#difference between solute and solvent
Text
youtube
#concentration terms#How to find mass percent of a solution#concentration terms mole concept#education#mass percent of a solution#molarity of a solution#molarity and molality problem#difference between solute and solvent#solute solvent and solution#Youtube
1 note
·
View note
Text
Liminal Physics 101 - What's wrong with the River?
I want to preface this theory/analysis by giving credit to the excellent, thought-provoking response left on my theory on the mechanism behind lyctoral thanergy generation by @greyhairedgeekgirl, because it inspired me to finally finish typing up this post.
There is a lot of conjecture contained within this theory but I've attempted to firmly root it in the terminology used by the characters in relation to the River, as well as how the River itself is described. My avenue of thought is closely related to that of @greyhairedgeekgirl, but I think my conclusion likely differs due to how I have chosen to interpret the definition of the River as a liminal space.
Anyway, onto the question I'm seeking to answer here: I feel that the answer to it lies in Harrow the Ninth, during the explanation we get in response to a question asked by John Gaius himself, and the veritably horrific implications of it.
“Harrowhark, what happens when somebody dies?”
“Thalergetic decay causes cellular death,” you said carefully, pressing the nail in harder, “which emits thanergy. The massive cell death that follows apopneumatism causes a thanergetic cascade, though the first bloom fades and the thanergy stabilises within thirty to sixty seconds.”
“What happens to the soul?”
“In the case of gradual death—senescence, illness … certain other forms—transition is automatic and straightforward. The soul is pulled into the River by liminal osmosis. In cases of apopneumatic shock, where death is sudden and violent, the energy burst can be sufficient to countermand osmotic pressure and leave the soul temporarily isolated. Whence we gain the ghost, and the revenant.”
Note how this explanation is structured in a sequential way that is likely deliberate:
We establish that thanergy is emitted by thalergetic decay: thalergy is characterised as life energy, produced by cell growth and reproduction. Thanergy is also said to be produced by cell death in the glossary of GtN, which to me indicates that the thalergy produced by a cell is in some way tied to it, beginning to decay into thanergy when the cell dies.
Massive cell death follows apopneumatism: the soul leaving the body results in mass cell death, resulting in the body's thalergy 'flipping' and rapidly decaying into thanergy.
Gradual death results in the soul being pulled into the River by liminal osmosis. Sudden and violent death results in a thanergetic energy burst sufficient to countermand (lit. revoke or cancel an order) osmotic pressure, leaving the soul temporarily isolated outside the River.
The soul leaves the body, the cellular thalergy begins to decay into thanergy in the absence of the soul, and the amount of thanergy produced results in the soul either being pulled into the River or being temporarily stranded.
River Terminology
liminal - occupying a position at, or on both sides of, a boundary or threshold; relating to a transitional or initial stage of a process. This word is used in reference to the River a lot.
apopneumatism - apo meaning 'from, away from' and pneumatism referring to the pneuma, or soul; this is the process of the soul coming away from the body. put simply, this is death.
liminal osmosis - osmosis is 'the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration)'; a solution is a solute dissolved in a solvent, meaning that osmosis is the process whereby a solution resolves the discrepancy in solute : solvent ratio between itself and another solution that are divided by a selectively-permeable membrane.
imagine you have two bodies of water, of unequal volume, one with more solute in it than the other: osmosis will result in the body with more solute gaining water from the body with less solute until the ratio of water : solute is equivalent in each body. it equalises their concentration of solute.
osmotic pressure - 'the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane', but it is also defined as 'the measure of the tendency of a solution to take in its pure solvent by osmosis'.
this is to say that osmotic pressure can serve as the current that pulls a soul into the river, if you assume that the river is a solution and the soul is a solvent. Alternatively, one could also consider the River as the selectively-permeable membrane dividing two solutions.
What does this mean?
Assume the following:
The world is a solution, solute dissolved in a solvent, and the soul is the solvent in that solution.
The River is a selectively-permeable membrane.
The River beyond that Abigail Pent speaks of is another solution.
The soul (solvent) is pulled through the River (selectively-permeable membrane) by osmotic pressure into the solution with less solvent in (the River beyond), except it can't, because that semi-permeable membrane has been rendered impermeable: why?
Solute concentration.
What is the solute?
You collected bits of dried wood—dried wood?—and
empty-coloured stones—stones?—from the banks of the River beyond death, and you collected armfuls of the sharply unkind osiers and tall, feathery plants, the ones with long fibrous stems as tall as you were and thin, tangled leaves. Filthy salt wind whipped your faces as you formed wards from the flotsam that grew, apparently, on the bank.
She stood before the coffin of the Sleeper, and gathered those white, soft, solid rips in her hands, and she popped the bubble, and the River came rushing in.
It came down around her in shreds, as light and insubstantial as drifts of spiderweb. The water sprayed through white holes, rushing in with a pounding roar: that brackish, bloodied water that only existed within the River. She was buoyed up by a spray of ice water and filth.
The River is described as brackish, it is associated with salt wind. Brackish means water with higher than average salinity, saltwater concentration, so let's assume our solute is salt.
What did John do when he became God? He introduced a copious amount of thanergy into the system, because murders generate more thanergy, enough to make souls unable to pass into the river, and used it to fuel himself.
He murdered Alecto. The salt-water creature: the first thalergetic planet he flipped. The water is the solvent, the solvent is the soul, salt is our solute, salt-water is our solution.
I was so close to cracking this third thing, the soul.
I’d realised there was the energy you produced from being alive and the energy you produced when you died, but the fact that energy was produced when you died meant there was another phase. I could get a corpse’s heart beating and get all the neurons firing in the brain, but it wasn’t producing the alive stuff anymore. It wasn’t an on-off switch.
“The body needs thalergy and a soul to keep the lights on. Anastasia’s tripod principle. Body plus thalergy, but no soul, is basically a very weird vegetable … after a while it gives up and shuts down.”
Nona the Ninth shows exactly what the soul is: the third thing, the on-off switch, the leg of the tripod. A body full of thalergy without a soul shuts down after a while because the thalergy isn't stable in the absence of a soul, and decays in its absence. Thalergy decay emits thanergy.
Thalergy is salt, water is the solvent, water is the soul, salt-water is the solution of a living creature: thalergy stabilised by a soul.
How does salt affect water?
A river is freshwater: it doesn't have high salinity. It is not salt-water.
What does salt do to water? It adds to its mass, makes it more buoyant. Buoyancy, or upthrust, is an upward force exerted by a fluid that opposes the weight of a partially or fully immersed object.
The Riverbed is studded with mouths that open at proximity of Resurrection Beasts, and no ghosts venture deeper than the bathyrhoic layer. Anyone who has entered a stoma has never returned. It is a portal to the place I cannot touch—somewhere I don’t fully comprehend, where my power and my authority are utterly meaningless. You’ll find very few ghosts sink as far as the
barathron.
Ghosts don't venture near the Riverbed. The Riverbed is studded with stoma. The stoma are mouths that open when Resurrection Beasts near them, and the Resurrection Beasts are the souls of murdered planets, the only souls that can sink that low; the stoma lead to a place John can't touch.
[...]“And that was a titanic effort on the part of Cassiopeia the First, who was brilliant and sensible and careful—she thought she could bait physical portions of the Resurrection Beast into the current. She was right. It followed her.”
They were writhing together, wild and excited—the current swirled in an agitated pandemonium—there was a massive sickening jolt, and the Mithraeum started to slide again, forward … tilting … sliding.
“We’re in the current now,” said Pyrrha calmly. “We’ll be pulled in, if the mouth doesn’t close.”
The current of the River leads to the stoma. The River is a semi-permeable membrane that leads to the River beyond, and the stoma are mouths in the Riverbed that lead to a place beyond the power of John. Osmosis pulls solvent, souls, through the membrane into the neighbouring solution.
Conclusion
You went en masse into the River, leaving your bodies behind to slump into C-curves—or at least, yours did, the rest of them stood—and crunched the silvery sand of the bank beneath your feet as the three saints led you both to assemble wards. No blood or flesh or bone here: the first two might be scavenged, the last swept away by the capricious tide. You collected bits of dried wood—dried wood?—and empty-coloured stones—stones?—from the banks of the River beyond death, and you collected armfuls of the sharply unkind osiers and tall, feathery plants, the ones with long fibrous stems as tall as you were and thin, tangled leaves.
The River holds no blood, flesh, or bone. But its waters are made brackish by a kind of salt: the thanergy of murdered billions. How can one make a ward from something unthanergetic, from dried wood and stones? It's impossible, unless they are suffused with thanergy, made pliable to a lyctoral touch.
When John murdered the planets and humanity in one fell stroke, he flooded with the River with enough thanergy that its buoyancy countermanded the osmotic process that draws souls into the River beyond. The River is full of ghosts gone mad: souls that should have moved beyond, but can't, because the current cannot carry them through the stoma, the thanergy working against its pull.
“A powerful necromancer at the peak of their game could last ten seconds in the River,” said God, pushing himself up to stand. “Soul magic is the great leveller. In the first few seconds their thanergy would all be stripped away … then their thalergy, and then their soul.”
The River strips away thanergy and thalergy, but it can only do so much: when its waters are already so permeated with thanergy, souls float, fail to sink to the depths and pass through it, carried by its current. They cannot reach the stoma because their souls are too light compared to that of the Resurrection Beasts, the thanergetic buoyancy pushing them back up.
What lies beyond the stoma isn't Hell, or rather, it is Hell: it is a place where John Gaius can't touch. It is where souls are meant to go. It is the River Beyond.
#the locked tomb#harrow the ninth spoilers#nona the ninth spoilers#tlt theories#tlt meta#hi it's me again i've gone even more insane :)
621 notes
·
View notes
Text
Models of the Particulate Nature of Matter
Hello everyone! Here are my notes on the first topic of CHEM HL for the IBDP! Hope they help <3

Lecture Notes
ATOMS, COMPOUNDS, MIXTURES
Atoms are the smallest particle of an element.
Elements are the primary constituents of matter, which cannot be chemically broken down into simpler substances.
Compounds consists of atoms of different elements chemically bonded together in a fixed ratio.
Mixtures contain more than one element or compound in no fixed ratio, which are not chemically bonded and so can be separated into physical methods.
CLASSIFYING MATTER
Elements exist with other elements, as chemical compounds which consist of different elements chemically bonded in a fixed ratio.
sodium chloride, is a white crystalline solid that is added to improve food, whereas sodium is a dangerously reactive metal that reacts violently with water, and chlorine is a toxic water.
Air is an example of homogeneous mixture as it has uniform composition and properties throughout. Additionally, a solution of salt in water and a metal alloy such as bronze, which is a mixture of copper and tin, are also homogeneous.
To form a homogeneous mixture, the inter-particle attraction within the different components mus be similar in nature to those between the components in the mixture.
Heterogeneous mixture, has a non-uniform composition and its properties are not the same throughout.
Does a mixture have the same classification at all scales?
SEPARATION OF MATTER
Filtration is used to separate a solid from a liquid or a gas. Solid is collected on the membrane and the filtrate containing the solute passes through.
Solvation is the process of attraction and association of molecules of a solvent with molecules or ions of a solute.
An example would be sand and salt as they have different solubilities
Residue is the mixture left in the filtrate after the procedure.
Distillation is used to separate a solvent from a solute.
The solvent has a lower boiling point than the solute, therefore it i collected as a gas and passes over the condensation sube, which is surrounded by cold water. This gas is condensed into the pure solvent, into a beaker at the end.
Paper chromatography is used to analyzed the composition of different solutions which are placed in the baseline. The aper is suspended to ensure that it is saturated as
The different components have different affinities for the water (or other solvent) in the paper.
This method can be used to investigate the different pigments in food colouring.
STATES OF MATTER Solid Liquid Gas Particles closely packed Particles more spaced Particles fully spread out Inter-particle forces strong, particles vibrate in position. Inter-particle forces weaker, particles can slide over each other. Inter-particle forces negligible, particles move freely. Fixed-shape No fixed shape No fixed shape Fixed volume Fixed volume No fixed volume
Temperature of the system is directly related to the average kinetic energy of the particles.
State of matter is determined by the strength of inter-particle forces that exist between the particles relative to this average kinetic energy: thus, if the inter-particle forces are sufficiently strong to keep the particles in position at a given temperature and pressure, the substance will be a solid. If not, it will be a liquid or a gas.
Example: which of the following has the highest average kinetic energy?
He at 100ºC
H2 at 200ºC
O2 at the 300ºC
H2O at 400ºC
Answer: 4, as the substance at the highest temperature has the highest average kinetic energy.
Fluids is another name for liquids and gases as they take the shape of their container. Diffussion, the process by which particles of a substance spread out more evenly occurs as a result of their random movement, occurring predominantly in these two fluid states.
KINETIC ENERGY (Ek)
Refers to the energy associated with movement or motion.
Ek:1/2mv^2
As the average kinetic energy of all particles at the same temperature is the same, there is an inverse relationship between mass and velocity. Therefore, particle with smaller mass will diffuse more quickly than those with greater mass at the same temperature.
solid (s)
liquid (l)
gas (g)
aqueous (aq)
Solutions are generally mixture of two or more components. The less abundant component is the solute, and he more abundant is the solvent. The solute can be solid, liquid or gas but the solvent is generally a liquid.
As the kinetic energy of particles increase, they will overcome the inter-particle forces and change states.
A solid changes to a liquid at a defined melting point, and a liquid changes to a gas at it’s boiling point
melting point ≠ freezing point
boiling point ≠ condensation point
Sublimation is the direct inter-conversion of a solid to a gas without going through the liquid state.
Deposition is the reverse of sublimation and occurs when a gas changes directly to a solid.
Boiling point is the temperature at which the vapour pressure is equal to the external pressure. As a liquid is heated, more particles enter the vapour state and the vapoure pressure increases, when the external pressure is lower, the vapour pressure needed to boil is reduced and so, boiling occurs at a low temperature.
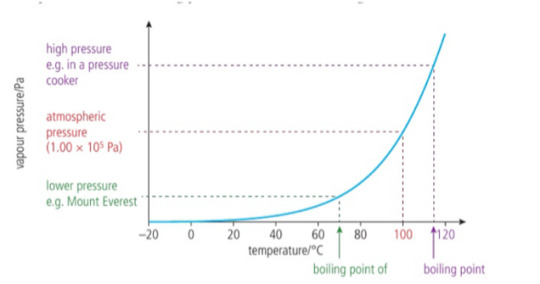
When the external pressure is lower, the vapour pressure needed to boil is reduced and so boiling occurs at a lower temperature.
KINETIC ENERGY AND TEMPERATURE.
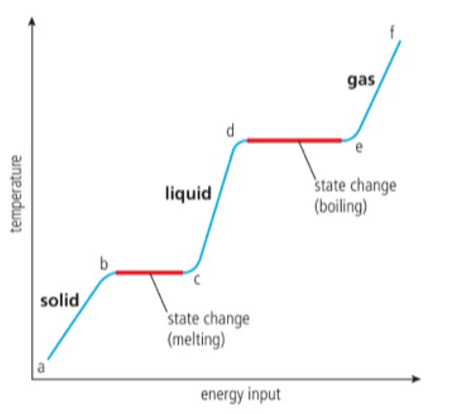
a-b, the solid is heated, the vibrational energy of its particles increases and so the temperature increases.
b-c This is the melting point, the vibrations are sufficiently energetic for the particles to move away from their fixated positions and form a liquid. Energy added, during this stage is used to break the inter-particle forces, not to raise the kinetic energy, so the temperature remains constant.
c-d As the liquid is heated, the particles gain kinetic energy and so the temperature increases.
d-e The boiling point, there is now sufficient energy to break all of the inter-particle forces and form a gas. It needs more energy than melting, as all the inter-particle forces must be broken.
e-f the gas is heated under pressure, the kinetic energy of particles continues to rise and so does temperature.
MELTING AND BOILING ARE ENDOTHERMIC PROCESSES.
KELVIN
The movement or kinetic energy of the particles of a substance depends on the temperature. If the temperature of a substance is decreased, the average kinetic energy of the particles also decreases.
-273ºC = 0ºK
0ºK= 273ºC
#study motivation#chaotic academia#academia#study#study tips#high school#studyblr#student#studyspo#ib#studyabroad#studying#studyspiration#study blog#student life#study movitation#study aesthetic#university#chemistry#organic chemistry#stem#stem academia#stemblr#women in stem#mathematics#science#stem student#biochemistry#math#ibdp
67 notes
·
View notes
Text
Molarity Converter: Simplifying Complex Chemical Conversions

In chemistry, it is often necessary to convert between different units of concentration to maintain consistency and accuracy across experiments. Whether you're working in a research lab or studying for your next chemistry exam, performing conversions between molarity and other concentration units can be complex and time-consuming. Thankfully, a molarity converter helps simplify these conversions, providing accurate results in seconds.
In this article, we'll explore the importance of unit conversions in chemistry, the common challenges faced, and how the molaritycalc.com molarity converter makes chemical conversions easier and more efficient.
Why Unit Conversions Matter in Chemistry
Chemistry is a discipline that frequently involves working with solutions of varying concentrations. Depending on the experiment or the field of study, chemists use different units of concentration, such as molarity, molality, mass percent, or normality, to express the quantity of solute dissolved in a solvent.
These conversions are essential for comparing solutions or preparing specific concentrations for experiments. For example:
Molarity (M) measures the concentration of a solute in a given volume of solution, expressed in moles per liter.
Molality (m) measures the concentration of a solute in terms of moles per kilogram of solvent.
Mass percent expresses the mass of the solute as a percentage of the total mass of the solution.
Each of these units provides valuable information for different types of experiments. However, when working with multiple solutions or when translating data between fields, it becomes necessary to convert between these units. Without a tool to automate the process, these conversions can become confusing, leading to errors that could impact experimental results.
The Challenges of Manual Conversions
For many chemistry students and professionals, performing these unit conversions manually is one of the most challenging and error-prone aspects of their work. Here are some common challenges that people face:
Complex formulas: Each unit of concentration requires a different formula for conversion. For example, converting molarity to molality involves knowing both the molarity and the density of the solution. The formulas themselves can be complicated, especially for someone who is new to chemistry or dealing with multiple solutions.
Time-consuming calculations: Converting between units like molarity, molality, and mass percent often requires multiple steps. This takes time and can slow down experimental progress, especially when working with large datasets.
Human error: With so many variables in play, it's easy to make a mistake during a manual conversion. Even a small error in calculations can throw off the results of an entire experiment or analysis.
These challenges underscore the importance of having a reliable and efficient molarity converter to simplify the process.
How the MolarityCalc Molarity Converter Simplifies Chemical Conversions
The molaritycalc molarity converter is specifically designed to make unit conversions fast and accurate. Whether you're converting molarity to molality, mass percent, or any other unit of concentration, the molarity converter can handle it all in just a few simple steps.
Here’s how the molarity converter on molaritycalc works:
Select the type of conversion: Begin by choosing the type of conversion you need. For instance, if you're converting from molarity to molality, you'll select this option from the dropdown menu on the calculator.
Input the required data: Depending on the conversion type, you'll need to input different values, such as the molarity, density, or mass of the solution. The molarity converter guides you through the input fields to ensure you enter all necessary information.
Instant conversion: After entering the data, click the "Convert" button, and the molarity converter will instantly provide the converted concentration in the desired unit. This eliminates the need for manual calculations and ensures that your conversions are accurate.
With molaritycalc, chemists and students can convert between different units of concentration quickly and with confidence, allowing them to focus on conducting experiments and analyzing results rather than spending valuable time on conversions.
Example: Converting Molarity to Molality in a Salt Solution
Let’s walk through a common conversion that researchers often encounter—converting molarity to molality in a salt solution. Suppose you're working with a sodium chloride (NaCl) solution that has a molarity of 2 M, and the density of the solution is 1.05 g/mL.
To convert molarity to molality, you would follow these steps using the molaritycalc molarity converter:
Input the molarity: In this case, 2 M.
Enter the density of the solution: 1.05 g/mL.
Calculate the molality: The molarity converter will automatically calculate the molality based on the input data.
The molarity converter provides the result in seconds, giving you the molality of the sodium chloride solution without requiring you to manually work through the complex formula.
Benefits of Using a Molarity Converter
The molaritycalc molarity converter offers several benefits for both students and professional chemists:
Accuracy: The molarity converter ensures that your conversions are precise. This reduces the risk of errors that could impact the outcome of experiments, especially when working with solutions of high or low concentrations.
Speed: Manual conversions take time, especially when working with multiple solutions or datasets. The molarity converter speeds up the process by providing instant results, allowing you to focus more on the experimental work itself.
Flexibility: The molarity converter can handle a wide range of conversions, from molarity to molality, normality, mass percent, and beyond. This versatility makes it useful in a variety of chemical fields, whether you're working in academic research, pharmaceutical development, or environmental analysis.
Ease of Use: The simple, intuitive interface of molaritycalc makes it easy to input the necessary data and perform conversions, even if you're new to chemistry or unfamiliar with the formulas.
Common Unit Conversions in Chemistry
Chemists frequently encounter several types of unit conversions, and having a tool that can handle these conversions is invaluable. Here are some common examples:
Molarity to Molality: This conversion is useful when working with solutions of varying concentrations and densities. Molality is often preferred in experiments involving temperature changes because it does not depend on the volume of the solution, which can fluctuate with temperature.
Molarity to Mass Percent: This conversion is essential when you need to express the concentration of a solution as a percentage of the total mass. Mass percent is particularly useful in industrial chemistry, where large quantities of chemicals are used.
Normality to Molarity: Normality (N) expresses the concentration of reactive units in a solution. It is often used in acid-base reactions and redox reactions. Converting between normality and molarity is necessary when comparing solutions or standardizing reactions.
Dilution Calculations: In many cases, chemists need to dilute a solution to a lower concentration. Using a molarity converter allows you to calculate the final molarity of the diluted solution quickly and accurately.
By using the molaritycalc molarity converter, you can perform all of these conversions and more with just a few clicks.
Conclusion
Unit conversions are a fundamental part of chemistry, and performing these conversions manually can be both time-consuming and prone to error. The molaritycalc molarity converter simplifies these conversions, allowing chemists and students to quickly and accurately convert between different units of concentration.
Whether you're converting molarity to molality, mass percent, or any other concentration unit, molaritycalc makes the process easy and efficient. By incorporating this tool into your chemical research or studies, you can save time, reduce errors, and improve the accuracy of your experimental work.
With the molaritycalc molarity converter at your disposal, complex chemical conversions are no longer a barrier to successful experimentation. Instead, they become simple, straightforward tasks that can be completed in seconds, allowing you to focus on what truly matters—advancing your knowledge of chemistry.
2 notes
·
View notes
Text
exist without permission
yet another internet cat that got to sit on a motorcycle has died, when will the madness stop.
wrote some self-indulgent canon x oc i'm not going to put in the tag. hella honestly, it's not very good or very ic, but it did get me to focus my brain on something.
könig x oc, sfw, 1.2k words
König loves rats. He has loved them since he was a kid, growing up in a rotten shithole in the part of Schladming where tourists didn’t fucking dare to go.
His girlfriend bought him two for his thirty-fourth birthday–a bonded pair of two fat boys, because males were the more affectionate sex in the species, one a little hooded fellow with a black mask of fur that rides down to his white shoulders and body, and a fat ball of black fur.
Rivka had led him into his rental’s second bathroom with a grim face like she was going to show him a body she’d dismembered and needed help disposing (and, being the foolish, loving fuck he is, he was already trying to scrape up different darkweb solvent solutions he’d found over the years to dissolve everything down to bonemeal–even though she would’ve known better).
“You have to be quiet, no sudden noises,” she’d warned him, and the little smile trying to tug at the corner of her thin mouth—revealing a slice of her sharp, silvery teeth—clued him in. She was excited. And there, in the bottom of the tub, was a spare bath towel, a bowl of water, and an overturned cardboard pet carrier. On the far side, facing the opposite wall, were two whiskered noses, stacked on top of one another.
König’s heart stopped beating so suddenly, he really thought it was some fatal shit. He’d spent most of the rest of the afternoon on the bathroom floor, cramped between tub and toilet, talking quietly to the young rats. Just dangling his hand near them, brushing his fingers over their soft, sleek backs, letting them get used to his voice, until they began to approach him. When they gripped the band of his watch, and then the cuff of his long sleeve to climb up his arm and explore, he let them.
He took a picture of them taking turns running the length of his thigh to his bent knee, and texted it to Rivka as she set up their big fucking enclosure in the spare room that served as his office.
Eventually, the rats navigate their way into the hood of his sweatshirt with shocking ease, and nibble the ends of his hair until they fall asleep. König doesn’t cry about it, but he weathers a sick-and-warm tantrum of awe that fills his gut and rises up his throat as if to kill him by bodily shock.
He loves rats.
And they love him.
+
When it’s time for Milf and Dilf to eat, they have to be separated, or they will beat the blind fuck out of one another. König has no idea where the behavior came from, and the breeder Rivka bought them from refused to answer calls after the fact. They show no aggression otherwise, so loving and dependent upon each other that a separation of an hour causes them to worry and sicken. It is simply at feeding time.
They’re largely free range rats, allowed to roam the house at liberty all day, and König only has to click his tongue to summon them from where they are playing, but it’s rare that he even has to. The little darling shits are so intelligent that they understand their schedule, and at 2000 he and the boys will more often than not end up going to the kitchen at the same time, the two of them darting between his feet. They race ahead, stop, look back to check on him, and race again.
They eat better than he normally would, if he didn’t cook for Rivka. Fancy rat food, a bisl of yogurt, kale. Sometimes, raspberries, if the fucking things don’t mold on him before he can even shove them in the fridge. They love sugar, but sugar will kill them.
Where König is too weak to deny him things that drip death into his body sweet-slow, the rats are helpless, and don’t know better, and can’t control themselves. He has to withhold for them.
Because they are small, because their eyes are so wet and kind. Because they want him and need him and depend upon him to take care of them.
Because he loves them.
And because they love him.
He scoops Dilf into his hoodie pocket, and Milf into his hood. He carries them to find Rivka—who is lying in the big bed on her side, with her legs crossed, buried in a smutty book delivered vis-a-vis Kindle screen—and hands off Milf and his bowl. She clicks her eyes up, and pushes into his hip with the ball of her foot. “Hi kultsi.”
“I’m taking Dilf with me to my computer,” he says. He stands. He waits.
It takes a second, but he can tell when the thought clicks in her head, like the slide of a bolt-action driving another bullet home. She folds her arm behind her head, disappearing the entire Kindle behind her back, and frowns her apology. “I’m not playing Sims tonight. My mods are all broken after the horse ranch expansion, so I just left my laptop on your shelf. But I’ll come watch you dick around, if you don’t mind.”
His expression snits slightly, but he forces it to smooth. Of course, he doesn’t fucking mind. What he fucking minds is not being near her, even if they aren’t talking or doing anything. He really fucking minds it when they’re both home and he doesn’t have her in his line of sight for longer than twenty minutes.
But Rivka minds it, too, and they always come to find each other when that minute mark draws near.
He runs his hand up her ankle to squeeze her toned, tattooed calf, and nods when he’s taken too long to respond as he chews apart his thoughts, her scope-glass eyes training on his face. “Sounds good. Just going to fuck around in Satisfactory for a while.”
“You and those fucking Swede games, Lee,” she grunts under her breath, shaking her head and turning back to her reading. Can frog march the Special Jaeger out of Finland, etc.
A smirk cuts across his warped mouth, and he bends down to kiss her shin. He leaves when he sees the corner of her mouth twitch again.
Dilf sits on his desk, going mad on his dinner, pausing only to wipe both paws over his face like a prayer to get yogurt off his fur. At one point he holds a little piece of kale in a small fist while he does it, and König about dies.
Maybe fifteen minutes later, Milf rides in on Rivka’s shoulder, looking sleepy. She pulls up her desk chair and kicks over the old milk crate she uses as an ottoman, setting herself up to König’s left. Once she’s settled, Milf stirs, standing on hind legs, sniffing the air, and Rivka automatically puts a hand on König’s thigh, creating a ramp for Milf to run down to reunite with Dilf.
And König settles in. His girlfriend reads something condemnably filthy next to him, glancing at his screen, and his rats sleep in a warm pile on his lap.
#my rat really loved yogurt and dried apricot and sugar snap peas but everything in moderation and he lived a long happy life#my work#and look at that rivka's first appearance and it's not very good whoops dkjlsdk next time will feel more like her
17 notes
·
View notes
Text
Band Incorrect Quote/Scenario -“I Love My Job…” (Hospital AU)
Well, with an incorrect-quote-worthy event at work, here’s another cursed extended band incorrect scenario in the cursed veterinary hospital AU with Styx and Squeeze… This time, rather than the mixed bunch in “Frencho Fryo Time”, or Squeeze by themselves as with the Daylight Savings time, this one features Styx by themselves, and the situation brings out their dramatic dynamic!
-It’s 10:00 AM, and a drug label has come back from reception to the pharmacy lab for the assistant team to refill a patient’s script of liquid furosemide*-
[*This is a diuretic medication, often used to prevent or remove fluid buildup around the heart and lungs (effusion), most commonly for patients in later stage heart disease]
Tommy Shaw: *Sees the label* “Is this a compound? There’s no recipe label printed.”
JY: “No, if you go in the cabinet above Dr. R’s desk, that one is pre-made in a solution, because it’s really unstable if we compound it in-house. All you have to do is just get a syringe and pull out however many cc’s the label says it’s for.”
Tommy Shaw: *Goes and looks in the cabinet* “JY, I can’t find it!”
JY: *Comes in, and finds the two boxes of it, unopened, but on the top shelf, and turned around so the labels face the back of the cabinet, unfortunately positioned in a way that set Tommy up for failure finding it* “There it is.” *Pulls one box down, and flips the other around.*
Tommy Shaw: “Really? Up there, turned around?”
JY: “I don’t know who put it away like that. It’s not supposed to be put away like that. Anyway, do you still want to fill it, or do you want me to fill it?”
Tommy Shaw: “I’ve never filled that one, but it seems easy enough, so I probably should.”
JY: “Okay. It’s not really any different than if you were doing the Cisapride solution.”
Tommy Shaw: *Perks up* “Oh, okay! Yeah, I can do that!”
JY: *Has an odd feeling that something is going to go wrong, despite the fact that Tommy has filled quite a few scripts before and that he usually has faith in Tommy to do things right, so he stays in the pharmacy hall in hopes he can be there to prevent whatever is about to go wrong*
-Less than two minutes later-
Tommy Shaw: *Has gotten the compounding bottle and syringe adapter tops at the ready, and is in the process of pulling the correct amount out of the stock bottle. As he is, he’s to get the syringe plunger to pull back due to the viscous nature of the furosemide solution. On autopilot, he disconnects the syringe and pulls some air into it, then puts it into the adapter top to create some extra air in the bottle to keep the plastic walls from getting sucked inward as the solution is pulled out, but forgets in his auto-pilot mode that he has a rather large, 25cc syringe, rather than a tiny 3 or 1cc syringe, thus, having the plunger pulled nearly halfway back with air is a lot more than usual. He starts to push the air into the bottle*
JY: *Does a double take, realizing after 5 cc’s what Tommy is unknowingly doing, and can see the sides of the bottle starting to bow outward* “Oh, don’t do that-!”
-Instantly, before anyone can even respond to JY’s warning, the air pressure in the bottle pushes the syringe adapter top out of the bottle neck and causes the solution to spray out of the bottle, across Tommy’s chest and arms, in his face, and all over the countertop and everything on it. It’s on the wall between the counter and the overhead cabinets, on the cabinet doors, and all over the lab sample tubes in the basket on the counter, as well as in the open box of microscope slides, and soaked into the towel that various bottles of cleaner and lab solvents sit on to protect the counter top from any leaks-
Dennis, John, and Chuck: *All gasp super loud*
Tommy Shaw: *Freezes with a look on his face of being a hundred percent done with everything as he sets the bottle down, which now only has about a quarter of the medication volume left in it, and wipes the droplets that are dangerously close to his eyes*
Dennis DeYoung: *Looks like he’s about to say something, or rather, shout something at Tommy*
JY: *Puts his hands up and very sternly shakes his head at Dennis, then turns and gives John and Chuck a similar warning look* “Don’t say anything...”
Tommy Shaw: *Inhales deeply with a wheeze and lets out a groaning sigh*
John Panozzo: *Walks out of the pharmacy hallway, because he’s trying hard not to laugh and knows he’s going to lose the battle*
Tommy Shaw: “I… don’t even know what I expected would happen when I did that… I don’t know why I did that… or what I was even thinking.” *Sighs again, setting the syringe and bottle down on the counter, and puts his hands down at his sides in defeat*
JY: *Grabbing some hand towels and rags out of the closet in the hallway* “Okay… it’s alright… we’ve got the other bottle. We’ll just clean all this up, use what we’ve got left in this one before opening the other, and we’ll let Alan Gratzer know that one of the bottles spilled and we’re on our last one. He doesn’t even have to know how it happened.”
Dennis DeYoung: *Eyes wide* “What do you mean he doesn’t-!?”
JY: *Trying so hard not to sigh in a way that sounds annoyed as he is* “Tommy, if you don’t have a spare shirt in your car, there’s that whole bin of spares in the bathroom. Please go wash that off and get changed, and we can make sure that goes in the next load of laundry.”
Tommy Shaw: *Looks alarmed* “Is this one of those drugs that are bad if you touch it?”
JY: “No, it doesn’t have effects on skin contact -and even if it did, it would only be an issue if you have blood pressure trouble. But you don’t need to be walking around covered in it.” *Waits until Tommy goes off before turning to Dennis* “I just said, it is NOT that big a deal, and Alan has WAY too much to do with tracking inventory -as long as nobody is stealing or consuming the drugs, he DOES NOT CARE how it happened. He just needs to know that we made a mistake, some was lost, and we need to order more. End of story!”
Chuck Panozzo: *Looks between both of them* “BYE.” *Walks off, and with the power of suggestion, goes back to the laundry room to see where the cycle is at and if he can make sure Tommy’s scrub top gets in the next load*
Dennis DeYoung: “Fine, then.” *Walks away toward treatment*
JY: *Groans to self as he sprays and wipes off the main part of the counter, then goes to get the other bottle of that particular medication solution so that he can fill the script and have that part over with before dealing with the more details areas that got splashed, deciding in his head that he’ll leave most of it for Tommy to clean when he gets back, but will deal with the tedious process of washing all the microscope slides and drying them so they don’t have water stains or streaks* “Ohh, I love my job, I love my job, I love my job… I do love my job. And my coworkers…”
#incorrect quotes#Incorrect band scenarios#situational meme#incorrect band scenario -Hospital AU edition (where Styx and Squeeze have been thrown through many a wild work experience)#(also implies a member of REO does inventory management)#insanity post#pardon my insanity#yes this happened last month#I was in JY’s place#no we did not have anyone being quite as dramatic as Dennis is here… which was probably a good thing for my coworker who did it#anyway… JY is like me… he really does love his job -but there are moments it drives him crazy!#(I’m about to go back to school and I’m gonna miss it though…)
2 notes
·
View notes
Text
Thinking about Liios and Estinien again. 😩
.
At the end of long days fighting, Estinien allowed Liios to help him doff his armor.
What was usually the work asked of a squire meant something else entirely when your equal was the one to do it for you, and Liios performed the task with a quiet devotion that made Estinien's heart ache with some unnamed, fierce emotion that had taken up residence in his ribcage for years.
First his gauntlets, then greaves, then the pauldrons, then the chest plate and tassets. Liios lifted them from him, unbuckling the belts and pulling them free with efficiency born of familiarity. He had gotten used to this new armor very quickly, Estinien thought. Though he had also been quick to learn with his old ones, the one stained with Nidhogg's blood, that was his undoing.
The pieces were mounted on the armor rack reserved for them. Liios looked them over with a craftsman's eye, cataloging the nicks and tears to fix. Then he returned to help Estinien out of his tabard, until he was unencumbered enough to do the rest himself.
While Estinien changed out of the sweat-soaked shirt for a fresh one, Liios rummaged for his toolkit and some alkahest. The usual repairs could be carried out with dark-matter crystals, but Estinien understood that the infrequent application of alkahests and solvents kept equipment as effective as when they were first made...
...when done by skilled hands.
If it had been anyone else, even if it was the Ishgardian masters of the forges themselves, Estinien would have yanked back the armor. Passed to him by Hraesvelgr, there was no way he could take such a risk with it. Even now he watched from across the room as Liios emptied the solutions and solvents into bowls, watched as he picked up the tools and crystals.
And only kept watching, as Liios mended first the chips and tears, then coated them. The movements were the same as they had been the last three times Liios had done it for him, months apart, so precise and regular Estinien could be certain they did not change between applications.
He did it in the same order as he had taken them off Estinien. Gauntlets, greaves, pauldrons, chest plate, tassets. The leather parts required a different solution, though Liios didn't reach for it. Instead he tested them, seemingly found them satisfactory, and set them aside in a moment.
Estinien left him to it, so he could go wash his face and the blood and Void dust out of his hair. When he returned, two chilled goblets of mint-flavored drinks in hands, Liios had finished.
Iceheart wore her challenges well, not unlike the woman Estinien had named the suit of armor after. She would never have the handsomeness of new steel, as she had seen battle even before the Dragonsong War, but as Estinien stood there and studied her, he felt the satisfying certainty of knowing she would bear him through several more decades yet. Perhaps even beyond it.
Liios, still seated at the table and now cleaning up his array of tools, looked up at him. He inclined his head in wordless request for his opinion, and Estinien nodded.
He put the drinks on the table where it had been cleared, and kissed Liios's cheek as he took the seat next to him. His not-lover hummed, long eyelashes fluttering as he returned the kiss with one of his own, then another one on Estinien's jaw, as was their habit.
They clacked their goblets together in silent cheer and relief at the end of another day, and drank.
#liios suvali#liiostinien#neither of them said one word to each other fr#the art of silence with bae
5 notes
·
View notes
Text
Transform Packaging Efficiency with Film Lamination Adhesives

In today's fast-paced world, efficiency and sustainability are more important than ever. For film manufacturers, print industry professionals, and product packaging designers, finding ways to reduce waste while improving efficiency is crucial. Enter film lamination adhesives—a game-changing solution that can transform your packaging processes. This blog post explores how film lamination adhesives can help you achieve these goals, making your packaging not just better but also greener.
Understanding Film Lamination Adhesives
Film lamination adhesives are specialized compounds designed to bond layers of film together, creating a single, cohesive material. Used extensively in packaging, these adhesives offer numerous benefits, including enhanced durability and improved barrier properties.
Why Use Film Lamination Adhesives?
Film lamination adhesives are essential for creating multi-layered packaging that protects products effectively. They provide a strong bond between different types of films, ensuring that the final product is both durable and functional.
Types of Film Lamination Adhesives
There are several types of film lamination adhesives available, each designed for specific applications. These include water-based, solvent-based, and solvent-free adhesives. Each type has its own set of advantages, making them suitable for various packaging needs.
Applications in Packaging
Film lamination adhesives are used in a wide range of packaging applications. From seed and pesticide packaging to dairy products and vacuum pouches, these adhesives offer versatile solutions for various industries.
Benefits of Film Lamination Adhesives
Using film lamination adhesives in your packaging processes can significantly reduce waste and improve efficiency. Here are some key benefits:
Enhanced Durability
One of the primary advantages of film lamination adhesives is their ability to create highly durable packaging. This means your products are better protected, reducing the risk of damage during transit.
Improved Barrier Properties
Film lamination adhesives help enhance the barrier properties of packaging, making it more resistant to moisture, oxygen, and other external factors. This is particularly important for products like dairy and vacuum-sealed items.
Versatility
Film lamination adhesives are incredibly versatile, making them suitable for a wide range of applications. Whether you're packaging seeds, pesticides, or consumer goods, these adhesives can meet your needs.
Improving Efficiency in Packaging
Efficiency is key to staying competitive in the packaging industry. Film lamination adhesives can streamline your processes, making them more efficient and cost-effective.
Faster Production Times
Film lamination adhesives can significantly speed up production times. By creating strong bonds quickly, they allow for faster assembly of multi-layered packaging, increasing your overall output.
Reduced Downtime
Using high-quality film lamination adhesives can reduce downtime caused by equipment malfunctions or material failures. This ensures a smoother production process and higher efficiency.
Cost Savings
By minimizing waste and improving production efficiency, film lamination adhesives can lead to significant cost savings. This makes them a smart investment for any packaging operation.
The Future of Packaging with Film Lamination Adhesives
The packaging industry is constantly evolving, and film lamination adhesives are at the forefront of this evolution. By adopting these advanced adhesives, you can stay ahead of the curve and ensure your packaging processes are both efficient and sustainable.
Innovations on the Horizon
Ongoing research and development in film lamination adhesives promise even more exciting innovations in the future. Stay tuned for new products and technologies that will further enhance your packaging capabilities.
Commitment to Sustainability
At [Your Company Name], we're committed to sustainability. Our film lamination adhesives are designed to minimize environmental impact while delivering superior performance. Join us in our mission to create a better, more sustainable future.
Taking the Next Step
Ready to revolutionize your packaging processes? Contact us today to learn more about our film lamination adhesives and how they can benefit your business. Our team of experts is here to help you find the perfect solution for your needs.
2 notes
·
View notes
Text
Kind of down again.
I’m physically exhausted, still, from last week but also still plugging along, trying to be active and get stuff done.

I had hoped Triton XL would be a fast, easy, total solution to removing Mattel head glue without damaging the paint and it hasn’t been, and that’s been a little bit of a let down.
I did do one more experiment (using Triton the same way I use T.A.) and again it wasn’t a magic answer. Results of an overnight soak in Triton-heavy distilled water are similar to the results of an overnight soak in T.A. concentrate. Better, but not gone.
Maybe changing the cleanser and water and letting her sit a while longer would get her totally clean, just like T.A., but Triton is difficult to rinse out (you really do need distilled water or you’re going to be rinsing for a WHILE) where T.A. isn’t and the less standing at the sink I have to do, the better [for me].
It feels like others testing Triton really, really want it to be more effective than T.A. and it isn’t. The biggest advantage Triton has is being safer for repainted faces.
Still, it is good that there is another option. You can choose between a solvent and a detergent, passive soaking or manual agitation, longer time frame with very little labor or shorter time frame with more labor, and get the same results.
I suspect Triton is more gentle on the vinyl, especially considering it’s less drying for the hair. T.A. does dry out doll hair.
I’m still let down. Everyone was so hyped up about it. I feel bad being like “The results have been the same.” It feels like harshing someone’s happy vibe.
I wanted it to work.

I don’t regret selling off all of my MH dolls still since this isn’t some magic glue-obliterating potion, so that’s good, I guess. I think I wouldn’t much regret getting rid of them anyway. I’m not sure I ever liked them in the first place, they were just different.
But really, I don’t tend to enjoy my dolls much. They’re all downstairs. I never spend time down there, so I never see my dolls.
I do wonder if that’s why I keep getting more and more dolls; because I can’t see them regularly. Or maybe it’s just habit.

Either way I’m just kind of discontent with life in general right now.
The past 15 years have been mostly in this one, unpainted room and I don’t see a way out.
11 notes
·
View notes
Photo


(hale appleman, they/he, cursed blood) To MICAH ALTMAN, the whole world looks like an open page. With a leap of faith, their ability of TRANSMUTATION grows a little stronger. They are a TWO-TAILED, MOSS GREEN PATAGONIA FOX shade aligned to NO ONE — but formerly of House Beltran. For 206 years, they have survived a world of magic with both their LIGHT-HEARTEDNESS and CAPRICE. They work as a POTIONS MASTER, but if they could change their fate, they’d want to BE USEFUL ONCE AGAIN.
basic stats // @cagliostrohq
There have always been plenty of things Micah did in their life very specifically because those things didn’t make sense, but working with potions had always made sense.
The transmutation skill came to them early in life, literally child’s play as they turned their friends’ toys into butterflies and back again. A powerful party trick, one that literally shaped the world around them, and eventually shaped Micah’s own future. A skill like theirs could be dangerous in so many hands, but the dreamshade had only ever wanted to help. Even in playfulness and pranks their motives weren’t cruel; whenever someone needed something, less useful items transformed into exactly what would help the most. Eventually, that meant Micah had every tool they ever needed to succeed, and crafting potions became their most useful practice yet, a skill they would go on to hone for decades — eventually, centuries.
Callum Byrne was the only other thing in Micah’s two hundred years of existence that’d ever made as much sense to them.
A proud spirit warrior from the House Beltran, Callum was not so proud when Micah met him. Drenched in sweat and shame, shuddering under a compulsion he did not know how to control, Callum came to Micah in pure desperation for help quelling the golden demon that coiled in his blood. Micah hesitated only briefly; they’d always been warned about cursed bloods, they’d heard the stories. But so too had humans and spirit warriors been told stories about those dangerous dreamshade, yet here Callum was. Desperate but earnest.
So, Micah did what they could to help. It was an uphill battle, but one they fought together. Trust came slowly but carefully, built brick by meticulous brick. Micah was only ever clear their remedies would be experimental, and Callum put his life and his sanity in their hands. Over a handful of years Micah didn’t manage to make much progress, only minor tinctures or solvents that, at best, muffled the worst of the cursed blood’s afflictions. The process was frustrating, but Micah never gave up. For Callum, they would never give up, even if it took another two hundred years.
A different opportunity presented itself far sooner than that, in the shape of magical contracts forged to fight the Boundary. In a matter of days, Callum was begging Micah to contract with him — if they could fight the Boundary together, maybe they could make steps towards a clearer solution for Callum in a much more tangible way. If they could fight the Boundary, they could fight Callum’s demons, and maybe, all together, they could win.
Micah agreed without hesitation, and as they did so they realized: the love they felt for Callum extended far beyond the fire-forged friendship the two had made over the past years.
Training together came easily to the pair, already so practiced in trust and now genuinely excited that they didn’t have to hide the closeness of their relationship, that between shade and warrior, that few contracted understood in the same way. The shade gave his magic freely and Callum used it expertly, a dance that felt as natural to the two as their friendship had for years. Micah didn’t pledge to Beltran in quite the same way or with the same depth they pledged themselves to Callum, but that didn’t necessarily matter. Not, at least, until House Beltran turned on them.
Micah’s potions had only ever been band-aids over bullet holes for the gold-flecked blood in Callum’s veins, and as the two started to shift their focus, the evolution of Micah’s experiments fell by the wayside. Time spent fighting all manner of beast and person didn’t help; it was never lost on Micah, the distant sheen of want in Callum’s eyes as he stood over an opponent, vanquished and bloodied. Micah could only ever drag him back to the light and pray. It was not a tactic that would last them the year they’d been granted to fight the Boundary.
The descent was abrupt, but Callum had already tasted the blood of others before, ultimately, he tasted Micah’s. A golden-webbed scar, branching from the indelible mark of Callum’s teeth, hides under a leather bracer Micah always wears on their left forearm.
Destroyed by what he’d done to the one person he trusted most, to the one person who trusted him the most, Callum did the only thing that made sense to him: he turned himself in. With a smile that didn’t reach his eyes nearly so well as the tears, he tried to get Micah to promise not to make a scene. Micah, naturally, did what they did best — and made a scene.
On the day of Callum’s execution, Micah begged the new boy-lord of House Beltran to spare their warrior, but no amount of scraped knees, tears, and groveling made any difference. All Micah managed to secure was a peaceful end for Callum, a cold comfort that left an equally cold vastness in its wake.
Micah left House Beltran the same day, feeling no loyalty to a house that’d turned its back on them, no matter the justifiable reason. Even as the scar on their forearm twisted and warped and started to impart upon Micah the very same side effects they’d seen Callum struggle with for years, Micah couldn’t forgive Beltran for putting him to death.
Adrift of purpose, Micah still hasn’t found the courage to pack up and leave the city just yet. That sense of usefulness and belonging they’d felt fighting alongside Callum sits in their veins as heavily as their curse, both longing to be fulfilled in very different capacities. For now, all Micah can manage is warring against their own curse, and sitting hesitantly by as they hope against hope to feel the same usefulness and flawless synergy they once felt with Callum. They hope against hope that even now that Callum is gone, the Boundary can be defeated, and even in death may Micah’s spirit warrior find some peace.
#cag: intro#about.#less of a bio and more 'the past handful of years'#bc i gotta be honest#i am Naut writing 206 years' worth of bio :]
6 notes
·
View notes
Text
Understanding the Differences Between Dry Cleaning and Laundry Services

Dry cleaning and laundry services are essential for keeping your clothes clean and well-maintained. However, it can be confusing to understand the differences between the two services. In this blog, we will explain the differences between dry cleaning and laundry services and help you choose the right service for your needs.
Dry cleaning is a specialized cleaning process that uses chemical solvents to remove dirt and stains from clothes. It is an ideal solution for delicate fabrics such as silk, wool, and cashmere, which can be damaged by water and soap. Dry cleaning is also useful for removing tough stains such as grease, oil, and ink.
On the other hand, laundry services use water, detergent, and mechanical action to clean clothes. It is an excellent solution for everyday clothes such as cotton, linen, and polyester. Laundry services can be used to clean clothes, bedsheets, curtains, and other washable fabrics.
When choosing a quality laundry service, there are several factors to consider. Here are the top 5 tips for choosing a quality laundry service:
Quality of service: Look for a laundry service that provides high-quality service. Check for online reviews, customer feedback, and ratings to evaluate the quality of service.
Range of services: Choose a laundry service that offers a wide range of services, including laundry, dry cleaning, ironing, and more. This will ensure that all your laundry needs are met.
Pricing: Compare the prices of different laundry services to find the best value for your money. Look for a service that provides transparent pricing and no hidden charges.
Convenience: Choose a laundry service that offers convenient pickup and delivery options. This will save you time and hassle of dropping off and picking up your laundry.
Customer service: Look for a laundry service that provides excellent customer service. They should be responsive to your queries, provide regular updates, and address any concerns you may have.
At Hamlet Laundry, we are committed to providing high-quality laundry and dry cleaning services to our customers in London. We offer a wide range of services, including laundry, dry cleaning, ironing, and more. Our services are available for all washable fabrics, including wedding dresses, curtains, and rugs.
We offer a convenient online order process through our website and mobile apps. You can easily schedule your pickup and delivery times, track your order, and receive an itemized invoice. We also offer a special discount of 30% on the first order for customers who place their order through our mobile apps.
2 notes
·
View notes
Text

Several Methods for Producing Cannabis Concentrates… and Some Pros and Cons
There are several methods for producing cannabis concentrates, each with its own set of pros and cons. Some common methods include:
Solvent-Based Extraction: This method involves using a solvent, such as butane, propane, or alcohol, to extract the desired compounds from the cannabis plant material. The solvent is passed through the plant material, which dissolves the desired compounds and separates them from the plant matter. The solution is then filtered and the solvent is removed, leaving behind a concentrated extract. This method can be efficient and can produce a high-quality product, but it can also be dangerous if not done properly, as solvents can be highly flammable.
CO2 Extraction: This method uses carbon dioxide to extract the desired compounds from the cannabis plant. The CO2 is pressurized and cooled to a liquid state, and then passed through the plant material. The pressure and temperature of the CO2 can be adjusted to selectively extract different compounds from the plant. This method is generally considered to be safe and produces a high-quality product, but it can be expensive and requires specialized equipment.
Rosin Pressing: This method involves applying heat and pressure to the cannabis plant material to extract the desired compounds. The plant material is placed between two heated plates or in a bag, and then pressure is applied to force the extract out. This method is generally considered to be safe and can produce a high-quality product, but it can be labor-intensive and may not be as efficient as other methods.
Water-Based Extraction: This method involves using water as the solvent to extract the desired compounds from the cannabis plant. The plant material is soaked in water, and the water is then filtered to separate the extract from the plant matter. This method is generally considered to be safe and can produce a high-quality product, but it can be less efficient and may not be suitable for extracting certain types of compounds.
Dry Sieve: This method involves using a mesh screen to separate the trichomes (the glandular hairs that contain the desired compounds) from the rest of the plant material. The plant material is agitated and the trichomes fall through the screen, leaving behind a concentrated extract. This method is generally considered to be safe and can produce a high-quality product, but it can be labor-intensive and may not be as efficient as other methods.
Enzyme-Assisted Extraction: This method involves using enzymes to break down the plant material and extract the desired compounds. The plant material is soaked in an enzyme solution, and the enzymes break down the plant material and release the desired compounds. This method is generally considered to be safe and can produce a high-quality product, but it can be less efficient and may not be suitable for extracting certain types of compounds.
Mechanical Separation: This method involves using mechanical means, such as grinding or shaking, to separate the trichomes from the rest of the plant material. The plant material is ground or shaken, and the trichomes fall off, leaving behind a concentrated extract. This method is generally considered to be safe and can produce a high-quality product, but it can be labor-intensive and may not be as efficient as other methods.
Butane Hash Oil (BHO) Extraction: This method involves using butane as the solvent to extract the desired compounds from the cannabis plant. The plant material is placed in a tube or container, and butane is passed through it to dissolve the desired compounds. The solution is then filtered and the butane is removed, leaving behind a concentrated extract. BHO extraction can produce a high-quality product, but it can be dangerous if not done properly, as butane is highly flammable.
Propane Hash Oil (PHO) Extraction: This method is similar to BHO extraction, but it uses propane as the solvent instead of butane. Propane is passed through the plant material to dissolve the desired compounds, and the solution is then filtered and the propane is removed. PHO extraction can produce a high-quality product, but it can also be dangerous if not done properly, as propane is highly flammable.
Isopropyl Alcohol Extraction: This method involves using isopropyl alcohol as the solvent to extract the desired compounds from the cannabis plant. The plant material is soaked in isopropyl alcohol, and the alcohol is then filtered to separate the extract from the plant matter. This method is generally considered to be safe, but it may not be as efficient as other methods and may not be suitable for extracting certain types of compounds.
Ethanol Extraction: This method involves using ethanol (grain alcohol) as the solvent to extract the desired compounds from the cannabis plant. The plant material is soaked in ethanol, and the ethanol is then filtered to separate the extract from the plant matter. Ethanol extraction can be efficient and can produce a high-quality product, but it may not be suitable for extracting certain types of compounds.
Steam Distillation: This method involves using steam to extract the desired compounds from the cannabis plant. The plant material is placed in a still, and steam is passed through it to dissolve the desired compounds. The steam is then cooled and the condensed liquid is collected, leaving behind a concentrated extract. Steam distillation can be efficient and can produce a high-quality product, but it may not be suitable for extracting certain types of compounds.
Lecithin-Assisted Extraction: This method involves using lecithin, a type of phospholipid, to help extract the desired compounds from the cannabis plant. The plant material is soaked in a solution containing lecithin, and the lecithin helps to dissolve the desired compounds. The solution is then filtered to separate the extract from the plant matter. Lecithin-assisted extraction can be efficient and can produce a high-quality product, but it may not be suitable for extracting certain types of compounds.
Supercritical Fluid Extraction (SFE): This method involves using a supercritical fluid, such as CO2, to extract the desired compounds from the cannabis plant. The supercritical fluid is passed through the plant material at high pressure, and the pressure and temperature of the fluid can be adjusted to selectively extract different compounds from the plant. SFE can produce a high-quality product, but it requires specialized equipment and can be expensive.
Sonic Extraction: This method involves using sound waves to extract the desired compounds from the cannabis plant. The plant material is placed in a chamber, and sound waves are applied to it to release the desired compounds. The extract is then collected and the plant matter is discarded. Sonic extraction can be efficient and can produce a high-quality product, but it requires specialized equipment and may not be suitable for extracting certain types of compounds.
The best method for producing cannabis concentrates will depend on the specific needs and goals of the producer.
By the Potmaster General, for Potsmart
2 notes
·
View notes
Text
WHAT ARE THE BEST TYPES OF PAINT TO USE IN SPRAY BOOTHS?
If you haven’t done it yet, you better get cracking. Solvent-based paints are out and you only have until next year to comply with new regulations banning solvent-based paints in spray booth operations. Fortunately, the move away from solvent-based paints to water-based paints is a win-win for both your business and the environment.
Working with waterborne paint and drying booths? If you’re looking for a unique method of spray painting that literally cuts airborne emissions in half, waterborne paint and paint drying booths are the perfect paint to use for your next automotive project.
When it comes to going waterborne, a lot of folks want to know what paint booth to buy or what waterborne drying system to add to their existing paint booth.
The Dangers of Solvent-Based Paint
Even in the best paint booth, Volatile Organic Compounds or VOCs pose enormous health risks according to the EPA. In solvent-based paints, practically 85% of the chemicals contained within are VOCs. The risks to your health include:
Irritation to the Eyes, Nose, and Throat
Headaches
Balance and Coordination Problems
Nausea
Liver Damage
Kidney Damage
Damage to the Central Nervous System
And Some Cause Cancer
In its place, paint booth operators are going to have to switch to water-based paints that are mostly water and only 10% contains VOCs. These changes are going into effect nationally next year (2017) and many states have their own VOC regulations.
Switch Now and Start Reaping the Benefits
When you consider all of the pluses to transition to water-based paints, it doesn’t make sense to wait until you absolutely have to switch. Your finishes look even better using water-based paint than they did using solvent-based paints, reflecting a truer color. You can save 50% on your energy costs. And your finishes are cleaner and much more even.
On top of that, you will increase productivity within your shop because you can push the cars through much faster. Our Waterborne paint system has adjustable jet speeds and in-booth controls.
How to effectively regulate humidity in a waterborne paint and drying booth
The main dissimilarity between waterborne paint and solvent paint is, without doubt, the distinctly different drying and curing process which each solution requires. Solvent-based paints are effectively cured through effective temperature control. On the flip side, the drying process for waterborne paints is effectively achieved by regulating humidity levels in a spray paint booth environment. As noted previously, your team will have to control the atmosphere in your unit to obtain the best paint job results. This means there will always be a need for increased airflow to enable the waterborne paint to dry up much faster. To be in a position of further regulating just how quickly such paints dry up, you may add either standard or even desert controllers in your booth.
Waterborne paint often necessitates “wet-on-wet” application. In simpler terms, this implies that the color applied is often different from the eventual dry color. So, it is always prudent to closely follow the paint manufacturer’s instructions to obtain the best possible paint job outcomes.
In terms of speed, auxiliary air movement systems such as waterborne jet systems and ceiling fans are the fastest drying, however, air turbulence may stir up dirt and debris if the paint booth or prep station is not clean. High powered spray booths that are designed to produce more cfm (cubic feet of air per minute) in the traditional draft pattern are a clean alternative to add-on blowers and fans but will take a little more time to dry since the draft us still not penetrating as close to the surface as a turbulent air system. The major benefit of waterborne paint booths with high CFM is the cleanliness of the job. In terms of drying waterborne paint, it may take a high powered downdraft paint booth a few minutes longer to flash, but if the job is clean, there is much more time saved overall since little or no buffing will be needed. By the same token, if a blower system can flash off waterborne paint very quickly but throws dirt or dust, the job may actually end up taking much longer in re-do’s and buffing time.
Essential Tips to Keep In Mind
Even though waterborne is safer than solvent, you cannot skimp out on protect eye and face-wear. You need to always wear a respirator whenever you’re in the booth because paint can linger in the air.
On occasion, waterborne paint will require you to do wet-on-wet application. This means the paint color you immediately apply may look different than the one that appears after it dries. However, some paints also have higher amounts of solid compositions. This means you can fully paint the part of vehicle with few coats.
Outside of having a full or half arch gas catalytic dryer, you should also use a handheld gas catalytic dryer to help quickly set the paint and prevent running or sagging.
In either case, the key to successfully drying waterborne paint is simple. Provide more airflow & above all, KEEP YOUR WORKSPACE CLEAN! A clean job is always the fastest
0 notes
Text
HDPE Bags: The Best HDPE Bags Wholesale Online
High-density polyethylene (HDPE) bags have become a staple in various industries due to their durability, versatility, and cost-effectiveness. Available in multiple sizes and thicknesses, these bags serve a wide range of applications, from packaging food and agricultural products to chemicals and construction materials. This article will dive into the benefits, features, and manufacturing processes of HDPE bags, while also shedding light on the best wholesale options available online for businesses and suppliers.
What Are HDPE Bags?
HDPE (High-Density Polyethylene) bags are made from a robust plastic material that is highly resistant to chemicals, moisture, and physical damage. This makes them perfect for packaging heavy-duty products such as grains, fertilizers, and chemicals. HDPE bags 50 kg variants are particularly popular for industrial use, as they provide enough strength to hold substantial quantities while maintaining their structural integrity.
HDPE bags come in a variety of sizes, and one of the most commonly sought-after capacities is the 50 kg HDPE bag, which is widely used in agriculture, construction, and bulk food storage. These bags offer an excellent balance between strength, durability, and affordability, making them a go-to solution for many industries.
The Benefits of HDPE Bags
HDPE bags offer several benefits over other types of packaging materials, such as paper or lower-grade plastics. Some of the key advantages include:
Strength and Durability: HDPE bags are exceptionally strong and can withstand heavy loads without tearing or breaking. This makes them ideal for packaging bulk items such as grains, fertilizers, and construction materials.
Chemical Resistance: HDPE is resistant to many acids, chemicals, and solvents, ensuring that the contents of the bag remain uncontaminated and safe.
Moisture Resistance: Unlike paper or fabric bags, HDPE bags do not absorb water, making them suitable for storing moisture-sensitive products.
Eco-friendly Options: Many manufacturers now offer recyclable and eco-friendly HDPE bags, aligning with global environmental sustainability efforts.
Cost-Effective: Given their durability and reusability, HDPE bags often prove to be more cost-effective in the long run compared to other materials.
HDPE Bags Manufacturing Process
The HDPE bags manufacturing process is a highly technical one that involves several stages of production. It begins with the polymerization of ethylene gas, which forms polyethylene resin. This resin is then melted and extruded into sheets of HDPE film, which are then cut and shaped into bags.
Once the material has been shaped into bags, they undergo additional processes such as heat-sealing, printing, and lamination to enhance durability and functionality. High-tech machinery and strict quality controls ensure that the bags produced meet stringent industry standards.
Popular Uses of HDPE Bags
HDPE bags serve a variety of uses in different industries, and their adaptability makes them a popular choice for packaging bulk items. Some common applications include:
Agricultural Packaging: HDPE bags 50 kg are widely used in agriculture to store grains, seeds, and fertilizers. The bags’ moisture resistance and durability make them ideal for long-term storage and transport.
Chemical and Fertilizer Packaging: HDPE bags are often employed to package chemicals and fertilizers because of their resistance to chemical degradation and their ability to hold heavy loads.
Construction Material Storage: In the construction industry, 50 kg HDPE bags are frequently used to store and transport materials like cement, sand, and gravel. Their strength ensures they won’t easily tear under heavy weights.
Food and Beverage Industry: These bags are also used for packaging large quantities of dry food items like rice, wheat, and flour. HDPE’s food-safe properties make it an ideal choice for this purpose.
HDPE Bags Price in India
When sourcing HDPE bags, particularly in large quantities for industrial or commercial purposes, understanding the HDPE bags price in India is crucial. Prices of HDPE bags can vary based on factors such as the size of the bag, thickness of the material, and any additional features like lamination or printing.
Typically, the price of HDPE bags 50 kg in India ranges from INR 15 to INR 50 per bag, depending on the quality and the supplier. Buying in bulk can significantly lower the price per bag, making it more economical for businesses to purchase wholesale. The 50 kg HDPE bag is especially in demand due to its ability to handle large quantities of material.
How to Choose the Best HDPE Bags Wholesale Online
Choosing the right HDPE bags supplier is crucial for ensuring that you get a high-quality product at a competitive price. When sourcing HDPE bags wholesale online, consider the following factors:
Reputation of the Supplier: Always opt for a reputable supplier who has positive reviews and a proven track record. A good supplier will offer not only competitive prices but also excellent customer service and timely delivery.
Product Range: Look for suppliers who offer a wide variety of HDPE bags in different sizes and thicknesses. This ensures you can find the right bag for your specific needs.
Customization Options: Some suppliers offer customizable bags with printing and branding options. This is especially beneficial for businesses looking to increase brand visibility.
Quality Control: Ensure that the supplier adheres to stringent quality control measures during the HDPE bags manufacturing process. This guarantees that the bags you receive are durable and meet industry standards.
Competitive Pricing: Compare prices across different suppliers to ensure that you're getting the best deal. Buying in bulk can significantly reduce costs, so look for suppliers offering wholesale rates.
Conclusion
HDPE bags are indispensable for many industries due to their strength, versatility, and cost-effectiveness. Whether you're in agriculture, construction, or the food industry, sourcing high-quality HDPE bags wholesale online can ensure that your packaging needs are met efficiently and economically. By understanding the HDPE bags manufacturing process, uses, and pricing, you can make informed decisions when purchasing the best HDPE bags for your business.
Frequently Asked Questions About HDPE Bags
1. What are HDPE bags used for?
HDPE bags are used for packaging a wide range of products, including agricultural goods like grains and fertilizers, construction materials such as sand and cement, and food products like rice and flour.
2. How are HDPE bags manufactured?
The HDPE bags manufacturing process involves the polymerization of ethylene gas to form polyethylene resin. This resin is then melted, extruded into sheets, and formed into bags through heat sealing and cutting.
3. What is the price of HDPE bags in India?
The HDPE Bags price in India varies based on size, quality, and features. On average, a 50 kg HDPE bag can range from INR 15 to INR 50 per bag. Bulk purchases can lead to more competitive pricing.
0 notes
Text
Thermo Adhesive Film for Bonding

Thermo adhesive films have emerged as an advanced bonding solution in various industries, offering superior performance, flexibility, and ease of application. These films are activated by heat, allowing for a seamless, strong, and durable bond between different materials. Whether used in automotive, electronics, textiles, or packaging industries, thermo adhesive films have become essential due to their versatile applications and high bonding efficiency.
One of the key advantages of thermo adhesive films is their ability to bond a wide range of materials, including plastics, metals, fabrics, and even wood. The film is applied to the surface, and when heat is used, it melts, creating a strong adhesive layer that binds the materials together. This process ensures a uniform bond without additional adhesives or mechanical fasteners, thus saving time and reducing material usage.
In industrial applications, thermo adhesive films provide high precision and consistency, making them ideal for automated production lines. Their use in electronics for assembling circuit boards or bonding protective layers showcases the importance of reliability in delicate applications. Additionally, thermo adhesive films contribute to eco-friendly practices, as they can be used without solvents, reducing harmful emissions.
Their flexibility and durability also make them suitable for consumer products like shoes, clothing, and home appliances, where a strong yet discreet bond is required. In conclusion, thermo adhesive films offer a cutting-edge, efficient, and environmentally friendly bonding solution, making them invaluable across industries.
0 notes
Text
Thermo Adhesive Film for Bonding

Thermo adhesive films have emerged as an advanced bonding solution in various industries, offering superior performance, flexibility, and ease of application. These films are activated by heat, allowing for a seamless, strong, and durable bond between different materials. Whether used in automotive, electronics, textiles, or packaging industries, thermo adhesive films have become essential due to their versatile applications and high bonding efficiency.
One of the key advantages of thermo adhesive films is their ability to bond a wide range of materials, including plastics, metals, fabrics, and even wood. The film is applied to the surface, and when heat is used, it melts, creating a strong adhesive layer that binds the materials together. This process ensures a uniform bond without additional adhesives or mechanical fasteners, thus saving time and reducing material usage.
In industrial applications, thermo adhesive films provide high precision and consistency, making them ideal for automated production lines. Their use in electronics for assembling circuit boards or bonding protective layers showcases the importance of reliability in delicate applications. Additionally, thermo adhesive films contribute to eco-friendly practices, as they can be used without solvents, reducing harmful emissions.
Their flexibility and durability also make them suitable for consumer products like shoes, clothing, and home appliances, where a strong yet discreet bond is required. In conclusion, thermo adhesive films offer a cutting-edge, efficient, and environmentally friendly bonding solution, making them invaluable across industries.
#thermoadhesivefilm#durableadhesivefilm#thermofilmforelectronics#thermobondingfilm#adhesivefilmsforpackaging
0 notes