#clinical guidelines
Explore tagged Tumblr posts
Text
youtube
#Renal cancer#diagnosis#treatment protocols#quality control#Chinese healthcare#oncology#nephrectomy#targeted therapy#immunotherapy#clinical guidelines#medical standards#multidisciplinary team#surgical oncology#radiotherapy#patient outcomes#standardized care#kidney cancer#medical guidelines#patient-centered care#rehabilitation.#Youtube
0 notes
Text
Antibiotic resistance and the One Health strategy: a review with scientific evidences
Background Antibiotic resistance is one of the most serious global threats to public health. Antibiotics, which are essential for the treatment of bacterial infections, have become progressively less effective due to the spread of resistant bacteria. This phenomenon not only compromises the ability to treat common infections, but also to perform surgical procedures and cancer treatments that…
#antibiotic resistance#antibiotics abuse#bacterial infection#bacterial strains#clinical guidelines#environmental pollution#global policies#nosocomial infection#One Health strategy#public health#sanitary surveillance
0 notes
Text
Achieving Excellence: Implementing Healthcare Standards in Practice

In the realm of healthcare, adherence to healthcare standards is paramount for ensuring quality care delivery and patient safety. From patient safety protocols to industry best practices, healthcare organizations strive to implement and maintain rigorous standards that uphold the highest levels of excellence in patient care.
Healthcare standards encompass a wide array of guidelines, protocols, and best practices aimed at standardizing processes, procedures, and protocols across healthcare settings. These standards serve as benchmarks for quality assurance and regulatory compliance, guiding healthcare professionals in delivering safe, effective, and consistent care to patients.
One of the cornerstones of healthcare standards is the implementation of patient safety protocols. These protocols are designed to minimize the risk of adverse events, medical errors, and preventable harm to patients. By adhering to established safety protocols, healthcare providers can create a culture of safety, mitigate risks, and enhance patient outcomes.
Furthermore, industry best practices play a crucial role in shaping healthcare standards and driving continuous improvement in patient care. These practices represent the collective wisdom and experience of healthcare professionals and organizations, offering proven strategies and methodologies for delivering high-quality, patient-centered care.
Standardization initiatives also play a significant role in advancing healthcare standards. These initiatives aim to harmonize processes, terminology, and technologies across healthcare systems, promoting interoperability, efficiency, and consistency in care delivery. By embracing standardization efforts, healthcare organizations can streamline operations, reduce errors, and improve care coordination.

Clinical guidelines are another essential component of healthcare standards, providing evidence-based recommendations for diagnosis, treatment, and management of various medical conditions. These guidelines serve as valuable tools for healthcare professionals, offering authoritative guidance on best practices and protocols for delivering optimal patient care.
Moreover, continuous quality improvement is integral to maintaining and enhancing healthcare standards over time. By systematically monitoring performance, identifying areas for improvement, and implementing evidence-based interventions, healthcare organizations can drive ongoing improvements in care quality, patient outcomes, and organizational efficiency.
In conclusion, achieving excellence in healthcare requires a steadfast commitment to implementing and upholding healthcare standards in practice. From patient safety protocols and industry best practices to standardization initiatives, clinical guidelines, and continuous quality improvement, these standards serve as guiding principles for delivering safe, effective, and patient-centered care. By embracing and adhering to these standards, healthcare organizations can foster a culture of excellence, promote patient safety, and drive continuous improvement in the delivery of healthcare services.
#Patient Safety Protocols#Industry Best Practices#Standardization Initiatives#Clinical Guidelines#Continuous Quality Improvement
0 notes
Text
Breaking Down the Complexities of Healthcare Standards

In the ever-evolving landscape of healthcare, adherence to Healthcare Standards is paramount to ensuring quality care, patient safety, and regulatory compliance. Let's delve into the key components of these standards and their significance in healthcare delivery:
Regulatory Compliance:
Healthcare providers must adhere to a myriad of regulations and guidelines set forth by regulatory bodies such as the FDA, CDC, and CMS. Regulatory compliance encompasses adherence to laws, rules, and standards governing healthcare practices, facilities, and services. Failure to comply with these regulations can result in penalties, fines, and reputational damage.
Clinical Guidelines:
Clinical guidelines are evidence-based recommendations developed to assist healthcare professionals in making informed decisions about patient care. These guidelines are derived from rigorous research and expert consensus and serve as a framework for standardizing clinical practice. By following clinical guidelines, healthcare providers can optimize patient outcomes, reduce variations in care, and improve quality and safety.
Quality Assurance:
Quality assurance in healthcare focuses on ensuring that processes and services meet predetermined standards of quality. It involves systematic monitoring, evaluation, and improvement of healthcare delivery to enhance patient outcomes and satisfaction. Quality assurance initiatives aim to identify areas for improvement, implement corrective actions, and measure the effectiveness of interventions.
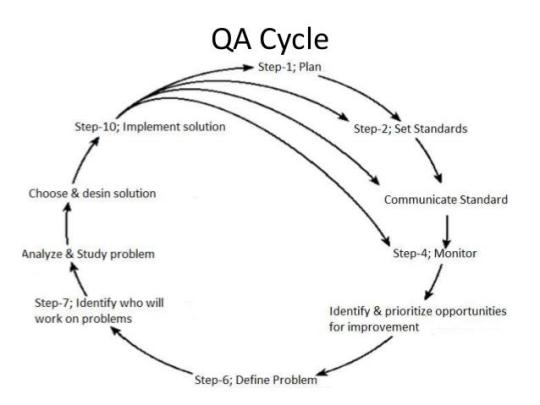
Patient Safety:
Patient safety is a fundamental aspect of healthcare standards, encompassing practices and protocols aimed at preventing harm to patients. Patient safety initiatives focus on reducing medical errors, preventing adverse events, and promoting a culture of safety within healthcare organizations. Strategies such as medication reconciliation, hand hygiene protocols, and error reporting systems contribute to enhancing patient safety.
Performance Metrics:
Performance metrics are quantitative measures used to assess the effectiveness, efficiency, and quality of healthcare services. These metrics provide insights into various aspects of healthcare delivery, including patient outcomes, resource utilization, and operational efficiency. By tracking performance metrics such as readmission rates, length of stay, and patient satisfaction scores, healthcare organizations can identify areas for improvement and drive continuous quality improvement.
In conclusion, Healthcare Standards encompass a broad spectrum of regulations, guidelines, and practices aimed at ensuring quality, safety, and compliance in healthcare delivery. By prioritizing regulatory compliance, adhering to evidence-based clinical guidelines, implementing robust quality assurance processes, promoting patient safety, and measuring performance through performance metrics, healthcare organizations can achieve excellence in patient care and service delivery.
0 notes
Text
Desperately trying to be hysterical all night again in advance of my doctor's appt in the morning because honestly I'm barely holding onto sanity as it is lmao
The amount of pre-appt research I do never stops turning up horrifying new pieces of information like this one:

Like my guy I can barely manage to stomach the ½ teaspoon of electrolytes I put in my water every day and you want me to eat SIX GODDAMN TIMES THAT MUCH??????
I would rather fling myself into a dying star I'm so fucking serious
#i have been frantically tracking my BP over the past few days and it goes tachcardic around 5min into standing up every time#that's not even include the at-risk measurements taken before that threshhold that aren't good they just aren't TACHYCARDIC#and then on top of it I'm basically just yo-yoing back and forth between full blown hypotension and tachycardia with rare moments of#quote unquote normal BP here and there#homestly it explains why i always shitty like who wouldn't#anyway I've got a 12 item list for my new pcp in the morning and I'm honestly fucking terrified because I don't know how I'll cope if they#blow me off yet again after everything I've done to protect myself#i literally can't keep living like this there's a really good chance i just throw myself off a bridge to be done with it and I'd rather not#anyway i think i've made a really good case with clinical treatment guidelines for 3-5 major medical interventions#and I'm so fucking desperate to get at least those covered#i need a new tilt table test i need rx fludrocortisone and IV saline/nutrition or prescription electrolytes and multi-vitamin#i need compression garment scripts and i need long-term PT and if I'm very lucky I will also get to need assessment of my stenosis/csf#i don't dare hope for a disability referral
15 notes
·
View notes
Text
Unga bunga saw a new condition this morning and I'm giddy to flesh out my knowledge w medical journals for fun heehee
#Creepy chatter#Hemosiderosis :3#Technically it's idiopathic pulmonary hemosiderosis but I'm starting base level and then going into spec manifestations#Still riding the high of my one and only ever case of diabetic myonecrosis#So unusual that there wasn't anything really in place to capture it#I BREAK the International Classification of Diseases 10th clinical modification#I FIND the myonecrosis I SLAP the guidelines around bc they didn't consider diabetic manifestations#The cool thing was the patient was THRIVING! :D what a kick ass doctor to whip that shit into nearly sub-clinical levels#Medical cw
3 notes
·
View notes
Text
elon musk did a nazi salute twice at the inauguration, and republicans are defending him.
trump revoked executive order 11246, which prohibited discrimination.
trump put all dei employees on leave to be fired.
trump blamed the dc plane crash on dei.
trump banned all lgbtq+ flags from being hung in government buildings.
trump ordered the pentagon to cancel celebration of mlk jr. day, black history month, women's history month, holocaust remembrance day, asian american pacific islander heritage month, lgbtq+ pride month, juneteenth, women's equality day, national hispanic heritage month, national disability employment awarenessmonth, and national american indian heritage month.
trump proposed removing all palestinians from gaza, turning the area into a vacation resort called “riviera of the middle east”.
trump posted an ai generated video showing what he hopes to turn palestine into, with a large golden statue of himself in the middle of it.
trump rolled back biden’s executive order to lower prescription drug costs for people using medicare and medicaid.
trump rescinded the $35 cap on insulin, and prices are expected to rise to $1500 a month.
trump ordered the national institutes of health to cancel their review panels on cancer research.
trump ended the guidelines to prevent ai misuse. the guidelines prevent many things, but notably it prevents production of ai child pornography.
when sean hannity asked trump about the economy, he said “i don’t care”, after campaigning with the economy as his main talking point.
trump has withdrawn the us from the world health organization.
trump is ordering health agencies to stop reporting on bird flu and halt publications of scientific reports.
trump’s epa is reversing the ban on asbestos, which causes deadly and rapidly-spreading cancer if exposed.
trump has pardoned over 1500 people who stormed the capitol on january 6th.
trump changed denali back to mount mckinley.
trump signed an executive order to rename the gulf of mexico to gulf of america.
trump shut down cbp one, an app which granted legal entry to 1 million+ immigrants.
trump has discussed introducing a “gold card”, which would allow the wealthiest people to buy us citizenship for $5 million usd.
trump is allowing ice raids at churches and elementary schools.
trump announced plans to declare a national emergency at the us-mexico border.
trump signed an executive order to expand the use of the death penalty.
trump disbanded the school safety board that works to prevent school shootings. it was comprised of survivors, educators, and gun violence prevention advocates and formed after the school shooting in parkland.
trump has threatened to invade panama to claim the panama canal.
trump withdrew from the paris climate act.
trump revoked all protections for transgender troops in the us military.
trump rescinded executive orders made by biden that benefited and protected women, lgbtq+ people, black americans, hispanic americans, asian americans, native hawaiians, and pacific islanders.
trump bombed iran 3 times without congressional approval.
trump is attempting to make it legal to refuse to hire or fire pregnant women.
trump pardoned 23 individuals convicted under the freedom of access to clinic entrances (FACE) act for their anti-abortion activism, including oftentimes violent protests at abortion clinics.
trump signed an executive order allowing deportation of foreign students who they believe express support for hamas or hezbollah.
trump announced that the us government will from here on out only recognize male and female as sexes. intersex is not legally recognized anymore.
trump is shutting down the lgbtq+ youth suicide hotline in july.
trump has told all schools and universities that they have two weeks to end all diversity initiatives, or he will cut federal funding. (as of feb 19, 2025)
trump told harvard to stop accepting immigrant students.
trump fired the staff of the federal aviation association after a deadly plane crash in dc.
trump has fired the heads of the tsa and coast guard, and gutted a key aviation safety advisory committee.
trump denied disaster relief funding for north carolina after tropical storm helene.
in georgia, a black woman named adriana smith is being kept alive on ventilators because she was 9 weeks pregnant when she died. she is legally brain dead. this was an exact plot in multiple episodes of the handmaid’s tale.
the state of louisiana just rolled back desegregation laws because of a petition from the department of justice.
the trump administration removed the federal government’s memorial to victims of gun violence. they took down 120 portraits of dead americans, including police officers and children.
the supreme court weakened the clean water act's limitations on raw sewage discharge into our water in a 5-4 ruling.
the official white house twitter account posted an “illegal alien deportation” asmr video where they did closeups of chains and the sound of ankle chains hitting the metal stairs of the airplanes deportees were being loaded onto.
on truth social, trump posted, “LONG LIVE THE KING!”.
at CPAC, a republican group called the “third term project” held a rally to support changing the constitution so trump can run for a third term. on their posters, they’re photoshopping his face onto julius caesar’s, seemingly forgetting what happened to julius caesar.
the trump administration paused health communications to prevent the fda from announcing food recalls.
the trump administration will not renew biden-era grants worth $1 billion that were aimed at boosting mental health services in schools.
the u.s. has surpassed 1,000 measles cases for the first time in five years, with 96% involving unvaccinated individuals or those with unknown vaccination status. rfk jr. has repeatedly claimed that measles can be treated with vitamin a.
republicans on tiktok are recreating elon’s salute to prove that it “wasn’t a nazi salute”, and they’re either doing it completely wrong because they know if they replicate it then it will actually be a salute, or they’re doing the proper salute and posting it online.
google and apple maps now display the gulf of mexico as “gulf of america”.
rfk jr. wants to ban SSRIs and put everyone on them into labor camps.
multiple state legislators are drafting bills to allow the punishment for abortion to be the death penalty.
andy ogles drafted a constitutional amendment to allow trump to be president for a third term.
the us senate confirmed russell vought, one of the main authors of project 2025, will lead the white house budget office.
nancy mace repeatedly used the t-slur during a congressional meeting, three times were out of spite.
andy biggs introduced a bill to abolish osha and completely eliminate federal workplace safety protections.
georgia republican congressman mike collins called for the deportation of new jersey born mariann budde, the bishop who urged trump to “have mercy” on the lgbtq+ community and immigrants during a service at the national cathedral.
florida republican anna paulina luna has introduced a bill to add trump to mount rushmore.
new york republican claudia tenney introduced a bill to make trump’s birthday a federal holiday.
west virginia republican delegate lisa white has introduced house bill 2712, which would remove rape and incest as exceptions for abortion, even for minors. you can call her at (304) 340- 3274 or email her at [email protected] and let her know your opinion on that.
there is a bill named the SAVE act which would require americans to provide their birth certificate, passport, or other citizenship documents every time they vote, and would require the last name on their driver’s license to match that of their birth certificate. this would prevent married women who have changed their last name from voting.
the u.s. government is considering suspending habeas corpus, which protects people from unlawful detention and ensures you receive due process.
bill h.r.1161, which is available publicly on congress.gov, would authorize trump to enter into negotiations to acquire greenland and to rename it to "red, white, and blueland".
six states (arizona, idaho, iowa, kansas, mississippi, and north dakota) are planning on challenging obergefell v. hodges, which would end same-sex marriage nationwide. about a dozen more states have representatives are also considering filing similar resolutions.
a bill to ban the mRNA vaccine has passed out of the house committee.
amazon revoked protections for lgbtq+ and black employees.
the cdc has removed their hiv prevention page.
the united states state department has officially changed its “travelers with special conditions” page which previously said “lgbtqi+ travelers” to “lgb travelers”, completely getting rid of the tqi+.
every single republican told us we were overreacting. trump swore he had nothing to do with project 2025 yet continues implementing details outlined in it. not a single person has the right to tell us we’re being dramatic anymore.
hope “cheaper eggs and gas” was worth it.
EDIT: i removed the “trump refused to swear on the bible” point because it was being taken as me being an offended christian. i’m not christian, im agnostic. the reason i included it in the first place is because he’s the first president in history to ever refuse to swear on ANYTHING. meanwhile his “conservative christian” followers had no issue with this, and decided to continue to scramble for excuses instead of admitting he may not be as religious as he claims he is. i figured taking that point out entirely is probably better than filling this with an explanation in the middle of the other important issues.
#*#allie talks#politics#us politics#fuck trump#trump administration#donald trump#trump#inauguration#current events#elon musk#fuck elon musk
68K notes
·
View notes
Text
Partner with Zenovel for strategic guidance in drug development and clinical operations. We bring deep expertise in managing complex drug trials and clinical studies, offering comprehensive clinical trial monitoring solutions, including highly effective risk based monitoring clinical trials. From GCP audits and QMS setup to regulatory affairs support and scientific overviews, Zenovel acts as your extended team, ensuring compliance, quality, and accelerated success for your innovative therapies.
#drug trials#clinical studies#drug development#good manufacturing practices guidelines#risk based monitoring clinical trials#clinical research center#quality assurance in clinical trials#life sciences
0 notes
Text
Important Lesson About Nursing Procedure For HESI TEST
The HESI A2 exam is a key component for admission into nursing and healthcare programs. It's designed to assess your knowledge and skills in areas such as math, reading, science, and English language usage. If you're looking to boost your HESI A2 score.
Feel free to join us at our private study group: HESI and TEAS Study Group 👈.

Here are some important objective-type questions about Anatomy & Physiology that has been encountered on the HESI test, which is often used for nursing school admissions or assessments.
What is the correct site for insulin injection? A. Deltoid muscle B. Gluteal muscle C. Abdomen D. Neck (Answer: C)
The abdomen is the preferred site for insulin injections.
Why the Abdomen?
Provides the fastest and most consistent absorption of insulin.
Easy to access and less affected by movement compared to other sites.
Allows for proper rotation of injection sites to prevent lipodystrophy (fat tissue damage).
Other Options:
Deltoid muscle (A): Used for intramuscular (IM) injections, not subcutaneous insulin.
Gluteal muscle (B): Less common for insulin due to slower absorption.
Neck (D): Not a safe or recommended site for any injections.
Other recommended subcutaneous injection sites for insulin include the thighs, upper arms, and buttocks, but the abdomen remains the best choice for quick and effective insulin absorption.
Which of the following is the best position for a patient in respiratory distress? A. Supine B. Prone C. Semi-Fowler’s D. Trendelenburg (Answer: C)
The best position for a patient in respiratory distress is C. Semi-Fowler’s.
This position involves the patient being reclined at a 30 to 45-degree angle, which helps to improve breathing by reducing the pressure on the diaphragm and allowing for better lung expansion. It is commonly used in respiratory distress, as it supports easier breathing compared to the other positions listed.
To get video lessons you can also go for YouTube videos about (HESI Test Practice Lesson 👈 and download HESI Test Prep App)
#health and wellness#healthcare#medicine#nutrition#physical health#nursing#hygiene#medical care#nursing school#nursing student#hesi study guideline#hesi test prep#hesi a2 test#hesi online classes#hesi prep#hesi practice test#nursing entrance exam#nursing home care#nursing test#nursing services#hospital#nursing homes west sussex#medical coding#surgery#clinic#health
0 notes
Text
Understanding ICH Guidelines: A Game-Changer in Global Pharma Regulations
The pharmaceutical industry operates under strict regulatory frameworks to ensure drug safety, efficacy, and quality. One of the most important global regulatory standards is the International Council for Harmonisation (ICH) Guidelines. These guidelines help unify regulatory requirements across different regions, making drug development and approval more streamlined.
What Are ICH Guidelines?
The ICH Guidelines were established to harmonize pharmaceutical regulations globally, ensuring that drugs meet consistent safety, quality, and efficacy standards. These guidelines are recognized by major regulatory agencies like:
✅ U.S. FDA (Food and Drug Administration) ✅ EMA (European Medicines Agency) ✅ PMDA (Pharmaceuticals and Medical Devices Agency - Japan)
By following ICH standards, pharmaceutical companies can avoid delays in approval and ensure compliance with multiple regulatory authorities simultaneously.
Key Areas Covered by ICH Guidelines
The ICH guidelines are categorized into four major groups:
🔹 Quality (Q-Series): Covers stability studies, pharmaceutical development, and quality risk management. 🔹 Safety (S-Series): Includes toxicology studies and non-clinical evaluations for drug safety. 🔹 Efficacy (E-Series): Focuses on clinical trial design, pharmacovigilance, and drug efficacy. 🔹 Multidisciplinary (M-Series): Includes common technical documents (CTD) and electronic submissions.
Why Are ICH Guidelines Crucial for Pharma Professionals?
✔ Regulatory Alignment: Ensures uniformity in drug approval across different regions. ✔ Time & Cost Efficiency: Reduces the need for duplicate clinical trials in different countries. ✔ Enhanced Patient Safety: Establishes strict safety protocols to minimize risks.
Deep Dive into ICH Guidelines
For a detailed exploration of ICH Guidelines and their impact on pharmaceutical research, check out this in-depth guide:
ICH Guidelines: A Comprehensive Guide to Global Pharmaceutical Harmonisation
This resource breaks down the complexities of ICH regulations and explains how they shape the future of drug development worldwide.
0 notes
Text
Texting all my nurse friends I got a B on my mental health clinical because, I've been in an inpatient facility before, and I was scared every day I'd be committed there against my will; and lord knows my parents won't understand. But I made it through!
Honorable mention to @keriweird and @weird-aunt for being the coolest RNs a person could ask for 🥹 I'm thankful to know you and thankful for your guidance.
#what ultimately dropped my grade was my tardies#I missed a total of 1.5 hours of clinical hours plus a missed assignment#so due to our school policy guidelines on attendance that is determined by the state board#I dropped 10%. but I still got an 86!!
2 notes
·
View notes
Text
4 Warning Signs of Colon Cancer You Should NEVER Ignore
#ColonCancer #CancerAwareness #HealthTips #EarlyDetection #CancerPrevention #HealthScreening #ImmunoOncology #CancerResearch #HealthyLiving #KnowTheSigns #CancerSymptoms #ProactiveHealth #ColonHealth #CancerScreening #HealthEducation #StayInformed #FarehaJamal #BioNTech #MedicalAdvice #FamilyHealth #WellnessJourney #HealthMatters #CancerTreatment #HealthyHabits #PreventiveCare
Hey there! Let’s talk about something important today—colon cancer. I know, it’s not the most glamorous topic, but it’s one of those things we really need to pay attention to, especially because it can be sneaky. Many cases of colon cancer don’t show symptoms early on, which is why knowing the warning signs is so crucial. I recently had a chat with Dr. Fareha Jamal, a Doctor of Pharmacy and…
#abdominal pain#American Cancer Society#BioNTech#blood in stool#bowel habits#cancer awareness#cancer prevention#cancer research#cancer risk factors#cancer screening#cancer screening guidelines#cancer treatment#cell binding assays#Colon Cancer#colon cancer symptoms#colorectal cancer#early detection of colon cancer#family history of cancer#Fareha Jamal#health tips#Healthy lifestyle#high-content screening#immuno-oncology#Mayo Clinic#proactive health management#unexplained weight loss#warning signs of colon cancer
0 notes
Text
5 Signs of Dehydration in Kids: What Every Parent Should Know

Children usually suffer from dehydration problems, and if not checked can soon lead to serious health problems. Kids are naturally more active and may forget to drink sufficient amounts of water, yet they need hydration for them to be healthy and in the process of development. Five Important Signs of Dehydration in Children That Parents Should Recognize:
1. Dry Skin or Mouth
Dry, flaky skin and a parched mouth are classic signs of dehydration. If your child's lips crack or their skin feels less elastic than usual, it is a sign that the body is lacking fluids to keep moist. Encourage them to sip water throughout the day and eat hydrating foods like watermelon or cucumber.
2. Fatigue and Irritability
Dehydration can greatly impact energy levels. If your child seems more tired, sluggish, or cranky than usual, it may be because of a lack of fluids. Without enough hydration, the body cannot function as it should, causing fatigue and mood swings.
3. Headaches
Headaches are a less common but important symptom of dehydration. When the body doesn't have enough water, it has decreased blood volume, thus reducing oxygen flow to the brain. If your child complains of headaches often or even during or after sports activities, make sure they get enough water.
4. Bad Breath
Dehydration causes a decrease in saliva production, which helps to clean the mouth and regulate bacteria. This can lead to an odor that is not pleasant. If your child has chronic bad breath, even with regular brushing, it may be time to increase their fluid intake.
5. Decreased Urination and Constipation
The child will rarely visit the bathroom and dark urine is an obvious sign of dehydration. For younger children, fewer wet diapers are a red flag. Dehydration also affects digestion, causing constipation in most cases. To prevent this, encourage your child to drink plenty of fluids and add fiber-rich foods to their diet.
Tips for Dehydration in Children
Provide Water Regularly: Ensure your child drinks water at all times, especially when they are physically active or the weather is hot.
Add Hydrating Foods: Fruits like oranges, melons, and strawberries have a lot of water and can fill up some of their fluid needs.
Develop a Habit: Teach the children to develop hydration habits, especially in younger kids who tend to forget drinking.
Steer Clear of High-Sugar Drinks: Hydrate with water or fresh juices instead of sodas and artificially flavored drinks.
Conclusion
Proper awareness and timely action can prevent dehydration in kids. With the knowledge of the five signs—dry skin or mouth, fatigue and irritability, headaches, bad breath, and decreased urination or constipation—you will be able to keep your child healthy and hydrated.
Hydration is very important for your child's overall well-being. Provide fluids easily, encourage frequent sips of water, and observe behavior for any sign of dehydration.
#best children clinic in ahmedabad#best pediatrician clinic in ahmedabad#children's clinic#childe specialist#best pediatrician ahmedabad#How do I know if my child is dehydrated?#How to tell if your child is dehydrated?#physical growth and development in childhood#What 5 things should you look for to identify dehydration?#What are the 4 ways to treat dehydration?#Will baby cry if dehydrated?#What are the guidelines for pediatric dehydration?
0 notes
Text
Enhancing Homeopathy Through Quality Case Reports: Insights from Dr. Saurav Arora's Webinar
Recently, Dr. Saurav Arora, an internationally acclaimed medical homeopath, conducted a webinar titled “The Art of Writing Scientific Cases in Homeopathy“. This enlightening session, available on this channel, delved into the critical need for high-quality, evidence-based case reports in homeopathy. (Link – https://youtu.be/399HlkzIGAo ) ##The Importance of Good Case Reports There is a…
#case record#cases in homeopathy#clinical research in homeopathy#homeopathic case reports#homeopathic guidelines for cases
0 notes
Text
Author Guidelines in Journal of Clinical & Medical Case Reports, Images (JCMCRI)
Authors submitting manuscripts to the Journal of Clinical & Medical Case Reports, Images (JCMCRI) are expected to adhere to the following ethical considerations:
Originality: The manuscript's content is original, and authors provide an author certificate and ethical statement confirming this. It has not been published or accepted for publication elsewhere, except for brief abstracts, communications, or conference proceedings.
Exclusivity: No portion of the submitted manuscript is being considered for publication elsewhere at the same time.
Review and Approval: The final version of the manuscript has been reviewed and approved by all authors, who have also signed an ethical statement form.
Permissions: Authors have obtained necessary permissions from their employers or universities to publish the submitted paper, and any copyrighted content included in the manuscript has been used with proper attribution and permission.
Image Manipulation: Authors adhere to CLIP principles regarding image manipulation and ensure that no unauthorized manipulation of images has occurred.
Any complaints regarding the violation of these ethical principles will be addressed according to the COPE flowchart.
Author Guidelines
JCMCRI aims to provide a platform for clinical and medical professionals, scientists, doctors, and academicians to publish the latest and emerging information, particularly focusing on unique, unusual, and rare cases that enhance the understanding of disease processes, diagnosis, and management in the field of medical sciences at an international level.
Review Process:
Manuscripts must be submitted exclusively to JCMCRI and should not have been previously published, accepted for publication elsewhere, or under consideration elsewhere.
After submission, manuscripts receive individual identification codes and undergo initial review by editors for suitability.
Manuscripts deemed suitable are sent for double-blind peer review by two or more expert reviewers.
Authors and reviewers remain anonymous to each other during the review process.
Authors receive reviewer comments and are directed to submit revised manuscripts along with a point-by-point response to reviewers' comments within the specified timeframe.
We prioritize speedy publication while ensuring a thorough review process.
Manuscript Templates: Please refer to the following manuscript templates for different types of submissions:
Case Reports
Word limit: up to 1500 words (excluding references) and abstract (150 words).
Sections: Abstract, Key-words, Introduction, Case report, Discussion, References, Tables, Legends.
Images should be submitted separately in jpg or jpeg format.
Case Series
Word limit: 1500 words for the main text and 200 words for the abstract (up to 15 references).
Includes reports involving more than 2 cases, retrospective or prospective.
Clinical Images
Limited to 3 images with a maximum of 200-300 words in the case or image description (3-5 references).
Mini Review
Word limit: not more than 3000 words.
Abstract: 150-250 words.
References: minimum of 15 to a maximum of 25, citing references within the past 5 years.
Editorial
Word limit: not more than 1000 words, with a minimum of 5 references and a maximum of 10.
Letter to the Editor
Word limit: not more than 1000 words, with a minimum of 5 references and a maximum of 10.
Authors can convey thoughts and raise concerns on recently published articles.
Guidelines for Manuscript Preparation:
Manuscripts should be in English and typewritten in 12-point Times New Roman font with one-inch margins.
Pages should be numbered consecutively on the top right corner, starting with the title page.
Follow the prescribed order for arranging the manuscript as per the provided template.
Authors are strongly encouraged to review the complete "Instructions for Authors" before submitting their manuscripts.
Title Page:
Type of manuscript (e.g., case report, images, letter to editor)
Title of the manuscript
Names of all authors/contributors with their highest academic degrees, designations, and affiliations
Department(s) and/or institution(s) to which the work should be credited
Corresponding author details including full address, email address, and phone number or mobile number
Abstract:
An unstructured abstract (not exceeding 300 words)
Keywords: 6-10 keywords related to the work
Introduction: Concise statement of the background, including relevant earlier work, suitably referenced
Case Report: Description of important materials and equipment, including manufacturer's name and location. Brief description of main clinical features and investigations
Discussion: Interpretation of results, logical presentation of the problem, and scope for further exploration
Conclusion
Summary of principal conclusions and wider implications
Authorship Criteria: All authors should contribute substantially to the concept/design of the study, acquisition/analysis/interpretation of data, drafting/revising the article, and final approval for publication.
Description of each author's contributions should be provided, with one author designated as 'guarantor'
Conflicts of Interest/Competing Interests:
Disclosure of any conflicts of interest with publication or mentioned institutions/products
Grant Information: Funding statement and sources of funding should be described
Acknowledgments: Recognition of acknowledgments and funding sources
References:
Numbered consecutively in order of first mention in the text
Identify references with Arabic numerals in square brackets
Journal titles should be abbreviated, except for non-indexed journals
Avoid using abstracts as references
Tables:
Each table should be headed with a short, descriptive caption.
Tables should be placed at appropriate locations within the text and numbered sequentially.
Tables should be formatted with horizontal lines only; vertical ruled lines are unnecessary.
Footnotes to tables should be indicated with a), b), c), etc., and typed on the same page as the table.
Figures:
Figures should be placed on separate pages, not inserted within the text.
All figures must be referred to in the text and numbered accordingly.
Photographs should be submitted as separate JPEG images.
Each figure must be accompanied by a legend explaining its contents.
Graphs and bar diagrams should preferably be prepared using Microsoft Excel and submitted as Excel graphs pasted in Word.
Keys to symbols, abbreviations, arrows, numbers, or letters used in the illustrations should be explained clearly in the legend, not on the illustration itself.
For more information regarding author guidelines: https://jcmcrimages.org/author-guidelines/
1 note
·
View note
Text
Ultimate Guide to Respiratory Tract Infections: Symptoms, Diagnosis, and Evidence-Based Treatments for URTIs and LRTIs
Upper Respiratory Tract Infections (URTIs) Introduction Respiratory tract infections (RTIs) encompass a wide range of conditions affecting the upper and lower respiratory tracts. They are common ailments that cause significant global morbidity and economic loss. This comprehensive guide covers everything you need to know about RTIs, from symptoms and diagnosis to evidence-based treatments and…
#Acute Bronchitis#Acute Bronchitis Management Guidelines#Bronchiolitis#Clinical Scores for Strep Throat#COPD Exacerbation#COPD Exacerbation Causes and Solutions#COVID-19 Symptoms#COVID-19 vs Influenza Symptoms#How to Treat Sinusitis and Pharyngitis#Lower Respiratory Tract Infections#Pharyngitis#Pneumonia#Pneumonia Diagnosis and Care#Respiratory Tract Infections#Respiratory Tract Infections Treatment#RSV Symptoms and Treatment#Sinusitis
0 notes