#cytochrome chain
Explore tagged Tumblr posts
Text
Cyanide inhibits cytochrome c oxidase in the mitochondria, which blocks the electron transport chain.
"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quote#plant physiology and development#nonfiction#textbook#cyanide#cytochrome#inhibition#mitochondria#electron transport chain
0 notes
Text
I wanna start a picrew chain because I've finally found one that has everything I've been looking for >:3
no guidelines, no nothing, just make what you look like or your ideal self :3
here the link
and here's me :DDD

tagging (/nf): @cytochrome-sea @sunfl0wersapphic @sparks-chaotic-cove @sunnymellow09 @disappointedcreeper @rivals-legacy
(hey mooties sorry for the tag on my alt lol)
#chaotic puppy posting#picrew chain#therianthropy#therian#therian community#otherkin community#otherkin#otherkinity#nonhumanity#nonhuman#nonhuman community#alterhuman community#alterhumanity#alterhuman
64 notes
·
View notes
Text
A new species of bacteria that functions like electrical wiring has recently been discovered on a brackish beach in Oregon. The species was named Candidatus Electrothrix yaqonensis in honor of the Yaquina tribe of Native Americans that once lived in and around Yaquina Bay, where the bacteria were found.
This species is a type of cable bacteria: rod-shaped microbes that are connected at both ends to one another to create a chain and which share an outer membrane, forming filaments several centimeters long. Cable bacteria are found in marine and freshwater sediments and, unusually among bacteria, are electrically conductive. This is due to their special metabolism, in which electrons generated by oxidizing sulfides in their deeper layers are sent to their surface layer, where they are received by oxygen and nitric acid.
The 25 species of cable bacteria known so far have been organized into two genera, Candidatus Electrothrix, which live in saltwater, and Candidatus Electronema, which live in fresh and brackish water. The new species discovered in this study has the genes and metabolic pathways of both the genera but is believed to be a bridge to an earlier branch of the Candidatus Electrothrix lineage, and so was classified as part of that genus.
The recently discovered species may provide new insights into how cable bacteria evolved and how they can function in diverse environments, Cheng Li, a postdoctoral researcher at Oregon State University and coauthor of the research, explained in a statement.
High Electrical Conductivity
Candidatus Electrothrix yaqonensis is distinct from existing cable bacteria in its appearance. Cable bacteria have outer shells that feature ridges, which spread out like mountains. The ridges of the new species are much thicker than those of previously known species, reaching an average thickness of about 228 nanometers, up to three times thicker than what has been seen before. The new species’ ridges are arranged in a spiral-like pattern on the surface of the filament, and their overall shape is more angular than that of other species.
But the most striking difference is that the new species’ filament is surrounded by a thick, transparent sheath. According to the authors of the paper outlining the discovery, this is a structure not previously seen. This sheath does not conduct electricity and is thought to protect the filament from the environment and foreign enemies.
A filament of Candidatus Electrothrix yaqonensis, the newly discovered species of cable bacteria. Photograph: Photograph: Oregon State University
Inside the new bacteria’s ridge is a fiber containing a nickel-centered metal complex, which functions as a “biological wire” that efficiently transports electrons along the filament. It is as if the structure itself was designed with an engineering intent.
The physical performance of the bacteria as a conductor is impressive. When the researchers placed microscopically isolated filaments on a gold electrode and applied a voltage, a graph showing the change in current and voltage produced a linear, symmetrical I-V curve—implying high electrical conductivity. The new species’ electrical resistance was approximately 370 kilo-ohms, which is equal to or better than that of known cable bacteria.
A Genetic Mosaic
Genomic analysis revealed that the new species has genetic features of both the Candidatus Electrothrix and Candidatus Electronema genera. This phenomenon, where genetically distinct material is intermingled within a single individual, is known as “mosaicism.” For example, this can be seen in the novel bacteria’s cytochrome, a type of protein involved in electron transport. Typically in the genus Candidatus Electrothrix, bacteria have a single heme (a complex composed of a divalent iron atom and a porphyrin). But the new species, like some other types of cable bacteria, is equipped with a cytochrome with two hemes.
This new species is also unique in the way it adapts to saline environments. Candidatus Electrothrix species, which live in saltwater, typically use an electron-transfer enzyme called “sodium-transporting NADH-quinone oxidoreductase (NQR)” to regulate osmotic pressure. But this enzyme is absent in Candidatus Electrothrix yaqonensis, which instead has several proteins— “sodium and proton exchange transporters (NHE)”—that exchange sodium ions and protons across the cell membrane. This is thought to be the result of adaptation to the unique environment of brackish water, where salinity fluctuates.
Further studies will reveal the mechanism of the unique sheath formation of Candidatus Electrothrix yaqonensis as well as the self-assembling process of its conductive fibers. According to the research team, this new species, because it combines high-electrical conductivity and environmental adaptability, has the potential to be used as a new material in the field of bioelectronics. Potentially it could help with the creation of biodegradable electronic devices and biosensors in the future. Its characteristics may also be useful for remediation of heavy metals and organic pollutants in sedimentary environments.
17 notes
·
View notes
Text
Cyanide Poison

Let's start by understanding exactly how cyanide kills you. In simple terms, cyanide prevents cells from using oxygen to make energy molecules.
The cyanide ion, CN-, binds to the iron atom in cytochrome C oxidase in the mitochondria of cells. It acts as an irreversible enzyme inhibitor, preventing cytochrome C oxidase from doing its job, which is to transport electrons to oxygen in the electron transport chain of aerobic cellular respiration. Now unable to use oxygen, the mitochondria can't produce the energy carrier adenosine triphosphate (ATP). Tissues that require this form of energy, such as heart, muscle cells, and nerve cells, quickly expend all their energy and start to die. When a large enough number of critical cells die, you expire as well. Death usually results from respiratory or heart failure.
Immediate aymptoms include headaches, nausea and vomiting, dizziness, lack of coordination, and rapid heart rate. Long exposure symptoms include unconsciousness, convulsions, respiratory failure, coma and death.
A person exposed to cyanide may have cherry-red skin from high oxygen levels, or dark blue coloring, from Prussian blue (iron-binding to the cyanide ion). In addition to this, skin and body fluids may give off an almond odor.
The antidotes for cyanide include sodium nitrite, hydroxocobalamin, and sodium thiosulfate.
A high dose of inhaled cyanide is lethal too quickly for any treatment to take effect, but ingested cyanide or lower doses of inhaled cyanide may be countered by administering antidotes that detoxify cyanide or bind to it. For example, hydroxocobalamin, natural vitamin B12, reacts with cyanide to form cyanocobalamin, which leaves the body in urine.
These antidotes are administrated via injection, or IV infusion.
Cyanide is actually a lot more common than you'd think. It's in pesticides, fumigants, plastics, and electroplating, among other things. However, not all cyanide are so poisonous. Sodium cyanide (NaCN), potassium cyanide (KCN), hydrogen cyanide (HCN), and cyanogen chloride (CNCl) are lethal, but thousands of compounds called nitriles contain the cyanide group, yet aren't as toxic. They still aren't terribly good for you, so I wouldn't go around ingesting other cyanide compounds, but they're not quite as dangerous as the lethal kind.
Thank you for reading, have a lovely day :)
#cyanide#poison#cyanide poison#tw poison#poisons#chemistry#?#if it counts lmao#crime#criminal#investigation#forensics#scienceblr#science#sherlock#sherlock holmes
49 notes
·
View notes
Text
Unraveling the Tapestry of Cellular Energy: A Comprehensive Voyage through the Electron Transport Chain 🧬⚙️
Prepare for a deep dive into the labyrinthine pathways of the Electron Transport Chain (ETC), where molecular machinations weave the intricate tapestry of cellular respiration. In this odyssey, we'll navigate the complexities with surgical precision, leaving no nuance unexplored.
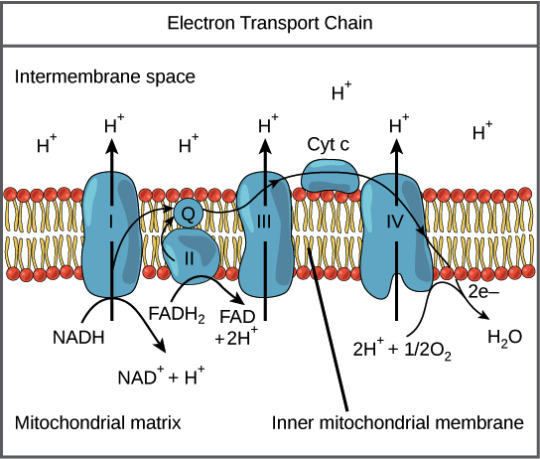
1. Prelude at Complex I (NADH Dehydrogenase):
The ETC's overture commences at Complex I, where NADH, a product of glycolysis and the Krebs cycle, surrenders its high-energy electrons. Traverse the serpentine route of flavin mononucleotide (FMN) and a succession of iron-sulfur clusters, witnessing the orchestrated dance that propels electrons toward the enigmatic ubiquinone (Q).
2. Interlude with Succinate (Complex II - Succinate Dehydrogenase):
As the symphony progresses, Complex II takes the stage with succinate as its protagonist. Succinate dehydrogenase, fueled by succinate from the Krebs cycle, orchestrates a parallel electron flow. Behold the ballet of electrons navigating iron-sulfur clusters and flavin adenine dinucleotide (FAD), converging upon ubiquinone (Q) in a seamless choreography.
3. Cytochrome Waltz (Complex III - Cytochrome bc1 Complex):
The narrative crescendos at Complex III, the cytochrome bc1 complex, where Q takes center stage. Through a series of mesmerizing redox reactions, Q gracefully shuttles electrons to cytochrome c. This transient dancer becomes the ethereal messenger, ferrying electrons with finesse towards the climactic rendezvous at Complex IV.
4. Grand Finale with Complex IV (Cytochrome c Oxidase):
In the climactic finale, Complex IV, personified by cytochrome c oxidase, awaits the electron ensemble. Watch as electrons, guided by a cascade of copper and iron centers, engage in a captivating pas de deux with molecular oxygen. Witness the alchemical metamorphosis as oxygen is humbly transmuted into water, marking the zenith of our electron saga.
5. Proton Symphony and ATP Synthesis:
Simultaneously, the proton symphony unfolds as protons, displaced during electron transit, accumulate in the intermembrane space. This sets the stage for a grand energy transfer. The finale crescendos with protons flowing back through ATP synthase, a molecular turbine, culminating in the synthesis of ATP—the lifeblood of cellular energy currency.
References:
1. Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2014). Molecular Biology of the Cell (6th ed.). Garland Science.
2. Nelson, D. L., Cox, M. M. (2017). Lehninger Principles of Biochemistry (7th ed.). W.H. Freeman and Company.
3. Berg, J. M., Tymoczko, J. L., Gatto, G. J. S., & Stryer, L. (2019). Biochemistry (8th ed.). W.H. Freeman and Company.
#science#biology#college#education#school#student#medicine#doctors#health#healthcare#molecules#chemistry#molecular biology
55 notes
·
View notes
Note
ermmie wormiee....can you explain how light dependant reactions work I'm genuinely kinda lost on that part of AP Bio like everything else makes sense but unit 3 (enzymes photosynthesis and cellular resp) is genuinely my OP

Ok, so I don’t know how much you have to know so let’s just get into it. It’s not that hard once you get it.
Also please keep in mind that I studied all of this in German so I’m sorry if some things are worded weirdly.
So, there are two photosystems (PSII and PSI) in plants. As you can see in this pic, the whole reaction starts with PSII, not PSI — that’s because PSI was discovered first. (But that’s not really important.)
Photosystems are able to absorb photons (that come from the light) and produce a high energy electron! As you can see in this pic, PSII absorbs the photon, creates a high energy electron and sends it through an electron transport chain (Plastoquinone - that’s a molecule btw) to the Cytochrome b6f complex and from there to PSI.
PSII uses water as an electron donor! That is very important because that’s how oxygen gets created as a by-product.
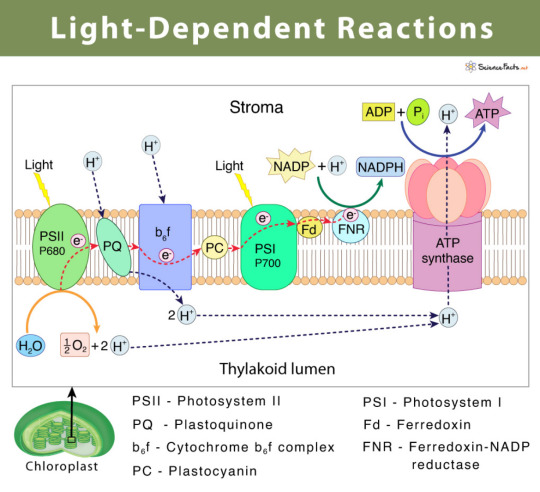
(sciencefacts.net)
PSI absorbs the photon again and produces another high energy electron which converts NADP+ to NADPH.
The big question is: how does the plant create ATP now?
That happens through non-cyclic photophosphorylation. See all those H+ protons? Whereas there is a bunch inside the thylakoid lumen, there isn’t much in the stroma.
That leads to a concentration compensation. So all those little protons that have been produced during the reaction actually go through the ATP-synthase into the stroma. Thus, the ATP-synthase creates ATP!
I also had to remember this, I don’t know if you do but if you have any questions about it, feel free to ask:
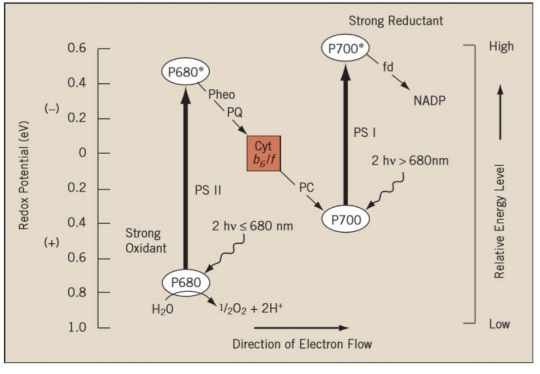
(istudy.pk)
All you need to know is basically:
Light hits PSII. Light = energy. PSII needs energy to turn electron (water = electron donor) into a high energy electron. High energy electron travels. H+ protons get produced during reactions. PSI needs energy. High energy electron converts NADP+ to NADPH (we need that for our Calvin cycle later). Bunch of protons travel through ATP-synthase —> we have ATP now!!!
I hope this makes somewhat sense. As I’ve mentioned, I had to remember all of this in German so yeah it might be a bit messy. If there’s anything unclear, feel free to send another ask!
19 notes
·
View notes
Text
#1299 What do mitochondria do?

What do mitochondria do? Mitochondria produce almost all of the chemical energy that cells need for all of their functions. Without the mitochondria, we can’t do anything. The word “mitochondria” was created in 1898 by a microbiologist called Carl Benda. He took the Greek word “mitos”, which means “thread”, and “khondrion”, which means “little granule”. Mitochondria is the plural form of the word. The singular form is mitochondrion. Carl Benda wasn’t the first person to discover mitochondria. They had first been observed by another German microbiologist called Albert von Kölliker. Kölliker was one of the first people to use a light microscope and Carl Benda used microscopes a lot, which rapidly became the new way of observing living organisms. He observed the mitochondria forming long chains, but he didn’t know what they did. Their purpose was only discovered in 1925. So, what do mitochondria do? Mitochondria probably evolved from independent bacteria. This is just a theory, but the thinking is that mitochondria were prokaryotic cells that were eaten by other cells. Prokaryotic cells are single celled organisms that don’t have a nucleus. They are able to produce energy and reproduce. They were probably eaten as food by eukaryote cells, which are single cells that do have a nucleus. At some point, the two developed a symbiotic relationship. The mitochondria were able to provide energy for the host cells and the host cells provided the mitochondria with food. So, the mitochondria in all of our cells were probably bacteria at some point, although, billions of years ago. Mitochondria have several functions. They produce energy for cell functions. The trigger cell death when a cell is ready to die. They regulate aging. They are very small. Each one is between 0.75 and 3 micrometers. A hair is about 80 micrometers. We have more than one mitochondria in each cell. There are up to 1,000. They move around in the cell and they can work in small groups, or they can combine to work in larger groups, depending on the task. They change shape to deal with the kind of nutrient they are going to eat. We have trillions and trillions of them in our bodies. Different types of cells have different numbers of mitochondria. Mature red blood cells don’t have any and liver cells have 2,000. The more energy a cell needs, the more mitochondria it has. Mitochondria can reproduce by cell division, just like bacteria, and when more are needed, they increase their numbers. The most important job for mitochondria, and probably the reason they ended up living inside our cells, is producing energy. When we eat food, it contains a lot of energy. That energy has ultimately come from the sun, been photosynthesized by plants, and then we either eat the plants or eat animals that have eaten the plants. However, we can’t directly use that energy to carry out processes in our bodies. The mitochondria convert that chemical energy into an energy form called adenosine triphosphate (ATP). This is the energy that our cells can use. The chemical energy is glucose and mitochondria oxidize the parts of the the glucose to make ATP, which powers all of the processes in our cells. Mitochondria also play a large role in cell death. Mitochondria release a protein called cytochrome c as they work. Once this protein has built up to significant levels, it triggers the cell to begin the process of shutting down. It seems odd that they would trigger cell death when that death will kill them as well. It seems that they do it to protect the overall organism. When there are too many damaged mitochondria, or when there is too much damage in a cell, the mitochondria can not function properly. This is when levels of cytochrome c really build up. It seems that the mitochondria have evolved to remove themselves when there is too much damage. I guess, we are the same. Mitochondria are also responsible for absorbing calcium and then holding it until it is needed. Our bodies need calcium for chemical activity in the nerves and muscles. Mitochondria help by hanging on to it for us. They can also warm us up when we are cold. When they burn glucose, they produce heat, which is why we get hot when we exercise. They can also create heat by burning brown fat. Adults don’t have a lot of brown fat, but mitochondria can use it when your core body temperature drops. Babies have a lot more brown fat than adults do. Mitochondria have a lot of functions. - #344 How did the Black Death change society? - #127 How does cancer kill people? - #345 What causes brain damage? - #1113 Do larger animals have a faster metabolism? - #343 How did the Black Death spread? Sources https://www.genome.gov/genetics-glossary/Mitochondria https://www.medicalnewstoday.com/articles/320875#disease https://medschool.ucla.edu/research/themed-areas/metabolism-research/mitochondria https://en.wikipedia.org/wiki/Mitochondrion https://en.wikipedia.org/wiki/Apoptosis https://bscb.org/learning-resources/softcell-e-learning/mitochondrion-much-more-than-an-energy-converter/ https://www.reddit.com/r/biology/comments/bfpbiu/how_was_mitochondria_integrated_to_the_host_cells/ https://en.wikipedia.org/wiki/Carl_Benda https://www.etymonline.com/word/mitochondria Image By Mariana Ruiz Villarreal LadyofHats - the diagram i made myself using adobe illustrator. as a source for the information i used the diagrams found here:, , , , , and ., Public Domain, https://commons.wikimedia.org/w/index.php?curid=6195050 Read the full article
0 notes
Text
Mitochondrial Dysfunction in Beckers Muscular Dystrophy
Introduction
Beckers Muscular Dystrophy (BMD) is a genetic neuromuscular disorder caused by mutations in the DMD gene, leading to defective dystrophin production. While dystrophin primarily serves as a structural protein, emerging evidence indicates its role in mitochondrial function and cellular metabolism. This article explores mitochondrial dysfunction in BMD, focusing on bioenergetics, oxidative stress, mitochondrial dynamics, and metabolic consequences.
Bioenergetic Impairment
Mitochondria are the primary energy-producing organelles, generating adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS). In BMD, mitochondrial bioenergetics are disrupted due to reduced dystrophin-associated glycoprotein complex (DGC) stability, affecting intracellular signaling and energy metabolism. Studies show that muscle fibers from BMD patients exhibit reduced ATP production, mitochondrial membrane potential (ΔΨm) depolarization, and decreased respiratory chain efficiency. Impaired complex I and complex IV activities have been reported, contributing to decreased oxidative phosphorylation and subsequent muscle weakness.
Oxidative Stress and ROS Accumulation
Mitochondria are a significant source of reactive oxygen species (ROS), which play dual roles as signaling molecules and contributors to oxidative damage. In BMD, excessive ROS production due to dysfunctional electron transport chain (ETC) exacerbates oxidative stress. Studies have demonstrated elevated lipid peroxidation, increased protein carbonylation, and mitochondrial DNA (mtDNA) damage in BMD-affected muscles. Reduced expression of key antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), further impairs the ability to counteract oxidative damage. The resulting oxidative burden contributes to muscle fiber degeneration, chronic inflammation, and apoptosis.
Mitochondrial Dynamics: Fission and Fusion Imbalance
Mitochondria continuously undergo fission and fusion processes to maintain cellular homeostasis. These dynamics are critical for mitochondrial quality control, ensuring the removal of damaged mitochondria via mitophagy. In BMD, an imbalance between fission and fusion leads to mitochondrial fragmentation and defective turnover. Key regulators such as dynamin-related protein 1 (DRP1) and mitofusin-2 (MFN2) exhibit altered expression, resulting in increased mitochondrial fission and reduced fusion. This dysregulation impairs mitochondrial network integrity, contributing to decreased ATP production and enhanced susceptibility to apoptosis.
Calcium Homeostasis and Mitochondrial Dysfunction
Dystrophin deficiency in BMD disrupts sarcolemmal stability, leading to aberrant calcium (Ca²⁺) handling. Elevated intracellular Ca²⁺ levels induce mitochondrial Ca²⁺ overload, impairing bioenergetic function and promoting mitochondrial permeability transition pore (mPTP) opening. mPTP dysregulation results in mitochondrial swelling, cytochrome c release, and apoptotic cascade activation. Additionally, excessive mitochondrial Ca²⁺ uptake alters ATP synthesis efficiency, exacerbating muscle fiber necrosis and degeneration.
Metabolic Alterations and Energetic Deficits
Skeletal muscle metabolism in BMD is characterized by a shift from oxidative to glycolytic energy production. Defective mitochondrial respiration forces muscle fibers to rely on glycolysis for ATP generation, leading to increased lactate accumulation and metabolic acidosis. This metabolic shift results in early fatigue, reduced endurance, and inefficient energy utilization. Transcriptomic analyses have identified downregulation of genes involved in fatty acid oxidation and tricarboxylic acid (TCA) cycle activity, further confirming the metabolic shift towards glycolysis. Such metabolic alterations compromise muscle function and regeneration capacity, contributing to disease progression.
Mitochondrial Quality Control and Mitophagy Defects
Mitophagy, a selective form of autophagy responsible for degrading damaged mitochondria, is impaired in BMD. The PINK1/Parkin pathway, essential for mitochondrial quality control, is downregulated in dystrophic muscle, leading to the accumulation of dysfunctional mitochondria. Defective mitophagy contributes to mitochondrial swelling, increased oxidative stress, and cellular energy deficits. Additionally, impaired mitophagy reduces the capacity for mitochondrial biogenesis, further exacerbating mitochondrial dysfunction and muscle pathology.
Conclusion
Mitochondrial dysfunction in BMD arises from bioenergetic impairments, oxidative stress, disrupted mitochondrial dynamics, altered Ca²⁺ homeostasis, metabolic deficits, and defective mitophagy. These abnormalities collectively contribute to muscle degeneration and disease progression. Understanding these mitochondrial defects provides valuable insights into the pathophysiology of BMD, emphasizing the need for targeted researc
h to mitigate mitochondrial dysfunction and improve muscle health in affected individuals.
#Beckers Muscular Dystrophy#Mitochondrial dysfunction in BMD#Oxidative stress in muscular dystrophy#Mitochondrial bioenergetics in BMD#ATP production in muscle disease#Reactive oxygen species (ROS) in BMD#Electron transport chain dysfunction#Mitochondrial DNA damage in BMD#Calcium homeostasis in muscular dystrophy#Mitochondrial fission and fusion imbalance#Mitophagy defects in BMD#Muscle fiber degeneration in BMD#Glycolytic metabolism in muscular dystrophy#Mitochondrial membrane potential disruption#Sarcolemmal instability in BMD#Superoxide dismutase (SOD) in muscle health#Mitochondrial permeability transition pore (mPTP)#Fatty acid oxidation in muscle disease#Dystrophin-associated glycoprotein complex (DGC)#Metabolic deficits in Beckers muscular dystrophy
0 notes
Text
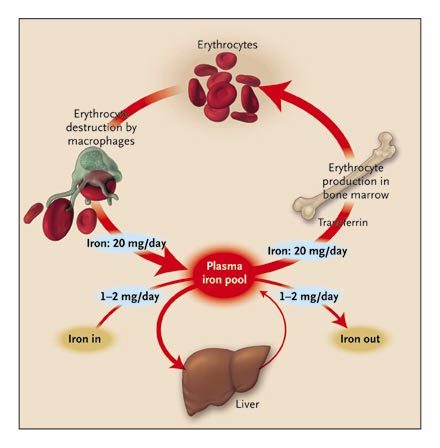
Iron regulation in the body Iron is a constituent of all living matter. Iron is a core factor in the electron transfer chain in the body; it is also a vital part of oxygen transport and iron storing molecules such as hemoglobin. It is also a component of host defense where it occurs in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Humans lack an effective mechanism to excrete surplus iron, though they are unsurpassed in iron conservation since they have unique homeostatic mechanisms that ensure iron level remains within normal parameters. These mechanisms regulate the absorption of iron from the duodenum and release it from macrophages and other iron-storing sites. The release of large amounts of iron into circulation may result in localized injury to the surrounding tissues. Iron exerts its toxic effects through the catalysis of free radical reactions leading to the production of free radicals such as peroxides that damage tissues. Furthermore, iron levels need to be maintained within physiological limits since infection is in part determined by the availability of iron for utilization by the invading microorganism. The bacteria have evolved complex mechanisms for uptake of iron from their environment that bind iron. An example of such mechanisms includes the secretion of organic molecules called siderophores that have a high affinity for iron. Desferrioxamine an iron binder is an example of such a siderophore. Other siderophores include yersiniabactin produced by Yersinia sp (Collins 2003, p.194). Studies carried out show that mice with iron overload are highly susceptible to a wide range of infections. This phenomenon has also been observed in human patients that have iron overload. (Jurado 1997, p. 888-890). Since little iron is lost from the body, iron regulation is through stepwise regulation of its absorption from the gut into the general circulation. Iron in the body is usually recycled and stored in various body stores (Ganz & Hershko 2000, p.2-4). Amplification of the absorption process occurs in states of iron deficiency whereas absorption is decreased in situations of iron overload. Through this mechanism, the body is able to maintain iron levels within normal limits (Finch et al 1978, p.335). Regulation of iron is an essential process since an excess of iron leads to end-organ damage while deficiency of iron results in cellular dysfunction and anemia. Regulation of the body’s iron stores is under the influence of specific iron regulating proteins. These proteins include transferrin, ferritin, ferroportin, and Hepcidin. Other proteins such as lactoferrin control iron levels through chelation to form iron-protein complexes (Waheed et al 1999, p.15). Total body iron A normal human body contains about 4 to 5g of Iron. Haemoglobin iron forms the bulk of total body iron, accounting for up to 60% of total body iron. Hemoglobin is major the functional form of iron in the body (Harris & Kellermeyer 1970,p 87). Approximately 10% of total body iron exists in the body as ferritin and hemosiderin. The liver stores iron as ferritin and this accounts for a third of total body iron. The remainder of iron is stored throughout the body tissues as myoglobin and in heme enzymes such as catalases, cytochromes, and peroxidizes and non-heme enzymes such as reductase, metalloflavoproteins, and ribonucleotide reductase. The stored iron exists in macrophages that populate the spleen, liver, and bone marrow. Iron occurs in minute quantities in circulation usually about 3-4mg (Donovan et al 2000, p.776-779). When red blood cells become senescent, they undergo lyses and liberate iron, which is incorporated in the synthesis of hemoglobin and other iron-dependent proteins. About 20mg of iron is recovered through this process. Daily losses of iron from the body are in minute quantities. It is estimated to be around 1-2mg of iron, which is readily replaced from the diet (Bothwell 1995, p.24) Figure 1: diagram showing Iron cycle in the human body (Source: Pietrangelo 2004, p.2383-2397). Iron Absorption The brush border of the epithelium of the intestinal villi of the duodenum and upper jejunum is the site of iron absorption in the human body. Iron is absorbed in form of heme, non-heme, ferric, or ferrous ions. Studies show that at least nine proteins are involved in the absorption process (Bothwell 1995, p.28). The amount of iron available in the body has an inverse relationship with the amount of iron absorbed. When body iron stores decrease, the amount of iron absorbed increases. When there is iron overload, the absorption of iron decreases. Dietary iron is mainly non-heme, which is readily absorbed depending on the presence of inhibitors or promoters. Inhibitors such as phytates and tannins decrease iron absorption while promoters such as ascorbic acid enhance absorption. Mucosal cells regulate iron absorption through various mechanisms: regulation of uptake across the brush border, retention of iron in the mucosal cell in a different form, and release of iron from the mucosal cell into plasma (Avunduk 2008, p. 427). Meat-derived iron is absorbed four times faster than vegetable-derived iron. An average adult consumes approximately 10 to 15mg of iron daily. Women lose more iron than men do. Iron is lost as “exfoliated epithelial cells of the gastrointestinal tract and skin, and menstrual blood loss in women” (Avunduk 2008, p. 427). The body lacks any physiological mechanism to excrete iron. As such, iron absorption should be finely controlled and regulated within the narrow physiological range. Iron Control Centre The liver is the center of control of iron homeostasis in the body. Control of iron levels in the body is essential since an overload results in organ damage and conditions like hemochromatosis. The deficiency of iron results in anemia usually referred to as iron deficiency anemia. The iron regulation process employs specialized sensors. The author writes, “Special sensors respond to the iron and stimulate the synthesis and release of the iron hormone Hepcidin, which is encoded by HAMP gene. Hepcidin circulates through the body and interacts with the iron exporter ferroportin expressed on the surface of iron-rich macrophages and intestinal cells. Because of this interaction, ferroportin is internalized and degraded. The unneeded iron remains in the cells where it is saved for future use in the form of ferritin. The diminished release of iron restores blood levels to the non-toxic range, thus reinsuring the stimulus for further Hepcidin synthesis and ferroportin gradually resumes its iron exporting activity”( Avunduk 2008, p. 428). The connection between Hepcidin and iron overload was first brought to light by Pigeon et al during the study of the response of the liver to iron overload. (Pigeon, Ilyin & Courselaud 2001,p. 16). They found out that the Hepcidin mRNA was induced by an iron overload of parenteral and dietary origin. Furthermore, they found out that induction of Hepcidin mRNA occurred when mice were treated with polysaccharides (LPS) to simulate an active bacterial infection. From these results, it was concluded that Hepcidin production was under the influence of iron and an immune system stimulus (polysaccharide). It was also observed that the Hepcidin gene is in the same loci as the upstream regulatory factor-2(USF-2) gene. Other gene knock-out studies reinforced the relation between USF-2gene and iron overload since mice that had their USF-2 gene knocked all developed hemochromatosis at the same time. (Nicholas, Bennoun &Devaux 2001, p. 12-14). It was further observed that these mice had no Hepcidin mRNA expressed. This led the authors to conclude that Hepcidin was a controller of iron absorption from the duodenum and upper jejunum and that it was a regulator of iron release from macrophages. It is the absence of Hepcidin that resulted in iron overload (hemochromatosis) since the gene coding for the Hepcidin gene is located in the same loci as the USF-2 gene. Mechanism of action of Hepcidin at the molecular level Inflammation and iron overload induce transcription of Hepcidin mRNA in the hepatocytes. Inflammatory mediators induce the production of cytokines such as IL-1, IL-6, TNF-β, and TNF-α. IL-6 acts by amplifying the transcription process. Hepcidin is produced in high amounts and released into circulation to be delivered to iron exporting cells located in the small intestine and spleen. Hepcidin then binds to the iron exporting protein called ferroportin (Fpn). When Hepcidin binds to ferroportin, it leads to activation of the Jak2 pathway and subsequent phosphorylation of ferroportin. The phosphorylated receptor is turn internalized and degraded by the lysosome hence impairing iron export from the affected cell into circulation (De Domenico 2007, p. 2569-2578.) It has been established that in absence of iron overload and inflammation, macrophages have little ferroportin on their surface. Read the full article
0 notes
Text
The Power of Methylene Blue: Enhancing Energy, Cognition, and Cellular Health

Methylene blue, a compound with a long history in medicine and scientific research, is gaining traction as a powerful biohacking tool. From boosting mitochondrial function to improving cognitive performance, methylene blue has a range of benefits that can support overall health and longevity. When paired with red light therapy, its effects can be even more profound.
What Is Methylene Blue?
Methylene blue is a synthetic compound that has been used for over a century in various medical applications, including as an antimicrobial and a treatment for methemoglobinemia (a condition that affects oxygen transport in the blood). More recently, research has uncovered its potential as a mitochondrial enhancer, cognitive booster, and neuroprotective agent.
Key Benefits of Methylene Blue
1. Mitochondrial Support & Energy Production
Methylene blue acts as an electron donor in the mitochondrial electron transport chain, helping to improve ATP production. This means more energy for your cells, leading to enhanced physical and mental performance.
2. Cognitive Enhancement & Neuroprotection
By increasing cellular energy and reducing oxidative stress, methylene blue has been shown to support brain health, improve memory, and protect against neurodegenerative conditions. It can also enhance focus, making it a powerful tool for productivity.
3. Anti-Inflammatory & Antioxidant Properties
Methylene blue helps neutralize reactive oxygen species (ROS) and reduces inflammation, which can support immune function and overall well-being.
4. Increased Oxygen Utilization & Blood Flow
By improving oxygen delivery to tissues, methylene blue can enhance endurance and cardiovascular function, making it beneficial for athletes and those looking to improve physical performance.
Methylene Blue & Red Light Therapy: A Powerful Combination
One of the most exciting synergies in biohacking involves combining methylene blue with red light therapy. Red and near-infrared light help activate cytochrome c oxidase, a key enzyme in the mitochondrial respiratory chain. Methylene blue amplifies this effect by optimizing electron flow and enhancing the efficiency of ATP production.
Together, these two therapies:
Improve cellular energy production
Support brain function and cognitive performance
Reduce oxidative stress and inflammation
Enhance tissue repair and recovery
For best results, take Methylene Blue Liquid Energy before red light therapy to maximize mitochondrial activation and overall benefits.
This advanced blend includes:
Methylene Blue:A powerful nootropic and cellular energy enhancer that boosts ATP production and supports cognitive function.
Mineral Oxide: Provides essential minerals that support various biochemical processes in the body, enhancing overall health along with charged oxygen that can benefit the electron transport chain (ETC).
Black Pepper Extract:Promotes bioavailability of Methylene Blue and Mineral Oxides.
How to Use Methylene Blue Safely
Dosage:Start with a low dose and gradually increase as tolerated. An average dose is 1 fl oz (30 mL) daily.
Timing:Best taken in the morning or early afternoon to avoid potential sleep disturbances due to increased energy levels.
Precautions:Methylene blue may interact with certain medications, particularly SSRIs and MAOIs. Always consult with a healthcare professional before use.
Final Thoughts
As your chiropractor in Macomb, MI, Methylene blue is a game-changer for those looking to enhance their energy, cognition, and cellular health. When combined with red light therapy, it creates a powerful synergy that optimizes mitochondrial function and overall well-being. If you’re looking for a high-quality methylene blue product, Methylene Blue Liquid Energy is an excellent choice to integrate into your health and performance regimen.
#chiropractor in macomb mi#chiropractic care macomb mi#top ranked chiropractor in macomb mi#affordable chiropractor in macomb mi#Methylene Blue Liquid Energy
0 notes
Text
The Science Behind Copper: Why It's Essential for Your Health
Copper, a trace mineral found in various foods and essential for the body's overall function, plays a significant role in maintaining health. Despite being required in small amounts, copper is indispensable for numerous physiological processes, including energy production, connective tissue formation, brain function, and the immune system. Understanding the science behind copper and its health benefits sheds light on why this mineral is vital for our well-being.
The Role of Copper in the Body
Copper is a cofactor for several enzymes known as cuproenzymes. These enzymes facilitate various biochemical reactions in the body. One of the critical functions of copper is its role in energy production. Copper is a component of cytochrome c oxidase, an enzyme in the mitochondria responsible for the final step in the electron transport chain, which generates ATP, the cell's primary energy currency.
Additionally, copper is crucial for the formation and maintenance of connective tissues. Lysyl oxidase, a copper-dependent enzyme, is essential for the cross-linking of collagen and elastin, two proteins that provide strength and elasticity to connective tissues like skin, bones, and blood vessels. This process is vital for wound healing, maintaining the integrity of blood vessels, and ensuring the strength of bones and cartilage.
Copper and the Nervous System
The nervous system relies heavily on copper for optimal function. Copper is involved in the synthesis of neurotransmitters, the chemical messengers that transmit signals between nerve cells. Dopamine-beta-hydroxylase, an enzyme that converts dopamine to norepinephrine, requires copper to function correctly. Norepinephrine is a neurotransmitter essential for mood regulation, alertness, and stress response.
Moreover, copper contributes to the formation and maintenance of myelin, the protective sheath around nerve fibers that ensures efficient transmission of nerve impulses. Copper deficiency can lead to neurological issues, including impaired motor coordination and cognitive function.

Copper's Role in the Immune System
A well-functioning immune system is crucial for defending the body against infections and diseases. Copper enhances the immune system by stimulating the production and activity of immune cells. It helps in the production of white blood cells, including neutrophils and macrophages, which are essential for phagocytosis—the process of engulfing and destroying pathogens.
Furthermore, copper possesses antimicrobial properties. It can kill or inhibit the growth of bacteria, viruses, and fungi, making it an essential element for maintaining immune health and preventing infections.
Sources of Copper and Daily Requirements
Copper is naturally present in a variety of foods. Good dietary sources include shellfish, seeds and nuts, whole grains, dark leafy greens, and organ meats. For most people, a balanced diet provides sufficient copper to meet daily needs. The recommended daily allowance (RDA) for copper varies by age, sex, and life stage, but for adults, it is approximately 900 micrograms per day.
Risks of Copper Deficiency and Toxicity
While copper deficiency is rare, it can occur, especially in individuals with certain genetic disorders, malabsorption issues, or those who rely on parenteral nutrition. Symptoms of deficiency include anemia, weakened immune response, neurological problems, and cardiovascular issues.
On the other hand, excessive copper intake can lead to toxicity, resulting in symptoms like abdominal pain, nausea, vomiting, and, in severe cases, liver damage and kidney failure. Therefore, it is essential to maintain a balanced intake of copper through diet and avoid excessive supplementation unless prescribed by a healthcare provider.

Conclusion
Copper's role in energy production, connective tissue formation, nervous system function, and immune health highlights its importance as an essential mineral. Ensuring adequate copper intake through a balanced diet can support these vital processes and improve overall health and well-being. Understanding the science behind copper underscores why this trace mineral is indispensable for maintaining a healthy body.
0 notes
Text

Ujjawala Ferrous
Ferrous Sulphate (as Fe) 19% Ujjawala Ferrous contains Iron (as Fe) 19% and Sulphur (as S) 10.5%. Iron plays vital role in photosynthesis and breakdown of carbohydrates. Iron play a significant role in various physiological and biochemical pathways in plants. It severs as components of many vital enzymes such as cytochromes of the electron transport chain, and it is thus required for a wide range of biological function in plants. It ensures normal growth of crops & high-quality yields and is suitable for all crops. Deficiency Symptoms: Iron deficiency results in yellowing between the leaf veins of young leaves. Browning of leaf edges also occurs in acid- loving plants. Dosage: Soil Application: Apply 10 kg of Ujjawala Ferrous per acre for all crops.
0 notes
Text
Sodium Cyanide Market Trends: Insights and Forecasts
Introduction Sodium cyanide is an odorless chemical compound with the formula NaCN. It is a white, water-soluble solid. NaCN has a wide range of industrial and other applications, but it is also notoriously toxic and has sometimes been used for suicide or murder. Let's explore some key facts about this dangerous chemical compound. Chemical Properties and Structure Sodium cyanide has the chemical formula NaCN and a molar mass of 49.01 g/mol. It dissociates in water to give hydroxide (NaOH) and cyanide (CN-) ions. The cyanide ion is linear with carbon and nitrogen separated by a triple bond. It is this CN- ion that is primarily responsible for NaCN 's high toxicity. NaCN is a white solid that melts at 563°C to give a colorless liquid. It is highly soluble in water. Toxicity and Mode of Action Cyanide is an inhibitor of cytochrome c oxidase, an important enzyme in the mitochondrial electron transport chain. By blocking this enzyme, cyanide essentially prevents aerobic respiration from taking place at the cellular level. This leads to a rapid depletion of oxygen to tissues and ultimately causes death due to hypoxia at the tissue and organ level. The lethal dose of NaCN for adult humans is reported to be 200-300 mg. However, as little as 1-5 grams can prove fatal. The primary symptoms of cyanide poisoning include headaches, dizziness, confusion, convulsions and cardiac arrest. Death by cyanide poisoning usually occurs within minutes to an hour. There is no antidote for cyanide poisoning. Treatment focuses on supportive measures in a hospital environment along with use of antidotes like sodium thiosulfate or dicobalt edetate to combat the effects of cyanide. Industrial Uses Despite its obvious toxicity, NaCN has many beneficial industrial applications primarily due to its ability to dissolve minerals containing precious metals like gold and silver. It is widely used for extraction of these metals via cyanidation process in mining operations. In this process, an aqueous solution of NaCN is used to leach gold from minerals into the water to facilitate separation and recovery of gold. NaCN is also used in some cleaning and metal surface treatment applications. Other uses include: Production of nylon - Sodium cyanide serves as an intermediate for adiponitrile, which is further used to make nylon 6,6. Case hardening - It is used in metallurgy for case hardening of steels to increase wear resistance and hardness. Alkylation - In organic chemistry, NaCN acts as an alkylating agent in production of compounds like acrylonitrile. Accidental and Intentional Poisonings There have been many accidental and intentional deaths reported due to sodium cyanide poisoning over the years. Accidental cases may occur due to occupational exposure in industries using cyanide or due to consumption of cyanide-containing products mistaken as food. Intentional poisonings with cyanide have been reported in cases of suicide or murder. Some high-profile murder cases have involved the use of NaCN by the perpetrator. Given the acute toxicity of cyanide ions, even small amounts ingested intentionally can prove rapidly lethal. Proper safety precautions are a must for any activities involving this deadly chemical salt. Regulation and Safe Handling Considering the high human toxicity of NaCN, it is designated as a schedule 2 substance under the Chemical Weapons Convention. Many countries have strict regulations governing its manufacture, transportation, storage and industrial usage. Workers directly handling NaCN must be properly trained in safety procedures like wearing recommended personal protective equipment. Leakage of cyanide solutions into the environment must also be prevented to avoid toxicity to other organisms. Overall, given the risks involved, sodium cyanide requires controlled and regulated use with utmost precautions taken at all stages to prevent accidental poisonings.
0 notes
Text
Biogenesis of cytochromes c and c1 in the electron transport chain of malaria parasites
BioRxiv: http://dlvr.it/T2C3pD
0 notes
Text
What Should You Know About Red Light Therapy Weight Loss?
Numerous studies have shown that red light treatment, a non-invasive method of low-level laser therapy, can aid in fat loss. While Red Light Body Contouring is not a magic bullet for weight reduction on its own, it has been used successfully by the biohacking community as a supplement to healthy eating and regular exercise to help people shed excess pounds more quickly.
Red light therapy is defined as.
Red light or Body Sculpting Treatmentsis a subset of LLLT that employs the application of bioactive visible and infrared light wavelengths to the affected area. Commonly used photon frequencies include the red end of the visible spectrum (630–660 nm) and the near infrared end (850 nm).
Red light therapy or Body Sculpting Non Surgicalimproves adenosine triphosphate (ATP) production by increasing the availability of oxygen at the fourth step of electron chain transport, when cytochrome c-oxidase is used. Then, localized fat reduction and improved body contouring are possible thanks to adenosine triphosphate.
Some research suggests that Red Light Therapy for Weight Losscan help the body get rid of excess fat by reducing fat cells and then releasing them through the body's natural waste elimination systems. The body's fat cell count drops as a result.
A clinical dermatologist can-do red-light treatment in the comfort of your own home or at a spa. Cost-effective and convenient, Red Light Therapy for Inflammationat home allow you to reap the weight loss advantages of LLLT without leaving the ease of your own home. Studies have revealed that red light treatment can aid with weight loss.
Red light therapy: any risks?
Although red light therapy has been demonstrated to be safe in separate trials, it is better to err on the side of caution. Before beginning a course of red-light therapy, it is wise to consult with your doctor.
A light exposure test should be done before beginning regular use of red-light treatment. This method entails illuminating a small patch of skin with the device's red and infrared lights to see if a reaction occurs.
Red light treatment, whether used at house or in a health club, has been shown in trials to have no serious adverse effects.
Is It Appropriate to Invest in Red Light Therapy?
Red Light Therapy Weight Loss will not be effective unless other crucial lifestyle parameters are also drastically improved. Maintaining a healthy body requires a commitment to both regular exercise and a well-rounded, nutritional diet. Fat loss is increased when combined with red light therapy compared to when either method is used alone.
As a non-invasive method of reducing fat and reshaping the physique, red light therapy has many advantages.
Red light therapy, when combined with a healthy diet and regular exercise, has been shown to be an effective, non-invasive method of accelerating weight loss. Because of the lack of serious safety issues, red light therapy is a great addition to your weight loss arsenal.
Waist, thigh, and hip circumference can all be significantly reduced with regular red light therapy sessions lasting only twenty minutes.
0 notes
Text
Mitochondrial Dysfunction in Primary Mitochondrial Disease
Introduction
Primary Mitochondrial Disease (PMD) refers to a group of genetic disorders resulting from defects in mitochondrial function. Mitochondria play a crucial role in energy production through oxidative phosphorylation (OXPHOS), and their dysfunction leads to a wide spectrum of clinical manifestations affecting multiple organ systems. PMD primarily arises from mutations in mitochondrial DNA (mtDNA) or nuclear DNA (nDNA) encoding mitochondrial proteins, resulting in impaired energy metabolism and increased cellular stress.
Pathophysiology of Mitochondrial Dysfunction
Mitochondrial dysfunction in PMD is primarily caused by defects in the electron transport chain (ETC), which is responsible for ATP synthesis. The ETC comprises five protein complexes embedded in the inner mitochondrial membrane. Mutations affecting these complexes disrupt ATP production, increase the production of reactive oxygen species (ROS), and lead to metabolic imbalances such as lactic acidosis.
Complex I (NADH: ubiquinone oxidoreductase) and Complex IV (cytochrome c oxidase) deficiencies are among the most common defects in PMD. These impairments reduce the efficiency of ATP production, leading to an energy crisis in high-demand tissues such as the brain, muscles, and heart. Additionally, defects in mitochondrial dynamics, including fission and fusion processes, further contribute to cellular dysfunction.
Genetic and Biochemical Basis
PMD is genetically heterogeneous, with mutations in over 350 known genes. These mutations can be inherited in a maternal, autosomal recessive, or dominant manner. Some commonly affected genes include:
MT-ND genes (encoding Complex I subunits)
SURF1 gene (involved in Complex IV assembly)
POLG gene (critical for mtDNA replication and maintenance)
PDHA1 gene (encoding a subunit of the pyruvate dehydrogenase complex)
Mutations in these genes impair the synthesis of key mitochondrial components, leading to energy production failure, oxidative stress, and apoptotic signaling.
Impact on the Nervous System
The nervous system is highly dependent on mitochondrial energy production, making it particularly susceptible to dysfunction. Mitochondrial defects in PMD often manifest as progressive neurodegenerative disorders, including:
Developmental delay and cognitive impairment
Seizures and epilepsy
Hypotonia and muscle weakness
Ataxia and movement disorders
Peripheral neuropathy
Histopathological findings in affected individuals often reveal spongiform degeneration, gliosis, and neuronal loss, particularly in the basal ganglia, cerebellum, and brainstem. These changes contribute to progressive neurological decline.
Effects on Other Organ Systems
Beyond the nervous system, mitochondrial dysfunction in PMD affects multiple organs due to the ubiquitous need for ATP. Key systemic manifestations include:
Musculoskeletal System: Myopathy, exercise intolerance, and rhabdomyolysis are common due to inadequate ATP supply for muscle contraction and maintenance.
Cardiovascular System: Cardiomyopathy, conduction abnormalities, and arrhythmias result from mitochondrial defects in cardiac muscle, leading to impaired contractility and electrical activity.
Metabolic System: Lactic acidosis and metabolic decompensation occur due to defective oxidative metabolism, leading to systemic energy deficits.
Gastrointestinal System: Dysmotility, feeding difficulties, and pancreatic dysfunction are observed, contributing to malnutrition and failure to thrive.
Endocrine System: Mitochondrial dysfunction affects hormone-producing glands, resulting in diabetes, hypothyroidism, and adrenal insufficiency.
Cellular and Molecular Consequences
Mitochondrial dysfunction in PMD leads to several cellular-level consequences, including:
Increased ROS production, causing oxidative stress and damage to lipids, proteins, and DNA.
Dysregulation of apoptosis, leading to premature cell death and tissue degeneration.
Defective calcium homeostasis, impairing neuronal and muscular function.
Impaired mitochondrial biogenesis, reducing the ability of cells to compensate for energy deficits.
Conclusion
Primary Mitochondrial Disease is a complex, multisystem disorder driven by genetic defects in mitochondrial function. The resulting energy production failure impacts the nervous, muscular, cardiovascular, metabolic, and endocrine systems, leading to severe clinical manifestations. Understanding the molecular and biochemical mechanisms underlying PMD is crucial for advancing diagnostic and research efforts. Continued investigation into mitochondrial biology and genetic contributors will enhance our knowledge of this debilitating disease.
#Mitochondrial#Dysfunction#PMD#Energy#Production#System#Defects#Complex#Leading#Mutations#ATP#These#Disease#Genetic#Resulting#Oxidative#Metabolic#Genes#Nervous
0 notes