#birefringence
Explore tagged Tumblr posts
Text

Albarran Cabrera —– Instagram
Opticks
Polarized #55454 "Mountain and lake", Pigments, gampi paper and gold leaf
#polarized#abstract photography#birefringence#concrete art#straight photography#unmanipulated photography#albarrancabrera#albarran cabrera#artists on tumblr#photographers on tumblr
219 notes
·
View notes
Text





birefringence in calcite
338 notes
·
View notes
Text
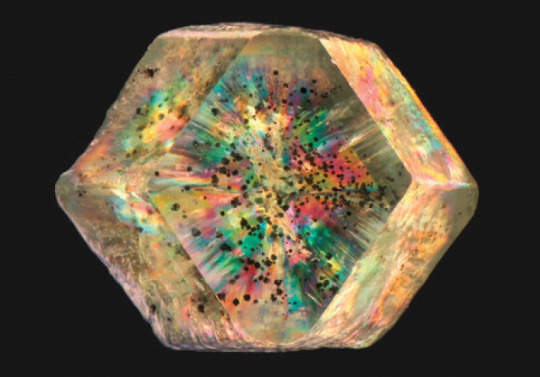
This demantoid is viewed through a polished window that is roughly parallel to the (110) crystal face. The sample was photographed with cross-polarized lighting and immersed in methylene iodide to high light the anomalous birefringence. The sample measures 8.8 mm across. Photomicrograph by Aaron Palke.
200 notes
·
View notes
Text

#alternative photography#scanography#original work#trippy#wallpaper#artistic photography#time#clock#birefringence
44 notes
·
View notes
Text
Birefringence Benefits
Shape of the white of the eye (the sclera) – at the back of the eyeball influences vision. Measuring changes in the scleral optical property called birefringence using a new tool called TRIPS-OCT brings promise of predicting short-sightedness and complications
Read the published research paper here
Still from video from work by Xinyu Liu and colleagues
Singapore Eye Research Institute, Singapore National Eye Centre, Singapore, Singapore
Video originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nature Biomedical Engineering, June 2023
You can also follow BPoD on Instagram, Twitter and Facebook
#science#biomedicine#immunofluorescence#neuroscience#biology#birefringence#sclera#eyeballs#eyesight#myopia#sciart
12 notes
·
View notes
Photo
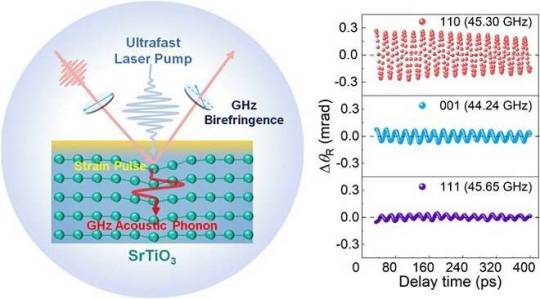
Coherent phonon-induced gigahertz optical birefringence realized in strontium titanate
Using ultrafast time-resolved pump detection technology, researchers led by Prof. Sheng Zhigao from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences have realized the gigahertz (GHz) frequency birefringence modulation induced by ultrafast coherent phonons in strontium titanate (SrTiO3) crystals.
According to the researchers, the operating frequency was found to be much higher than the cutoff frequency of the commercially available photoelastic modulators.
The study was published in Advanced Science.
A special material with birefringence can shape light. The photoelastic modulator based on birefringence modulation technology is one of the core components of modern optical technology. At present, most photoelastic modulators use the mechanical stress provided by piezoelectric materials to drive photoelastic crystals to achieve birefringence modulation, and their operating frequency is limited by the resonant frequency of photoelastic/piezoelectric crystals, which is generally in the order of kilohertz (kHz). Therefore, there is an urgent need to develop birefringent materials and modulation techniques with GHz operating frequency.
Read more.
13 notes
·
View notes
Text
i'm not saying i'm gonna write the sequel to An Opaque Heart but god, how do i keep coming up with ways to make their lives even worse
#🐍#is the engine of A Birefringent Mind me going 'hey wouldnt it be fucked up if'#i'm not saying the answer is yes#jokes aside i think the real reason i'm not chomping at the bit to write#i killed off so many characters i'd need to introduce a bunch of OCs to have a plot#mini squiggles
11 notes
·
View notes
Text

new silly just dropped. This is Optic Calcite Birefringe, she’s one of Selenite’s groupmates
yes I know the word calcite is missing an e
#rain world#rw#cam draws things#oc things#optic calcite birefringe#rw iterator oc#rw iterator#iterator oc
11 notes
·
View notes
Text
Pretty

#bunch of forams under cross polarized light#vpoc posting homework again... what has the world come to#SUPER bright birefringence though. very cool
2 notes
·
View notes
Text
7 exams down, 1 to go
#so the electrodynamics section was quite as awful as previous years#however he did include a question at the end about birefringence which isn't something we learnt in this course at all#we learnt about it in Optics which was in the other foundations of physics module#so at least I could write something#but I didn't add a note saying that it wasn't part of the course#I think I've done alright overall#I'm just glad this is over and I never have to do electrodynamics again#the final exam is on monday and is classical mechanics and quantum theory#panda's post
7 notes
·
View notes
Text

TSRNOSS. Page 241. Sorry about the break in continuity, but my files got jumbled, and this came up instead of 109, which latter is probably lurking somewhere in those files. I'll get around to finding it.
#plant#herbivore#growth stunting#saturated fatty acids#cholesterol production#protein intake#urinary calcium#flow birefringence#biotechnology#protein expression#cell death#tropics#bacterial burden#cursive#handwriting#theoretical biology#notebooks#manuscript#viscosity
0 notes
Text
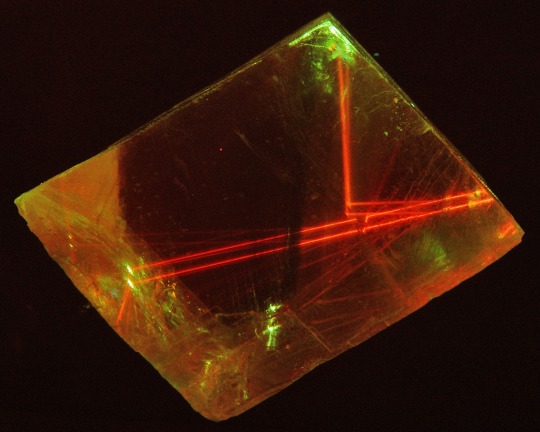
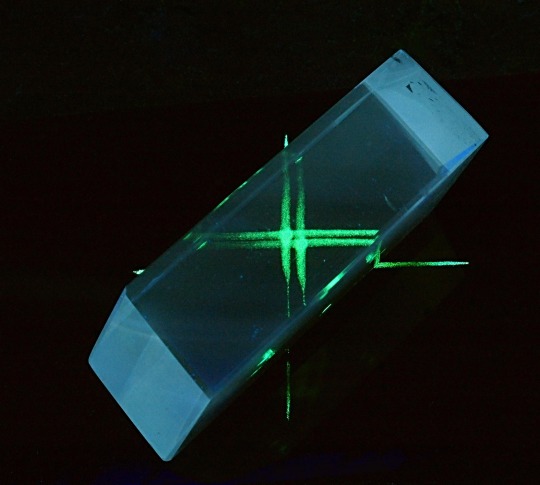
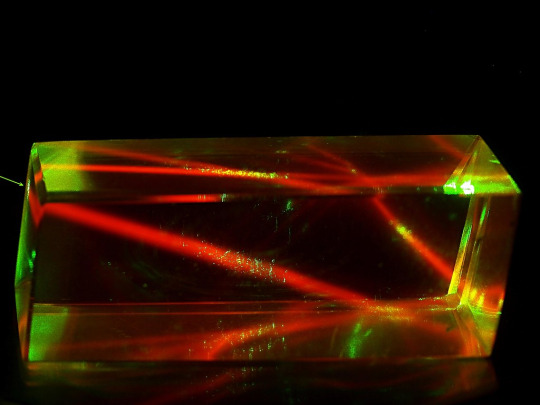

birefringence & fluorescence from lasers through calcite
#one beam enters. two beams leave. said like a wrestling announcer#lasers#calcite#birefringence#fluorescence#geology#gems
243 notes
·
View notes
Text
Birefringent Crystals
Birefringence is an optical property exhibited by materials whose refractive index varies with the polarization and direction of light propagation. These optically anisotropic crystal materials are said to be birefringent crystals. OST Photonics offers several birefringent crystals such as YVO4 and a-BBO crystals, etc.
What Are the Applications of Birefringent Crystal?
Birefringent crystal is an important photoelectric functional crystal material, which is widely used in the field of optical polarizer, optical modulation and nonlinear optical technology.
FAQS about Birefringent Crystals
What Is Birefringence?
When a beam of light strikes an interface of crystal, it typically generates two refracted beams, which is known as the phenomenon of birefringence.
Which Crystals Have Birefringence?
Calcite(CaCO3), YVO4, Alpha-BBO, Quartz, MgF2 and LiNbO3 crystals exhibit birefringence.

1 note
·
View note
Note
Do you have any neat calcites?
Do I ever!! Here are just a few of my favorites.

Here’s a specimen of huge brown barite crystals on a druzy of yellow calcite, from Elk Creek South Dakota.
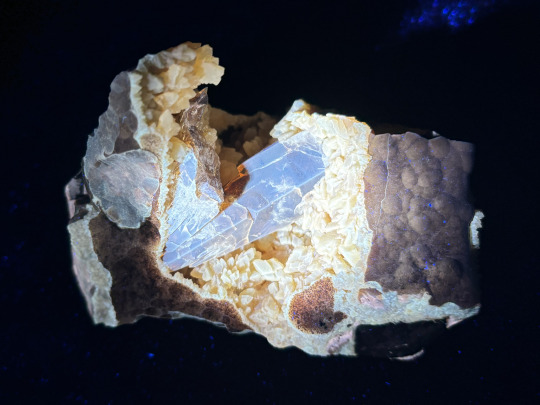
Under a long wave UV light (365nm), the calcite fluoresces yellow, and the barite fluoresces pale blue.

The barite also has very strong yellow-green phosphorescence, meaning it glows in the dark for a while after the UV light is removed!

This is mangano calcite, a form of calcite that gets its faint pink color from manganese.

This specimen features lenticular crystals and a cool “pagoda” formation!

And of course, here it is showing off what mangano calcite is most famous for: its gorgeous orange-pink fluorescence.

Here is some classic optical calcite! Although it’s often called "Iceland Spar", this particular specimen is from Mexico.

Optical calcite is known for its birefringence, an optical effect in which it doubles the image behind it! This is because calcite’s crystal structure polarizes the light passing through it, splitting it into horizontal and vertical wavelengths. All calcite technically does this; we can just see it happening in optical calcite because it is very clear and easy to see through.

This beautiful, water-clear specimen of dogstooth calcite crystals is from Linwood Mine in Iowa. It features very distinct phantoms!

Phantoms form when a thin layer of some other mineral begins growing on the surface of the crystal. As the calcite continues to grow, that layer becomes trapped inside of the crystal, becoming a faint record of the crystal’s size and shape when it was much younger! Calcite phantoms are especially interesting because their image is distorted by birefringence.

This piece is a columnar calcite from Fujian Provence, China! Note the uncommon column shapes of the crystals.

It fluoresces a lovely orange red!

And of course my favorite calcite of all! This specimen is cobaltocalcite from Morocco. While that hot pink color looks totally dyed and fake, it’s actually completely natural! Cobaltocalcite gets its distinctive color from atoms of cobalt in its molecular structure.
There's more calcite in my collection, but these are the best, I think!
347 notes
·
View notes
Photo
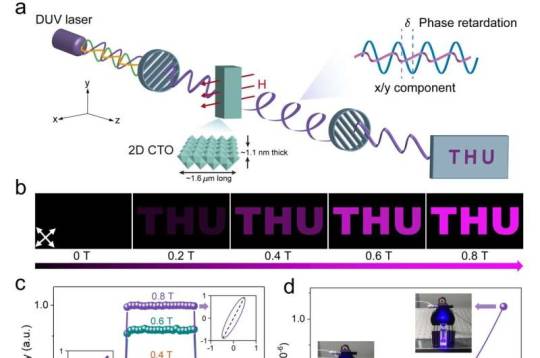
Deep-ultraviolet birefringent hydrogel based on 2D cobalt-doped titanate
A birefringence based light modulator that works in the wavelength region of < 350 nm plays a vital role in DUV beam shaping, high density data storage, semiconductor micro-nano processing and photolithography. Actually, a series of DUV birefringent materials, including single crystals of α-BBO, MgF2, Ca(BO2)2, and α-SnF2, have thus been made and commercially used. However, these birefringent elements have fixed birefringence, limiting their capability of continuous light modulation.
Liquid crystals (LCs) are another kind of birefringent materials, of which birefringence is tunable via the molecular alignment by external electrical or magnetic stimuli. Up to now, the commonly used LCs are mainly based on organic molecules or polymers, which are not stable under DUV light due to photochemical degradation effects. Meanwhile, DUV can also induce free radicals in some organic groups, and initiate their polymerization, which disorders the alignment and the resultant birefringence of LC. Therefore, organic LC cannot modulate DUV light.
In a new paper published in Light Science & Application, three teams of scientists, led by Professor Hui-Ming Cheng and Associate Professor Baofu Ding from Shenzhen Institute of Advanced Technology, CAS, Professor Wei Cai from Xi'an Research Institute of High Technology, Professor Bilu Liu from Tsinghua University, China, cooperatively synthesized two-dimensional (2D) inorganic cobalt-doped titanate (CTO) LC by using a wet chemical method. The 2D LC has large magnetic & optical anisotropy as well as high transmittance of > 70% in the wavelength of 300 ~ 350 nm, which enables the transmitted DUV modulation in a magnetic and portable way (Fig. 1).
Read more.
8 notes
·
View notes
Text
Those are absolutely beautiful!! And the different colors!! 🤩 is the blue caused by crystal defects then? Instead of a specific element?
Ohhhh I know nothing about zircon vs. cubic zirconia except, I assume cubic zirconia is in the cubic system? and zircon is… tetragonal? (if I remember correctly 😂) I’m definitely curious to know more!
Please know I would LOVE to see any microscope pictures!!!
And just for fun, this is the atlas of zircon textures (which I used to categorize my tiny zircons):
Some of the zoning is really pretty, especially in magmatic zircons.
just-thoughts-about-gems (this is the place to ask your gemstone questions)
#I think birefringence helped with differentiation between zircons and apatites?#but I don’t remember anything about DR#not to sound like a bad mineralogist#but i mainly used scanning electron microscopy for this project#instead of optical mineralogy#so i don’t remember much about the optical properties#ohhh I love rutile!! I’ve mainly seen it as an inclusion in quartz though#I have a big chunk that sits on my desk and the rutile sparkles when the sun hits it#also emerald inclusions? 👀#and thermochron is very fun!#i did thermal history modeling#using He profiles (since He is produced when U and Th decay)#and then the distribution of U and Th and He within individual grains#can be used to construct a thermal path#I had to learn A LOT about diffusion#but luckily I didn’t have to write the model 😂#also please know that I am so excited about these topics#anything you want to share I will gobble up#geology rocks#gemology time!
21 notes
·
View notes