gray-matter-in-a-teacup
13 posts
This blog is a compilation of knowledge on various subjects, for Entertainment and Educational purposes. Here you will find mentalism, music, science, crime, psychology, self help, robotics, language, botany, art history, and much, much more. Pretty much everything. Enjoy!
Don't wanna be here? Send us removal request.
Text
ok kids repeat after me
vinegar and bleach makes chlorine gas, which is highly toxic
ammonia and bleach makes chloramine, which is highly toxic
rubbing alcohol and bleach makes chloroform, which is highly toxic
hydrogen peroxide and vinegar makes peracetic/peroxyacetic acid, which can be highly corrosive
be careful about your cleaning products and dont get yourself injured or potentially killed ok
590K notes
·
View notes
Text
HEY. WRITEBLR
PUNCTUATION.
THE PUNCTUATION GUIDE.
A fantastic website for punctuation. It is easy to read and short enough to comprehend! It has all three types of dashes! It explains quotation marks! It explains punctuation for formal and informal writing! GOOD RESOURCE.
316 notes
·
View notes
Text
when the academic article is so good it has you giggling and kicking your feet
11K notes
·
View notes
Text




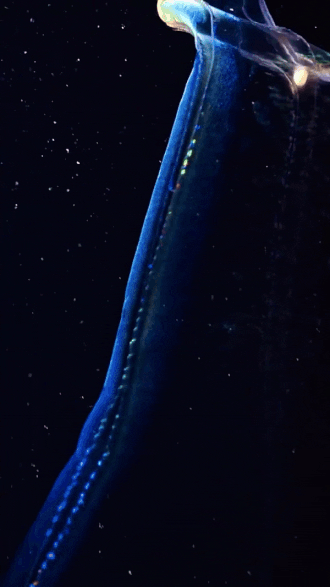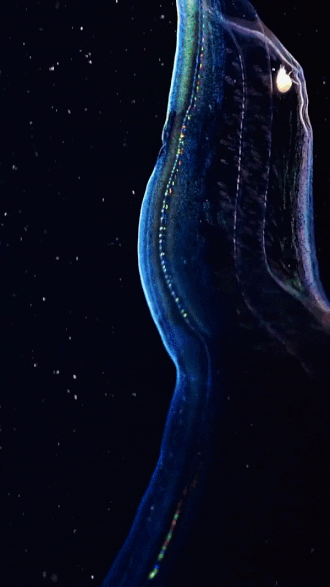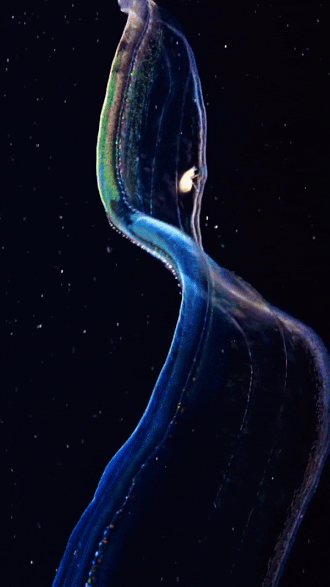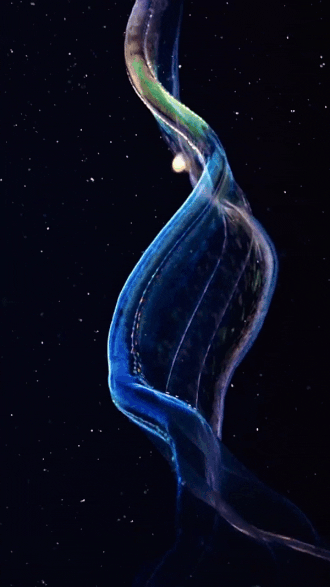
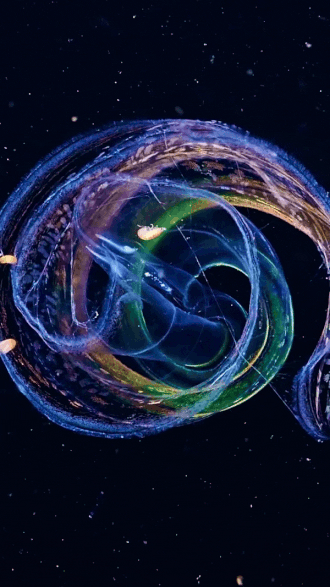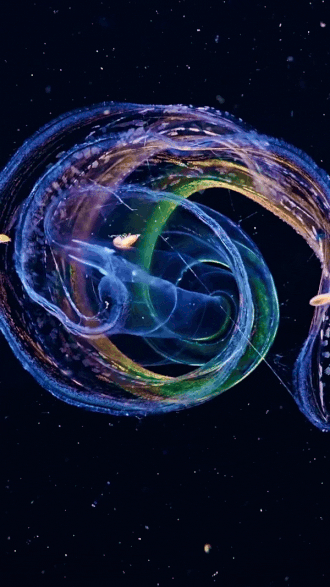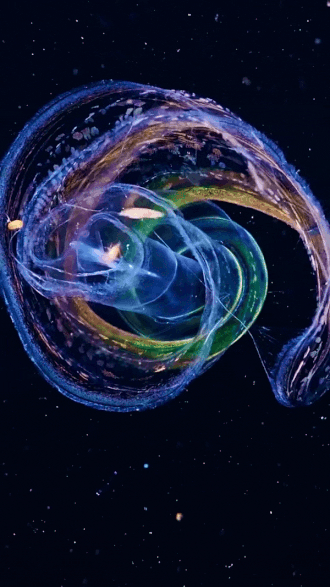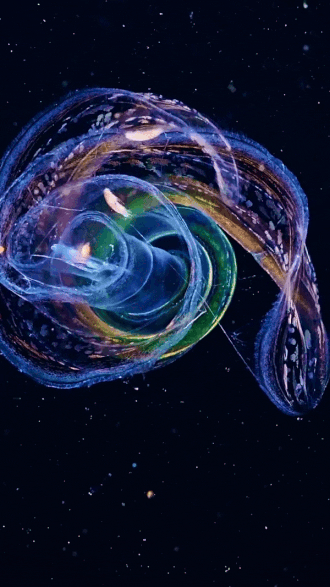
the venus girdle (cestum veneris) | aquatilis_expedition on ig
43K notes
·
View notes
Text
Remember: behind every robot that turns evil is an engineer who specifically installed red LEDs into the eyes just for this occasion
121K notes
·
View notes
Text
favourite thing in the world is when the pages of a book go all soft and yellowy and the edges are slightly fuzzy and rounded. these books couldn’t give you a papercut if you tried they’ve been loved too much. they love you too much
32K notes
·
View notes
Text
Cyanide Poison

Let's start by understanding exactly how cyanide kills you. In simple terms, cyanide prevents cells from using oxygen to make energy molecules.
The cyanide ion, CN-, binds to the iron atom in cytochrome C oxidase in the mitochondria of cells. It acts as an irreversible enzyme inhibitor, preventing cytochrome C oxidase from doing its job, which is to transport electrons to oxygen in the electron transport chain of aerobic cellular respiration. Now unable to use oxygen, the mitochondria can't produce the energy carrier adenosine triphosphate (ATP). Tissues that require this form of energy, such as heart, muscle cells, and nerve cells, quickly expend all their energy and start to die. When a large enough number of critical cells die, you expire as well. Death usually results from respiratory or heart failure.
Immediate aymptoms include headaches, nausea and vomiting, dizziness, lack of coordination, and rapid heart rate. Long exposure symptoms include unconsciousness, convulsions, respiratory failure, coma and death.
A person exposed to cyanide may have cherry-red skin from high oxygen levels, or dark blue coloring, from Prussian blue (iron-binding to the cyanide ion). In addition to this, skin and body fluids may give off an almond odor.
The antidotes for cyanide include sodium nitrite, hydroxocobalamin, and sodium thiosulfate.
A high dose of inhaled cyanide is lethal too quickly for any treatment to take effect, but ingested cyanide or lower doses of inhaled cyanide may be countered by administering antidotes that detoxify cyanide or bind to it. For example, hydroxocobalamin, natural vitamin B12, reacts with cyanide to form cyanocobalamin, which leaves the body in urine.
These antidotes are administrated via injection, or IV infusion.
Cyanide is actually a lot more common than you'd think. It's in pesticides, fumigants, plastics, and electroplating, among other things. However, not all cyanide are so poisonous. Sodium cyanide (NaCN), potassium cyanide (KCN), hydrogen cyanide (HCN), and cyanogen chloride (CNCl) are lethal, but thousands of compounds called nitriles contain the cyanide group, yet aren't as toxic. They still aren't terribly good for you, so I wouldn't go around ingesting other cyanide compounds, but they're not quite as dangerous as the lethal kind.
Thank you for reading, have a lovely day :)
#cyanide#poison#cyanide poison#tw poison#poisons#chemistry#?#if it counts lmao#crime#criminal#investigation#forensics#scienceblr#science#sherlock#sherlock holmes
49 notes
·
View notes
Text
it’s not ‘talking to myself’ it’s called a soliloquy you fuck
113K notes
·
View notes
Text
🧑🏻🔬THE PERIODIC TABLE OF CHEMICAL ELEMENTS👩🏿🔬
(Note: I am not a chemist, just someone who enjoys science, if I made any errors please let me know, thank you and I hope you enjoy reading this)

Ever wonder about this bad boy? While here's a quick crash course on it and some of it groups!
What is it?
The Periodic Table is an arrangement of rows and columns used to display and predict what elements exist and their properties based upon where they fall.
The farther right and down you go, the "heavier" the elements get as they have more Protons, going from 1 Proton for Hydrogen to as of now 118 Protons for Oganesson. Each Collum, excluding the Transition Metals and Rare Earth Series, have similar chemical properties.
Along with this, excluding the Transition Metals, as you go left to right the number of Valence Electrons increases by 1 up to 8 before flipping around back to 1. Valence Electrons can be thought of as the Hands of Atoms, being how they interact and react with each other; a free valence electron spot being a place another atom can bond to like an open hand, while an occupied valence electron spot is like a full hand slapping away other potential electrons.
Each valence group, along with the Transition Metals, builds up the base of all of Chemistry and the universe. So lets have a look at a few of them, shall we?
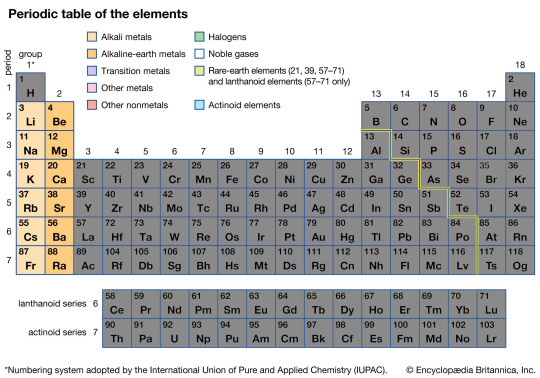
The Alkaline-Earth Metals
The Alkaline-Earth Metals are the two first columns of the Periodic table, and are often sub-divided into the Alkaline Metals and Alkaline Earth Metals. Both groups however do share some properties, often being softer metals that are extremely reactive.
Most famously, they react with water to produce Hydrogen and some heat amount of heat. On the more reactive side such as Lithium and Sodium, this means upon contact with water they will explode, sending red hot metal flying with a violent blast. On the less extreme end such as Magnesium or Calcium, they can be used as ways to produce Hydrogen in small quantities.
Not only are they reactive with water, but also air, oxidizing rapidly in the air and in the case of Magnesium having an extremely violent and energetic reaction when lit on fire.
They also all like to bond with chlorine to produce salts such as Sodium chloride, or more commonly known as humble Table Salt.
After the neat uniformity of the Alkaline-Earth metals, we reach,

The Transition Metals
The Transition Metals are not transgender metals, but rather a large collection of various metals that don't as neatly fit into rows or columns as the other Elemental groupings. Most notably, they have no Valence Electron Shell patterns, meaning you often have to search out the specific Transition Metal you are working with to know how many spots it has still open. They are by far the largest single group of elements within the Periodic Table, and chances are if you think of a metal it will be a transition metal.
It includes some famous stars such as Iron, Gold, Copper, Titanium, Lead, Zinc, Osmium, Tungsten, and Silver. As you can tell from the diverse cast of elements, all of these have wildly varying properties; take the strength and hardness of Iron and Tungsten compared Gold and Zinc as an example.
However they are not totally dissimilar as they are all still metals, meaning they share the properties of Metals. These include high thermal and electrical conductivity, liking to gobble up ions, are highly ductile (the ability to be pulled and bent into wire without breaking), form Cations (positively charged ions), and other such Metal traits.
Truly a party of metals, followed by,

The Other Metals
As the name would suggest, these are some of the other metals and can be thought of as an extension of the Transition Metals.
However their proximity to the other groups gives a few of these metals such as Aluminum more unique chemical behaviors compared to the standard transition metals, which is why sometimes you'll see some disagreement and debate about which ones, if any, should be placed into the next group,

The Metalloids
The Metalloids border between the Metals and the Non Metals, and as such are unique in having properties of both Metals and Non Metals.
Most famous of the Metalloids is arguably Silicon, which makes of the base of the modern world through its semiconductor properties in circuit boards and chips like the one bring used to allow you to read these very words.
Another famous, or perhaps infamous, Metalloid is Arsenic, used in as many poisoning murders for its toxic lethality as paintings and dyes for its beautiful vibrant green hue when turned into a pigment.
And if Arsenic is a bit too deadly for your liking, Gallium is always an option, used in old vacuum tube era computing and lighting. Along with its vintage past, it also has a melting point of 85.58°F or 29.76°C, just shy of room temperature; meaning if you hold a piece of Gallium in your hand, it will melt into a liquid - and unlike Mercury, it is non toxic making it far safer to play with, although it does stick to glass and stain objects so be careful if you do play with it to not let it touch something you don't want getting stained.
And as hinted just before, let us introduce the one and only,
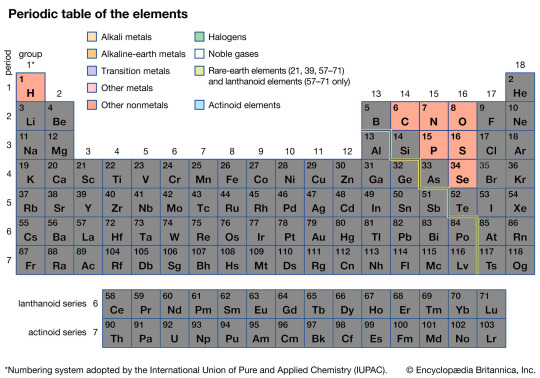
The Non Metals
The Non Metals are a small but extremely unique and important group. All of these elements as the name would suggest sharing the properties of being Non Metals, meaning they are all for the most part poor thermal and electrical conduct, have poor ductility, and form Anions (negatively charged ions).
If you are biologist of any kind, you may also notice this group contains many elements crucial for organic life; the three most important of which without doubt being Carbon, Oxygen, and Hydrogen.
Along with Nitrogen and a few other elements, these elements firm the basis of all life on Earth. From the air you breathe being a Nitrogen-Oxygen mix, to your cells being based on Carbon, to the very DNA that created you. All of it based on Carbon, Oxygen, Nitrogen, and Hydrogen.
And while Oxygen may have taken the honor of the name of Oxidation, it has nothing on the next group,
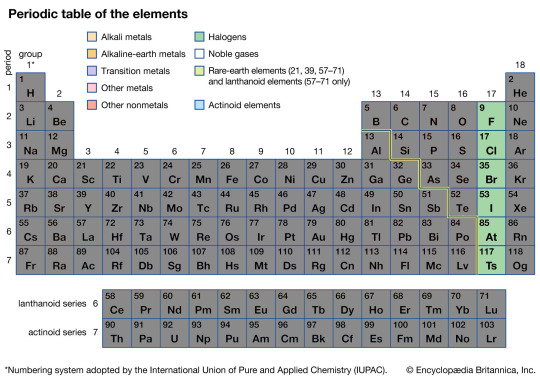
The Halogens
The Halogens, due to missing just one election to be totally stable, are some of the most violently reactive elements on the entire Periodic Table, with Fluorine even known to be able to eat through glass if given enough time.
It might be surprising to hear then that the Halogens also produce some of the most chemically stable compounds known on Earth, such as Polytetrafluoroethylene (PTFE), A.K.A., Teflon. This is because when these elements create compounds they become stable, meaning to break them apart you need an equal amount of energy to do so, and the energy requirement for Halogen compounds is often massive, making them chemically hardy.
Outside of Teflon and eating glass, the less reactive of the Halogens also are used in medicine as a way to sterilize an area. Most commonly and famously used would be Iodine, which not only has a beautiful purple hue, but is also used in surgeries to sterilize equipment and areas before operations, saving countless lives from infections every day.
In contrast to the reactive Halogens, we have next,
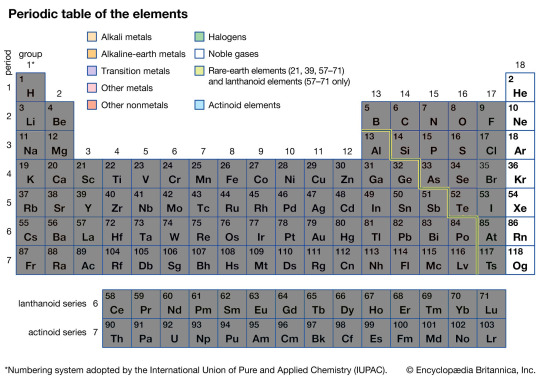
The Nobel Gases
The Nobel Gases like any true Nobels with wealth and status don't like to get involved in the common peasantry's squabbles. In the world of chemicals and elements, this means their full valence electron shells don't stubbornly refuse to chemically react with any other element, and the compounds they do form are often unstable and want to fall apart back into a Nobel Gas and whatever common element it wound up stuck with.
While this lack of reacting does make them rather boring from a reaction perspective, it does make them extremely useful when you do not want things chemically reacting by acting as a buffer between other elements; most easily seen in high end car lights, often filled with Xeon or other Nobel Gases to make sure no matter how hot the light gets it doesn't react chemically and degrade.
Along with protecting lights, they can also be used for lights. Due to their unreactive nature in even high energy environments, it makes them perfect to be used to fill a sealed glass tube that has power shot through it; otherwise known as a Neon Light. However despite the name, not all Neon Lights are filled with Neon, only the orange-red ones are. Other colors such as blues and greens come from the other Nobel Gasses depending on their spectrum emissions.
And on light and degradation, last but not least we have,
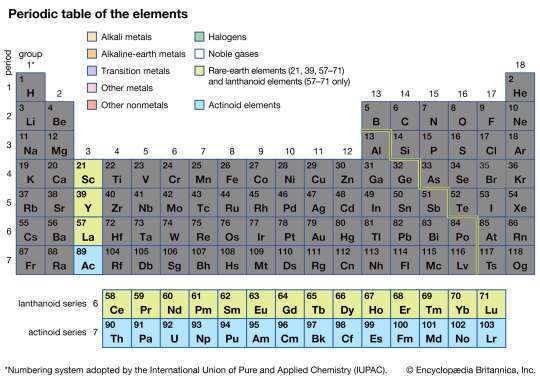
The Rare Earth Metals
The Rare Earth Metals can be and often are split into two smaller groups, the upper Lanthanide series and the lower Actinide series.
The upper Lanthanides are all rather chemically similar, meaning they can often be treated somewhat interchangeably in a chemical sense. Their most notable use is without a doubt their ferro magnetic properties, making some of the most powerful magnets on Earth barring electro-magnets. Neodymium magnets are by far the most famous of them, being known to be able to leap across across tables and smash fingers if not handled carefully, while holding up hundreds of pounds of weight.
In contrast, the lower Actinide series is the domain of many of the Radioactive elements and sees the boundary line between the natural and artificial elements. All of them are radioactive to various degrees with decreasing half life lengths.
It is here Thorium and Uranium is found, being the heart of both terrifying bombs and the cleanest sources of energy known to mankind. Beyond Uranium, all the elements are artificial and must be made by humans in breeder reactors.
Despite their association with atom bombs and nuclear reactors, many of these elements also have far more mundane uses. Americium is used in common household Smoke Detectors, saving untold numbers of lives and dollars in property through early warnings of smoke and fire. Other ones are used by NASA and other space agencies in Radioisotope thermoelectric generators or R.T.G.s, used to power spacecraft like Voyager 2 and the Mars Curiosity Rover thousands of miles away from Earth where conventional solar panels would be too heavy, unwieldy, and inefficient so far away from the Sun.
152 notes
·
View notes
Text
Deducing With Disorders Pt. 1
Dissociative Disorders: TW - Mentions/Descriptions of Dissociation
Credit to @sleuth2k7 for the idea
So quick disclaimer: I am not a certified psychologist, I am giving generalized advice for deducing with disorders that I have been diagnosed with based on my personal experience. Nothing is one-size-fits-all.
When I was 15 I was diagnosed with derealization/depersonalization disorder. DR/DP is one of three types of dissociative disorders spanning approximately 1%-2% of the population, and as with all dissociative disorders, involves repeated episodes of disconnection from one's sense of self and surroundings. With DR/DP specifically, I experience long episodes of what I can only describe as having a VR headset glued on where you can only watch what's happening to your character, and you just have this certainty nothing in this world is real, occasionally being removed from the driver's seat entirely and placed further back in the VR world by some part of your brain that belongs to you on every count except your own.
TLDR: Am I real? Are you? How to deduce when you aren't sure if you're real is the question.
Answer: I personally do reality checks to start, and I incorporate deduction into that. Am I real? Well does 2+2 still equal 4? Do those baggy pants plus that ill-fitting suit still mean that this teenager is wearing his dad's clothes? Probably. Let's find out.
So when we're reconnecting to ourselves and our surroundings, we look for things that verify our experience as truly our own. Make it a game. Certain deductions are worth certain points, it can help to have a friend to play with. A profession is worth 5, a relationship status is worth 10, etc. No friend? No problem. The classic grounding method of listing 5 things you see, 4 you hear, 3 you can touch, 2 you can smell, and 1 thing you can taste can be built upon to be a more mindful exercise that you don't just run right through.
When listing your sensory observations, use your list to then deduce things about the person. For example, I used to smoke, quit like 2 or 3 years ago, but my ex would always be able to know I had been smoking when I was within 20 feet of her. She could see the nicotine stains, the smudge of ash on my thumb from flicking the cigarette, the lump of a crumpled cigarette pack in my pocket, and not just the obvious pieces of information, she saw the cologne in my bag, and the look on the faces of my friends who would smoke with me before school. For sound she may have heard the dry smoker's cough, the crackling of a lit cigarette, the spritz of a bottle of cologne before I walked up to her, and the click of a lighter in my fidgeting hands. She would reach out and touch the grainy bits of ash on my shirt, and feel the tremors in my hand, and the movement of the pack in my pocket. Obviously, she could smell the tar and smoke of the cigarette, even under the smell of my cologne. Even if I had brought a change of clothes, sprayed copious amounts of cologne, and washed my hands, she could always taste it when we kissed.
All this is of course the long-winded way to say that mindfulness is a invaluable tool when deducing with a disorder that separates you from reality. Hopefully, you can use these mindfulness techniques in conjunction with abductive reasoning to reconnect with yourself and your surroundings. I apologize for the brevity of the post, but rest assured the quality and frequency of posts should be back up to standard soon.
13 notes
·
View notes
Text
Skip Google for Research
As Google has worked to overtake the internet, its search algorithm has not just gotten worse. It has been designed to prioritize advertisers and popular pages often times excluding pages and content that better matches your search terms
As a writer in need of information for my stories, I find this unacceptable. As a proponent of availability of information so the populace can actually educate itself, it is unforgivable.
Below is a concise list of useful research sites compiled by Edward Clark over on Facebook. I was familiar with some, but not all of these.
⁂
Google is so powerful that it “hides” other search systems from us. We just don’t know the existence of most of them. Meanwhile, there are still a huge number of excellent searchers in the world who specialize in books, science, other smart information. Keep a list of sites you never heard of.
www.refseek.com - Academic Resource Search. More than a billion sources: encyclopedia, monographies, magazines.
www.worldcat.org - a search for the contents of 20 thousand worldwide libraries. Find out where lies the nearest rare book you need.
https://link.springer.com - access to more than 10 million scientific documents: books, articles, research protocols.
www.bioline.org.br is a library of scientific bioscience journals published in developing countries.
http://repec.org - volunteers from 102 countries have collected almost 4 million publications on economics and related science.
www.science.gov is an American state search engine on 2200+ scientific sites. More than 200 million articles are indexed.
www.pdfdrive.com is the largest website for free download of books in PDF format. Claiming over 225 million names.
www.base-search.net is one of the most powerful researches on academic studies texts. More than 100 million scientific documents, 70% of them are free
303K notes
·
View notes
Text
Was loosing my mind in the middle of psych class yesterday because I was reading some articles on basket weaving techniques (dont ask i dont have an explanation i should have been paying attention), which lead me to checked the invasive plants directory for my state (dont ask again i dont have an answer for this either), and realized that some of the most invasive plants in my area are perfect for basket making
Which obviously let me to literally get so jittery and excited about my prospects of plant picking and basket making i was quite literally bouncing in my seat
now i have a bundle of invasive plants ripped up from corners of my college campus drying in the garage
no point to this story except to remind you to get deranged about things, it makes your life more fun
23 notes
·
View notes