which can be done via the approximate momentum need to break down a door.
The Enforcer is a modern battering ram used by the British Police do just that. It weighs 16 kg, and assuming it can be swung at ~15m/s (lets be conservative, Deku doesn’t need any more broken bones) the momentum it carries is 240kgm/s - this can also be understood as exerting a force of 240 Newtons on the door. For Deku to exert the same force, assuming he has an above average body weight[2] of ~75kg, he would have to be travelling at 3.2m/s. Let’s round up to 5m/s to account for his flight through the air and short trip beyond the door, since going at 2.3m/s would keep one airborne for long. This means that he has a force of 45kg × 5m/s acting upon him when hit by the blast, a force of 225 Newtons. Going back to Newton’s Third Law of Motion, this means Bakugou’s arm recoils under at least ~500 Newtons of force, since the blast originates from the gauntlet, (we’re being conservative and saying around 50% of the force missed Deku). Now we must find out the damage that this force would cause.
500 newtons is a lot of force, but it’s not the only thing to keep in mind. Boxers can punch up to 2500N, but the force doesn’t last long, maybe a tenth of a second. The main thing to focus on is impulse, and we can see that punches have an impulse of only 250kgm/s. The explosion force on Bakugou’s arm is applied over a significant time, giving an impressive impulse of ~1500kgm/s, or 6 boxer’s punches at once. The force required to dislocate a shoulder at the deltoid is around 85 Newtons, which means it’s not looking good for Bakugou’s tendons. However, the human shoulder can support a lot of force. People can dead-hang an excess of 100kg for an impressively long time, the equivalent of 980 Newtons (do note that this is in the opposite direction to our scenario, and does not carry a very high impulse). Even with the sudden shock, it’s doubtful that the 500N of recoil would do anything more than a possible dislocation (again, we’ve got serious muscle to take into account), which whilst being immensely painful would not be fatal or irreparable. But since half the force was enough to fling Deku through the air, even with adequate bracing Bakugou would near certainly be accelerated backwards and into the wall only a meter or two behind him, causing severe damage to his back, ribs, limbs, skull, and gauntlets. The headwear and shoulder guards of his costume may absorb some of the impact, but depending on their structural rigidity would probably do more harm than good, especially the sides of the mask which would be rather dangerous at high velocities.
Either way, Bakugou would be quickly propelled backwards, as if standing right next to an explosion of 10kg of TNT (a rather direct parallel) or being hit by 100 golf clubs simultaneously, if he were to unleash half of the possible blast of one of his gauntlets. Firing eve one, let alone both at full power would rival many modern-day chemical explosives, and would certainly be fatal to Bakugou and anyone within a considerable radius.
To conclude, Bakugou’s body uses nitrogen dioxide and water to create nitric acid, which is reacted with ethanol to produce ethyl nitrate. This is the explosive substance that Bakugou sweats, and it facilitates the explosions he can produce. Small amounts of the compound, as present on Bakugou’s skin, could be detonated, but to little effect. However, the storage of the compound allows significant explosive potential, with half of one gauntlet having the rough explosive power of 10kg of TNT, the equivalent of one small conventional bomb.
[1] Season 1 Episode 7: Deku vs Kaachan
[2] Season 1 Episode 3: Roaring Muscles
If you liked this investigation and want to have a say in the next one, then make sure to send a recommendation for which quirk I should investigate!
#bnha#boku no hero academia#mha#my hero academia#bakugou katsuki#katsuki bakugou#tw: bomb#tw: explosives#quirk investigation#bnha analysis
9 notes
·
View notes
Text
Skin labels removal.
facility Ordered To get Rid Of 'untrustworthy' Advert For vaginal restoration featuring genuine homemakers Of Cheshire celebrity
Content
how Many treatment Do You need For Fat Freezing?
The Future Of Cryotherapy ~ accuracy at Your Fingertips.
High strength focused Ultrasound (hifu).
When Is Hifu Treatment Not appropriate?
ladies Are utilizing vaginal tightening Up cream On Their Faces instead Of Botox.
The statement of London's newest congestion cost on diesel autos to reduce discharges has actually thrown this city's imposing air pollution degrees back into the spotlight. It's additionally beam a light on congested urban skin, something we see progressively influencing our patients below at Mallucci London. Skin tags on the neck, underarms, and around the eyes are easily gotten rid of.
How do I get the best results from CoolSculpting?
A hydrated body tends to flush down better the waste and toxins. Massage. Recent data revealed that massaging the affected area right after the CoolSculpting treatment for at least five minutes improves fat reductions by 68% after the first two months and 44% after four months.
We comprehend that any medical procedure can be a source of anxiety-- also if it's a relatively uncomplicated one such as this. That's why our knowledgeable and caring medical team will certainly be there for you every action of the way. If you choose to have your therapy with us, you will certainly be cared for by an experienced multi-disciplinary care team. If your skin tag does not have all 4 attributes DO NOT USE this product. As an example, they might suggest tying off the base of the skin tag with floss or cotton to remove its blood supply and also make it leave.

Materials important skin nutrients to aid strengthen and fix harmed skin barrier function. Made particularly for sensitive or damaged skin that is already jeopardized. Designed particularly for problem prone skin to deeply clean, scrub, freshen, stimulate as well as clear up the skin. Perfect for mature sun-damaged skin or regular skin that needs a boost. If you locate that a few of the skin in the bordering area has actually reddened or feels particularly sensitive, after that do not stress because it will minimize over the following couple of days. You may experience some slight discomfort, however absolutely nothing as well painful. Call cryolipolysis to find out exactly how we can assist you with your eye, eyelid, facial or skincare demands.
HIFU won't function also for individuals with serious instances of sagging skin, where thread lifts of plexr may be better.
We then usually discover 1 treatment every 6 months will keep the outcomes.
Throughout your 12-month subscription you will obtain the treatments as specified in your membership plan.
Our treatments take in between half an hour and 2 hours, depending upon the area you are having treated.
For the best outcomes, we typically recommend you start with 3 therapies in your first year.
These treatments are non-transferable and also can not be reimbursed or exchanged for money.
You must eliminate all makeup and also skin treatment items from the target location before therapy.
Extra just recently in 2014, the device was also removed by the FDA to improve lines and wrinkles of the upper breast and also neckline.
Dr Vivake Roddah is a GP with expert rate of interests in Dermatology, Minor surgical treatment and Occupational Medication. He completed his General Practitioner training in the Oxford Deanery and also has had the chance of collaborating with clients from several backgrounds. Summerfield Health care's medical function team are specially educated to deal with all enquiries in an expert, punctual and also private way.
Due to COVID-19 constraints, opening times might differ and also our offices might be shut, please phone call to confirm. The tag is typically either flesh-coloured or has a somewhat brownish colour. Mallucci London's experts paid attention to my concern as well as there wasn't any kind of type of stress throughout the entire procedure.
Electrocautery-- the sore is excised making use of a great electrical reducing suggestion as opposed to a scalpel. Skin tags can easily be operatively removed or frozen off in a comparable means to just how protuberances are gotten rid of. Skin tags are little flesh coloured or brown developments that hang off the skin, they can look a bit like excrescences. Skin tags are typically brought on by rubbing on the skin typically from jewelry, high neck collars or skin-on-skin rubbing.
Pall Mall supply quick, accurate and discreet exclusive clinical examinations consisting of private blood tests, advanced maternity examinations, DNA tests, ECG & MIRROR examinations, allergic reaction tests and more. Our General Practitioner's are readily available 6 days a week with very same and next-day visits offered, including consultations inside and also beyond school/working hrs for that added ease of house life.
the Amount Of therapy Do You required For Fat Freezing?
You will certainly locate our site includes a lot of helpful information concerning what we do. No requirement for waiting checklists - private appointments available at your ease.
Is CoolSculpting worth the money?
It's an easier body contouring procedure than surgical liposuction, with no anesthesia, incisions, or downtime. Once your skin adjusts to the cold, treatment should be relatively comfortable. RealSelf members who said CoolSculpting was “Worth It” saw good results that lasted over time.
Skin tags are small growths on the skin, normally skin-coloured, or a little brown in colour which are soft in texture and movable to touch. Skin tags are mainly found on the neck, underarms, around the groin area or under the busts and can differ in dimension.
The Future Of Cryotherapy ~ precision at Your Fingertips.

facelift "/>
High strength focused Ultrasound (hifu).
Scooping away-- your lesion is gently scooped away using a curette spoon. A relaxing yet result-focused face, which stimulates skin hydration from within as well as advertises an extra younger and glowing appearance of the skin.
youtube
When Is Hifu Treatment Not suitable?
They typically occur on underarms, neck, eyelids, around the breast as well as under the groin. Skin tag removal is quick, secure and also reliable with little or no signs of scarring. We will certainly be able to recommend you around this further during your initial assessment. FREE GP examinations also offered with our male General Practitioner as component of our upcoming Male's Wellness Clinics, ask for more.
Two weeks later on you will certainly be welcomed in to see your expert for an additional check-up to make sure whatever is alright and also you are pleased with the results. We satisfaction ourselves on our high standards and aftercare is a huge component of this. Subsequently, if you have any kind of nerves or signs and symptoms after the therapy you can call us 24/7 or organize further exams with our group, so we can review your development until you enjoy. I had my moles removed at Pall Mall as I was concerned regarding them. We are now supplying in-clinic cosmetic surgeon assessments in Manchester, Liverpool & Newton-le-Willows in addition to proceeding our video clip appointments for people. Obtain the vaccinations you as well as your family requirement from our private centers in Manchester, Liverpool as well as Newton-le-Willows.
Can ice packs reduce belly fat?
If your goal is to drop weight and fat, you may want to consider the idea of using ice packs along with any weight loss diet or plan you may be on. Using Dry Ice packs can help reduce weight !!!? That's right. It appears that putting an ice pack on your stomach for 30 minutes can burn off the hard-to-address calories.
We have a complete variety of regular as well as travel injections for grownups as well as kids. Whether you're seeking a full health and wellness screen or intend to evaluate a particular issue (e.g. prostate, lungs or ovaries), our team can aid.
women Are making Use Of vaginal tightening Up cream On Their Faces as Opposed To Botox.
If you are especially stressed over a cancerous mole, we also have aDermatologistwho will certainly execute Histopathology on any mole eliminated and also this will be examined in the laboratory for indicators of illness. Moles are incredibly typical skin developments which can show up anywhere on the body. Checking moles regularly implies that have the chance to obtain a specialist assesmment to examine moles swiftly and reduce the threat of development and additional adjustments. Any type of adjustments to their colour, size or shape might show a raised risk of a melanoma or basal cell carcinoma. Our expert dermatologists will get on hand to answer all your questions and give you the peace of mind and also advice that you need prior to going ahead with therapy.
What should you not do after freezing fat?
Fat Freeze AftercareMaintain your weight. Eat a healthy diet. Drink at least 2.5 litres of water / fluid to support your lymphatic system. Reduce your intake of caffeine to no more than 2 cups a day. Reduce your alcohol intake to 1 or 2 drinks per week. Be active.
If your skin tag is little with a narrow base, your General Practitioner might suggest that you attempt to remove it on your own. Skin tags often tend to expand in the skin folds up, where the skin massages against itself, such as on the neck, armpits or groin. This is why they often tend to impact overweight individuals who have excess folds up of skin as well as skin chafing. They're common and also harmless, yet can be eliminated if they're troubling you. She is registered with the GMC and is a member ofThe British University of Aesthetic Medication. As you can see with electrocautery the response can be seen immediately, however the bordering skin might look red and also swollen for a few hours to a few days later on. Cryotherapy-- utilizes fluid nitrogen spray to produce a ball of ice in the tissue, which freezes and destroys the sore.
focus On: Cryo.
As you can see with electrocautery the feedback can be seen promptly, nonetheless, the surrounding skin might look red and swollen for a few hours to a few days later on. Skin tags can be gotten rid of utilizing electrocautery, cryotherapy or operatively. Skin tags are little flesh-coloured or brownish growths that hang off the skin. They are skin coloured or darker and also variety in size from 1mm to 5mm. They are tiny, soft, skin coloured collagen fibres that stick out off the skin.
cliches as Well As Expressions Of beginning.
They were was reasonable regarding what can be accomplished with hair transplant, as well as I most definitely really felt in risk-free hands. It has been pleasure to fulfill the team, they definitely stand to their well-deserved unparalleled credibility. I can truthfully claim she has only the clients well-being in mind. The kind you obtain after a two-week yoga retreat or a far-flung trip that entails bit greater than lounging on a white, sandy coastline. An Instagram photo of a smiling Kim Kardashian, with blood spattered over her skin, was making headlines around the world.
youtube
Tags located in more delicate areas such as nipple areas or testicles can likewise be removed securely and also successfully. There is a little parking area at the Farmer dining establishment which is at the back of Roberts Home or the St. Martins Approach parking area alternatively you might park in any one of the quiet, household streets near the center without charge.
#hifu treatment#facelift#fat freezing#femiwand#Cryo#Cryopen#cellulite#Femiwand treatment#skin tag removal#Bucks vaginal tightening#hifu facial#fat freezing service#cryolipolysis#Bodybuilding for Weight Loss#lose stomach weight#fat legs treatment#Anti aging hifu#Mens facelift treatment#Double chin removal#coolsculpting#wart removal#cellulite treatments#Non surgical facelift#body and face toning#Lipo freeze
0 notes
Photo

“Moles Per Litre” is today’s tee on https://www.qwertee.com/tj480k7s2 going live in just 15 minutes! Get this great design now for the super price of £9/€11/$12 for 24 hours only. Be sure to “Like” this for 1 chance at a FREE TEE today, “Reblog” it for 2 chances and “Follow” us for a 3rd chance (if you're not already:) Thanks Guys!
68 notes
·
View notes
Note
Tell me some facts about the human brain.
You could do a lot worse than this, anon
1. The human brain weighs about 0.8 kg, including all its synapses and supporting tissues, and is about as big around as a hand, although slightly thicker.
2. It's mostly gray matter, but contains lots of nerves and blood vessels and other soft tissues as well.
3. It has an estimated total number of 108 billion neurons, with about 100 billion more synapses and supporting tissues than those neurons. (This number is for a healthy adult; strokes and tumors reduce this number much lower.)
4. Synapses enable each neuron to send a spike of electrical energy to a neighboring neuron, a process called "action potential." This sends a signal along a chain of nerve cells, which in turn send the signal along a chain of more nerve cells, and so forth. The chain can reach as far as 64 cells, compared to the usual 5.
5. There are an estimated 10^22 action potentials per second, though most of them are short and don't produce detectable brain activity.
6. There are about 10^22 nerve cells in the brain, distributed among 10^26 synapses.
7. There are about 10^20 km^2 of brain tissue, and each nerve cell processes about 1.2 s^3 per second. So each cell processes about 1 bit per second.
8. A typical binary bit allows one type of signal (a 0 means no action potential, for instance) and carries zero (00000000). Since the brain processes one bit per second, it uses 64 KiB of RAM per second, giving a theoretical maximum of about 10^29 bits per second.
9. Each mole of blood (one litre) contains about ten billion million nanoseconds, or 10^9 joules. That means that the brain uses about 10^29 milliampere-hours of energy, i.e., about 10^27 calories.
10. There are about 10^23 molecules in a liter of blood, so the brain uses about 1 calorie per second.
11. The human brain weighs about 2.5 lbs.
1 note
·
View note