#clinical trial monitor salary
Explore tagged Tumblr posts
Text
Clinical Research Monitoring: A Guide to Clinical Monitoring
Clinical research monitoring is a vital part of clinical trials and it involves various activities to ensure the safety and accuracy of the data collected. It is important that the clinical trial is conducted in a way that meets regulatory standards, protects human studies participants, and minimizes potential risks to their health and well-being. Clinical trial monitoring can include activities such as auditing study sites, evaluating data for accuracy and completeness, review of protocols and amendments, reviewing case report forms (CRFs), identifying any deviations from the standard operating procedures (SOPs) or protocols, managing corrective action plans (CAPs), following up on safety reports, tracking progress against enrollment goals and much more. Apart from evaluating data quality, clinical research monitoring also ensures compliance with all regulatory standards like GCP (Good Clinical Practices) ICH (International Conference on Harmonization), FDA regulations and local laws. In addition to this ongoing monitoring throughout a study's duration, there may be audits conducted by sponsors or regulatory authorities at any time during or after completion of a clinical trial. All these efforts are dedicated towards ensuring that the results obtained from a clinical trial are accurate, reliable and applicable for use in making medical decisions.
Steps to Clinical Monitoring
1. Establish an Effective Monitoring Plan: Ensure that the plan is comprehensive and contains all applicable elements, such as the types of monitoring activities to be conducted, frequency of monitoring visits, data collection methods, and specific criteria for acceptable performance.
2. Develop Appropriate Documentation: Design protocol-specific monitoring tools and forms to document information from site visits including source documents, data collection instruments, case report forms (CRF). In addition, develop a Monitoring Log or Tracking System which will enable better accountability for study activities.
3. Execute Monitors’ Visits: Depending on the complexity of the trial and regulatory requirements, conduct pre-study qualification visits (PSQV), pre-initiation visits (PIV), initiation visits (IVs), periodic monitoring visits (PMV) and close out visits (COV). During each visit, ensure that good clinical practice is followed at all times by reviewing source documents and data collection instruments. Review patient enrollment logs to ensure accuracy and record any discrepancies in the visit report.
4. Report Findings: Generate detailed yet concise reports per each monitor's visit with clear recommendations for corrective actions if required; provide professional feedback to investigators regarding their performance; identify any areas of noncompliance with protocol requirements or applicable regulations; recommend training or educational sessions when appropriate; track all follow up activities related to corrective actions taken in response to findings from monitors' visits; ensure that essential documentation is complete before closing out a particular study site.
5. Quality Assurance: Validate accuracy of tracking systems used by monitors during their visits; assess risk associated with various deficiencies identified during monitoring process; carry out periodic internal audits/assessments to ensure compliance with established SOPs/guidelines related to clinical research monitoring activities; take preventive measures based on audit/assessment results in order to strengthen internal quality system processes.
Types of Clinical Trial Monitoring
1. Types of Clinical Research Monitoring: Clinical research monitoring is the process to assess the quality and integrity of clinical trial data and ensure compliance with applicable regulatory requirements. It can be done through three primary methods: onsite monitoring, centralized or remote monitoring, and risk-based approaches.
2. Onsite Monitoring: Onsite monitoring is considered the "gold standard" for clinical research monitoring, as it requires the presence of a monitor at a study site during the entire duration of a trial. The monitor will typically review source documentation such as patient records, lab results, and investigational product dispensing logs to assess accuracy and conformance with study protocols and good clinical practices (GCP). The monitor also interviews staff members responsible for conducting the trial to verify that procedures are being followed properly.
3. Centralized or Remote Monitoring in Clinical Trials: Centralized or remote monitoring enables sponsors to conduct clinical research monitoring without needing to send someone onsite to each study location. This is accomplished by using technology such as web portals, video conferencing, and virtual meetings that allow monitors to remotely review data from various sites simultaneously and quickly flag any issues that arise. Additionally, centralized/remote monitoring allows sponsors to be more proactive in identifying potential risks associated with a trial prior to sending monitors onsite for an assessment.
4. Risk-Based Approaches: Risk-based approaches use data analytics tools such as descriptive statistics and predictive algorithms to identify potential trends or outliers in clinical trial data that may represent heightened risk of noncompliance with GCPs or other regulations. By leveraging technology, these approaches can help sponsors identify issues earlier in the course of a trial so they can take corrective action before something goes wrong.
5. Benefits of Clinical Research Monitoring: Utilizing effective clinical research monitoring strategies helps ensure that trials are conducted ethically, safely, correctly according to protocol standards, within timelines agreed upon with regulatory authorities, and within budget constraints set out by sponsors/CROs/investigators/other stakeholders involved in a study’s execution.. Clinical research monitors act as an independent third party who are able to provide objective insight into how studies are being conducted across multiple sites which helps minimize errors due to bias from investigators or other personnel who may have vested interests in outcomes associated with their studies.. In addition, effective clinical research monitoring helps ensure patient safety by providing oversight about how drugs or medical devices used in trials are administered as well as ensuring patient confidentiality is maintained throughout the course of a study.. Lastly, robust clinical research monitoring protocols help reduce costs associated with delays caused by errors made during trials which can add up significantly over time if not avoided through proper oversight methods both pre-study start up until closeout occurs after all enrolled patients have completed their participation in a given trial
Clinical Research Monitoring Guide
1. Understand the Basics of Clinical Research Monitoring: Clinical research monitoring is a key part of the clinical research process, ensuring the safety and accuracy of results. It involves periodically assessing study sites to confirm that data is being collected properly, according to ethical and legal requirements, as per Good Clinical Practice (GCP) guidelines.
2. Know What Types of Studies are Monitored: Clinical research monitoring can be used for a variety of studies, including clinical trials, observational studies, epidemiologic studies, and public health surveys. It is important to know what type of study you are monitoring in order to ensure that the appropriate procedures are followed.
3. Understand How to Monitor a Study Site: The primary goal of clinical research monitoring is to confirm that the protocol and informed consent form have been followed properly at each site. This requires a thorough review of all relevant documents such as case report forms (CRFs), source documentation (e.g., physician notes), internal audit reports (audit trails), and external quality assurance reports. Additionally, it involves evaluating compliance with GCP guidelines during study visits or remote reviews, as well as conducting interviews with staff members to assess how they are handling data collection and reporting processes.
4. Become Familiar With Regulatory Requirements: In addition to GCP guidelines, there may be applicable regulations from local governments or other institutions that must be adhered to when conducting clinical research monitoring activities. Understanding these regulations is essential for ensuring compliance with applicable laws and regulations related to clinical research activities.
5. Develop an Effective Monitoring Plan: An effective monitoring plan should include a detailed timeline for visiting sites, information about any specific areas where focused attention is required (e.g., enrolling/randomizing patients or managing adverse events), and plans for auditing/reviewing data generated by the study site(s). Additionally, it should incorporate measures for controlling risk associated with data collection processes so that issues can be identified early on in the study process before they become problematic later on down the line.
Clinical Research Monitor Job
The job of a Clinical Research Monitor is to ensure that clinical trials are conducted ethically, safely and in compliance with established standards. The primary responsibility of the monitor is to protect the rights, safety and well-being of the human subjects enrolled in the trial. Duties typically include developing protocols for clinical studies; coordinating study start up activities; conducting site visits; monitoring data for timeliness, accuracy and completeness; auditing files for regulatory compliance; managing investigator queries/issues; preparing visit reports; reviewing update protocols related to study operations; resolving issues raised through audit reports or other sources; providing technical guidance to sites regarding protocol implementation or study conduct; and escalating complex issues or potential risks as needed.
Clinical Research Monitor Salary
Salaries for this position tend to vary depending on education level, experience and geographical location but can range from $60,000 per year for entry level positions up to around $90,000 per year for more experienced professionals. In addition to salary many employers also offer benefits such as paid vacation days, health insurance plans and retirement packages.
Resources for Clinical Research Monitoring
1. National Institutes of Health (NIH): Clinical Research Monitoring
This link provides information on NIH's guidelines for monitoring clinical research, which include topics such as the roles and responsibilities of the investigator, data safety monitoring boards, and protocols for reporting unanticipated problems and adverse events.
2. National Institutes of Health (NIH): Guide to Clinical Research Monitoring
This comprehensive guide walks readers through all aspects of clinical research monitoring, including topics such as study design, randomization strategies, regulatory compliance requirements, data management, monitoring plans and reports, quality improvement initiatives, and safety assessments.
3. US Food and Drug Administration (FDA): Guidelines for Clinical Trials Monitoring
This resource from the FDA outlines the importance of effective monitoring in clinical trials and provides an overview of the different roles within a clinical trial as well as details about essential elements for implementation of an effective monitoring strategy such as risk assessments and adverse event tracking.
4. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
ICH has developed standards that provide a set of harmonized technical requirements for clinical trials conducted across countries in the European Union (EU), Japan, and US with an emphasis on quality assurance and safety monitoring during trials.
5. Association of Clinical Research Professionals (ACRP)
ACRP's guidelines provide best practice recommendations for conducting clinical research studies in accordance with applicable regulations and standards to ensure patient safety monitoring during studies as well as data integrity throughout the process from start to finish.
6. Pharmaceutical Research & Manufacturers of America (PhRMA)
The PhRMA guidelines provide an overview of expectations around clinical research activities with respect to ethics, data integrity, safety reporting, resource allocation and more. It defines roles and responsibilities of all those involved in overseeing a clinical trial such as a Clinical Research Monitor or CRA who has primary responsibility for ensuring that the protocol is implemented correctly throughout a study’s duration
Clinical Research Monitoring Review
1. What is the main purpose of clinical research monitoring?
A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards
B) To ensure that data collected during a research study is accurate and reliable
C) To evaluate the safety of participants enrolled in a research trial
D) To oversee the financial management of a research project
Answer: A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards. Clinical Research Monitors are responsible for ensuring compliance with Good Clinical Practice guidelines, protecting participant privacy, verifying data accuracy, and evaluating protocol deviations. In addition, they may also be involved in reviewing participant eligibility requirements, conducting site assessments, providing training to investigators and staff on proper study procedures, as well as monitoring progress towards completion of all requirements of the study.
2. What type of individuals typically serve as clinical research monitors?
A) Physicians
B) Nurses
C) Regulatory specialists
D) All of the above
Answer: D) All of the above. Clinical Research Monitors can come from various backgrounds such as medical doctors (MDs), nurses (RNs), pharmacists (RPhs), regulatory specialists (e.g., Regulatory Affairs Professionals or Paralegals), or biostatisticians/data analysts who have experience in clinical trials and understand local regulations related to human subject protection. Each monitor has specific job duties depending on their education and experience, such as assessing compliance with regulatory guidance or analyzing data sets for accuracy, completeness, integrity, or validity.
3. What kind of activities do clinical research monitors need to perform?
A) Protocol reviews or verifications
B) Ensuring appropriate documentation completion
C) Site visits to observe investigator conduct
D )All of the above
Answer: D )All of the above. Clinical Research Monitors need to perform several activities including protocol reviews or verifications; ensuring appropriate documentation completion; site visits to observe investigator conduct; liaising between sponsors and sites; assisting with resolving issues associated with adverse events; reviewing case report forms for completeness, accuracy, consistency and correctness; evaluating subject safety throughout enrollment process;and writing reports detailing their findings at each visit.
4. What is one benefit gained from having an effective Clinical Research Monitor on-site? A) Reduced risk for legal liability stemming from negligence
B) Improved protocol adherence by investigators
C) Increased patient engagement during trial period
D )All of the above
Answer: D) All of the above . An effective Clinical Research Monitor encompasses several benefits such as reduced risk for legal liability stemming from negligence due to thorough oversight and accurate record keeping; improved protocol adherence by investigators through continued communication between sponsor representatives and researchers on-site regarding best practices; increased patient engagement during trial period due to more detailed explanations about potential risks/benefits offered by having monitor on-site ; and improved efficiency when dealing with complex protocols that require multiple levelsof oversight due to familiarity with protocol specifics which decreases time spent troubleshooting errors or unclear instructions..
5. How often should Clinical Research Monitors visit a particular site?
A) Weekly B) Biweekly C) Monthly D) Quarterly
Answer: C) Monthly . It is recommended that Clinical Research Monitors visit sites at least once per month in order to maintain active surveillance over ongoing studies at each location while also providing timely feedback regarding any issues discovered while on-site visits are taking place within a shorter timeframe if needed based upon changes made midstream or other unanticipated circumstances which might require immediate attention by sponsor personnel.
#clinical monitoring#clinical trial monitoring#remote monitoring clinical trials#clinical research monitor#clinical research monitor salary#risk based monitoring in clinical trials#clinical trial monitor#clinical trial monitor salary#monitoring of clinical trials by industry sponsors#centralized monitoring clinical trials#clinical research monitoring#clinical site monitoring#clinical trial monitoring services#clinical trial remote monitoring#clinical trials monitor#medical monitor clinical trial#risk based monitoring clinical trials#clinical monitor#clinical research monitor jobs#clinical trials monitoring#clinically validated blood pressure monitors for home use#monitoring in clinical trials#clinical monitoring services#clinical trial monitor jobs#journal of clinical monitoring and computing#monitoring clinical trials#remote monitoring in clinical trials#central monitoring clinical trials#clinical data monitoring#clinical monitoring jobs
0 notes
Text
Leading 10 Careers for Phlebotomy Technicians: Unlock Your Future in Healthcare
Top 10 Careers for Phlebotomy Technicians: Unlock Your Future in Healthcare
Embarking on a career as a phlebotomy technician opens a world of exciting opportunities in the ever-growing field of healthcare. Phlebotomy technicians are key players in the medical community, specializing in drawing blood and assisting in laboratory tests. If you’re passionate about healthcare and searching for a fulfilling career, this article will explore the top 10 careers for phlebotomy technicians, along with practical tips and insights to help you navigate your future successfully.
Benefits of Pursuing a career in Phlebotomy
Before diving into specific career paths, let’s discuss the benefits of becoming a phlebotomy technician:
High Demand: The need for skilled phlebotomy technicians continues to rise as healthcare facilities expand.
Job Stability: With the healthcare industry steadily growing, job security is an attractive aspect of this field.
Good Compensation: Phlebotomists can expect competitive salaries, frequently enough with room for advancement.
Variety of Work environments: opportunities exist in hospitals, clinics, laboratories, donation centers, and more.
1. Registered Nurse (RN)
Phlebotomy technicians often advance to become registered nurses. As RNs, they handle patient care, administer medications, and coordinate treatments.Additional training and education are required for this role.
2. Medical laboratory Technician (MLT)
MLTs perform various laboratory tests and analyze blood specimens. having phlebotomy experience is essential, as it allows for a smoother transition into laboratory settings.
3. Blood Bank Technician
Blood bank technicians focus on the collection,testing,and processing of blood donations. They play a vital role in ensuring a safe blood supply.
4. Healthcare Compliance Officer
For those interested in the administrative aspect, becoming a healthcare compliance officer can be a rewarding career. This role involves ensuring healthcare providers adhere to laws and regulations.
5. Patient Care Technician (PCT)
PCTs assist nurses and doctors in providing care to patients, including drawing blood and monitoring vitals. This position allows for direct patient interaction, making it an excellent choice for those passionate about patient care.
6. Phlebotomy Supervisor
With experience, phlebotomy technicians can move into supervisory roles, managing teams of phlebotomists and ensuring standard procedures are followed.
7. Physician’s Assistant (PA)
PAs are crucial members of healthcare teams, providing diagnosis and treatment under physician supervision. Phlebotomy experience can give aspiring PAs an edge during the submission process.
8. Clinical Research Coordinator
Clinical research coordinators oversee clinical trials,ensuring they adhere to regulatory standards. Phlebotomy skills are beneficial in collecting and processing specimens.
9. Medical Assistant
As medical assistants, professionals support healthcare providers with administrative tasks and direct patient care, including drawing blood and preparing patients for examinations.
10. forensic technician
Forensic technicians collect and analyze physical evidence from crime scenes, including blood samples. This unique career path offers a blend of science and law enforcement.
Practical Tips for Aspiring Phlebotomy Professionals
Get Certified: Obtain certification from recognized organizations like the National Phlebotomy Association (NPA) or American Society of Phlebotomy Technicians (ASPT).
Gain Experience: Internships and volunteer positions in healthcare settings can considerably enhance your resume.
Network: Connect with professionals in the field through online platforms like LinkedIn or local community events.
Stay Informed: Follow industry developments and advancements to keep your skills updated.
Case Study: A Day in the Life of a Phlebotomy technician
Meet Jane, a certified phlebotomy technician working in a busy urban hospital. her day begins with the morning rounds in which she draws blood for various tests ordered by healthcare providers. Jane emphasizes the importance of interpersonal skills in her role; making patients feel comfortable is key to success. Throughout her day, she interacts with other healthcare professionals, consolidating her path towards becoming a medical laboratory technician.
First-Hand Experience: testimonials from Phlebotomy Technicians
Let’s hear from two phlebotomy technicians about their careers:
“I love being a phlebotomist! Each day is different, and I enjoy meeting new patients. It’s gratifying to know that my work directly impacts their healthcare.” – Sarah T., Phlebotomy Technician
“The skills I learned in phlebotomy have opened many doors for me. I’m currently applying for nursing school and I couldn’t have done it without starting as a phlebotomist.” – Mark R., Phlebotomy Technician
Conclusion: Your Future Awaits
A career in phlebotomy can be the stepping stone to a fulfilling path in healthcare. The roles highlighted in this article show the diverse possibilities available. By obtaining the right training and certifications, gaining experience, and staying engaged in the healthcare community, you can unlock your future in this meaningful field. Embrace the opportunities that come your way and take the next step toward a rewarding career with endless potential!
youtube
https://phlebotomytechnicianschools.net/leading-10-careers-for-phlebotomy-technicians-unlock-your-future-in-healthcare/
0 notes
Text
Open Your Future: Top Phlebotomy Jobs in NYC - Opportunities & Insights
Unlock Your Future: Top Phlebotomy Jobs in NYC – Opportunities & Insights
Phlebotomy is an essential part of the healthcare industry, offering significant opportunities for those looking to establish a fulfilling career. In New York City, the demand for skilled phlebotomists is on the rise, creating a wealth of job openings and career advancement opportunities. This article delves into the top phlebotomy jobs in NYC, the benefits of pursuing this career, insights into the job market, and practical tips for success.
Understanding Phlebotomy and Its Importance
Phlebotomy involves drawing blood for tests, transfusions, donations, or research. Phlebotomists play a crucial role in patient care by ensuring accurate blood collection and handling.
Ensures quality blood samples for diagnostic testing
Maintains patient safety and comfort
Helps diagnose and monitor health conditions
Top Phlebotomy Job Opportunities in NYC
New York City has a myriad of healthcare institutions offering various phlebotomy positions. Here’s a look at some of the top roles currently available:
1. Hospital Phlebotomist
Working in hospitals can provide exposure to a broader range of patients and medical conditions. Hospital phlebotomists often work with emergency cases and require strong skills in patient interaction.
2. Outpatient Clinic Phlebotomist
Outpatient clinics offer a more structured environment. Phlebotomists in these settings typically have scheduled appointments, making their work more routine.
3. Mobile Phlebotomist
Mobile phlebotomy services are gaining popularity, especially post-pandemic. These professionals travel to patients’ locations, offering convenience and enhancing patient care for those who can’t travel.
4. Research Lab Phlebotomist
In research settings, phlebotomists collect samples for clinical trials and studies. This role requires attention to detail and often involves strict protocols.
5. Blood Donation Center Phlebotomist
Working at blood banks and donation centers can be fulfilling, allowing you to contribute directly to community health by ensuring an adequate blood supply.
Job Title
Setting
Typical Salary Range
Hospital Phlebotomist
Hospital
$40,000 – $55,000
Outpatient Clinic Phlebotomist
Clinic
$38,000 – $52,000
Mobile Phlebotomist
Field
$42,000 – $60,000
Research Lab Phlebotomist
Laboratory
$45,000 – $65,000
Blood Donation Center Phlebotomist
Donation Center
$39,000 – $54,000
Benefits of a Career in Phlebotomy
A career in phlebotomy offers numerous benefits, making it an attractive option for many individuals:
High Demand: With the growing need for healthcare services, phlebotomists are always in demand.
Quick Entry: Training programs can be completed in a matter of months, allowing you to enter the workforce relatively quickly.
Job Satisfaction: The ability to help patients and contribute to their health can lead to a rewarding work experience.
Career Advancement: With additional training, you can pursue advanced roles in healthcare.
Practical Tips for Aspiring Phlebotomists
If you’re interested in pursuing a career in phlebotomy, consider the following tips:
1. Get Proper Training
Enroll in a certified phlebotomy training program, which typically includes coursework and clinical practice.
2. Obtain Certification
Certifications from bodies like the National Phlebotomy Association (NPA) or the American Society for Clinical Pathology (ASCP) can enhance your credibility.
3. Gain Hands-on Experience
Look for internships or volunteer opportunities in healthcare settings to build your skills and resume.
4. Network Within the Industry
Join healthcare and phlebotomy professional groups to connect with industry professionals and learn about job openings.
5. Stay Updated
Continuously seek additional training and education to stay current with the latest techniques and technologies in phlebotomy.
Case Study: Successful Phlebotomist in NYC
Meet Sarah, a 28-year-old phlebotomist who started her career right out of a community college. After completing her certification, she secured a job at a local hospital in Manhattan.
Within two years, Sarah advanced to the role of lead phlebotomist, overseeing a team and contributing to training new staff. She shares, “The best part of my job is interacting with patients and knowing that I play a role in their healthcare journey.”
Insights into the Job Market
New York City’s job market for phlebotomists is thriving due to the increasing healthcare needs of its diverse population. Here’s what the data shows:
The NYC metro area expects a 17% growth in phlebotomy jobs by 2026.
Phlebotomists typically earn between $35,000 and $65,000 depending on experience and the healthcare setting.
Flexibility in work hours is common, with many phlebotomists working part-time or on a flexible schedule.
Conclusion
Phlebotomy offers promising job opportunities in New York City, contributing significantly to the healthcare industry. With the right training, certification, and experience, you can unlock a rewarding career that not only provides financial stability but also the satisfaction of helping others. Whether you choose to work in a bustling hospital, a serene outpatient clinic, or as a mobile phlebotomist, the future of phlebotomy in NYC is bright. Start your journey today and become a vital part of the healthcare system.
youtube
https://phlebotomycertificationcourse.net/open-your-future-top-phlebotomy-jobs-in-nyc-opportunities-insights/
0 notes
Text
Why Clinical SAS Certification is Crucial for Career Advancement in Clinical Research
In the rapidly evolving world of clinical research, data is at the heart of decision-making. Clinical trials, which evaluate the safety and efficacy of new medical treatments, generate vast amounts of data. Analyzing this data effectively is crucial not only for advancing scientific knowledge but also for ensuring patient safety and meeting regulatory requirements. This is where Clinical SAS certification plays a pivotal role.
SAS (Statistical Analysis System) is one of the most widely used statistical software tools in the pharmaceutical, biotechnology, and healthcare industries for analyzing clinical trial data. Clinical SAS certification is a formal acknowledgment of your expertise in using SAS for clinical data analysis, and it is increasingly seen as a key factor in career advancement. In this article, we will explore why Clinical SAS certification is essential for anyone looking to progress in clinical research and the benefits it brings to both individuals and organizations.
1. Increasing Demand for SAS Professionals in Clinical Research
As clinical trials become more complex and data-driven, the demand for skilled professionals who can manage and analyze clinical data has skyrocketed. The pharmaceutical, biotech, and healthcare industries rely heavily on data to make critical decisions regarding drug development, medical device approval, and patient safety.
Given the increasing volume and complexity of clinical trial data, proficiency in Clinical SAS is a must-have skill. Professionals who are certified in Clinical SAS are in high demand because they possess the specialized knowledge required to clean, analyze, and interpret clinical trial data accurately. SAS is known for its ability to handle large datasets, perform complex statistical analyses, and generate reproducible results — all essential for clinical research.
Having Clinical SAS certification helps professionals stand out in a competitive job market. It demonstrates to employers that you have the technical expertise and practical skills necessary to handle the challenges posed by modern clinical trials. Whether you are looking to join a clinical research organization (CRO), a pharmaceutical company, or a healthcare institution, SAS-certified professionals are seen as valuable assets.
2. Improved Job Opportunities and Higher Salaries
The pharmaceutical and biotechnology sectors are among the highest-paying industries for professionals with statistical analysis skills. Clinical SAS certification opens the door to a range of job opportunities, including roles like:
SAS Programmer: Writing and optimizing SAS code to process clinical trial data.
Biostatistician: Analyzing clinical trial data using SAS to draw conclusions about treatment efficacy and safety.
Data Manager: Overseeing the collection, organization, and quality control of clinical data using SAS.
Clinical Research Associate (CRA): Managing and monitoring clinical trial data to ensure compliance and accuracy.
According to industry reports, individuals with SAS certification often earn higher salaries compared to their non-certified peers. On average, SAS-certified professionals in clinical research can expect to see an increase in salary, with many companies offering attractive compensation packages to attract qualified candidates. The certification serves as a signal to employers that you have the specialized knowledge needed to handle complex data analysis tasks, making you a more competitive candidate.
3. Expertise in Handling Complex Clinical Trial Data
Clinical trials generate massive amounts of data, often from multiple sites and across different stages of treatment. Clinical SAS is specifically designed to handle such large, complex datasets efficiently. By earning Clinical SAS certification, professionals gain expertise in key areas of data management and statistical analysis, including:
Data Cleaning and Transformation: Ensuring that clinical trial data is accurate, complete, and ready for analysis. SAS provides powerful tools for detecting and correcting errors in the data.
Statistical Analysis: Performing sophisticated statistical analyses like survival analysis, regression modeling, and hypothesis testing using SAS.
Reporting and Visualization: Generating clear, reproducible tables, listings, and graphs that summarize the results of clinical trials. SAS offers robust options for creating high-quality reports that meet regulatory standards.
Compliance and Standardization: SAS is widely used in the industry because it complies with regulatory standards like the FDA and EMA. Being proficient in SAS helps professionals navigate the complex regulatory environment of clinical research.
These advanced skills are essential for interpreting clinical trial results accurately and ensuring that the data meets the necessary scientific and regulatory standards. Professionals who have mastered SAS are more likely to contribute to the success of clinical trials by ensuring that the analysis is both accurate and efficient.
4. Ensuring Regulatory Compliance and Data Integrity
Clinical trials are subject to strict regulatory requirements. The FDA (Food and Drug Administration) and EMA (European Medicines Agency), along with other global regulatory bodies, have rigorous standards for clinical trial data. To ensure that the data is both valid and reliable, it must be analyzed and reported using approved methods and tools. Clinical SAS certification ensures that professionals are equipped with the knowledge to meet these regulatory requirements.
SAS is widely recognized by regulatory agencies as a trusted tool for data analysis in clinical trials. It is used to generate the statistical reports required for regulatory submissions, such as the Clinical Study Report (CSR) and Statistical Analysis Plan (SAP). SAS-certified professionals are familiar with the industry standards and best practices for data analysis, which significantly reduces the risk of errors and helps ensure that clinical trial results are reproducible and compliant with regulatory guidelines.
5. Enhanced Career Growth and Professional Development
Clinical SAS certification not only boosts your job prospects but also accelerates your career growth. As the demand for data-driven decision-making in clinical trials grows, SAS-certified professionals are well-positioned for career advancement. Certification demonstrates a high level of competence and can lead to opportunities for higher-level positions, including managerial and leadership roles in clinical research.
Moreover, certification is an investment in your professional development. It signals a commitment to continuing education and staying up-to-date with the latest tools and techniques in clinical data analysis. For professionals looking to transition into higher-paying roles or expand their job responsibilities, Clinical SAS certification is an essential credential that can open doors to new opportunities.
6. Hands-On Experience with the Latest SAS Tools and Techniques
One of the key components of SAS certification training is gaining practical, hands-on experience with SAS software. This experience is invaluable when it comes to applying SAS tools and techniques to real-world clinical trial data. Certification courses typically cover a wide range of topics, from basic SAS programming to more advanced statistical methods, giving professionals a well-rounded skill set that can be applied immediately in their roles.
SAS-certified professionals are not just proficient in basic programming skills but also adept at using advanced techniques such as:
Macro programming to automate repetitive tasks and improve efficiency.
Advanced statistical modeling for analyzing complex clinical trial data.
Data visualization tools to present findings in a clear and meaningful way.
These hands-on skills make SAS-certified professionals more effective in their roles and better equipped to tackle the challenges of modern clinical research.
Conclusion
Clinical SAS certification is an essential asset for anyone looking to advance their career in clinical research. It equips professionals with the advanced statistical and programming skills needed to handle complex clinical trial data, ensures regulatory compliance, and boosts job opportunities and earning potential. As the clinical research industry continues to grow and evolve, the demand for skilled SAS professionals will only increase.
By obtaining SAS certification training, you not only enhance your technical expertise but also position yourself as a leader in the field of clinical data analysis. Whether you're a statistician, data manager, programmer, or clinical research associate, Clinical SAS certification can propel your career forward, ensuring that you are well-prepared for the challenges of modern clinical trials and research.
0 notes
Text
The Essential Guide to Understanding the Roles and Responsibilities of Registered Nurses
Title: The Essential Guide to Understanding the Roles and Responsibilities of Registered Nurses
Meta Title: A Complete Breakdown of the Roles and Responsibilities of Registered Nurses
Meta Description: Discover the key responsibilities of registered nurses, their day-to-day tasks, and the importance of their role in healthcare. Learn what it takes to become a registered nurse and excel in this rewarding profession.
Introduction: Registered nurses play a crucial role in the healthcare system, providing essential care to patients in various settings. From hospitals to clinics, nursing homes, and even schools, registered nurses are at the forefront of patient care, promoting health and well-being. In this comprehensive guide, we will break down the roles and responsibilities of registered nurses, highlighting the key tasks they perform, the skills required, and the impact they have on the lives of patients.
Roles and Responsibilities of Registered Nurses:
1. Patient Care: Registered nurses are responsible for providing direct care to patients, including taking vital signs, administering medication, and assisting with daily activities. They assess patients’ conditions, create care plans, and monitor their progress throughout their stay in the healthcare facility.
2. Educator: Registered nurses often play the role of educator, teaching patients and their families about their conditions, medications, and self-care techniques. They explain medical procedures, offer emotional support, and promote health literacy to empower patients to take control of their health.
3. Advocate: Registered nurses act as advocates for their patients, ensuring that their needs are met and their rights are respected. They communicate with other healthcare professionals to coordinate care, address any concerns or issues that may arise, and advocate for the best possible outcomes for their patients.
4. Administrator: Registered nurses may also take on administrative responsibilities, such as managing patient records, scheduling appointments, and coordinating care between different healthcare providers. They are responsible for ensuring that all aspects of patient care run smoothly and efficiently.
5. Researcher: Registered nurses may engage in research activities to improve patient care and outcomes. They participate in clinical trials, collect data, and contribute to evidence-based practice to advance the field of nursing and healthcare as a whole.
Benefits and Practical Tips for Registered Nurses:
– Competitive salary and benefits – Job security and opportunities for advancement – Fulfilling career that makes a difference in people’s lives – Continual learning and professional development opportunities
Case Study: Sarah is a registered nurse working in a busy hospital emergency department. She is responsible for triaging patients, administering medication, and coordinating care with the medical team. Sarah’s quick thinking and attention to detail have saved many lives, earning her the respect and admiration of her colleagues and patients.
First-hand Experience: As a registered nurse, I have had the privilege of caring for patients from all walks of life. Every day is a new challenge, but the rewards of helping others in their time of need make it all worthwhile. The skills and compassion I have developed as a nurse have made me a better healthcare provider and a better person.
Conclusion: Registered nurses play a vital role in the healthcare system, providing compassionate care to patients and making a difference in their lives. By understanding the roles and responsibilities of registered nurses, we can appreciate the dedication and hard work they put into their profession. Whether you are considering a career in nursing or seeking healthcare services, remember the essential role that registered nurses play in promoting health and well-being for all.
By following this comprehensive guide, you can gain a better understanding of what it takes to excel in this fulfilling profession and contribute to the betterment of society through your work as a registered nurse.
youtube
https://nursingcertificationcourses.com/the-essential-guide-to-understanding-the-roles-and-responsibilities-of-registered-nurses/
0 notes
Text
Clinical Research Coordinators Hiring at Prabhans Clinical Services Kanpur | Oncology & Cardiology Experience Preferred Prabhans Clinical Services in Kanpur is actively hiring Clinical Research Coordinators and Senior Clinical Research Coordinators for multiple vacancies. Open Positions Prabhans Clinical Services is hiring for the following roles: 1. Clinical Research Coordinator (2 Vacancies) Location: Kanpur, Uttar Pradesh Experience: Minimum 1-2 years in clinical research, preferably in Oncology or Cardiology. Salary: ₹12,000 - ₹18,000 per month (based on experience) This role requires candidates to assist in managing clinical trials, ensuring compliance with regulatory guidelines, and working directly with patients and study teams. Those with prior experience in Oncology or Cardiology will be given preference. [caption id="attachment_105755" align="aligncenter" width="640"] Clinical Research Coordinators Hiring at Prabhans Clinical Services Kanpur[/caption] 2. Senior Clinical Research Coordinator (2 Vacancies) Location: Kanpur, Uttar Pradesh Experience: Minimum 3-5 years in clinical research with a specialization in Cardiology or Oncology. Salary: ₹18,000 - ₹25,000 per month (based on experience) Senior Clinical Research Coordinators will oversee trial management and coordinate with multiple stakeholders, including sponsors and investigators. The ideal candidate will have robust experience in clinical documentation, patient monitoring, and regulatory submissions. Key Responsibilities For both positions, responsibilities include: Coordinating clinical trial activities in accordance with Good Clinical Practice (GCP) and ICH guidelines. Collecting, documenting, and maintaining clinical trial data. Ensuring timely enrollment of patients and adherence to trial protocols. Assisting in the preparation of clinical study reports. Managing communications between sponsors, study teams, and regulatory bodies. Monitoring patient safety and ensuring compliance with ethical standards. Senior Clinical Research Coordinators will also lead project management tasks, mentor junior coordinators, and oversee study timelines and budgets. How to Apply Interested candidates can apply by sending their updated CVs to: Email: [email protected] & [email protected]
0 notes
Text
Exploring Opportunities: B Pharmacy Career and Beyond
Exploring Opportunities-: B Pharmacy Career and Beyond
B Pharmacy, or Bachelor of Pharmacy, is a field of study that offers a wide range of career opportunities both in India and abroad. The pharmaceutical industry is a vital sector, contributing significantly to the economy and the overall health and well-being of people. In this blog, we will delve into the scope of a B Pharmacy degree, various job options available after completing the course, their respective salaries, and opportunities in government sectors.
Scope of Pharmacy in Foreign Countries
B Pharmacy graduates have a promising scope in foreign countries. The global pharmaceutical industry constantly seeks skilled professionals to work in research, manufacturing, quality control, regulatory affairs, and marketing. Countries like the United States, Canada, Australia, the United Kingdom, and several European nations have a high demand for qualified pharmacists. Obtaining relevant licenses and certifications is crucial for securing positions in these countries.
B Pharmacy Jobs: A Comprehensive List
After completing a B Pharmacy degree, there is a diverse range of job opportunities available. Here's a list of potential job roles:
Pharmacist: Working in retail pharmacies, hospitals, or clinics, dispensing medications and providing healthcare advice to patients.
Clinical Research Associate: Conducting research trials on drugs, ensuring their safety and efficacy.
Drug Inspector: Monitoring and regulating the quality and safety of drugs in pharmaceutical manufacturing units.
Medical Writer: Creating content related to pharmaceuticals for publications, regulatory documents, or marketing materials.
Quality Control/Quality Assurance Analyst: Ensuring the quality and safety of pharmaceutical products through various testing and inspection procedures.
Regulatory Affairs Manager: Managing the process of getting drugs approved by regulatory authorities.
Sales and Marketing Executive: Promoting and selling pharmaceutical products to healthcare professionals or directly to consumers.

After B Pharmacy Jobs and Salary
The salary after completing a B Pharmacy degree varies based on the job role, experience, and location. Here's an overview of potential salaries for freshers:
Pharmacist: ₹2.5 - ₹4 lakhs per annum
Clinical Research Associate: ₹3 - ₹5 lakhs per annum
Quality Control/Quality Assurance Analyst: ₹2.5 - ₹4 lakhs per annum
Medical Writer: ₹2.5 - ₹4.5 lakhs per annum
Sales and Marketing Executive: ₹2.5 - ₹4.5 lakhs per annum
B Pharmacy Jobs in the Government Sector
Government jobs are highly sought after due to job security, attractive pay scales, and additional perks. B Pharmacy graduates can find opportunities in various government sectors:
Drug Inspector: Salary ranges from ₹6 - ₹8 lakhs per annum.
Government Pharmacist: Salary varies by state and position, typically ranging from ₹4 - ₹6 lakhs per annum.
Public Health Officer: Salary varies based on experience and responsibilities, generally ranging from ₹5 - ₹8 lakhs per annum.
Exploring Further: Courses and Opportunities
After B Pharmacy, pursuing further education is a great way to enhance your career prospects. Some popular courses include:
MBA in Pharmaceutical Management: Combining business skills with pharmaceutical knowledge for managerial roles in the industry.
Master of Pharmacy (MPharm): Specializing in areas such as pharmacology, pharmaceutical chemistry, pharmaceutics, etc., for advanced research and academic roles.
Conclusion
A B Pharmacy degree offers a plethora of career options, both in India and abroad. Whether you choose to work in the private sector, government, or pursue further education, the opportunities are diverse and rewarding. Stay informed, upgrade your skills, and explore the vast landscape of possibilities that a career in pharmacy presents.
0 notes
Text
Clinical Research Courses

Clinical research is an important aspect of the healthcare industry, as it helps develop new drugs and treatments for various diseases. If you're interested in pursuing a career in clinical research, there are many courses available that can provide you with the necessary training and education. In this article, we'll explore the different clinical research courses available and how they can benefit aspiring professionals in the field.
Some of the popular clinical research courses available are:
Clinical Research Associate (CRA) Program: Focuses on monitoring clinical trials, ensuring compliance with regulatory guidelines, and managing data collection and analysis
Ideal for individuals who want to work as clinical research associates, project managers, or data managers
Clinical Data Management (CDM) Program:Focuses on the collection, management, and analysis of clinical trial data
CDM professionals ensure accuracy and completeness of data collected during clinical trials, and play a crucial role in the drug development process
Clinical Trial Management Program: Provides specialized training in managing clinical trials, from study design to execution
Ideal for individuals who want to work as clinical trial managers, project managers, or clinical operations professionals
Pharmacovigilance Program:Focuses on monitoring the safety of drugs during clinical trials and post-marketing
Ideal for individuals who want to work in drug safety or regulatory affairs
Medical Writing Program:
Focuses on writing clinical trial documents such as protocols, clinical study reports, and regulatory submissions
Ideal for individuals who want to work in medical writing or regulatory affairs
Benefits of pursuing a clinical research course:
Gain knowledge and skills required to excel in the field
Enhance employability, as the healthcare industry is always on the lookout for skilled professionals in this field
Lead to lucrative career opportunities, as clinical research professionals are in high demand and can command competitive salaries
In conclusion, pursuing a clinical research course can open up many opportunities and help you make a significant impact in the healthcare industry. There are various courses available that cater to different areas of clinical research, and you can choose the one that aligns with your interests and career aspirations. Invest in your future by enrolling in a clinical research course today.
#Clinical Research Courses#Best clinical research courses in India#Best clinical research Institute in India#Clinical research Institute#Clinical Research Courses In Pune
0 notes
Text
It’s a match!

Summary: You meet Duncan on a dating app and the two of you decide to go out for dinner together. Warning: this contains smut! :)
From the writer: Hey guys, this is the first-ever fic I’ve written about Duncan from House of Cards! I really like this and I hope you do as well. If you enjoyed this, all likes and reblogs are appreciated + check out more fics I’ve written on my masterlist!
Word count: 2,425
Left, left, left, right, left, right, right— it’s a match! A cute man named Duncan with brown hair and blue eyes. He lives in the city, not too far from your home in Alexandria. He’s a politician, or at least claims to be on his profile; he’s looking for a relationship but would also like to have fun and see where this dating app takes him. Perfect, you think. Before you could even send ‘hi,’ a message from Duncan comes through. He is wondering if you would like to meet up for dinner sometime this week and even offers to pick you up. You reply back, saying you would love to meet up with him although you live out of his way, so he shouldn’t bother to pick you up. Also, you don’t really like it when you get picked up on the first date. If your date is annoying, weird, or boring you never like to stick around— a friend is always willing to fake an emergency call for you to give you an out.
The time agreed for dinner was tonight at seven-thirty. There’s a Capital Grille downtown, and Duncan offers to call and make a reservation. While everything is falling into place for tonight, your mind wanders to what you should wear. Thankfully, you take Fridays off of work, so you’re not going to be bombarded with patients or meetings today. It’s very important, first impressions— you wouldn’t want to look tired or worn-out like after a long day of work. After circling around your walk-in closet a couple times, you decide on a black jumpsuit with a v-neck and a halter-style neckline. The legs are wide-cut and there’s a thin band of rhinestones around the entire waist. A matching pair of black, open-toed Louboutin heels tie the look together nicely.
Slowly, you make your way down the stairs to your underground garage. Whoever built this house initially went all-out— big columns surrounding the entire house complete with first and second-floor wraparound porches. A garage aboveground wouldn’t go with the aesthetic of this Antebellum South style mansion. Sometimes, it’s difficult to pick your car of the day, but today is not one of those days. Between your Mercedes S550, Porsche 911 Carrera S Cabriolet, Audi S3, and the Rolls-Royce Phantom, the Porsche takes the cake for tonight. The weather is nice tonight, you could let the top down and cruise up the highway for your short ride to D.C. Plus, the black exterior with black detailing, then red from the mechanical details behind the wheels matches your outfit perfectly.
Eventually, you find your way to the Capital Grille downtown. It’s already dark outside, but you manage to spot the valet horseshoe just in time before missing the turn completely. You exchange your keys for a valet ticket, then walk through the door held for you by the hosts. As you look up to see if your date has arrived, you spot Duncan at a booth near the side of the restaurant. You walk over and he stands to greet you, offering a small hug before the both of you sit.
“So, Ms. (y/l/n),” he says, but you cut him off before he could continue.
“Dr. (y/l/n),” you say with a smile, raising your glass of ice water to your lips to take a small sip. Duncan quickly apologizes and corrects himself, giving a warm smile back in return; he mentally kicks himself for not remembering after reading your profile. Duncan goes on and says he’s wondering about your hobbies, but could see why you might not have many as caring for patients takes up a large chunk of time. It wouldn’t be so bad if you were only a dermatologist, you go on, but you also run a successful clinical trial research lab in your office as well. Having to continually monitor patients is a huge time-suck.
“What do you do, Duncan?” you ask, glancing up to meet his brilliant blue eyes. He explains that he is a politician and many of his family members are prominent people around the city as well. Sure, you think you’ve seen Duncan in an interview or read about him in an article or two, you recall. After making small talk about each other’s careers for a bit longer, you begin to delve into what intrigued you about the other. What ‘made you swipe,’ per se. For you, Duncan seemed nice and cute as well as successful from what he described in his profile. You look for men with a similar drive and ambition as you or else you may get bored, you say. Similarly, Duncan says he was attracted to your success and ambition in your field of work. He even remarks that he has visited your practice before, but seen your nurse practitioner rather than you. As you continue to chat with Duncan, you begin to wonder if he would ride back home with you. Sure, he may be looking for a relationship, but you’re not sure you’d like to spend time getting to know this man if he’s a not a good fuck. There’s plenty of cuter, more successful men in D.C. for you to spend your time on if Duncan can’t make it happen. Sometimes, nice guys are nice because they need to compensate for something.
“So, did you drive here?” you ask, gliding your finger across the top of your water glass in circles.
“Oh, no, I Ubered in case we wanted to get a bottle of wine,” he says.
Perfect, you think. You could offer Duncan a ride back to his place or offer him a ride to your place. How could he resist at that point? After dinner has come to an end, you and Duncan make your way out to the valet horseshoe again. He holds the door for you and offers to wait with you while your car is being pulled up.
“I could give you a ride home,” you say with a smile. He initially declines, but then graciously accepts your offer when he opens the Uber app and finds that no rides are available near him. A loud, high-pitched whirring noise fills the alcove, and you could tell your car close. The turbo engine causes the noise to be more high-pitched than a regular engine as the turbines spin ten times faster than a regular engine would allow. You step forward and hand the valet boy your ticket from where the top would be if it was up.
“This is you?” Duncan says, raising his eyebrows and smiling. You make your way to the driver’s side door— the valet boy left it open for you, then closes it behind you as you tuck your feet inside. Duncan opens the passenger side door and climbs in. As soon as you pull out from the restaurant’s front and stop to turn on the road, you begin to speak.
“Why don’t you come home with me?” you ask, turning on your turn signal to indicate the right turn you’re about to make. There’s a line of cars passing by for now, so you turn to Duncan in anticipation of his response. There is not much hesitation before he nods his head yes and a smile curls around his face.
To no surprise on Duncan’s end, he is now pulling up towards an amazing home. What really gets him is the underground garage. What a nice touch, he thinks to himself. He knows for sure she’ll never be after his money— perhaps he’s going for hers at this point. Although that may be a joke with himself, for now, he knows that the car he’s currently sitting in is half a year’s salary at his job now. For the current object of his attention, this is just frivolous spending money. For her, a quarter-million dollars is nothing, especially in a garage with three more amazing, beautiful cars. All cars are shiny black— each with stunning, custom details.
You press a button near the rearview mirror and the garage begins to close. The trip up the stairs to the first floor of your house is longer than you would like it to be, especially with Duncan on your mind. He stands behind you on the stairs, as a gentleman should— ready to catch the lady if she slips or tumbles. Once you’ve made it upstairs, you don’t bother walking all the way to your room, you sit down on the couch in your living room. Duncan soon follows, sitting right next to you, resting his hand on your thigh. As he leans in for a kiss, you surge forward to meet his lips. His scruffy facial hair tickles your face, but you pull away from his lips as it begins to scratch. Men with facial hair are amazing, but you prefer it to be a little longer so it could be smoother rather than prickly. Nothing against Duncan himself so far, though— amazing kisser, that man. His hands move from your shoulders down to your waist, and he attempts to tug your jumpsuit up, probably thinking it’s a shirt.
“Oh, I got that,” you say, reaching behind you to unzip the zipper from the back of your outfit. As the zipper becomes undone, the neckline to your outfit loosens and a sleeve begins to fall from your shoulder. Duncan unbuttons his own shirt and allows that to fall to the floor, then peels off his undershirt and throws it across the room. You let your sleeves slip all the way down your arms, then off your fingertips until the top of your jumpsuit pools at your waist, leaving you sitting on the couch in your bra and pants. Kicking off your shoes, you pull your jumpsuit down further so you would only be in your bra and underwear. After seeing this, Duncan makes haste to unbuckle his pants and belt, then casts those to the side as well. Clearly, he was excited to be here as there is a prominent bulge present in his boxers, straining against the thin fabric. Duncan’s soft hands meet your unclothed waist— this gives you the chills as be begins to kiss you again, scruffy facial hair and all. Now, you’re beginning to feel a growing sense of urgency pool between your legs, and you’re eager to explore what Duncan has to offer.
“Why don’t we move this to the bedroom?” he asks after breaking his string of kisses. You agree and stand up to lead Duncan to your bedroom. After arriving at the master suite, it doesn’t take long for both of you to climb on the bed. Duncan’s beneath you as you lay on top of him, legs straddled above his waist, brushing over his solid erection. Your lips are still crashing together, but your mouth grows wider with every kiss, accepting more of Duncan with every movement. One of his hands travel to your back while the other begins to massage one of your breasts, then you feel your bra unhook and fall in front of you. Duncan catches it with the hand that was occupied with your breast, then he throws it off the bed. After this, you reach to tug down your underwear, then cast it aside like your bra. Duncan’s erection springs up from his boxers, and it’s a clear sign of relief when he is freed from the confines of his tight underwear. After exchanging a few more passionate kisses, you reach for his long, thick member and line it up with your entrance. He lets out a few small groans as you touch him. As you begin to sink down on him, he bucks his hips up and thrusts himself further in, yet you’re not all the way adjusted to how he feels in you. As you rock your hips over him a few times, the feeling of him stretching your walls all the way begins to sink in— there’s not a space inside untouched by Duncan, you’re completely full. You shift back and forth on him, causing the pressure due to him being more and less present in alternating sides inside you. The most pleasure comes from moving your hips forward, having him press back inside you near your most sensitive areas. After a few more minutes of this, Duncan leans forward and tells you to lay down.
He’s now between your thighs with your legs wrapped around his waist, ankles crossing behind him. Now, he’s pounding into you, skin hardly slapping against skin. For a while, it was a dull sensation— but, time after time, it’s now biting and harsh every time he smacks himself against you, balls slapping against your wet heat repeatedly for the past few minutes now. You could tell where he was inside of you by tracking where the significantly thicker head was from the rest of his cock, the suction caused by this letting a lewd popping sound release through the air every time he pulls completely out and then re-enters. Duncan then decided to place a pillow under your hips, allowing for him to angle down inside you to hit your sweet spot. Not long after this, you feel your orgasm is near. Tightening your grip on Duncan with your legs, you begin to rock your hips in sync with every thrust he makes, chasing your own pleasure. As his movements become somewhat erratic and varying from his usual thrust force, he either shifts slightly or slams even harder into you. After a few deep breaths and hard thrusts, Duncan releases himself inside you, pushing himself even deeper inside, almost brushing your cervix. Following this, your orgasm washes over you, clenching around his cock and drawing his warmth even deeper inside you. Muscle contractions move up his cock, squeezing him for anything he has left.
Slowly, Duncan exits you, followed by a trail of his cum leaking from your entrance. He looks down and licks his lips at the sight of you laying down in front of him, full of him.
“You’re staying over,” you say, no room to interpret that as a question due to your commanding tone.
“I couldn’t leave if I wanted to, baby, you’re my ride home,” he says, laying down next to you and smiling.
“I don’t want to, though. You actually don’t have to take me back to my place at all.”
///
*** I’ve tagged you if you asked to be on my tag list or if I thought you would be interested in this fic based on interactions with my other fics/posts! Let me know if you would like to be added or removed from my tag list. :)
Tag list: @langdonsoceaneyes @ms-mead @daydreamingofcody @psychobitchtess @swampwitchh13 @ahstmblrupdates @forgivemelucifer @jocelynscloset @ccodyfern @sammy-samm @xavierplymptonsangel @lilithrmorningstar1 @slashersrus @im-the-music-whore @isometimeswrite132 @gingersnapped13 @recklessmoannn @nickiechao11 @dani5216 @antichristsqueen666 @nightsblackroses @bitchchatter
#duncan shepard#duncan shepherd x reader#duncan shephard imagine#duncan shepherd fanfiction#duncan shepherd fanfic#duncan shepherd smut#house of cards duncan shepherd#duncan shepherd house of cards#duncan shepherd x you
77 notes
·
View notes
Text
Medical Research Jobs
Medical research jobs provide individuals with the opportunity to make significant contributions to the advancement of medicine and healthcare. These positions involve conducting clinical trials, laboratory research, and analyzing data in order to develop new treatments for diseases. Working in medical research requires a high level of scientific knowledge, analytical skills, and critical thinking. Professionals in this field often collaborate with other researchers from different disciplines to conduct studies that aim to improve patient care and treatment options. Medical researchers also work closely with doctors, nurses, and other healthcare professionals so they can share their findings and better understand patient needs. With such a wide range of responsibilities, medical researchers play an essential role in driving innovation in the medical industry. The advancement of modern medicine is dependent on the insights that these professionals provide through their investigations. As such, medical research jobs offer ambitious individuals the chance to make a meaningful contribution to humanity’s ever-evolving knowledge of health and well-being.
Clinical Research Associate (CRA): Designs and implements clinical trials according to established protocols. Monitors the progress of trials and reports results to principal investigators. Salary range from $50,000 to $90,000 per year. CRA Certification through CCRPS requires completion of a 8-week course on research principles and methodology plus two years of experience in clinical research related activities such as site monitoring or data management.
Clinical Research Coordinator: This position involves coordinating the clinical aspects of research studies, such as collecting and organizing data, tracking recruitment of study participants, managing patient information, and monitoring research protocols. Salary range: $43,000 to $55,000 per year. Get CRC Certification through CCRPS: Certified Clinical Research Professional (CCRP).
Clinical Trials Manager (CTM): A Clinical Trials Manager is responsible for overseeing the entire clinical trial process from start to finish. They ensure the compliance of all study protocols while managing timelines and budgets. Salary range: $70,000-$90,000 per year; Certification through CCRPS: Certified Clinical Trial Manager (CCTM).
Medical Research Lab Technician: Responsible for performing laboratory tests according to established protocols in order to aid in medical research projects. Salary range: $35,000 to $45,000 per year. Certification through CCRPS: Certified Laboratory Technician (CLT).
Bioinformatics Scientist: Utilizes computer technology and statistical analysis in order to analyze biological data derived from experiments in medical research labs. Salary range: $75,000 to $100,000 per year Certification through CCRPS: Certified Bioinformatics Scientist (CBS).
Medical Writer: Writes and edits materials for clinical trials and other medical research projects, including study protocols and reports for publication or regulatory submission purposes. Salary range: $60,000 to $80,000 per year Certification through CCRPS: Certified Medical Writer (CMW).
Regulatory Affairs Manager: Manages the regulatory affairs process by ensuring that medical products adhere to applicable regulations throughout their development and commercialization phases. Salary range: $95,000 to $125,000 per year Certification through CCRPS for Certified Regulatory Affairs Manager (CARM)
Regulatory Affairs Specialist: Develops regulatory strategies for the submission of clinical trial applications to regulatory agencies, monitors global regulations governing clinical trial design and implementation and tracks changes in legislation affecting the development process of drugs, biologics or medical devices; coordinates communication between sponsors, investigators and regulatory authorities; prepares high-quality documents such as study protocols and amendments for submissions to regulatory bodies; participates in meetings with regulatory agencies worldwide; performs literature searches related to drug development topics; assists with ongoing maintenance of regulatory files as required by regulations. Salary range from $55,000 to $100,000 per year depending on experience level. Pharmacovigilance Certification through CCRPS requires completion of a 8-week course on research principles and methodology plus five years minimum experience in pharmaceutical industry or related fields with emphasis on Regulatory Affairs functions.
#medical research jobs#research medical center jobs#medical research job#medical research jobs near me#medical researcher job#medical researcher jobs#jobs in medical research#medical researcher job description#oracle medical research jobs#medical research assistant jobs#atlanta center for medical research jobs#bill & melinda gates medical research institute jobs#clinical research jobs for foreign medical graduates#coriell institute for medical research jobs#entry level medical research jobs#feinstein institute for medical research jobs#fomat medical research jobs#gates medical research institute jobs#high paying medical research jobs#highest paying medical research jobs#how to get a job in medical research#hunter medical research institute jobs#jobs in medical research field#maine medical center research institute jobs#medical cannabis research jobs#medical clinical research jobs#medical device clinical research jobs#medical device research and development jobs#medical device research jobs#medical doctor research jobs
1 note
·
View note
Text
Why Do Nuclear Medicine Jobs and Nuclear Pharmacy Jobs Matter?
Introduction: there's a very strong relationship between nuclear technology and medical research. Several medical therapies and treatments have been done using nuclear medical machines for a long time. For example, some elements of medical nuclear use are vital in cancer research and treatment. That shows how important it is to have fully qualified and skilled medical personnel in the medical industry.
What’s Nuclear Medicine?
Nuclear medicine is a specialized medical branch that applies radioactive substances to diagnose and treat diseases. It is common in diagnosing and treating several cancers and other diseases. Nuclear has grown with the improvements in medical treatments.
Applications and Importance of Nuclear Medicine
(a). It is essential while identifying, diagnosing, and treating several diseases.
(b). Nuclear medicine processes are vital in treating hyperthyroidism, thyroid cancer, lymphomas, bone pain, and most cancer types.
(c). It's vital while studying the state or condition of the heart and lungs.
(d). It's helpful while trying
(e). It's important in the study of the respiratory system. It can help tell blood flow issues, show infection sources, and best treatments.
(f). It's applied in bone scanning. It helps to know the condition of the breaks, fractures, injuries, ligaments, tendons, etc.
(g). It's vital in the study of the brain to tell electrical signals, health, and to see abnormal brain function.
(h). It's vital to know if there are post-surgery complications. It can help track thyroid function and activity.
With nuclear medicine jobs and all related fields, there are many choices for all skilled personnel. From research to actual practice in highly developed working environments.
What’s Nuclear Pharmacy?
Nuclear pharmacy is a highly-skilled and special pharmaceutical industry practice. It handles the preparation of radioactive materials that enhance and promote health. It is via the safe and effective use of radioactive drugs that diagnose and treat specific disease conditions.
Applications and Importance of Nuclear Pharmacy
(a). Handling radiopharmaceuticals while ensuring quality control in clinical trials and use.
(b). They are responsible for patient-specific radiopharmaceutical doses for diagnostic imaging and for therapeutic processes in hospitals and medical facilities.
(c). Safely prepare, store and handle radioactive medications used to diagnose and treat specific health conditions. Radioactive medicines help diagnose and treat most cancers by targeting a specific area.
(d). A nuclear pharmacy prepares patients for treatment with radioactive medications. It monitors them to see if there are any signs of adverse reactions.
(e). It ensures the safe disposal of nuclear medical waste and material from the hospital premises. That ensures no one is put to harm or faces the danger of medical nuclear radiation.
A nuclear pharmacy job needs a qualified medical professional trained to handle nuclear items. Besides these, there are other aligned nuclear fields like nuclear security jobs or nuclear physics jobs. It is very important to get the most highly qualified experts in each field.
Summary: a comprehensive directory or database of all nuclear field-related jobs helps avail vital information. Whether stepping into nuclear engineer jobs or nuclear construction jobs, there is only one resonant database. It is safe and vital to validate all nuclear handling professionals.
For more info :-
Nuclear Engineer Jobs
Nuclear Engineering Salary
Nuclear Construction Jobs
Nuclear Power Plant Jobs
Nuclear Medicine Jobs
Nuclear Pharmacy Jobs
Visit our Social media sites :-
Source URL :- https://sites.google.com/view/nukejobsinc/home
0 notes
Text
Clinical Specialist, North and West Germany
Clinical Specialist, North and West Germany
Job title: Clinical Specialist, North and West Germany Company: Masimo Job description: Job Description: Region: North and western Germany Duties and Responsibilities: Accompanying applications (trial… order of measurement parameters and products) in the field of non-invasive patient monitoring at the customer’s site… Expected salary: Location: Hamburg Job date: Sun, 28 Nov 2021 07:24:47…
View On WordPress
0 notes
Text
Clinical Trial Coordinator Recruitment at Homi Bhabha Cancer Hospital and Research Centre, New Chandigarh Looking for an opportunity to work in clinical research? Homi Bhabha Cancer Hospital and Research Centre, a renowned institution under the Department of Atomic Energy, is hiring for the position of Clinical Trial Coordinator. This is an excellent opportunity for candidates with a background in life sciences and clinical research to work in a dynamic environment. Read on to learn about the qualifications, job responsibilities, and how to apply for this role. Position: Junior Clinical Trial Coordinator Interview Date: 30th September 2024 Location: Homi Bhabha Cancer Hospital and Research Centre, Medicity, SAS Nagar, New Chandigarh, Punjab Reporting Time: 09:30 A.M. to 11:00 A.M. Number of Posts: 2 Salary Range: ₹30,000 to ₹35,000 per month Key Qualifications: Education: Bachelor's in Science (B.Sc.) or Pharmacy (B. Pharm) with a degree/diploma in Clinical Research Methodology. Experience: A minimum of 1 year of experience in conducting clinical trials is preferred. Age Limit: Up to 35 years. Candidates with relevant experience in clinical trial coordination, especially those familiar with trial methodologies and protocols, are encouraged to apply. Experience in a hospital or research setting will be an added advantage. Job Responsibilities As a Clinical Trial Coordinator, you will play a pivotal role in overseeing and managing clinical trials. Your key responsibilities include: Coordinating and managing all aspects of clinical trials in accordance with ethical guidelines. Ensuring compliance with trial protocols, maintaining accurate records, and monitoring patient participation. Collaborating with research teams, doctors, and other healthcare professionals to facilitate the smooth operation of the trial. Managing trial documentation, including informed consent forms and data collection reports. Ensuring that trials meet regulatory requirements and reporting trial progress to the supervising department. This position offers a significant opportunity to gain hands-on experience in clinical research while contributing to impactful cancer research at a prestigious institution. Data Entry Operator Position Also Available In addition to the Clinical Trial Coordinator position, Homi Bhabha Cancer Hospital and Research Centre is also hiring Data Entry Operators. Interview Date: 1st October 2024 Location: Homi Bhabha Cancer Hospital and Research Centre, New Chandigarh Number of Posts: 2 Salary Range: ₹22,500 per month Age Limit: Up to 27 years Qualifications: Any graduate with good typing skills and proficiency in MS Office. A minimum of 1-year experience in a related field is preferred. How to Apply Eligible candidates can attend the walk-in interview at the H.R.D. Department of Homi Bhabha Cancer Hospital and Research Centre, New Chandigarh. The interview will be held at Medicity, SAS Nagar, Punjab. Please ensure that you bring the following documents with you to the interview: Updated resume Passport-sized photograph Originals and photocopies of your PAN card, Aadhaar card, and all educational and experience certificates. If there are a large number of applicants, a multiple-choice questionnaire (MCQ) test may be conducted. Shortlisted candidates will then proceed to the interview round. Norms may be relaxed for deserving candidates with relevant experience. [caption id="attachment_104517" align="aligncenter" width="640"] Clinical Trail Coordinator Recruitment Homi Bhabha Cancer Hospital and Research Centre[/caption] About Homi Bhabha Cancer Hospital and Research Centre Homi Bhabha Cancer Hospital and Research Centre is a unit of Tata Memorial Centre (TMC), a grant-in-aid institution under the Department of Atomic Energy, Government of India. The hospital is at the forefront of cancer research and treatment in India, offering state-of-the-art facilities and innovative treatment protocols. This recruitment drive is managed by M/s Principle Security & Allied Services Pvt.
Ltd., an outsourcing agency providing manpower services to Homi Bhabha Cancer Hospital & Research Centre. Contact Information For any queries regarding the recruitment process, feel free to reach out to the following contacts: Email: [email protected], [email protected] Phone: 18005721201 or 01602810091 (EXTN: 3616)
0 notes
Text
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
By: Jason Karimi, WeedPress Contributor Title: The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial Sourced From: weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/ Published Date: Sun, 21 Mar 2021 23:04:30 +0000
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0246990
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin ,
Benjamin Shechet,
Colin Hennigan,
Rebecca Matthews,
Amy Emerson,
Rick Doblin
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin,
Benjamin Shechet, …

x
Published: March 17, 2021
https://doi.org/10.1371/journal.pone.0246990
Article
Authors
Metrics
Comments
Media Coverage
Peer Review
Abstract
Introduction
Methods
Results
Discussion
Conclusions
Supporting information
Acknowledgments
References
Reader Comments (0)
Figures
Abstract
Importance
There is a pressing need for development of novel pharmacology for the treatment of Posttraumatic Stress Disorder (PTSD). Given increasing use of medical cannabis among US military veterans to self-treat PTSD, there is strong public interest in whether cannabis may be a safe and effective treatment for PTSD.
Objective
The aim of the present study was to collect preliminary data on the safety and potential efficacy of three active concentrations of smoked cannabis (i.e., High THC = approximately 12% THC and < 0.05% CBD; High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD) compared to placebo in the treatment of PTSD among military veterans.
Methods
The study used a double-blind, cross-over design, where participants were randomly assigned to receive three weeks of either active treatment or placebo in Stage 1 (N = 80), and then were re-randomized after a 2-week washout period to receive one of the other three active treatments in Stage 2 (N = 74). The primary outcome measure was change in PTSD symptom severity from baseline to end of treatment in Stage 1.
Results
The study did not find a significant difference in change in PTSD symptom severity between the active cannabis concentrations and placebo by the end of Stage 1. All three active concentrations of smoked cannabis were generally well tolerated.
Conclusions and relevance
The present study is the first randomized placebo-controlled trial of smoked cannabis for PTSD. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment, but no active treatment statistically outperformed placebo in this brief, preliminary trial. Additional well-controlled and adequately powered studies with cannabis suitable for FDA drug development are needed to determine whether smoked cannabis improves symptoms of PTSD.
Trial registration
Identifier: NCT02759185; ClinicalTrials.gov.
Figures

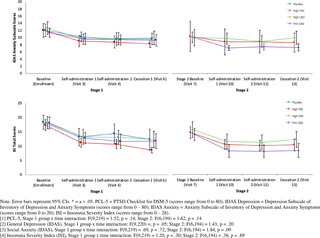











Citation: Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. (2021) The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS ONE 16(3): e0246990. https://doi.org/10.1371/journal.pone.0246990
Editor: Bernard Le Foll, Centre for Addiction and Mental Health, CANADA
Received: February 11, 2020; Accepted: January 26, 2021; Published: March 17, 2021
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All non-identifiable, relevant data are currently attached in the Supporting Information files.
Funding: Authors BY, RD, AE, MB, PR, and SS received Grant Number: RFA#135, an award funded by the Colorado Department of Public Health and Environment (CDPHE): https://www.colorado.gov/pacific/cdphe/approved-medical-marijuana-research-grants The study was also partially funded by the sponsor, The Multidisciplinary Association for Psychedelic Studies (MAPS): https://maps.org/research/mmj/ The sponsor designed the protocol with input from from MB, SS, and PR. The sponsor monitored the data quality, conducted data analysis, contributed to decision to publish, and assisted with preparation of manuscript through critical review.
Competing interests: Author MBM is an employee of Canopy Growth Corporation, during which time he has received stock options, serves on the Board of Directors for AusCann Group Holdings Limited, was a prior employee of Zynerba Pharmaceuticals, and has received consulting fees from Tilray Inc. Author ML serves on the scientific advisory board for FSD Pharma and has received consulting fees from Greenwich Biosciences, Zynerba Pharmaceuticals, and Tilray Inc in the past two years. Authors RD, BY, JW, BS, CH, RM, and AE receive salary from the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) non-profit research and educational organization. Author SS receives salary from the Scottsdale Research Institute, which is a private LLC and has no shareholders. The Academic Editor, BLF, co-authored “The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda” (https://pubmed.ncbi.nlm.nih.gov/32360455/) with one of the authors, MBM. This article was a result of a meeting where a large number of investigators came together to discuss clinical trial outcomes with representative from NIH and FDA. No other relationship between this author and the Academic Editor exists. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Posttraumatic Stress Disorder (PTSD) is a serious, worldwide public health problem. In the United States the lifetime prevalence of PTSD in the general population is between 6 and 10% [1,2], and between 13 and 31% in US military veterans [2,3]. PTSD is typically a chronic condition [4,5], and is associated with high rates of psychiatric and medical co-morbidity, disability, suffering, and suicide [4,6–8]. Food and Drug Administration (FDA)-approved pharmacological treatments for PTSD are currently limited to two selective serotonin reuptake inhibitors (SSRIs): sertraline and paroxetine, which have significantly lower effect sizes (SMD between -.28 and -.56) compared to trauma-focused psychotherapy (SMD between -1.01 and -1.35) [9,10]. Indeed, the current Department of Defense (DoD) and Department of Veterans Affairs (VA) best practice guidelines for treatment of PTSD recommend psychotherapy over pharmacotherapy [11]. However, the majority of military veterans with PTSD who receive one of the best practices psychotherapies for PTSD, which were determined efficacious through clinical trials, do not remit or reduce symptoms below clinical thresholds by the end of treatment [12,13].
There is a strong public interest, particularly among Patients with PTSD, clinicians, and researchers, in whether cannabis can be an effective pharmacological treatment option for individuals with PTSD, or a safe alternative treatment for patients who do not respond to current front-line treatment. Cross-sectional and prospective studies document the widespread use of cannabis by individuals with PTSD [14,15]. Moreover, veterans with PTSD who do not show remission following standard treatment are more likely to use cannabis following completion of PTSD treatment [16]. Two recent prospective studies of patients using cannabis to self-treat PTSD provide evidence that whole plant cannabis can produce short [17] and long-term relief of PTSD symptoms [18].
There is some preclinical evidence that at least two of the active compounds in cannabis, delta-9-tetrahydrocannabinol (THC; the primary constituent responsible for intoxication from cannabis) and cannabidiol (CBD; one of the non-intoxicating cannabinoids in cannabis), can positively impact processes that underly PTSD pathology [19]. Specifically, administration of CBD in rats and mice dampens cue-elicited fear responses [20,21], while administration of THC and THC+CBD appears to block reconsolidation of fear memory [22]. Likewise, both THC and CBD when administered alone facilitate fear extinction learning [23,24], which is a critical component for recovery from PTSD [25,26]. This work suggests that THC and/or CBD could modify how patients with PTSD experience and respond to reminders of trauma.
In addition to cannabis’ potential to perhaps modify mechanisms that maintain the core psychopathology of PTSD, early phase clinical data on isolated cannabinoid constituents in humans suggest that active components of cannabis might provide acute relief from specific symptoms of PTSD. For example, two open-label studies and one randomized placebo controlled trial found that administration of low doses of a THC analogue led to improvements in self-reported subjective sleep quality, decreased frequency of nightmares, and improvements in self-reported overall well-being among those with PTSD [27–29].
While these data appear promising, the potential therapeutic effects of smoked, herbal cannabis on PTSD have not been examined in a randomized, placebo controlled trial. Military veterans with PTSD are overwhelmingly choosing smoked cannabis to self-treat PTSD and related conditions [30]. Moreover, herbal cannabis varies significantly across plants in its THC and CBD content [29]. While both cannabinoids could hold therapeutic value, unlike THC, CBD is non-intoxicating and does not carry significant risk of abuse [30]. In addition, CBD may temper the anxiogenic effects of THC in cannabis preparations that contain both CBD and THC [31,32]. It is unclear whether THC, CBD, or some combination of compounds may lead to greater reductions in PTSD symptoms with better safety profiles compared to other combinations. In addition, previous clinical studies rely entirely on standardized dosing, rather than test more naturalistic and generalizable ad libitum dosing regimens. This is a major limitation of previous research because there is substantial individual variability in cannabinoid tolerability [31]. Indeed, military veterans who use cannabis for PTSD tend to self-titrate to much larger doses than those tested in research studies [32,33].
The primary objective of the present study was to conduct a randomized placebo-controlled trial to assess the safety and potential efficacy of smoked, herbal cannabis for the treatment of PTSD in military veterans. Specifically, the study was designed to examine the independent effects of ad libitum use of up to 1.8 grams/day of three active preparations of smoked cannabis: (i) High THC, (ii) High CBD, and (iii) one-to-one ratio of THC and CBD (THC+CBD) against placebo on PTSD symptoms in a sample of veterans with PTSD.
Methods
Trial design
The trial protocol can be found at https://maps.org/research-archive/mmj/MJP1-Protocol-Amend4-oct-13-2015.pdf. The study received ethics approval from the Copernicus Group Independent Review Board (IRB) and was conducted in accordance with all local and Federal laws and regulations, including obtaining written informed consent from all study participants. The study included a randomized, double-blind, placebo-controlled, crossover trial of smoked cannabis containing three different concentrations of THC and CBD, and placebo. The cross-over design included two stages with four treatment groups in Stage 1 (High THC, High CBD, THC+CBD, and placebo) and re-randomization into three active treatment groups in Stage 2 (High THC, High CBD, and THC+CBD). The primary aim of the study was to determine whether change in PTSD symptom severity at the end of Stage 1 (primary study endpoint) differed by condition. The crossover design allowed for additional comparisons of within-subject and between-subject differences in safety and preliminary efficacy across the two Stages and allowed for assessment of participants’ preference for cannabis concentrations assigned in either Stage 1 vs. Stage 2. Each stage included three weeks of ad libitum use up to 1.8 grams/day of the assigned treatment followed by a two-week cessation period. This upper limit was necessary due to the outpatient setting for self-administration and the Schedule 1 controlled substance status of cannabis.
Primary outcome and safety assessments were conducted at baseline (visit 0), end of treatment in Stage 1 (visit 5; primary study endpoint), following the Stage 1 cessation period/Stage 2 baseline (visit 7), and end of treatment in Stage 2 (visit 12). Self-reported assessment of withdrawal symptoms was conducted at screening, baseline, and weekly during the two-week cessation periods following each stage of treatment (visits 6, 7, 13, 14). Secondary outcomes were assessed throughout the study before/after treatment and cessation periods.
Participants.
Study participants were recruited using community-based advertisements, presentations, and website advertisements. Study inclusion and exclusion criteria were as follows:
Inclusion Criteria. Individuals were eligible for study enrollment if they (1) were a US military veteran, (2) met DSM-5 (APA, 2013) criteria for PTSD with symptoms of at least six months in duration (index trauma did not have to be related to military service), (3) had PTSD of at least moderate severity based on a CAPS-5 score of = >25 at baseline assessment, (4) were at least 18 years of age, (5) reported they were willing and able to abstain from cannabis use two-weeks prior to baseline assessment, which would be verified by urine toxicology screens at screening and baseline, and agreed to abstain from using non-study cannabis during the trial, (6) were stable on any pre-study medications and/or psychotherapy prior to study entry, and (7) agreed to comply with study procedures.
Exclusion criteria. Study participants were excluded if they (1) were pregnant, nursing, or of child bearing potential and not practicing effective means of birth control, (2) had a current or past serious mental illness (e.g., personality disorder, psychotic disorder) determined by the SCID-5-RV [34], or self reported a positive family history (first-degree relative) of psychotic or bipolar disorder (3) were determined at high risk for suicide based on the C-SSRS [35], (4) had allergies to cannabis or other contraindication for smoking cannabis, (5) had a current diagnosis or evidence of significant or uncontrolled hematological, endocrine, cerebrovascular, cardiovascular, coronary, pulmonary, gastrointestinal, immunocompromising, or neurological disease, (6) met DSM-5 criteria for moderate-severe Cannabis Use Disorder on the CUDIT-R (= >11), (7) screened positive for any illicit substance other than cannabis during the two-week screening, or (7) were unable to provide informed consent.
Randomization and blinding.
The Stage 1 randomization list utilized blocks to ensure equal treatment assignments, and the Stage 2 randomization utilized multiple validated randomization lists that re-randomized participants in a blinded manner. The randomization procedure specified that participants would be randomized to treatment conditions using small block randomization in a 1:1:1:1 ratio in Stage 1 and then be re-randomized into two of the three active cannabis conditions (THC, CBD, THC+CBD) with a 1:1 ratio in Stage 2. Randomization in Stage 2 excluded the participant’s Stage 1 treatment condition. As placebo was not an option in Stage 2, placebo participants were randomized 1:1 between High THC and High CBD, but were not given the option to be randomized to THC + CBD in order to facilitate simpler programming of the web-based randomization system. This two-step randomization resulted in an unbalanced distribution of Stage 2 participants overall across active dose groups. In order to maintain the blind, a central electronic database was utilized for randomization based on validated computer-generated lists.
All study staff (with the exception of the Randomization Monitor and Drug Product Packaging Technician) and participants were blinded to condition assignments. The blind could only be broken for an individual participant if there was a clinically or medically urgent emergency requiring knowledge of the participant’s condition assignment. This emergency unblinding required approval from the site PI and Coordinating Investigator. Likewise, the unblinded Randomization Monitor could provide dose assignment through the electronic randomization system. Randomization information was only available within the web-based randomization system and only viewable by the designated Randomization Monitor.
Interventions.
Study drug was obtained from the National Institute on Drug Abuse (NIDA). Four concentrations of cannabis from NIDA included: High THC = approximately 12% THC and < 0.05% CBD); High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD. Samples of each batch were tested and confirmed for their concentration levels by an independent third-party analytical testing laboratory in Phoenix, Arizona. The independent testing lab found in two separate analyses that the High THC batch was just 9%, with the other batches very close to what was reported by NIDA.
At the beginning of each stage, participants were asked to visit the clinic site for four hours on two successive days and self-administer under supervision of study staff one dose of the cannabis preparation that they were randomly assigned to in that Stage. Vital signs for safety were collected during these visits (i.e., blood pressure, pulse). The study provided participants a total of 37.8 grams (1.8 grams/day)for the three-week ad libitum treatment period along with a metal pipe for treatment delivery (smoked). Participants were asked to refrain from using non-study cannabis, and return any remaining study cannabis that was not used each week. When study drug was returned the clinic team weighed the returned cannabis to calculate participants’ average use in grams per day during the treatment period in each stage. Participants were asked to refrain from any cannabis use during a two-week cessation period (between stages), then were re-randomized into one of three active treatment groups. All study participants were provided the option to enroll in an open label extension (Stage 3) with the cannabis of their choice in the same amount they returned unused in Stages 1 and 2 so participants had no disincentives to returning unused amounts. The results of Stage 3 are not reported here.
Demographic measures.
Baseline demographic information included age, sex, race/ethnicity, education, employment status. Other baseline measures included: whether the index trauma was combat-related, body mass index (BMI), risk for sleep apnea (STOP-bang) [36], and risk for cannabis use disorder (CUDIT-R) [37].
Safety measures.
Adverse Events (AEs) were assessed at baseline, during the introductory session, self-administration session, end of treatment, and before/after cessation in each stage by asking participants to self-report any side effects experienced over the past week. All AEs were coded by Systems Organ Class. The study physician then rated all AEs by severity (mild, moderate, severe) and study relatedness (i.e., possibly related, probably related, not related). AEs rated possibly related and probably related were collapsed into one “related” category.
Additional safety measures included the 15-item Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) and the Columbia-Suicide Severity Rating Scale (CSSR-S) (Posner et al., 2011). The MWC was administered at screening, baseline, and each week following cessation of Stages 1 and 2 (visits 6, 7, 13, 14). The CSSR-S was self-administered at all study visits.
Outcome measures.
The primary outcome of the current study was change in PTSD symptom severity from baseline (visit 0) to end of the three-week treatment period in Stage 1 (visit 5) using the Clinician-Administered PTSD Scale for DSM-5 Total Severity Score (CAPS-5) [38]. The CAPS-5 is a semi-structured clinician interview, and is well-validated for determining PTSD diagnoses consistent with the Diagnostic and Statistical Manual of Mental Disorders, Version 5 (DSM-5) and assessing change in symptom severity over time [39]. PTSD diagnosis is based on meeting the DSM-5 symptom cluster criteria (minimum threshold of symptoms with a score ≥ 2) with a qualifying criterion A index trauma. The CAPS-5 Total Severity Score is calculated by summing the total score for each of the four symptom categories to assess past-month PTSD symptoms on a specific traumatic event: intrusion (Category B), Avoidance (Category C), Mood and Cognition (Category D), and Hyperarousal (Category E). CAPS-5 Total Severity scores range from 0–80, where higher scores indicate worse PTSD severity.
Secondary outcome measures included a modified version of the 20-item self-report PTSD Checklist for DSM-5 (PCL-5) [40], which was changed to assess for past week symptoms, the 20-item general depression subscale and 5-item anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms’ (IDAS) [41], the 80-item self-report Inventory of Psychosocial Functioning (IPF) [42], and the 7-item self-report Insomnia Severity Index (ISI) [43]. Secondary outcome measures were collected at baseline (visit 0 and visit 7), self-administration (visit 4 and visit 10), before cessation (visit 6 and visit 13), and after cessation (visit 7 and visit 14) in both Stage 1 and Stage 2. Total and subscale scores were calculated for each measure.
Other measures.
The validity of study blinding to active or inactive treatment in Stage 1 was assessed by asking participants and clinicians to independently guess whether the participant was randomized to an active (High THC, High CBD, THC+CBD) or inactive (placebo) treatment group at the end of Stage 1. At the end of Stage 2, participants were asked whether they preferred the treatment to which they were assigned in Stage 1 or Stage 2.
Table 1 includes a summary of all assessments by visit.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 1. Summary of assessments by visit.
https://doi.org/10.1371/journal.pone.0246990.t001
Study power.
The primary study aim was to gather preliminary data on the safety and potential efficacy of different cannabis preparations to treat PTSD among veterans. In the absence of published effect sizes for the impact of THC, CBD, or THC+CBD on CAPS-5 scores, the target sample size was chosen to allow detection of an effect size of 0.4 or greater (small to medium effect) based on between group differences in the primary outcome measure (i.e., change in total CAPS-5 severity score from baseline to the end of Stage 1 active treatment phase). Power analysis suggested that 76 completing participants (n = 19 per group) would be needed to detect an effect size of d = 0.4 at 82% power and .05 significance level. Enrollment and randomization continued until 76 participants completed the Stage 1 outcome assessment. Eighty participants were enrolled and 76 partcipants completed Stage 1.
Statistical analyses.
Descriptive statistics were performed to test the normality of baseline measures on the total study sample and across each treatment group to ensure adequate randomization. Means, medians, and frequencies were calculated, and within-subject and between-group differences were tested for categorical variables using chi-square tests and t-tests or analysis of variance (ANOVA) for continuous variables.
Safety was analyzed by tabulating the frequency, severity, and relatedness to treatment of AEs. A Chi-square test was used to assess for differences in frequency of AEs across groups. An AE was counted once per subject for each assessment period.
The primary outcome was analyzed using ANOVA to test for between-group differences in change in Total PTSD Severity scores from baseline to end of treatment in Stage 1 (CAPS-5 visits 0 and 7). Secondary outcomes were analyzed using a series of additional ANOVAs to test for between-group differences in change scores from baseline to end of treatment for Stage 1 (CAPS-5 visits 0 and 7; secondary measures visits 0 and 6) and Stage 2 (CAPS-5 visits 7 and 12; secondary measures visits 7 and 13). All dependent variables were tested for normality, and summarized by both mean and median values by group. Within-subject change scores were tested for each treatment group using a series of t-tests. Tukey’s pairwise comparisons were used to test for group differences in change scores between all pairs of treatment conditions in Stage 1 and Stage 2. Analyses were conducted consistent with an intent-to-treat (ITT) framework, where all available data from randomized participants who received at least one week’s supply of study drug (N = 80) were summarized for baseline characteristics and entered into the models. However, the use of ANOVA tests only allowed for analysis of change in participants who completed outcome assessments (N = 76 for primary outcome analysis).
Results
Sample characteristics
A total of 261 individuals completed screening and 51% met eligibility criteria for study inclusion. Eighty participants were enrolled and randomized into one of four treatment groups (n = 20 per group), of which 76 participants completed the Stage 1 outcome assessment. In Stage 2, a total of 74 participants were re-randomized into High THC (n = 29), High CBD (n = 27), or THC+CBD (n = 18). There were no significant Stage 1 treatment assignment differences in demographics or baseline scores on the primary and secondary outcome variables (i.e., CAPS-5, PCL-5, IDAS Social Anxiety, IDAS Depression, IPF, and ISI). Sample demographics and baseline characteristics are summarized in Table 2.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 2. Baseline characteristics by treatment group.
https://doi.org/10.1371/journal.pone.0246990.t002
Treatment adherence and attrition
Rates of engagement and completion are summarized in the Consort Diagram (Fig 1). During Stage 1, 3 participants (3.8%) did not complete endpoint outcome assessments. After Stage 1, 6 participants (7.5%) did not continue into Stage 2. Of the 74 participants who were re-randomized into Stage 2, 3 (4.1%) discontinued treatment due to an AE, and 7 total (9.5%) did not complete Stage 2 endpoint outcome assessments. The overall attrition rate for the percent of randomized participants who dropped out before completing Stage 2 outcome assessments, was 16.3%.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 1. Consort flow diagram.
https://doi.org/10.1371/journal.pone.0246990.g001
Cannabis use in grams
In Stage 1, there was no statistically significant difference between groups in total grams of smoked cannabis/placebo during the three-week treatment period (21 days) across the treatment groups (F [3, 71] = 2.23, p = .09). Mean (SD) grams of smoked cannabis/placebo used by each treatment group in Stage 1 were as follows: placebo (M = 8.4, SD = 10.1), High THC (M = 14.6, SD = 10.4), High CBD (M = 14.3, SD = 13.0), THC+CBD (M = 8.2, 6.8).
In stage 2, there was a significant group difference in total grams of smoked cannabis (F [2, 64] = 3.42, p = .04), such that participants in the THC+CBD group used significantly more cannabis (M = 17.6, SD = 10.6), compared to participants randomized to High THC (M = 10.7, SD = 10.9), or High CBD (M = 9.3, SD = 10.5).
Assessment of study blind
In Stage 1, 60% of placebo participants accurately guessed assignment to an inactive treatment, 58% of High CBD participants accurately guessed that they were in an active condition, and 100% of participants in the High THC and THC+CBD groups accurately guessed assignment into an active treatment condition. Similar results were found for clinicians. In Stage 1, forty-five percent of clinicians accurately guessed placebo participants’ assignment in an inactive treatment, 16% accurately guessed High CBD participants’ assignment into an active treatment, and 100% accurately guessed that participants assigned to High THC or THC+CBD were randomized into an active treatment. Therefore, the study blind was appropriately upheld only when participants were assigned to High CBD or placebo conditions, but was not upheld when participants were assigned to High THC or High THC/CBD.
Treatment preference
At the end of Stage 2, participants who completed final assessments (n = 74) indicated their preference for either their blinded Stage 1 or Stage 2 treatment assignment. Twenty-five participants (34%) indicated a preference for a Stage 1 or Stage 2 assignment to High THC, 10 participants (13%) indicated a preference for a Stage 1 or Stage 2 assignment to High CBD, 26 participants (35%) indicated a preference for a Stage 1 or Stage 2 assignment to THC+CBD, and 4 participants (5%) indicated a preference for a Stage 1 assignment to placebo. Two participants (3%) equally preferred their Stage 1 and Stage 2 treatment assignments.
Safety outcomes
Adverse events.
All Adverse Events (AEs) reported during Stage 1 are summarized in Table 3. Number of participants who reported at least one AE did not significantly differ by treatment group in either Stage 1 (p = .38) or Stage 2 (p = .27). Thirty-seven of 60 participants who received THC, CBD, or THC+CBD during Stage 1 (61.7%) reported at least one treatment-related AE by the end of Stage 1. In Stage 2, Forty-five of the 74 participants who received THC, CBD, or THC+CBD (60.8%) reported at least one treatment-related AE during Stage 2. Three of 80 participants (3.8%) reported an unrelated Serious Adverse Event (SAE) during the study, specifically heart palpitations (n = 1; THC+CBD, Stage 1 cessation period), pulmonary embolism (n = 1, High THC, Stage 2), and abscess (n = 1, High CBD, Stage 2). One participant (THC+CBD) discontinued treatment during the introductory session in Stage 1 due to an AE, and two participants discontinued treatment during the introductory session in Stage 2 due to an AE (High CBD and High THC conditions). Across both Stages, 13 total participants terminated from the study early due to an AE (8.4%). The most common AEs reported (i.e., those with >10% frequency) were cough (12.3%), followed by throat irritation (11.7%) and anxiety (10.4%). Emergency unblinding was never used in the study.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 3. Number of participants with adverse events by systems organ class/preferred terms and treatment-relatedness.
https://doi.org/10.1371/journal.pone.0246990.t003
One participant who received CBD in Stage 1 (5.0%) reported treatment-related suicidal ideation. One participant from each treatment condition (3.6% – 5.9%) reported treatment-related suicidal ideation in Stage 2.
Cannabis withdrawal symptoms.
Fig 2 summarizes mean withdrawal symptom scores on the MWC by group at Stage 1 and Stage 2 baseline, end of treatment, and following 1-week of cessation. All treatment groups reported mean withdrawal symptoms in the moderate range (Mean score = 32–38) at baseline assessment (prior to initiating treatment in Stage 1). All treatment groups showed a significant reduction in withdrawal symptoms from baseline to the end of the treatment phase of Stage 1. Only participants assigned to High THC in Stage 1 reported a significant increase in mean self-reported withdrawal symptoms after one week of cessation from the assigned treatment in Stage 1 (Δ = 12.6, SD = 11.41, p = .0004). There was no significant change in withdrawal symptoms from the end of Stage 2 treatment to one-week follow-up.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 2. Mean total marijuana withdrawal scores by treatment condition across Stage 1 & Stage 2.
https://doi.org/10.1371/journal.pone.0246990.g002
Primary efficacy outcome
PTSD symptom severity (CAPS-5).
Results of the analysis of change in total PTSD symptom severity on the CAPS-5 are summarized in Table 4 for both Stage 1 (primary outcome) and Stage 2. In Stage 1, there was no significant between-group difference in CAPS-5 Total Severity scores between treatment groups [F(3, 73) = 1.85, p = .15]. All four treatment groups, including placebo, achieved significant within-subject reductions in total CAPS-5 Total Severity scores from Stage 1 baseline (visit 0) to end of treatment (visit 5). Specifically, participants who received placebo in Stage 1 reported a mean reduction of 13.1 points (SD = 12.10, p < .001, d = -1.30), participants who received High THC reported a mean reduction of 15.2 points (SD = 11.3, p < .0001, d = -1.99), High CBD participants reported a mean reduction of 8.4 points (SD = 10.09, p < .05, d = -.79), and THC+CBD participants reported a mean reduction of 8.5 points (SD = 9.88, p < .05, d = -.83).

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 4. Mean (SD)/Median (IQR) and analysis of change in CAPS-5 total severity scores by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t004
Secondary efficacy outcomes
The results of the study’s secondary efficacy outcomes are summarized in Table 5.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 5. Mean (SD)/Median (IQR) and analysis of group change in PCL-5, IDAS social anxiety, IDAS general depression, IPF, and ISI by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t005
Self-reported PTSD symptoms, PCL-5.
In Stage 1, there was no significant difference in PCL-5 change scores between treatment groups from baseline to end of Stage 1.
In Stage 2, mean change in PCL-5 scores significantly differed by treatment condition [F(2, 63) = 4.06, p = .02]. Specifically, there was a significant difference between High CBD and THC+CBD in PCL-5 change scores, with participants who received THC+CBD reporting greater reductions in PTSD symptoms on the PCL-5 (Δ = -16.4, SD = 16.0, p < .001, d = -1.43) compared to participants who received High CBD (Δ = -9.1, SD = 11.0, p = .02, d = -.67).
IDAS general depression & social anxiety subscales.
In Stage 1, there were no significant differences between treatment conditions in either change in IDAS General Depression or IDAS Social Anxiety scores.
In Stage 2, treatment groups significantly differed in IDAS Social Anxiety mean change scores [F(2, 63) = -3.08, p = .05] and IDAS General Depression mean change scores [F(2, 63) = 3.76, p = .03]. Specifically, participants in the THC+CBD condition in Stage 2 reported significant pre-post reductions in IDAS Social Anxiety scores (Δ = – 2.8, SD = 3.90, p = .04, d = -.70), and participants in the High THC (Δ = -9.0, SD = 11.1, p < .01, d = -.90) and THC+CBD treatment conditions (Δ = -13.4, SD = 10.0, p < .0001, d = -1.68) reported significant reductions in IDAS General Depression scores in Stage 2.
ISI insomnia.
In Stage 1, there was no significant difference between treatment conditions in mean change in total insomnia symptoms on the ISI.
In Stage 2, there was no significant difference between treatment groups in mean change scores in total insomnia symptoms on the ISI.
Psychosocial functioning, IPF.
In Stage 1, there was no significant between-group difference in mean in overall psychosocial functioning (IPF total score).
In Stage 2, there was no significant difference between treatment conditions in IPF mean change scores.
Posthoc analysis
CAPS-5 subscale scores B, C, D, and E.
As a follow-up to the primary outcome analysis, posthoc analyses were conducted to test the effects of treatment group on change in each of the primary symptom domains of PTSD (i.e., intrusions, avoidance, negative thoughts and emotions, hyperarousal) using the CAPS-5 B, C, D, and E subscale scores. The posthoc analysis for subscale scores used the same analytic approach as the analysis for primary and secondary outcomes.
PCL-5, IDAS social anxiety, IDAS general depression, and ISI longitudinal analysis.
Mixed-models for repeated measures (MMRM) were computed to test for group differences over time for all secondary outcome assessments that were measured at more than two time points within each stage. The use of MMRM models allowed all randomized participants’ data to be analyzed within the models based on the missing-at-random assumption (MAR).
Posthoc results.
In Stage 1, there was no significant difference between groups in mean change for any of the subscale scores on the CAPS-5 [Subscale B, F(3,73) = 1.58, p = .20; Subscale C, F(3,73) = 1.06, p = .37; Subscale D, F(3,73) = 2.26, p = .09; Subscale E, F(3,73) = .84, p = .48].
In Stage 2, there was a significant difference between groups in mean change on the CAPS-5 C (avoidance) [F(2,64) = 4.95, p = .01] and CAPS-5 D (negative thoughts and emotions) [F(2,64) = 8.60, p < .001] subscales. Specifically, there was a significant difference between participants who received High CBD and THC+CBD in mean change in CAPS-5 C (avoidance) subscale scores (Δ = 1.9, 95% CI: 0.38, 3.35, d = -.97) and CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 5.2, 95% CI = 2.04, 8.26, d = -1.13), and between High THC and THC+CBD group participants in mean change in CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 4.1, 95% CI: 1.09, 7.15, d = -1.01). In Stage 2, there were no significant differences between groups in mean change on the CAPS-5 B (intrusions) [F(2,64) = 2.80, p = .07] or CAPS-5 E [F(2,64) = 1.13, p = .33] (hyperarousal) Subscales.
Results of the MMRM analyses testing group differences over time in PCL-5, IDAS Social Anxiety, IDAS General Depression, and ISI appear in Fig 3.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 3. Post hoc Mixed Models for Repeated Measures (MMRM) testing change in PCL-5, IDAS depression, IDAS anxiety, and ISI scores over time.
https://doi.org/10.1371/journal.pone.0246990.g003
Only IDAS Social Anxiety scores in Stage 2 had a significant time x treatment effect, such that social anxiety showed a quadratic drop in Stage 2 among those in the THC+CBD conditions and those in the High THC and High CBD conditions did not show change over time. All other models failed to find a significant difference between groups over time.
Discussion
The present study served as the first randomized placebo-controlled trial of smoked cannabis for symptoms of PTSD in US military veterans. Study-related AEs were generally mild to moderate, and did not significantly differ by treatment condition. The study failed, however, to find a significant effect of treatment condition on the primary efficacy outcome, change in total PTSD severity on the CAPS-5 from baseline to end of Stage 1. All treatment groups (placebo, High CBD, High THC, THC+CBD) achieved statistically significant reductions in PTSD severity on the CAPS-5 in Stage 1, with effect sizes for change in mean PTSD severity ranging between d = .83 (High CBD) and d = 1.34 (High THC). These effect sizes are much larger than effect sizes reported for symptom change in other psychopharmacology trials for PTSD. For example, a 2018 meta-analysis reported standardized mean differences between .33 and .97 across PTSD pharmacology trials. The average length of trials reported in the 2018 meta-analysis lasted approximately ten weeks, whereas the current trial’s primary endpoint was evaluated after only three weeks of treatment.
The study’s failure to detect a significant difference between groups in Stage 1 could perhaps be explained by several confounding factors. First, the study sample included participants with a history of cannabis use. The recruitment of active cannabis users might have increased the potential for biased responding. Given the topical nature of the current trial and its relevance for public policy on medical cannabis, participants might have been biased to report positive effects regardless of condition. Despite many participants already having experience with the drug, nearly half of those receiving placebo believed that they received active cannabis. Prior expectations about cannabis’ effects might explain why even those in the placebo condition reported larger than average reductions in PTSD symptoms after only 3 weeks of treatment.
Second, many participants reported significant cannabis withdrawal symptoms at the time of randomization and early in Stage 1. Nearly half of the study sample (43%, n = 34) were positive for THC at study screening and 23% (n = 18) remained positive for THC at Stage 1 baseline, which would suggest chronicity of previous use or continued cannabis use during the two week washout from screening to baseline (lack of compliance with two week abstinence inclusion criteria). Total cannabis withdrawal symptoms averaged in the moderate range for all treatment groups at the start of Stage 1 (despite two weeks of abstinence prior to randomization), then generally reduced to the mild to moderate range by the end of treatment in Stage 1. Participants who received High THC in Stage 1 reported a significant increase in withdrawal following one week of cessation from Stage 1 treatment, which averaged in the moderate range following cessation. While groups did not differ in cannabis withdrawal ratings, the presence of withdrawal and trends in change could confound (or help explain) interpretation of results. We cannot rule out that cessation of cannabis use (in the placebo condition) or reversal of withdrawal (in the THC and THC+CBD conditions) might have partially been responsible for significant within-subjects change. Moreover, participants randomly assigned to receive High THC in Stage 1 had CUDIT total scores (indicating cannabis use disorder risk) nearly two times greater than participants who were assigned to other active treatment conditions. This is a major confound and limitation of the current study.
Third, total exposure to smoked cannabis was lower than anticipated and might not equate to a full therapeutic dose. In Stage 1, cannabis use in grams ranged from 8.2g (THC+CBD) to 14.6g (high THC) over three weeks (.39g/day to .69g/day on average), despite all participants having access to up to 37.8g over the three week period (1.8g/day). Average cannabis quantity that most cannabis users consume is difficult to estimate from epidemiological studies due to differences in cannabis potency and route of administration. However, large scale studies of medicinal cannabis users treating chronic pain and anxiety report average daily use in ranges closer to 1-3g/day [44,45]. Likewise, two smaller studies that assessed military veterans who use medical cannabis to self-treat PTSD reported median daily use of .85g to 1.14g/day [46] and average use of 3.8g/day [33]. We suspect that participants might have used less cannabis in the current study because of differences between the cannabis available for research trials and the quality of cannabis sold commercially. Several participants spontaneously reported to study staff that the smoke from the cannabis that was provided was “harsher” than they were used to. This difference might also be attributable to the two-week cessation periods mitigating tolerance.
Finally, the present study did not include a placebo arm in Stage 2, which limited the analyses that could be employed in order to take advantage of the crossover design. This significantly limited power to find significant differences across groups. The unexpectedly large response to placebo (d = 1.30), coupled with the small sample size per condition, meant that the current study was underpowered to detect significant differentiation from placebo. If the very large placebo response observed in this study remains consistent in future studies, a trial that tests only one preparation of cannabis (e.g., only high THC cannabis) against placebo would still need a total sample size of nearly one thousand participants (n = 479 per group) to achieve a statistically significant result. However, including a placebo run-in stage to identify and exclude placebo responders in any future trials could substantially reduce this total sample size [47].
Despite these limitations, there were several notable findings. While the study’s primary outcome assessment failed to find differentiation from placebo in PTSD symptom change on the CAPS-5, all participants showed a positive response to treatment in a very brief time period. In addition, side effects were general mild and transient. One of the largest concerns from providers regarding self-treatment of PTSD with cannabis is that it may exacerbate PTSD symptoms. While the current study’s treatment duration was too brief to identify long-term risk, two-tailed significance tests did not show evidence of symptom exacerbation in any condition. These data provide preliminary evidence of safety of short-term ad libitum cannabis use in this population.
While Stage 2 results should be interpreted with caution given the possible carry-over effects and unbalanced randomization across active dose groups, the study did identify some statistically significant differentiation between groups when particiants were re-randomized to only the three active conditions. Given that Stage 2 THC+CBD participants consisted of only those who received active treatment in Stage 1, this cohort might be providing a window into the effects of slightly longer active cannabis treatment. The positive response to treatment evidenced by participants receiving THC+CBD in Stage 2 suggest that it is possible that a longer active treatment period might be necessary to achieve treatment gains that could outperform placebo. It is equally plausible, however, that greater reductions in CAPS-5 severity scores in Stage 2 among THC and THC+CBD treatment groups were due to attenuation of cannabis withdrawal symptoms, as all treatment groups experienced a 2-week cessation period between Stage 1 and Stage 2. This effect would be consistent with prior literature suggesting that symptoms of cannabis use disorder (CUD) can interfere in successful recovery from PTSD [48].
The current study is unique, in that it trialed whole plant cannabis preparations, rather than single molecule extracts or synthetic pharmaceutical cannabinoids. In addition to reporting change in structured assessments of symptoms, the study results provide critical information about participant preference for cannabinoid preparations when exposed to different whole plant THC and CBD ratios. Consistent with previous work [46], participants in the current study reported a general preference for cannabis types that included significant quantities of THC. This effect might be explained by a signal of efficacy, or simply due to the intoxicating and reinforcing effects of high THC cannabis. Nevertheless, the demand for testing cannabis as a therapeutic for PTSD has largely been driven by military veteran advocacy groups, and yet development of cannabinoid therapeutics has not focused on trialing cannabis preparations that military veterans currently use to self-treat their symptoms. Input from stakeholders on which cannabinoids to trial as cannabis-based medicine for military veteran-specific conditions is certainly justified.
Results from the current trial provide invaluable information for future cannabinoid trials for PTSD. While the null findings raise questions about the utility of continuing to trial whole plant cannabis for the treatment of PTSD, the study found that whole plant, smoked cannabis was generally well tolerated and did not lead to deleterious effects in most participants after 3 weeks of ad libitum use. These safety results are consistent with recent real-world evidence studies of whole plant cannabis for PTSD [17,18]. However, those studies both found evidence of potential efficacy as well. Given that many veterans with PTSD are already using cannabis to self-treat their symptoms, identifying which preparations and with which method of administration are most beneficial and/or are least harmful is critical. These future studies will need to take active steps to ensure appropriate blinding despite the intoxicating nature of the drug. While the CBD and placebo conditions were appropriately blinded in the current study, participants and assessors could accurately guess condition when participants received THC-based treatments. Enrolling novice or naïve users, or limiting total THC content to sub-intoxicating doses could improve these blinding issues. Conversely, if higher THC doses are indeed therapeutic, other designs that are less reliant on active blinding, such as randomized withdrawal, might be warranted. Researchers should also consider including objective surrogate endpoint assessments (e.g., physiology, biological specimens, neuroimaging) as secondary indicators of treatment response. As none of these have been validated for a PTSD population as surrogate endpoints, the present study and future studies must utilize the “gold-standard” semi-structured clinical interview for PTSD severity, which is the CAPS-5.
Future studies would also likely benefit from a longer treatment period similar to more traditional medication trials (e.g., 12-weeks), and if including a placebo comparator should plan for a potentially larger than normal placebo response. To mitigate placebo, researchers might consider: 1) including a placebo run-in period prior to randomization to attempt to identify placebo responders, 2) powering the trial for a potentially larger than typical placebo effect, and/or 3) excluding participants with strong apriori beliefs about cannabis’ therapeutic effects [e.g., prescreening with expectancy measures, such as Devilly & Borkovec’s Credibility/Expectancy Questionnaire (CEQ)]. For generalizability to female veterans and civilians with PTSD, these studies should also attempt to recruit a greater number of female participants, as the current study’s sample was overwhelmingly male. Finally, future studies would also greatly benefit from access to high quality flower cannabis. The flower preparations provided through the NIDA drug supply program include all parts of the plant (instead of just buds) and are only available in specific cannabinoid ratios. Studies that test high quality buds in various phenotypes with variable potencies of cannabinoids and terpene ratios would more closely mirror what is available within state-sponsored medical cannabis programs.
Conclusions
The present study failed to find a significant group difference between smoked cannabis preparations containing High CBD, High THC, and THC+CBD against placebo in regards to their impact on PTSD symptoms. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment. The failure to differentiate treatment groups from placebo is likely attributable to the higher than average treatment response in the placebo condition and to the shorter than average duration of treatment. Higher powered studies that attempt to mitigate the effect of pronounced placebo appear warranted.
Supporting information
S1 Checklist –
Showing 1/15: pone.0246990.s001.DS_Storesorry, we can’t preview this file1 / 15figshare
S1 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s001
(DS_STORE)
S2 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s002
(DOC)
S1 File.
https://doi.org/10.1371/journal.pone.0246990.s003
(CSV)
S2 File.
https://doi.org/10.1371/journal.pone.0246990.s004
(TXT)
S3 File.
https://doi.org/10.1371/journal.pone.0246990.s005
(TXT)
S4 File.
https://doi.org/10.1371/journal.pone.0246990.s006
(TXT)
S5 File.
https://doi.org/10.1371/journal.pone.0246990.s007
(TXT)
S6 File.
https://doi.org/10.1371/journal.pone.0246990.s008
(TXT)
S7 File.
https://doi.org/10.1371/journal.pone.0246990.s009
(TXT)
S8 File.
https://doi.org/10.1371/journal.pone.0246990.s010
(TXT)
S9 File.
https://doi.org/10.1371/journal.pone.0246990.s011
(TXT)
S10 File.
https://doi.org/10.1371/journal.pone.0246990.s012
(TXT)
S11 File.
https://doi.org/10.1371/journal.pone.0246990.s013
(TXT)
S12 File.
https://doi.org/10.1371/journal.pone.0246990.s014
(XLSX)
S13 File.
https://doi.org/10.1371/journal.pone.0246990.s015
(PDF)
Acknowledgments
We thank Scott Hamilton and Ilsa Jerome for their assistance in quality checking all of the study data.
References
1. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder. Curr Opin Psychiatry. 2015;28: 307–311. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
2. Gradus JL. Epidemiology of PTSD. In: National Center for PTSD. 2014.
3. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28: 307–11. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
4. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62: 617. pmid:15939839
View Article
PubMed/NCBI
Google Scholar
5. Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149: 671–675. pmid:1575259
View Article
PubMed/NCBI
Google Scholar
6. Perkonigg A, Kessler RC, Storz S, Wittchen H-U. Traumatic events and post-traumatic stress disorder in the community: prevalence,risk factors and comorbidity. Acta Psychiatr Scand. 2000;101: 46–59. pmid:10674950
View Article
PubMed/NCBI
Google Scholar
7. Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62 Suppl 17: 16–22. Available: http://www.ncbi.nlm.nih.gov/pubmed/11495091.
View Article
Google Scholar
8. Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro III A, Parker VA, et al. Burden of Medical Illness in Women With Depression and Posttraumatic Stress Disorder. Arch Intern Med. 2004;164: 1306. pmid:15226164
View Article
PubMed/NCBI
Google Scholar
9. Merz J, Schwarzer G, Gerger H. Comparative Efficacy and Acceptability of Pharmacological, Psychotherapeutic, and Combination Treatments in Adults With Posttraumatic Stress Disorder. JAMA Psychiatry. 2019;76: 904. pmid:31188399
View Article
PubMed/NCBI
Google Scholar
10. Forman-Hoffman V, Middleton JC, Feltner C, Gaynes BN, Weber RP, Bann C, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update. 2018 [cited 11 Oct 2019]. Available: https://www.ncbi.nlm.nih.gov/books/NBK525132/.
View Article
Google Scholar
11. Management of Post-Traumatic Stress Working Group. VA/DOD clinical practice guideline for management of post-traumatic stress. 2017.
12. Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67: 194–200. Available: http://www.ncbi.nlm.nih.gov/pubmed/10224729. pmid:10224729
View Article
PubMed/NCBI
Google Scholar
13. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consult Clin Psychol. 1992;60: 748–56. Available: http://www.ncbi.nlm.nih.gov/pubmed/1401390. pmid:1401390
View Article
PubMed/NCBI
Google Scholar
14. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153: 369–375. pmid:8610824
View Article
PubMed/NCBI
Google Scholar
15. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136: 162–165. pmid:24412475
View Article
PubMed/NCBI
Google Scholar
16. Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychol Addict Behav. 2011;25: 485–491. pmid:21261407
View Article
PubMed/NCBI
Google Scholar
17. LaFrance EM, Glodosky NC, Bonn-Miller M, Cuttler C. Short and Long-Term Effects of Cannabis on Symptoms of Post-Traumatic Stress Disorder. J Affect Disord. 2020;274: 298–304. pmid:32469819
View Article
PubMed/NCBI
Google Scholar
18. Bonn-Miller MO, Brunstetter M, Simonian A, Loflin MJ, Vandrey R, Babson KA, et al. The Long-Term, Prospective, Therapeutic Impact of Cannabis on Post-traumatic Stress Disorder. Cannabis Cannabinoid Res. 2020.
View Article
Google Scholar
19. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14. pmid:28813324
View Article
PubMed/NCBI
Google Scholar
20. Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. 2010;207: 105–111. pmid:19800921
View Article
PubMed/NCBI
Google Scholar
21. Gomes F V, Reis DG, Alves FH, Corrêa FM, Guimarães FS, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT 1A receptors. J Psychopharmacol. 2012;26: 104–113. pmid:21148020
View Article
PubMed/NCBI
Google Scholar
22. Stern CAJ, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25: 958–965. pmid:25799920
View Article
PubMed/NCBI
Google Scholar
23. Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226: 781–792. pmid:23307069
View Article
PubMed/NCBI
Google Scholar
24. Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64: 396–402. pmid:22796109
View Article
PubMed/NCBI
Google Scholar
25. Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36: 23–31. pmid:23260015
View Article
PubMed/NCBI
Google Scholar
26. Yehuda R, LeDoux J. Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron. 2007;56: 19–32. pmid:17920012
View Article
PubMed/NCBI
Google Scholar
27. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15: 84–88. pmid:19228182
View Article
PubMed/NCBI
Google Scholar
28. Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51: 585–588. pmid:25467221
View Article
PubMed/NCBI
Google Scholar
29. Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, Open-Label, Pilot Study of Add-On Oral Δ9-Tetrahydrocannabinol in Chronic Post-Traumatic Stress Disorder. Clin Drug Investig. 2014;34: 587–591. pmid:24935052
View Article
PubMed/NCBI
Google Scholar
30. Loflin M, Babson K, Sottile J, Norman S, Gruber S, Bonn-Miller M. A Cross-Sectional Examination of Choice and Behavior of Veterans with Access to Free Medicinal Cannabis. Am J Drug Alcohol Abuse. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
31. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. European Journal of Internal Medicine. Elsevier B.V.; 2018. pp. 12–19. pmid:29307505
View Article
PubMed/NCBI
Google Scholar
32. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
33. Loflin M, Earleywine M, Bonn-Miller M. Medicinal versus recreational cannabis use: Patterns of cannabis use, alcohol use, and cued-arousal among veterans who screen positive for PTSD. Addict Behav. 2017;68. pmid:28088054
View Article
PubMed/NCBI
Google Scholar
34. First MB, Williams JBW, Karg RSK, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA Am Psychiatr Assoc. 2015. pmid:32821383
View Article
PubMed/NCBI
Google Scholar
35. Posner K, Brent D, Lucas C, Gould M, Stanley B., Brown G., et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008.
36. Shahid A, Wilkinson K, Marcu S, Shapiro CM. STOP-Bang Questionnaire. STOP, THAT and One Hundred Other Sleep Scales. Springer New York; 2011. pp. 371–383. https://doi.org/10.1007/978-1-4419-9893-4_92
37. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110: 137–143. pmid:20347232
View Article
PubMed/NCBI
Google Scholar
38. Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). [Assessment]. 2013.
View Article
Google Scholar
39. Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30: 383–395. pmid:28493729
View Article
PubMed/NCBI
Google Scholar
40. Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28: 1379–1391. pmid:26653052
View Article
PubMed/NCBI
Google Scholar
41. Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 2007;19: 253–268. pmid:17845118
View Article
PubMed/NCBI
Google Scholar
42. Bovin MJ, Black SK, Rodriguez P, Lunney CA, Kleiman SE, Weathers FW, et al. Development and validation of a measure of PTSD-related psychosocial functional impairment: The Inventory of Psychosocial Functioning. Psychol Serv. 2018;15: 216–229. pmid:29723024
View Article
PubMed/NCBI
Google Scholar
43. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34: 601–8. pmid:21532953
View Article
PubMed/NCBI
Google Scholar
44. Turna J, Simpson W, Patterson B, Lucas P, Van Ameringen M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J Psychiatr Res. 2019;111: 134–139. pmid:30738930
View Article
PubMed/NCBI
Google Scholar
45. Ware MA, Wang T, Shapiro S, Collet JP. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain. 2015;16: 1233–1242. pmid:26385201
View Article
PubMed/NCBI
Google Scholar
46. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
47. Lee S, Walker JR, Jakul L, Sexton K. Does elimination of placebo responders in a placebo run-in increase the treatment effect in randomized clinical trials? A meta-analytic evaluation. Depress Anxiety. 2004;19: 10–19. pmid:14978780
View Article
PubMed/NCBI
Google Scholar
48. Bonn-Miller MO, Boden MT, Vujanovic A a., Drescher KD. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychol Trauma Theory, Res Pract Policy. 2011;5: 193–200.
View Article
Google Scholar
Curated by Thc 420 Hemp
Via https://weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/
source https://lauragfollett.weebly.com/blog/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial
0 notes
Text
Clinical Research Associate Salary - What's the pay for a clinic research associate?
Here's What You Need to Know to Get a Clinical Research Associate Job
What's the pay for a clinic research associate?: $61-$110K
A Clinical Research Associate (or Monitor) is hired either in-house (“the trial site”) or externally (by the sponsor or CRO) to do review clinical trial data and ensure that investigational therapies are tested ethically and scientifically through performing site visits that review files like patient medical notes in order to ensure quality of trial data. The catch 22 of clinical research associate jobs is that the ICH GCP guidelines require both education AND experience in order to work in this role, so you getting your foot in the door is tough. Once you get experience, your education (i.e. certifications or degrees showing understanding of additional responsibilities) can help promotes you quickly through the CRA career ladder.
How to become or get promoted as a clinical research associate?
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate.
How much does a Clinical Research Associate make in the United States?
The average Clinical Research Associate salary in the United States is $61-110K. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, the number of years you have spent in your profession.
Clinical Research Associate (CRA) Salary
Per Payscale “An entry-level Clinical Research Associate (CRA) with less than 1 year experience can expect to earn an average total compensation (includes tips, bonus, and overtime pay) of $55,588 based on 203 salaries. An early career Clinical Research Associate (CRA) with 1-4 years of experience earns an average total compensation of $66,245 based on 1,118 salaries. A mid-career Clinical Research Associate (CRA) with 5-9 years of experience earns an average total compensation of $76,086 based on 294 salaries. An experienced Clinical Research Associate (CRA) with 10-19 years of experience earns an average total compensation of $81,540 based on 157 salaries. In their late career (20 years and higher), employees earn an average total compensation of $83,342.”
Determine CRA Salary by location using Payscale
Salary: Clinical Research Associate
Research Associate Salary resource: Click here to see the CRA Salary Range from 1,800+ employers
Clinical Research Associates Pay: Clinical Research Associate Salary in United States (by city)
Durham, NC - $97K
New York, NY, Irvine, CA, Houston, TX - $95K
Philadelphia, PA - $92K
Atlanta, GA - $84K
Raleigh, NC - $82K
Chicago, IL - $79K
Senior Clinical Research Associate Salary
The average salary of a Senior Senior Clinical Research Associate is ~105K per year ($54/hour, $2k/week, $9k/month). This can range from $81k to $139k.
Starting Salary of a Clinical Research Associate Position is between $60,000 and $65,000
Some employers may prefer hiring entry-level clinical research associates with less experience in clinical research so they can learn their job functions by training under Senior CRAs or CRA IIs.
Clinical Research Associate II Salary is $86,677 / yr
CRA II @ PPD: $84,733/yr
CRA II @ PRA Health Sciences: $96,017/yr
CRA II @ IQVIA: $79,412/yr
CRA II @ ICON: $100,000/yr
Comment below how much you get paid and what helped you get promoted!
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate.
#Medpace Clinical Research Associate Salary#medpace clinical research associate salary#clinical research associate salary#senior clinical research associate salary#clinical research associate salary entry level#regional clinical research associate salary#clinical research associate salary in south africa#in house clinical research associate salary#clinical research associate job salary#clinical research associate salary chicago#clinical research associate salary parexel#senior clinical research associate salary range#abbott clinical research associate salary#clinical research associate merck salary#clinical research associate philippines salary#clinical research associate salary california#clinical research associate salary nc#clinical research associate salary quintiles india#clinical research associate salary seattle#covance clinical research associate salary#salary for clinical research associate in usa#salary of clinical research associate in canada#clinical research associate salary australia#clinical research associate salary canada#clinical research associate salary montreal#clinical research associate salary new york#clinical research associate salary us#clinical research associate starting salary#clinical research associate job description and salary#clinical research associate salary dubai
1 note
·
View note
Text
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
By: Jason Karimi, WeedPress Contributor Title: The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial Sourced From: weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/ Published Date: Sun, 21 Mar 2021 23:04:30 +0000
https://journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0246990
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin ,
Benjamin Shechet,
Colin Hennigan,
Rebecca Matthews,
Amy Emerson,
Rick Doblin
The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial
Marcel O. Bonn-Miller,
Sue Sisley,
Paula Riggs,
Berra Yazar-Klosinski,
Julie B. Wang,
Mallory J. E. Loflin,
Benjamin Shechet, …

x
Published: March 17, 2021
https://doi.org/10.1371/journal.pone.0246990
Article
Authors
Metrics
Comments
Media Coverage
Peer Review
Abstract
Introduction
Methods
Results
Discussion
Conclusions
Supporting information
Acknowledgments
References
Reader Comments (0)
Figures
Abstract
Importance
There is a pressing need for development of novel pharmacology for the treatment of Posttraumatic Stress Disorder (PTSD). Given increasing use of medical cannabis among US military veterans to self-treat PTSD, there is strong public interest in whether cannabis may be a safe and effective treatment for PTSD.
Objective
The aim of the present study was to collect preliminary data on the safety and potential efficacy of three active concentrations of smoked cannabis (i.e., High THC = approximately 12% THC and < 0.05% CBD; High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD) compared to placebo in the treatment of PTSD among military veterans.
Methods
The study used a double-blind, cross-over design, where participants were randomly assigned to receive three weeks of either active treatment or placebo in Stage 1 (N = 80), and then were re-randomized after a 2-week washout period to receive one of the other three active treatments in Stage 2 (N = 74). The primary outcome measure was change in PTSD symptom severity from baseline to end of treatment in Stage 1.
Results
The study did not find a significant difference in change in PTSD symptom severity between the active cannabis concentrations and placebo by the end of Stage 1. All three active concentrations of smoked cannabis were generally well tolerated.
Conclusions and relevance
The present study is the first randomized placebo-controlled trial of smoked cannabis for PTSD. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment, but no active treatment statistically outperformed placebo in this brief, preliminary trial. Additional well-controlled and adequately powered studies with cannabis suitable for FDA drug development are needed to determine whether smoked cannabis improves symptoms of PTSD.
Trial registration
Identifier: NCT02759185; ClinicalTrials.gov.
Figures






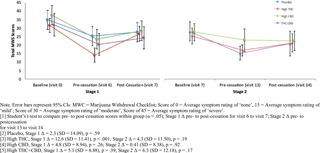






Citation: Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. (2021) The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS ONE 16(3): e0246990. https://doi.org/10.1371/journal.pone.0246990
Editor: Bernard Le Foll, Centre for Addiction and Mental Health, CANADA
Received: February 11, 2020; Accepted: January 26, 2021; Published: March 17, 2021
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Data Availability: All non-identifiable, relevant data are currently attached in the Supporting Information files.
Funding: Authors BY, RD, AE, MB, PR, and SS received Grant Number: RFA#135, an award funded by the Colorado Department of Public Health and Environment (CDPHE): https://www.colorado.gov/pacific/cdphe/approved-medical-marijuana-research-grants The study was also partially funded by the sponsor, The Multidisciplinary Association for Psychedelic Studies (MAPS): https://maps.org/research/mmj/ The sponsor designed the protocol with input from from MB, SS, and PR. The sponsor monitored the data quality, conducted data analysis, contributed to decision to publish, and assisted with preparation of manuscript through critical review.
Competing interests: Author MBM is an employee of Canopy Growth Corporation, during which time he has received stock options, serves on the Board of Directors for AusCann Group Holdings Limited, was a prior employee of Zynerba Pharmaceuticals, and has received consulting fees from Tilray Inc. Author ML serves on the scientific advisory board for FSD Pharma and has received consulting fees from Greenwich Biosciences, Zynerba Pharmaceuticals, and Tilray Inc in the past two years. Authors RD, BY, JW, BS, CH, RM, and AE receive salary from the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) non-profit research and educational organization. Author SS receives salary from the Scottsdale Research Institute, which is a private LLC and has no shareholders. The Academic Editor, BLF, co-authored “The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda” (https://pubmed.ncbi.nlm.nih.gov/32360455/) with one of the authors, MBM. This article was a result of a meeting where a large number of investigators came together to discuss clinical trial outcomes with representative from NIH and FDA. No other relationship between this author and the Academic Editor exists. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Posttraumatic Stress Disorder (PTSD) is a serious, worldwide public health problem. In the United States the lifetime prevalence of PTSD in the general population is between 6 and 10% [1,2], and between 13 and 31% in US military veterans [2,3]. PTSD is typically a chronic condition [4,5], and is associated with high rates of psychiatric and medical co-morbidity, disability, suffering, and suicide [4,6–8]. Food and Drug Administration (FDA)-approved pharmacological treatments for PTSD are currently limited to two selective serotonin reuptake inhibitors (SSRIs): sertraline and paroxetine, which have significantly lower effect sizes (SMD between -.28 and -.56) compared to trauma-focused psychotherapy (SMD between -1.01 and -1.35) [9,10]. Indeed, the current Department of Defense (DoD) and Department of Veterans Affairs (VA) best practice guidelines for treatment of PTSD recommend psychotherapy over pharmacotherapy [11]. However, the majority of military veterans with PTSD who receive one of the best practices psychotherapies for PTSD, which were determined efficacious through clinical trials, do not remit or reduce symptoms below clinical thresholds by the end of treatment [12,13].
There is a strong public interest, particularly among Patients with PTSD, clinicians, and researchers, in whether cannabis can be an effective pharmacological treatment option for individuals with PTSD, or a safe alternative treatment for patients who do not respond to current front-line treatment. Cross-sectional and prospective studies document the widespread use of cannabis by individuals with PTSD [14,15]. Moreover, veterans with PTSD who do not show remission following standard treatment are more likely to use cannabis following completion of PTSD treatment [16]. Two recent prospective studies of patients using cannabis to self-treat PTSD provide evidence that whole plant cannabis can produce short [17] and long-term relief of PTSD symptoms [18].
There is some preclinical evidence that at least two of the active compounds in cannabis, delta-9-tetrahydrocannabinol (THC; the primary constituent responsible for intoxication from cannabis) and cannabidiol (CBD; one of the non-intoxicating cannabinoids in cannabis), can positively impact processes that underly PTSD pathology [19]. Specifically, administration of CBD in rats and mice dampens cue-elicited fear responses [20,21], while administration of THC and THC+CBD appears to block reconsolidation of fear memory [22]. Likewise, both THC and CBD when administered alone facilitate fear extinction learning [23,24], which is a critical component for recovery from PTSD [25,26]. This work suggests that THC and/or CBD could modify how patients with PTSD experience and respond to reminders of trauma.
In addition to cannabis’ potential to perhaps modify mechanisms that maintain the core psychopathology of PTSD, early phase clinical data on isolated cannabinoid constituents in humans suggest that active components of cannabis might provide acute relief from specific symptoms of PTSD. For example, two open-label studies and one randomized placebo controlled trial found that administration of low doses of a THC analogue led to improvements in self-reported subjective sleep quality, decreased frequency of nightmares, and improvements in self-reported overall well-being among those with PTSD [27–29].
While these data appear promising, the potential therapeutic effects of smoked, herbal cannabis on PTSD have not been examined in a randomized, placebo controlled trial. Military veterans with PTSD are overwhelmingly choosing smoked cannabis to self-treat PTSD and related conditions [30]. Moreover, herbal cannabis varies significantly across plants in its THC and CBD content [29]. While both cannabinoids could hold therapeutic value, unlike THC, CBD is non-intoxicating and does not carry significant risk of abuse [30]. In addition, CBD may temper the anxiogenic effects of THC in cannabis preparations that contain both CBD and THC [31,32]. It is unclear whether THC, CBD, or some combination of compounds may lead to greater reductions in PTSD symptoms with better safety profiles compared to other combinations. In addition, previous clinical studies rely entirely on standardized dosing, rather than test more naturalistic and generalizable ad libitum dosing regimens. This is a major limitation of previous research because there is substantial individual variability in cannabinoid tolerability [31]. Indeed, military veterans who use cannabis for PTSD tend to self-titrate to much larger doses than those tested in research studies [32,33].
The primary objective of the present study was to conduct a randomized placebo-controlled trial to assess the safety and potential efficacy of smoked, herbal cannabis for the treatment of PTSD in military veterans. Specifically, the study was designed to examine the independent effects of ad libitum use of up to 1.8 grams/day of three active preparations of smoked cannabis: (i) High THC, (ii) High CBD, and (iii) one-to-one ratio of THC and CBD (THC+CBD) against placebo on PTSD symptoms in a sample of veterans with PTSD.
Methods
Trial design
The trial protocol can be found at https://maps.org/research-archive/mmj/MJP1-Protocol-Amend4-oct-13-2015.pdf. The study received ethics approval from the Copernicus Group Independent Review Board (IRB) and was conducted in accordance with all local and Federal laws and regulations, including obtaining written informed consent from all study participants. The study included a randomized, double-blind, placebo-controlled, crossover trial of smoked cannabis containing three different concentrations of THC and CBD, and placebo. The cross-over design included two stages with four treatment groups in Stage 1 (High THC, High CBD, THC+CBD, and placebo) and re-randomization into three active treatment groups in Stage 2 (High THC, High CBD, and THC+CBD). The primary aim of the study was to determine whether change in PTSD symptom severity at the end of Stage 1 (primary study endpoint) differed by condition. The crossover design allowed for additional comparisons of within-subject and between-subject differences in safety and preliminary efficacy across the two Stages and allowed for assessment of participants’ preference for cannabis concentrations assigned in either Stage 1 vs. Stage 2. Each stage included three weeks of ad libitum use up to 1.8 grams/day of the assigned treatment followed by a two-week cessation period. This upper limit was necessary due to the outpatient setting for self-administration and the Schedule 1 controlled substance status of cannabis.
Primary outcome and safety assessments were conducted at baseline (visit 0), end of treatment in Stage 1 (visit 5; primary study endpoint), following the Stage 1 cessation period/Stage 2 baseline (visit 7), and end of treatment in Stage 2 (visit 12). Self-reported assessment of withdrawal symptoms was conducted at screening, baseline, and weekly during the two-week cessation periods following each stage of treatment (visits 6, 7, 13, 14). Secondary outcomes were assessed throughout the study before/after treatment and cessation periods.
Participants.
Study participants were recruited using community-based advertisements, presentations, and website advertisements. Study inclusion and exclusion criteria were as follows:
Inclusion Criteria. Individuals were eligible for study enrollment if they (1) were a US military veteran, (2) met DSM-5 (APA, 2013) criteria for PTSD with symptoms of at least six months in duration (index trauma did not have to be related to military service), (3) had PTSD of at least moderate severity based on a CAPS-5 score of = >25 at baseline assessment, (4) were at least 18 years of age, (5) reported they were willing and able to abstain from cannabis use two-weeks prior to baseline assessment, which would be verified by urine toxicology screens at screening and baseline, and agreed to abstain from using non-study cannabis during the trial, (6) were stable on any pre-study medications and/or psychotherapy prior to study entry, and (7) agreed to comply with study procedures.
Exclusion criteria. Study participants were excluded if they (1) were pregnant, nursing, or of child bearing potential and not practicing effective means of birth control, (2) had a current or past serious mental illness (e.g., personality disorder, psychotic disorder) determined by the SCID-5-RV [34], or self reported a positive family history (first-degree relative) of psychotic or bipolar disorder (3) were determined at high risk for suicide based on the C-SSRS [35], (4) had allergies to cannabis or other contraindication for smoking cannabis, (5) had a current diagnosis or evidence of significant or uncontrolled hematological, endocrine, cerebrovascular, cardiovascular, coronary, pulmonary, gastrointestinal, immunocompromising, or neurological disease, (6) met DSM-5 criteria for moderate-severe Cannabis Use Disorder on the CUDIT-R (= >11), (7) screened positive for any illicit substance other than cannabis during the two-week screening, or (7) were unable to provide informed consent.
Randomization and blinding.
The Stage 1 randomization list utilized blocks to ensure equal treatment assignments, and the Stage 2 randomization utilized multiple validated randomization lists that re-randomized participants in a blinded manner. The randomization procedure specified that participants would be randomized to treatment conditions using small block randomization in a 1:1:1:1 ratio in Stage 1 and then be re-randomized into two of the three active cannabis conditions (THC, CBD, THC+CBD) with a 1:1 ratio in Stage 2. Randomization in Stage 2 excluded the participant���s Stage 1 treatment condition. As placebo was not an option in Stage 2, placebo participants were randomized 1:1 between High THC and High CBD, but were not given the option to be randomized to THC + CBD in order to facilitate simpler programming of the web-based randomization system. This two-step randomization resulted in an unbalanced distribution of Stage 2 participants overall across active dose groups. In order to maintain the blind, a central electronic database was utilized for randomization based on validated computer-generated lists.
All study staff (with the exception of the Randomization Monitor and Drug Product Packaging Technician) and participants were blinded to condition assignments. The blind could only be broken for an individual participant if there was a clinically or medically urgent emergency requiring knowledge of the participant’s condition assignment. This emergency unblinding required approval from the site PI and Coordinating Investigator. Likewise, the unblinded Randomization Monitor could provide dose assignment through the electronic randomization system. Randomization information was only available within the web-based randomization system and only viewable by the designated Randomization Monitor.
Interventions.
Study drug was obtained from the National Institute on Drug Abuse (NIDA). Four concentrations of cannabis from NIDA included: High THC = approximately 12% THC and < 0.05% CBD); High CBD = 11% CBD and 0.50% THC; THC+CBD = approximately 7.9% THC and 8.1% CBD, and placebo = < 0.03% THC and < 0.01% CBD. Samples of each batch were tested and confirmed for their concentration levels by an independent third-party analytical testing laboratory in Phoenix, Arizona. The independent testing lab found in two separate analyses that the High THC batch was just 9%, with the other batches very close to what was reported by NIDA.
At the beginning of each stage, participants were asked to visit the clinic site for four hours on two successive days and self-administer under supervision of study staff one dose of the cannabis preparation that they were randomly assigned to in that Stage. Vital signs for safety were collected during these visits (i.e., blood pressure, pulse). The study provided participants a total of 37.8 grams (1.8 grams/day)for the three-week ad libitum treatment period along with a metal pipe for treatment delivery (smoked). Participants were asked to refrain from using non-study cannabis, and return any remaining study cannabis that was not used each week. When study drug was returned the clinic team weighed the returned cannabis to calculate participants’ average use in grams per day during the treatment period in each stage. Participants were asked to refrain from any cannabis use during a two-week cessation period (between stages), then were re-randomized into one of three active treatment groups. All study participants were provided the option to enroll in an open label extension (Stage 3) with the cannabis of their choice in the same amount they returned unused in Stages 1 and 2 so participants had no disincentives to returning unused amounts. The results of Stage 3 are not reported here.
Demographic measures.
Baseline demographic information included age, sex, race/ethnicity, education, employment status. Other baseline measures included: whether the index trauma was combat-related, body mass index (BMI), risk for sleep apnea (STOP-bang) [36], and risk for cannabis use disorder (CUDIT-R) [37].
Safety measures.
Adverse Events (AEs) were assessed at baseline, during the introductory session, self-administration session, end of treatment, and before/after cessation in each stage by asking participants to self-report any side effects experienced over the past week. All AEs were coded by Systems Organ Class. The study physician then rated all AEs by severity (mild, moderate, severe) and study relatedness (i.e., possibly related, probably related, not related). AEs rated possibly related and probably related were collapsed into one “related” category.
Additional safety measures included the 15-item Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) and the Columbia-Suicide Severity Rating Scale (CSSR-S) (Posner et al., 2011). The MWC was administered at screening, baseline, and each week following cessation of Stages 1 and 2 (visits 6, 7, 13, 14). The CSSR-S was self-administered at all study visits.
Outcome measures.
The primary outcome of the current study was change in PTSD symptom severity from baseline (visit 0) to end of the three-week treatment period in Stage 1 (visit 5) using the Clinician-Administered PTSD Scale for DSM-5 Total Severity Score (CAPS-5) [38]. The CAPS-5 is a semi-structured clinician interview, and is well-validated for determining PTSD diagnoses consistent with the Diagnostic and Statistical Manual of Mental Disorders, Version 5 (DSM-5) and assessing change in symptom severity over time [39]. PTSD diagnosis is based on meeting the DSM-5 symptom cluster criteria (minimum threshold of symptoms with a score ≥ 2) with a qualifying criterion A index trauma. The CAPS-5 Total Severity Score is calculated by summing the total score for each of the four symptom categories to assess past-month PTSD symptoms on a specific traumatic event: intrusion (Category B), Avoidance (Category C), Mood and Cognition (Category D), and Hyperarousal (Category E). CAPS-5 Total Severity scores range from 0–80, where higher scores indicate worse PTSD severity.
Secondary outcome measures included a modified version of the 20-item self-report PTSD Checklist for DSM-5 (PCL-5) [40], which was changed to assess for past week symptoms, the 20-item general depression subscale and 5-item anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms’ (IDAS) [41], the 80-item self-report Inventory of Psychosocial Functioning (IPF) [42], and the 7-item self-report Insomnia Severity Index (ISI) [43]. Secondary outcome measures were collected at baseline (visit 0 and visit 7), self-administration (visit 4 and visit 10), before cessation (visit 6 and visit 13), and after cessation (visit 7 and visit 14) in both Stage 1 and Stage 2. Total and subscale scores were calculated for each measure.
Other measures.
The validity of study blinding to active or inactive treatment in Stage 1 was assessed by asking participants and clinicians to independently guess whether the participant was randomized to an active (High THC, High CBD, THC+CBD) or inactive (placebo) treatment group at the end of Stage 1. At the end of Stage 2, participants were asked whether they preferred the treatment to which they were assigned in Stage 1 or Stage 2.
Table 1 includes a summary of all assessments by visit.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 1. Summary of assessments by visit.
https://doi.org/10.1371/journal.pone.0246990.t001
Study power.
The primary study aim was to gather preliminary data on the safety and potential efficacy of different cannabis preparations to treat PTSD among veterans. In the absence of published effect sizes for the impact of THC, CBD, or THC+CBD on CAPS-5 scores, the target sample size was chosen to allow detection of an effect size of 0.4 or greater (small to medium effect) based on between group differences in the primary outcome measure (i.e., change in total CAPS-5 severity score from baseline to the end of Stage 1 active treatment phase). Power analysis suggested that 76 completing participants (n = 19 per group) would be needed to detect an effect size of d = 0.4 at 82% power and .05 significance level. Enrollment and randomization continued until 76 participants completed the Stage 1 outcome assessment. Eighty participants were enrolled and 76 partcipants completed Stage 1.
Statistical analyses.
Descriptive statistics were performed to test the normality of baseline measures on the total study sample and across each treatment group to ensure adequate randomization. Means, medians, and frequencies were calculated, and within-subject and between-group differences were tested for categorical variables using chi-square tests and t-tests or analysis of variance (ANOVA) for continuous variables.
Safety was analyzed by tabulating the frequency, severity, and relatedness to treatment of AEs. A Chi-square test was used to assess for differences in frequency of AEs across groups. An AE was counted once per subject for each assessment period.
The primary outcome was analyzed using ANOVA to test for between-group differences in change in Total PTSD Severity scores from baseline to end of treatment in Stage 1 (CAPS-5 visits 0 and 7). Secondary outcomes were analyzed using a series of additional ANOVAs to test for between-group differences in change scores from baseline to end of treatment for Stage 1 (CAPS-5 visits 0 and 7; secondary measures visits 0 and 6) and Stage 2 (CAPS-5 visits 7 and 12; secondary measures visits 7 and 13). All dependent variables were tested for normality, and summarized by both mean and median values by group. Within-subject change scores were tested for each treatment group using a series of t-tests. Tukey’s pairwise comparisons were used to test for group differences in change scores between all pairs of treatment conditions in Stage 1 and Stage 2. Analyses were conducted consistent with an intent-to-treat (ITT) framework, where all available data from randomized participants who received at least one week’s supply of study drug (N = 80) were summarized for baseline characteristics and entered into the models. However, the use of ANOVA tests only allowed for analysis of change in participants who completed outcome assessments (N = 76 for primary outcome analysis).
Results
Sample characteristics
A total of 261 individuals completed screening and 51% met eligibility criteria for study inclusion. Eighty participants were enrolled and randomized into one of four treatment groups (n = 20 per group), of which 76 participants completed the Stage 1 outcome assessment. In Stage 2, a total of 74 participants were re-randomized into High THC (n = 29), High CBD (n = 27), or THC+CBD (n = 18). There were no significant Stage 1 treatment assignment differences in demographics or baseline scores on the primary and secondary outcome variables (i.e., CAPS-5, PCL-5, IDAS Social Anxiety, IDAS Depression, IPF, and ISI). Sample demographics and baseline characteristics are summarized in Table 2.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 2. Baseline characteristics by treatment group.
https://doi.org/10.1371/journal.pone.0246990.t002
Treatment adherence and attrition
Rates of engagement and completion are summarized in the Consort Diagram (Fig 1). During Stage 1, 3 participants (3.8%) did not complete endpoint outcome assessments. After Stage 1, 6 participants (7.5%) did not continue into Stage 2. Of the 74 participants who were re-randomized into Stage 2, 3 (4.1%) discontinued treatment due to an AE, and 7 total (9.5%) did not complete Stage 2 endpoint outcome assessments. The overall attrition rate for the percent of randomized participants who dropped out before completing Stage 2 outcome assessments, was 16.3%.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 1. Consort flow diagram.
https://doi.org/10.1371/journal.pone.0246990.g001
Cannabis use in grams
In Stage 1, there was no statistically significant difference between groups in total grams of smoked cannabis/placebo during the three-week treatment period (21 days) across the treatment groups (F [3, 71] = 2.23, p = .09). Mean (SD) grams of smoked cannabis/placebo used by each treatment group in Stage 1 were as follows: placebo (M = 8.4, SD = 10.1), High THC (M = 14.6, SD = 10.4), High CBD (M = 14.3, SD = 13.0), THC+CBD (M = 8.2, 6.8).
In stage 2, there was a significant group difference in total grams of smoked cannabis (F [2, 64] = 3.42, p = .04), such that participants in the THC+CBD group used significantly more cannabis (M = 17.6, SD = 10.6), compared to participants randomized to High THC (M = 10.7, SD = 10.9), or High CBD (M = 9.3, SD = 10.5).
Assessment of study blind
In Stage 1, 60% of placebo participants accurately guessed assignment to an inactive treatment, 58% of High CBD participants accurately guessed that they were in an active condition, and 100% of participants in the High THC and THC+CBD groups accurately guessed assignment into an active treatment condition. Similar results were found for clinicians. In Stage 1, forty-five percent of clinicians accurately guessed placebo participants’ assignment in an inactive treatment, 16% accurately guessed High CBD participants’ assignment into an active treatment, and 100% accurately guessed that participants assigned to High THC or THC+CBD were randomized into an active treatment. Therefore, the study blind was appropriately upheld only when participants were assigned to High CBD or placebo conditions, but was not upheld when participants were assigned to High THC or High THC/CBD.
Treatment preference
At the end of Stage 2, participants who completed final assessments (n = 74) indicated their preference for either their blinded Stage 1 or Stage 2 treatment assignment. Twenty-five participants (34%) indicated a preference for a Stage 1 or Stage 2 assignment to High THC, 10 participants (13%) indicated a preference for a Stage 1 or Stage 2 assignment to High CBD, 26 participants (35%) indicated a preference for a Stage 1 or Stage 2 assignment to THC+CBD, and 4 participants (5%) indicated a preference for a Stage 1 assignment to placebo. Two participants (3%) equally preferred their Stage 1 and Stage 2 treatment assignments.
Safety outcomes
Adverse events.
All Adverse Events (AEs) reported during Stage 1 are summarized in Table 3. Number of participants who reported at least one AE did not significantly differ by treatment group in either Stage 1 (p = .38) or Stage 2 (p = .27). Thirty-seven of 60 participants who received THC, CBD, or THC+CBD during Stage 1 (61.7%) reported at least one treatment-related AE by the end of Stage 1. In Stage 2, Forty-five of the 74 participants who received THC, CBD, or THC+CBD (60.8%) reported at least one treatment-related AE during Stage 2. Three of 80 participants (3.8%) reported an unrelated Serious Adverse Event (SAE) during the study, specifically heart palpitations (n = 1; THC+CBD, Stage 1 cessation period), pulmonary embolism (n = 1, High THC, Stage 2), and abscess (n = 1, High CBD, Stage 2). One participant (THC+CBD) discontinued treatment during the introductory session in Stage 1 due to an AE, and two participants discontinued treatment during the introductory session in Stage 2 due to an AE (High CBD and High THC conditions). Across both Stages, 13 total participants terminated from the study early due to an AE (8.4%). The most common AEs reported (i.e., those with >10% frequency) were cough (12.3%), followed by throat irritation (11.7%) and anxiety (10.4%). Emergency unblinding was never used in the study.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 3. Number of participants with adverse events by systems organ class/preferred terms and treatment-relatedness.
https://doi.org/10.1371/journal.pone.0246990.t003
One participant who received CBD in Stage 1 (5.0%) reported treatment-related suicidal ideation. One participant from each treatment condition (3.6% – 5.9%) reported treatment-related suicidal ideation in Stage 2.
Cannabis withdrawal symptoms.
Fig 2 summarizes mean withdrawal symptom scores on the MWC by group at Stage 1 and Stage 2 baseline, end of treatment, and following 1-week of cessation. All treatment groups reported mean withdrawal symptoms in the moderate range (Mean score = 32–38) at baseline assessment (prior to initiating treatment in Stage 1). All treatment groups showed a significant reduction in withdrawal symptoms from baseline to the end of the treatment phase of Stage 1. Only participants assigned to High THC in Stage 1 reported a significant increase in mean self-reported withdrawal symptoms after one week of cessation from the assigned treatment in Stage 1 (Δ = 12.6, SD = 11.41, p = .0004). There was no significant change in withdrawal symptoms from the end of Stage 2 treatment to one-week follow-up.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 2. Mean total marijuana withdrawal scores by treatment condition across Stage 1 & Stage 2.
https://doi.org/10.1371/journal.pone.0246990.g002
Primary efficacy outcome
PTSD symptom severity (CAPS-5).
Results of the analysis of change in total PTSD symptom severity on the CAPS-5 are summarized in Table 4 for both Stage 1 (primary outcome) and Stage 2. In Stage 1, there was no significant between-group difference in CAPS-5 Total Severity scores between treatment groups [F(3, 73) = 1.85, p = .15]. All four treatment groups, including placebo, achieved significant within-subject reductions in total CAPS-5 Total Severity scores from Stage 1 baseline (visit 0) to end of treatment (visit 5). Specifically, participants who received placebo in Stage 1 reported a mean reduction of 13.1 points (SD = 12.10, p < .001, d = -1.30), participants who received High THC reported a mean reduction of 15.2 points (SD = 11.3, p < .0001, d = -1.99), High CBD participants reported a mean reduction of 8.4 points (SD = 10.09, p < .05, d = -.79), and THC+CBD participants reported a mean reduction of 8.5 points (SD = 9.88, p < .05, d = -.83).

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 4. Mean (SD)/Median (IQR) and analysis of change in CAPS-5 total severity scores by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t004
Secondary efficacy outcomes
The results of the study’s secondary efficacy outcomes are summarized in Table 5.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Table 5. Mean (SD)/Median (IQR) and analysis of group change in PCL-5, IDAS social anxiety, IDAS general depression, IPF, and ISI by treatment & stage.
https://doi.org/10.1371/journal.pone.0246990.t005
Self-reported PTSD symptoms, PCL-5.
In Stage 1, there was no significant difference in PCL-5 change scores between treatment groups from baseline to end of Stage 1.
In Stage 2, mean change in PCL-5 scores significantly differed by treatment condition [F(2, 63) = 4.06, p = .02]. Specifically, there was a significant difference between High CBD and THC+CBD in PCL-5 change scores, with participants who received THC+CBD reporting greater reductions in PTSD symptoms on the PCL-5 (Δ = -16.4, SD = 16.0, p < .001, d = -1.43) compared to participants who received High CBD (Δ = -9.1, SD = 11.0, p = .02, d = -.67).
IDAS general depression & social anxiety subscales.
In Stage 1, there were no significant differences between treatment conditions in either change in IDAS General Depression or IDAS Social Anxiety scores.
In Stage 2, treatment groups significantly differed in IDAS Social Anxiety mean change scores [F(2, 63) = -3.08, p = .05] and IDAS General Depression mean change scores [F(2, 63) = 3.76, p = .03]. Specifically, participants in the THC+CBD condition in Stage 2 reported significant pre-post reductions in IDAS Social Anxiety scores (Δ = – 2.8, SD = 3.90, p = .04, d = -.70), and participants in the High THC (Δ = -9.0, SD = 11.1, p < .01, d = -.90) and THC+CBD treatment conditions (Δ = -13.4, SD = 10.0, p < .0001, d = -1.68) reported significant reductions in IDAS General Depression scores in Stage 2.
ISI insomnia.
In Stage 1, there was no significant difference between treatment conditions in mean change in total insomnia symptoms on the ISI.
In Stage 2, there was no significant difference between treatment groups in mean change scores in total insomnia symptoms on the ISI.
Psychosocial functioning, IPF.
In Stage 1, there was no significant between-group difference in mean in overall psychosocial functioning (IPF total score).
In Stage 2, there was no significant difference between treatment conditions in IPF mean change scores.
Posthoc analysis
CAPS-5 subscale scores B, C, D, and E.
As a follow-up to the primary outcome analysis, posthoc analyses were conducted to test the effects of treatment group on change in each of the primary symptom domains of PTSD (i.e., intrusions, avoidance, negative thoughts and emotions, hyperarousal) using the CAPS-5 B, C, D, and E subscale scores. The posthoc analysis for subscale scores used the same analytic approach as the analysis for primary and secondary outcomes.
PCL-5, IDAS social anxiety, IDAS general depression, and ISI longitudinal analysis.
Mixed-models for repeated measures (MMRM) were computed to test for group differences over time for all secondary outcome assessments that were measured at more than two time points within each stage. The use of MMRM models allowed all randomized participants’ data to be analyzed within the models based on the missing-at-random assumption (MAR).
Posthoc results.
In Stage 1, there was no significant difference between groups in mean change for any of the subscale scores on the CAPS-5 [Subscale B, F(3,73) = 1.58, p = .20; Subscale C, F(3,73) = 1.06, p = .37; Subscale D, F(3,73) = 2.26, p = .09; Subscale E, F(3,73) = .84, p = .48].
In Stage 2, there was a significant difference between groups in mean change on the CAPS-5 C (avoidance) [F(2,64) = 4.95, p = .01] and CAPS-5 D (negative thoughts and emotions) [F(2,64) = 8.60, p < .001] subscales. Specifically, there was a significant difference between participants who received High CBD and THC+CBD in mean change in CAPS-5 C (avoidance) subscale scores (Δ = 1.9, 95% CI: 0.38, 3.35, d = -.97) and CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 5.2, 95% CI = 2.04, 8.26, d = -1.13), and between High THC and THC+CBD group participants in mean change in CAPS-5 D (negative thoughts and emotions) subscale scores (Δ = 4.1, 95% CI: 1.09, 7.15, d = -1.01). In Stage 2, there were no significant differences between groups in mean change on the CAPS-5 B (intrusions) [F(2,64) = 2.80, p = .07] or CAPS-5 E [F(2,64) = 1.13, p = .33] (hyperarousal) Subscales.
Results of the MMRM analyses testing group differences over time in PCL-5, IDAS Social Anxiety, IDAS General Depression, and ISI appear in Fig 3.

Download:
PPT PowerPoint slide
PNG larger image
TIFF original image
Fig 3. Post hoc Mixed Models for Repeated Measures (MMRM) testing change in PCL-5, IDAS depression, IDAS anxiety, and ISI scores over time.
https://doi.org/10.1371/journal.pone.0246990.g003
Only IDAS Social Anxiety scores in Stage 2 had a significant time x treatment effect, such that social anxiety showed a quadratic drop in Stage 2 among those in the THC+CBD conditions and those in the High THC and High CBD conditions did not show change over time. All other models failed to find a significant difference between groups over time.
Discussion
The present study served as the first randomized placebo-controlled trial of smoked cannabis for symptoms of PTSD in US military veterans. Study-related AEs were generally mild to moderate, and did not significantly differ by treatment condition. The study failed, however, to find a significant effect of treatment condition on the primary efficacy outcome, change in total PTSD severity on the CAPS-5 from baseline to end of Stage 1. All treatment groups (placebo, High CBD, High THC, THC+CBD) achieved statistically significant reductions in PTSD severity on the CAPS-5 in Stage 1, with effect sizes for change in mean PTSD severity ranging between d = .83 (High CBD) and d = 1.34 (High THC). These effect sizes are much larger than effect sizes reported for symptom change in other psychopharmacology trials for PTSD. For example, a 2018 meta-analysis reported standardized mean differences between .33 and .97 across PTSD pharmacology trials. The average length of trials reported in the 2018 meta-analysis lasted approximately ten weeks, whereas the current trial’s primary endpoint was evaluated after only three weeks of treatment.
The study’s failure to detect a significant difference between groups in Stage 1 could perhaps be explained by several confounding factors. First, the study sample included participants with a history of cannabis use. The recruitment of active cannabis users might have increased the potential for biased responding. Given the topical nature of the current trial and its relevance for public policy on medical cannabis, participants might have been biased to report positive effects regardless of condition. Despite many participants already having experience with the drug, nearly half of those receiving placebo believed that they received active cannabis. Prior expectations about cannabis’ effects might explain why even those in the placebo condition reported larger than average reductions in PTSD symptoms after only 3 weeks of treatment.
Second, many participants reported significant cannabis withdrawal symptoms at the time of randomization and early in Stage 1. Nearly half of the study sample (43%, n = 34) were positive for THC at study screening and 23% (n = 18) remained positive for THC at Stage 1 baseline, which would suggest chronicity of previous use or continued cannabis use during the two week washout from screening to baseline (lack of compliance with two week abstinence inclusion criteria). Total cannabis withdrawal symptoms averaged in the moderate range for all treatment groups at the start of Stage 1 (despite two weeks of abstinence prior to randomization), then generally reduced to the mild to moderate range by the end of treatment in Stage 1. Participants who received High THC in Stage 1 reported a significant increase in withdrawal following one week of cessation from Stage 1 treatment, which averaged in the moderate range following cessation. While groups did not differ in cannabis withdrawal ratings, the presence of withdrawal and trends in change could confound (or help explain) interpretation of results. We cannot rule out that cessation of cannabis use (in the placebo condition) or reversal of withdrawal (in the THC and THC+CBD conditions) might have partially been responsible for significant within-subjects change. Moreover, participants randomly assigned to receive High THC in Stage 1 had CUDIT total scores (indicating cannabis use disorder risk) nearly two times greater than participants who were assigned to other active treatment conditions. This is a major confound and limitation of the current study.
Third, total exposure to smoked cannabis was lower than anticipated and might not equate to a full therapeutic dose. In Stage 1, cannabis use in grams ranged from 8.2g (THC+CBD) to 14.6g (high THC) over three weeks (.39g/day to .69g/day on average), despite all participants having access to up to 37.8g over the three week period (1.8g/day). Average cannabis quantity that most cannabis users consume is difficult to estimate from epidemiological studies due to differences in cannabis potency and route of administration. However, large scale studies of medicinal cannabis users treating chronic pain and anxiety report average daily use in ranges closer to 1-3g/day [44,45]. Likewise, two smaller studies that assessed military veterans who use medical cannabis to self-treat PTSD reported median daily use of .85g to 1.14g/day [46] and average use of 3.8g/day [33]. We suspect that participants might have used less cannabis in the current study because of differences between the cannabis available for research trials and the quality of cannabis sold commercially. Several participants spontaneously reported to study staff that the smoke from the cannabis that was provided was “harsher” than they were used to. This difference might also be attributable to the two-week cessation periods mitigating tolerance.
Finally, the present study did not include a placebo arm in Stage 2, which limited the analyses that could be employed in order to take advantage of the crossover design. This significantly limited power to find significant differences across groups. The unexpectedly large response to placebo (d = 1.30), coupled with the small sample size per condition, meant that the current study was underpowered to detect significant differentiation from placebo. If the very large placebo response observed in this study remains consistent in future studies, a trial that tests only one preparation of cannabis (e.g., only high THC cannabis) against placebo would still need a total sample size of nearly one thousand participants (n = 479 per group) to achieve a statistically significant result. However, including a placebo run-in stage to identify and exclude placebo responders in any future trials could substantially reduce this total sample size [47].
Despite these limitations, there were several notable findings. While the study’s primary outcome assessment failed to find differentiation from placebo in PTSD symptom change on the CAPS-5, all participants showed a positive response to treatment in a very brief time period. In addition, side effects were general mild and transient. One of the largest concerns from providers regarding self-treatment of PTSD with cannabis is that it may exacerbate PTSD symptoms. While the current study’s treatment duration was too brief to identify long-term risk, two-tailed significance tests did not show evidence of symptom exacerbation in any condition. These data provide preliminary evidence of safety of short-term ad libitum cannabis use in this population.
While Stage 2 results should be interpreted with caution given the possible carry-over effects and unbalanced randomization across active dose groups, the study did identify some statistically significant differentiation between groups when particiants were re-randomized to only the three active conditions. Given that Stage 2 THC+CBD participants consisted of only those who received active treatment in Stage 1, this cohort might be providing a window into the effects of slightly longer active cannabis treatment. The positive response to treatment evidenced by participants receiving THC+CBD in Stage 2 suggest that it is possible that a longer active treatment period might be necessary to achieve treatment gains that could outperform placebo. It is equally plausible, however, that greater reductions in CAPS-5 severity scores in Stage 2 among THC and THC+CBD treatment groups were due to attenuation of cannabis withdrawal symptoms, as all treatment groups experienced a 2-week cessation period between Stage 1 and Stage 2. This effect would be consistent with prior literature suggesting that symptoms of cannabis use disorder (CUD) can interfere in successful recovery from PTSD [48].
The current study is unique, in that it trialed whole plant cannabis preparations, rather than single molecule extracts or synthetic pharmaceutical cannabinoids. In addition to reporting change in structured assessments of symptoms, the study results provide critical information about participant preference for cannabinoid preparations when exposed to different whole plant THC and CBD ratios. Consistent with previous work [46], participants in the current study reported a general preference for cannabis types that included significant quantities of THC. This effect might be explained by a signal of efficacy, or simply due to the intoxicating and reinforcing effects of high THC cannabis. Nevertheless, the demand for testing cannabis as a therapeutic for PTSD has largely been driven by military veteran advocacy groups, and yet development of cannabinoid therapeutics has not focused on trialing cannabis preparations that military veterans currently use to self-treat their symptoms. Input from stakeholders on which cannabinoids to trial as cannabis-based medicine for military veteran-specific conditions is certainly justified.
Results from the current trial provide invaluable information for future cannabinoid trials for PTSD. While the null findings raise questions about the utility of continuing to trial whole plant cannabis for the treatment of PTSD, the study found that whole plant, smoked cannabis was generally well tolerated and did not lead to deleterious effects in most participants after 3 weeks of ad libitum use. These safety results are consistent with recent real-world evidence studies of whole plant cannabis for PTSD [17,18]. However, those studies both found evidence of potential efficacy as well. Given that many veterans with PTSD are already using cannabis to self-treat their symptoms, identifying which preparations and with which method of administration are most beneficial and/or are least harmful is critical. These future studies will need to take active steps to ensure appropriate blinding despite the intoxicating nature of the drug. While the CBD and placebo conditions were appropriately blinded in the current study, participants and assessors could accurately guess condition when participants received THC-based treatments. Enrolling novice or naïve users, or limiting total THC content to sub-intoxicating doses could improve these blinding issues. Conversely, if higher THC doses are indeed therapeutic, other designs that are less reliant on active blinding, such as randomized withdrawal, might be warranted. Researchers should also consider including objective surrogate endpoint assessments (e.g., physiology, biological specimens, neuroimaging) as secondary indicators of treatment response. As none of these have been validated for a PTSD population as surrogate endpoints, the present study and future studies must utilize the “gold-standard” semi-structured clinical interview for PTSD severity, which is the CAPS-5.
Future studies would also likely benefit from a longer treatment period similar to more traditional medication trials (e.g., 12-weeks), and if including a placebo comparator should plan for a potentially larger than normal placebo response. To mitigate placebo, researchers might consider: 1) including a placebo run-in period prior to randomization to attempt to identify placebo responders, 2) powering the trial for a potentially larger than typical placebo effect, and/or 3) excluding participants with strong apriori beliefs about cannabis’ therapeutic effects [e.g., prescreening with expectancy measures, such as Devilly & Borkovec’s Credibility/Expectancy Questionnaire (CEQ)]. For generalizability to female veterans and civilians with PTSD, these studies should also attempt to recruit a greater number of female participants, as the current study’s sample was overwhelmingly male. Finally, future studies would also greatly benefit from access to high quality flower cannabis. The flower preparations provided through the NIDA drug supply program include all parts of the plant (instead of just buds) and are only available in specific cannabinoid ratios. Studies that test high quality buds in various phenotypes with variable potencies of cannabinoids and terpene ratios would more closely mirror what is available within state-sponsored medical cannabis programs.
Conclusions
The present study failed to find a significant group difference between smoked cannabis preparations containing High CBD, High THC, and THC+CBD against placebo in regards to their impact on PTSD symptoms. All treatment groups, including placebo, showed good tolerability and significant improvements in PTSD symptoms during three weeks of treatment. The failure to differentiate treatment groups from placebo is likely attributable to the higher than average treatment response in the placebo condition and to the shorter than average duration of treatment. Higher powered studies that attempt to mitigate the effect of pronounced placebo appear warranted.
Supporting information
S1 Checklist –
Showing 1/15: pone.0246990.s001.DS_Storesorry, we can’t preview this file1 / 15figshare
S1 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s001
(DS_STORE)
S2 Checklist.
https://doi.org/10.1371/journal.pone.0246990.s002
(DOC)
S1 File.
https://doi.org/10.1371/journal.pone.0246990.s003
(CSV)
S2 File.
https://doi.org/10.1371/journal.pone.0246990.s004
(TXT)
S3 File.
https://doi.org/10.1371/journal.pone.0246990.s005
(TXT)
S4 File.
https://doi.org/10.1371/journal.pone.0246990.s006
(TXT)
S5 File.
https://doi.org/10.1371/journal.pone.0246990.s007
(TXT)
S6 File.
https://doi.org/10.1371/journal.pone.0246990.s008
(TXT)
S7 File.
https://doi.org/10.1371/journal.pone.0246990.s009
(TXT)
S8 File.
https://doi.org/10.1371/journal.pone.0246990.s010
(TXT)
S9 File.
https://doi.org/10.1371/journal.pone.0246990.s011
(TXT)
S10 File.
https://doi.org/10.1371/journal.pone.0246990.s012
(TXT)
S11 File.
https://doi.org/10.1371/journal.pone.0246990.s013
(TXT)
S12 File.
https://doi.org/10.1371/journal.pone.0246990.s014
(XLSX)
S13 File.
https://doi.org/10.1371/journal.pone.0246990.s015
(PDF)
Acknowledgments
We thank Scott Hamilton and Ilsa Jerome for their assistance in quality checking all of the study data.
References
1. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder. Curr Opin Psychiatry. 2015;28: 307–311. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
2. Gradus JL. Epidemiology of PTSD. In: National Center for PTSD. 2014.
3. Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28: 307–11. pmid:26001922
View Article
PubMed/NCBI
Google Scholar
4. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62: 617. pmid:15939839
View Article
PubMed/NCBI
Google Scholar
5. Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry. 1992;149: 671–675. pmid:1575259
View Article
PubMed/NCBI
Google Scholar
6. Perkonigg A, Kessler RC, Storz S, Wittchen H-U. Traumatic events and post-traumatic stress disorder in the community: prevalence,risk factors and comorbidity. Acta Psychiatr Scand. 2000;101: 46–59. pmid:10674950
View Article
PubMed/NCBI
Google Scholar
7. Breslau N. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J Clin Psychiatry. 2001;62 Suppl 17: 16–22. Available: http://www.ncbi.nlm.nih.gov/pubmed/11495091.
View Article
Google Scholar
8. Frayne SM, Seaver MR, Loveland S, Christiansen CL, Spiro III A, Parker VA, et al. Burden of Medical Illness in Women With Depression and Posttraumatic Stress Disorder. Arch Intern Med. 2004;164: 1306. pmid:15226164
View Article
PubMed/NCBI
Google Scholar
9. Merz J, Schwarzer G, Gerger H. Comparative Efficacy and Acceptability of Pharmacological, Psychotherapeutic, and Combination Treatments in Adults With Posttraumatic Stress Disorder. JAMA Psychiatry. 2019;76: 904. pmid:31188399
View Article
PubMed/NCBI
Google Scholar
10. Forman-Hoffman V, Middleton JC, Feltner C, Gaynes BN, Weber RP, Bann C, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update. 2018 [cited 11 Oct 2019]. Available: https://www.ncbi.nlm.nih.gov/books/NBK525132/.
View Article
Google Scholar
11. Management of Post-Traumatic Stress Working Group. VA/DOD clinical practice guideline for management of post-traumatic stress. 2017.
12. Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67: 194–200. Available: http://www.ncbi.nlm.nih.gov/pubmed/10224729. pmid:10224729
View Article
PubMed/NCBI
Google Scholar
13. Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. J Consult Clin Psychol. 1992;60: 748–56. Available: http://www.ncbi.nlm.nih.gov/pubmed/1401390. pmid:1401390
View Article
PubMed/NCBI
Google Scholar
14. Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153: 369–375. pmid:8610824
View Article
PubMed/NCBI
Google Scholar
15. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136: 162–165. pmid:24412475
View Article
PubMed/NCBI
Google Scholar
16. Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychol Addict Behav. 2011;25: 485–491. pmid:21261407
View Article
PubMed/NCBI
Google Scholar
17. LaFrance EM, Glodosky NC, Bonn-Miller M, Cuttler C. Short and Long-Term Effects of Cannabis on Symptoms of Post-Traumatic Stress Disorder. J Affect Disord. 2020;274: 298–304. pmid:32469819
View Article
PubMed/NCBI
Google Scholar
18. Bonn-Miller MO, Brunstetter M, Simonian A, Loflin MJ, Vandrey R, Babson KA, et al. The Long-Term, Prospective, Therapeutic Impact of Cannabis on Post-traumatic Stress Disorder. Cannabis Cannabinoid Res. 2020.
View Article
Google Scholar
19. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14. pmid:28813324
View Article
PubMed/NCBI
Google Scholar
20. Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. 2010;207: 105–111. pmid:19800921
View Article
PubMed/NCBI
Google Scholar
21. Gomes F V, Reis DG, Alves FH, Corrêa FM, Guimarães FS, Resstel LB. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT 1A receptors. J Psychopharmacol. 2012;26: 104–113. pmid:21148020
View Article
PubMed/NCBI
Google Scholar
22. Stern CAJ, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015;25: 958–965. pmid:25799920
View Article
PubMed/NCBI
Google Scholar
23. Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). 2013;226: 781–792. pmid:23307069
View Article
PubMed/NCBI
Google Scholar
24. Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, et al. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64: 396–402. pmid:22796109
View Article
PubMed/NCBI
Google Scholar
25. Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36: 23–31. pmid:23260015
View Article
PubMed/NCBI
Google Scholar
26. Yehuda R, LeDoux J. Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron. 2007;56: 19–32. pmid:17920012
View Article
PubMed/NCBI
Google Scholar
27. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15: 84–88. pmid:19228182
View Article
PubMed/NCBI
Google Scholar
28. Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51: 585–588. pmid:25467221
View Article
PubMed/NCBI
Google Scholar
29. Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, Open-Label, Pilot Study of Add-On Oral Δ9-Tetrahydrocannabinol in Chronic Post-Traumatic Stress Disorder. Clin Drug Investig. 2014;34: 587–591. pmid:24935052
View Article
PubMed/NCBI
Google Scholar
30. Loflin M, Babson K, Sottile J, Norman S, Gruber S, Bonn-Miller M. A Cross-Sectional Examination of Choice and Behavior of Veterans with Access to Free Medicinal Cannabis. Am J Drug Alcohol Abuse. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
31. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. European Journal of Internal Medicine. Elsevier B.V.; 2018. pp. 12–19. pmid:29307505
View Article
PubMed/NCBI
Google Scholar
32. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
33. Loflin M, Earleywine M, Bonn-Miller M. Medicinal versus recreational cannabis use: Patterns of cannabis use, alcohol use, and cued-arousal among veterans who screen positive for PTSD. Addict Behav. 2017;68. pmid:28088054
View Article
PubMed/NCBI
Google Scholar
34. First MB, Williams JBW, Karg RSK, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA Am Psychiatr Assoc. 2015. pmid:32821383
View Article
PubMed/NCBI
Google Scholar
35. Posner K, Brent D, Lucas C, Gould M, Stanley B., Brown G., et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008.
36. Shahid A, Wilkinson K, Marcu S, Shapiro CM. STOP-Bang Questionnaire. STOP, THAT and One Hundred Other Sleep Scales. Springer New York; 2011. pp. 371–383. https://doi.org/10.1007/978-1-4419-9893-4_92
37. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110: 137–143. pmid:20347232
View Article
PubMed/NCBI
Google Scholar
38. Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). [Assessment]. 2013.
View Article
Google Scholar
39. Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30: 383–395. pmid:28493729
View Article
PubMed/NCBI
Google Scholar
40. Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, et al. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (PCL-5) in veterans. Psychol Assess. 2016;28: 1379–1391. pmid:26653052
View Article
PubMed/NCBI
Google Scholar
41. Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 2007;19: 253–268. pmid:17845118
View Article
PubMed/NCBI
Google Scholar
42. Bovin MJ, Black SK, Rodriguez P, Lunney CA, Kleiman SE, Weathers FW, et al. Development and validation of a measure of PTSD-related psychosocial functional impairment: The Inventory of Psychosocial Functioning. Psychol Serv. 2018;15: 216–229. pmid:29723024
View Article
PubMed/NCBI
Google Scholar
43. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34: 601–8. pmid:21532953
View Article
PubMed/NCBI
Google Scholar
44. Turna J, Simpson W, Patterson B, Lucas P, Van Ameringen M. Cannabis use behaviors and prevalence of anxiety and depressive symptoms in a cohort of Canadian medicinal cannabis users. J Psychiatr Res. 2019;111: 134–139. pmid:30738930
View Article
PubMed/NCBI
Google Scholar
45. Ware MA, Wang T, Shapiro S, Collet JP. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain. 2015;16: 1233–1242. pmid:26385201
View Article
PubMed/NCBI
Google Scholar
46. Loflin MJE, Babson K, Sottile J, Norman SB, Gruber S, Bonn-Miller MO. A cross-sectional examination of choice and behavior of veterans with access to free medicinal cannabis. Am J Drug Alcohol Abuse. 2019; 1–8. pmid:31135227
View Article
PubMed/NCBI
Google Scholar
47. Lee S, Walker JR, Jakul L, Sexton K. Does elimination of placebo responders in a placebo run-in increase the treatment effect in randomized clinical trials? A meta-analytic evaluation. Depress Anxiety. 2004;19: 10–19. pmid:14978780
View Article
PubMed/NCBI
Google Scholar
48. Bonn-Miller MO, Boden MT, Vujanovic A a., Drescher KD. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychol Trauma Theory, Res Pract Policy. 2011;5: 193–200.
View Article
Google Scholar
Curated by Thc 420 Hemp
source https://weedpress.wordpress.com/2021/03/21/the-short-term-impact-of-3-smoked-cannabis-preparations-versus-placebo-on-ptsd-symptoms-a-randomized-cross-over-clinical-trial/
1 note
·
View note